Abstract
Prescriptive BW charts can facilitate discrimination between normal and abnormal birthweight. This study aimed to develop a prescriptive BW chart specific to Asian populations and assess its utility in predicting infant mortality. A retrospective cohort study was conducted using data from Taiwan National Health Insurance Research Database and National Birth Reporting Database. This study included 2 956 475 live-born singleton infants born to healthy mothers with uncomplicated pregnancies in Taiwan from January 1, 2004, to December 31, 2019. BW percentiles were estimated from GA 24–42 weeks by ranking the data in ascending order and calculating the percentile values based on the relative position of each observation within the dataset. Infant mortality rates were calculated for different GA groups, and optimal BW percentile cutoffs for predicting mortality were determined. A total of 2,255,989 infants (77.6%) from low-risk pregnancies were included in the development of the BW chart. Sex-specific BW percentiles were calculated. Optimal cutoff for predicting mortality were identified as follows: below the 22nd percentile or above the 96th percentile for extremely preterm infants (GA: 24–27 + 6 weeks), below the 11th percentile or above the 98th percentile for very preterm infants (GA: 28–31 + 6 weeks), below the 9th percentile or above the 99th percentile for moderately preterm infants (GA: 32–33 + 6 weeks), below the 8th percentile or above the 98th percentile for late preterm infants (GA: 34–36 + 6 weeks), and below the 7th percentile or above the 100th percentile for term infants (GA: > 37 weeks). A prescriptive BW chart was developed using data from a large population of Asian infants from low-risk pregnancies. BW percentiles were determined to predict infant mortality. Clinicians can utilize this approach to provide effective consultations to parents and improve decision-making processes.
Keywords: Birthweight, Fetal growth, Reference charts, Prescriptive BW chart, Risk factors, Small-for-gestational-age, Infant mortality
Subject terms: Medical research, Risk factors
Introduction
Newborn birth weight (BW) is a crucial metric in the fields of obstetrics and neonatology and is the most commonly used indicator of fetal growth in these fields1. Newborns can be classified into the following categories according to their BW: small for gestational age (SGA), appropriate for gestational age (AGA), or large for gestational age (LGA). An SGA newborn has a relatively high risk of neonatal complications, including neonatal asphyxia, meconium aspiration syndrome, hypoglycemia, bronchopulmonary dysplasia, and neurodevelopmental disorders2–5. An LGA newborn has a relatively high risk of obstetric complications, traumatic delivery, hypoglycemia, hyperbilirubinemia, polycythemia, neonatal asphyxia, longer hospital stay, and neonatal death6,7. Over time, both SGA and LGA infants are at risk of metabolic syndrome, arterial hypertension, dyslipidemia, coronary disease, and type 2 diabetes mellitus8–12. Currently, most researchers and clinicians use BW in the 10th percentile as a cutoff for SGA and use BW exceeding the 90th percentile as a cutoff for LGA. However, using BW at the 10th percentile as the sole cutoff for classifying SGA in all gestational age may not always be optimal to identify high risk infants13,14. Therefore, we analyzed our data and try to identify neonates who may be at high risk of adverse outcomes based on infant mortality, across different gestational age groups.
To differentiate between typical BW and abnormal BW, BW charts must be developed. A descriptive chart, also known as a reference or population chart, can be developed using data obtained from an unselected population without the exclusion of individuals with risk factors for intrauterine growth restriction. By contrast, a prescriptive chart describes standard for fetal size by using data obtained from a low-risk population that has been carefully selected by adhering to specific exclusion criteria15. Studies have demonstrated that the implementation of prescriptive BW charts can enhance the identification of infants who are at a relatively high risk of unfavorable neonatal outcomes16.
Existing prescriptive BW charts have limitations in terms of their population representation. For example, a study conducted in the Netherlands focused primarily on infants of Dutch descent, with limited representation of infants of other ethnicities, including Asians17. However, infant BW may be influenced by environmental and genetic factors. For example, the BW standards in the United States differ from those in China18,19. Besides, in Taiwan, the existing BW standards for Taiwanese infants are based on data from a relatively small sample of newborns born between January 1, 1998, and December 31, 200220. Given these limitations, it is crucial to develop a new and more representative BW standard for Taiwanese infants.
The present study developed a prescriptive BW chart by using the data of newborns from low-risk pregnancies in Taiwan. This chart was used to evaluate the optimal BW percentiles for identifying infant mortality in various gestational age (GA) groups.
Materials and methods
This retrospective cohort study used data from the Taiwan National Birth Reporting Database (NBRD) and the National Health Insurance Research Database (NHIRD). The NBRD was established in 1995 and has been published online since 2004. The NBRD includes data on all live births and stillbirths that are delivered in hospitals or clinics; data on these births are reported by medical professionals. GA was estimated on the basis of the date of the last menstrual period and early ultrasonography performed by an obstetrician. For this study, we used birth data for the period 2004–2019, which were released for research purposes by the Taiwan Health Promotion Administration. The NHIRD is maintained by the National Health Insurance Administration and contains the claims data from the National Health Insurance program, which was established on March 1, 1995, and provides universal coverage to 99.9% of Taiwan’s 23.69 million residents. We used the unique identification (ID) numbers assigned to each resident in Taiwan to establish links between the 2 nationwide databases. The studies involving human participants were reviewed and approved by The Institutional Review Board of Taichung Veterans General Hospital, No. CE23059C (Date of approval: February 27, 2023).
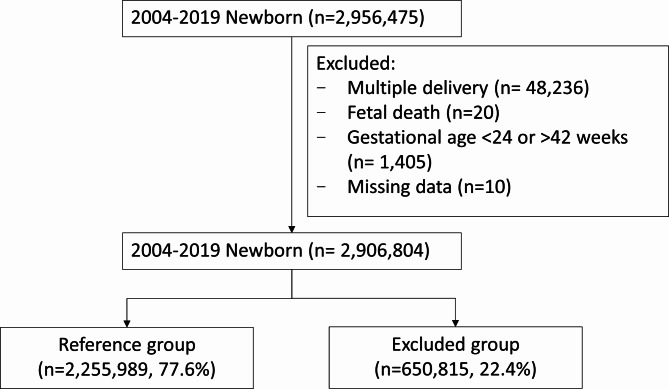
In this study, we analyzed the data of newborns who were delivered between January 1, 2004, and December 31, 2019, and had a GA between 24 and 42 weeks. We excluded stillbirths, newborns from multiple gestations, and newborns with missing data (eg, missing BW, GA, or sex data; Fig. 1). The raw data of all newborns and their mothers were analyzed. We excluded newborns born to mothers with risk factors for SGA or excessive fetal growth (650,815); the risk factors included hypertensive disorders (31,104), diabetes (56,855), other preexisting maternal medical conditions (433,048), and medical conditions related to the pregnancy (96,679). Additionally, we excluded newborns with congenital diseases (201,343). Finally, the low-risk study population (2,255,989) comprised live-born singleton infants born to healthy mothers who had uncomplicated pregnancies. The data of this population were used to develop a prescriptive BW chart. We calculated BW percentiles for male and female infants separately from the 24th week. We compared our BW chart with the INTERGROWTH-21st chart and the chart developed by Hoftiezer et al. (hereafter referred to as the Netherlands chart).
Fig. 1.
Flowchart of study population selection. Conditions of the mothers in the excluded group: diabetes mellitus (n = 56 885); hypertension (n = 31 104); and other maternal medical conditions: hyperlipidemia (n = 54 015), chronic kidney disease (n = 1446), seizure (n = 10 007), cerebrovascular accident (n = 7373), psychiatric disease (n = 57 191), human immunodeficiency virus (n = 322), malignant neoplasm (n = 14 774), uterine malformation (n = 285), obesity (n = 15 372), systemic lupus erythematosus (n = 7891), rheumatoid arthritis (n = 7563), Sjogren syndrome (n = 17 659), ankylosing spondylitis (n = 10 518), psoriatic arthritis/psoriasis (n = 10 896), systemic sclerosis (n = 349), dermatomyositis (n = 1233), polymyositis (n = 611), autoimmune thyroiditis (n = 8630), antiphospholipid syndrome (n = 922), ulcerative colitis (n = 1568), Crohn disease (n = 30 242), thyroid disease (n = 154 313), juvenile idiopathic arthritis (n = 531), and anemia (n = 19 337). Congenital disorders in newborns in the excluded group: Congenital disorders (201 343): congenital malformation (n = 198 480), and inborn errors of metabolism (n = 2863). Medical conditions related to pregnancy (96 679): gestational hypertension (n = 4089), pre-eclampsia or eclampsia (n = 9759), gestational diabetes mellitus (n = 39 498), placenta previa or abruptio placentae (n = 39 736), placenta accrete (n = 394), polyhydramnios (n = 1570), and TORCH infection (n = 1633).
Using our derived BW chart, we calculated BW percentiles for estimating infant mortality rates in the following GA groups: extremely preterm (GA: 24–27+6 weeks), very preterm (GA: 28–31+6 weeks), moderately preterm (GA: 32–33+6 weeks), late preterm (GA: 34–36+6 weeks), and term (GA: ≥ 37 weeks). The optimal BW threshold for determining SGA and LGA in each GA group was calculated according to infant mortality. We used the Youden Index21 to identify the optimal BW threshold; the optimal BW threshold was determined to be the value that could maximize the Youden index (ie, the value that could maximize sensitivity + specificity – 1)22.
Statistical analysis
All data were analyzed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). A P value of < 0.05 was considered to indicate statistical significance. We estimated the BW distribution for male and female newborns separately. The BW percentiles calculated for every week of gestation starting from the 24th week. Percentiles were calculated by ranking the data in ascending order and determining the position of each value relative to the dataset. The percentile rank was computed using the formula 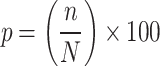 , where n is the rank and N is the total number of observations. Baseline characteristics were analyzed using descriptive statistics. Chi-square test was used to compared between reference and excluded group. The Youden Index can be defined as J = maxc
, where n is the rank and N is the total number of observations. Baseline characteristics were analyzed using descriptive statistics. Chi-square test was used to compared between reference and excluded group. The Youden Index can be defined as J = maxc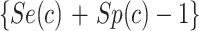 , where J ranges from 0 (indicating complete overlap of biomarker distributions for the diseased and healthy populations) to 1 (indicating complete separation of biomarker distributions for the diseased and healthy populations). Moreover, c* represents the criterion for choosing the optimal threshold value21.
, where J ranges from 0 (indicating complete overlap of biomarker distributions for the diseased and healthy populations) to 1 (indicating complete separation of biomarker distributions for the diseased and healthy populations). Moreover, c* represents the criterion for choosing the optimal threshold value21.
Results
Prescriptive BW chart
We included a total of 2,906,804 infants born in Taiwan between 2004 and 2019 in this study. However, we excluded 650,815 infants (22.4%) because of high-risk pregnancy factors; thus, the remaining 2,255,989 (77.6%) infants constituted our low-risk population for our analysis, and we used their data to develop our prescriptive BW chart. Table 1 presents the baseline characteristics of the study population and the excluded population. Compared with the study population, the excluded population contained higher proportions of infants who were born to mothers aged > 35 years; were delivered through cesarean section; were born preterm; had low BW; and had high perinatal mortality, neonatal mortality, and infant mortality rates.
Table 1.
Characteristics of study population.
| Characteristic | Reference group | Excluded group | Total (n = 2,906,804) | P value |
|---|---|---|---|---|
| (n = 2,255,989, 77.6%) | (n = 650,815, 22.4%) | |||
| n (%) | n (%) | n | ||
| Maternal age | < 0.001 | |||
| > 35 | 1,865,784 (82.7) | 484,833 (74.5) | 2,350,617 | |
| ≥ 35 | 390,205 (17.3) | 165,982 (25.5) | 556,187 | |
| Year | < 0.001 | |||
| 2004–2011 | 1,145,010 (50.8) | 286,277 (44) | 1,431,287 | |
| 2012–2019 | 1,110,979 (49.2) | 364,538 (56) | 1,475,517 | |
| Gender | < 0.001 | |||
| Female | 1,092,683 (48.4) | 301,211 (46.3) | 1,393,894 | |
| Male | 1,163,306 (51.6) | 349,604 (53.7) | 1,512,910 | |
| Delivery method | < 0.001 | |||
| Vaginal delivery | 1,531,110 (67.9) | 385,852 (59.3) | 1,916,962 | |
| Cesarean section | 724,879 (32.1) | 264,963 (40.7) | 989,842 | |
| Gestational age, wk | < 0.001 | |||
| 24–27 + 6 | 1817 (0.1) | 3408 (0.5) | 5225 | |
| 28–31 + 6 | 5740 (0.3) | 7324 (1.1) | 13,064 | |
| 32–33 + 6 | 9764 (0.4) | 7790 (1.2) | 17,554 | |
| 34–36 + 6 | 118,790 (5.3) | 55,351 (8.5) | 174,141 | |
| ≥ 37 | 2,119,878 (94) | 576,942 (88.6) | 2,696,820 | |
| Birthweight, g | < 0.001 | |||
| < 1000 | 1749 (0.1) | 3616 (0.6) | 5365 | |
| 1000–1999 | 17,922 (0.8) | 17,768 (2.7) | 35,690 | |
| 2000–2999 | 836,347 (37.1) | 254,471 (39.1) | 1,090,818 | |
| 3000–3999 | 1,364,258 (60.5) | 361,453 (55.5) | 1,725,711 | |
| ≥ 4000 | 35,713 (1.6) | 13,507 (2.1) | 49,220 | |
| Perinatal mortality | < 0.001 | |||
| Death 24 h | 18 (0) | 9 (0) | 27 | |
| Death 2–7 days | 16 (0) | 7 (0) | 23 | |
| Death 8–28 days | 1541 (0.1) | 605 (0.1) | 2146 | |
| Death 29–365 days | 3080 (0.1) | 2460 (0.4) | 5540 | |
| Death > 365 days | 2313 (0.1) | 1827 (0.3) | 4140 | |
| Alive | 2,249,021 (99.7) | 645,907 (99.2) | 2,894,928 |
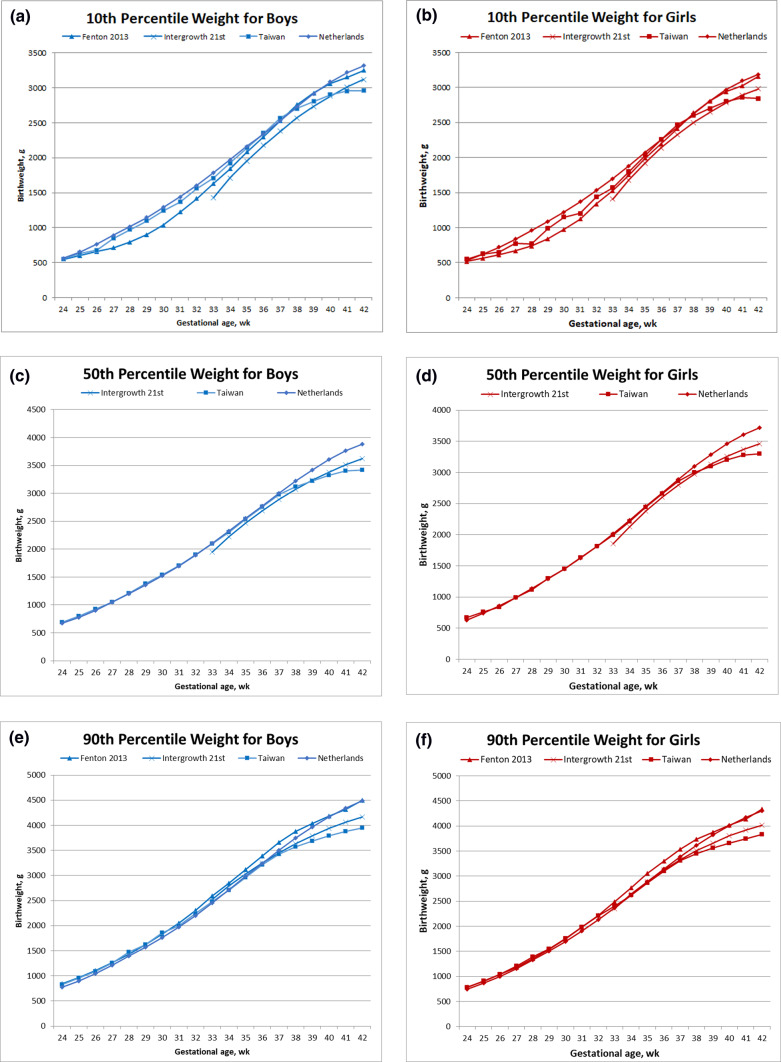
We created sex-stratified BW percentile graphs (3rd, 10th, 50th, 90th, and 97th percentiles) from our study population (Fig. 2). The results revealed that as GA increased, the percentile curves exhibited a steady and smooth rise. Moreover, at each GA, male infants exhibited higher BW values than did female infants in all percentile curves. The highest BW gains occurred between 36 and 37 weeks of gestation, and the BW growth rates decelerated after 37 weeks.
Fig. 2.
New Taiwan birthweight charts vs alternative weight charts. Comparison of proposed Taiwan birth weight chart, stratified by birth weight percentiles for male and female infants, with other charts developed using data of low-risk infants. (A) 10th percentile weight for boys; (B) 10th percentile weight for girls; (C) 50th percentile weight for boys; (D) 50th percentile weight for girls; (E) 90th percentile weight for boys; (F) 90th percentile weight for girls.
Comparison of BW chart with previously published charts
Figure 2 illustrates comparisons of our BW chart, stratified by BW percentiles for male and female infants, with the INTERGROWTH-21st and Netherlands charts. We observed that at GAs of 24–37, our 10th, 50th, and 90th percentile BW values for both male and female infants exhibited similar trends to those in the Netherlands chart. However, after a GA of 37 weeks, our percentile BW values were than those in the Netherlands chart. Furthermore, we determined that at GAs of < 37 weeks, our 10th and 50th percentile BW values for both male and female infants were higher than those in the INTERGROWTH-21st chart; however, after a GA of 40 weeks, the BW values in our chart were lower. We also noted that before a GA of < 37 weeks, our 90th percentile BW values were similar to those in the INTERGROWTH-21st chart; nevertheless, after a GA of 38 weeks, our chart exhibited smaller values than those in the INTERGROWTH-21st chart.
Association between birth weight percentiles and infant mortality
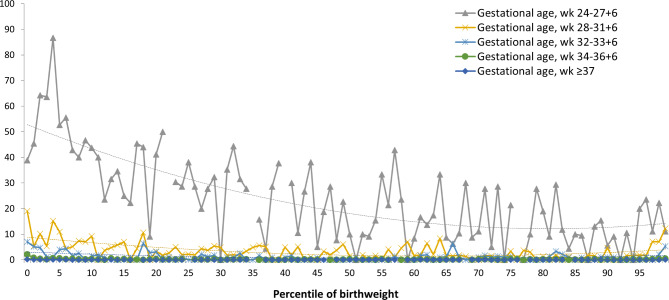
Figure 3 presents infant mortality rates calculated according to the BW percentiles for each GA group. The infant mortality rate was highest in the extremely preterm group and decreased gradually as GA increased. The mortality rate curve derived for each GA group exhibited a “reversed-J” shape. In addition, we observed that BW below the 22nd percentile (OR: 2.14; 95% CI: 1.62–2.82) or above the 96th percentile (OR: 2.67; 95% CI: 1.21–5.89) was the optimal cutoff for detecting infant mortality in the extremely preterm group. We also noted that BW below the 11th percentile (OR: 3; 95% CI: 3.0–2.1) or above the 98the percentile (OR: 5.81; 95% CI: 2.54–13.11) was the optimal cutoff for detecting infant mortality in the very-preterm group. BW below the 9th percentile (OR: 4.78; 95% CI: 2.91–7.86) or above the 99th percentile (OR: 7.9; 95% CI: 2.63–23.75) was the optimal cutoff for detecting infant mortality in the moderately preterm group. Moreover, BW below the 8th percentile (OR: 5.56) or above the 98th percentile (OR: 4.03; 95% CI: 1.75–9.3) was the optimal cutoff for detecting infant mortality in the late-preterm group. Finally, BW below the 7th percentile (OR: 3.75; 95% CI: 3.07–4.59) or above the 100th percentile (OR: 3.69; 95% CI: 2.09–6.51) was the optimal cutoff for detecting infant mortality or LGA, respectively, in the term group. Sex-stratified analysis was also done, and result was showed in Table 2.
Fig. 3.
Infant mortality between different gestational age groups.
Table 2.
BW percentile cutoffs stratified by sex.
| Gestational age, week/group | BW percentile cutoffs | aOR | 95% CI | |
|---|---|---|---|---|
| 24–27 + 6 | Total | < 22 | 2.14 | 1.62–2.82 |
| > 96 | 2.67 | 1.21–5.89 | ||
| Male | < 33 | 2.87 | 2.1–3.91 | |
| > 96 | 0.71 | 0.32–1.57 | ||
| Female | < 22 | 4.57 | 3.26–6.41 | |
| > 96 | 0.51 | 0.19–1.33 | ||
| 28–31 + 6 | Total | < 11 | 3.0 | 2.1–4.29 |
| > 98 | 5.81 | 2.54–13.31 | ||
| Male | < 11 | 4.15 | 2.55–6.75 | |
| > 94.9 | 1.12 | 0.51–2.45 | ||
| Female | < 16 | 2.56 | 1.65–3.97 | |
| > 98 | 5.24 | 2.64–10.4 | ||
| 32–33 + 6 | Total | < 9 | 4.78 | 2.91–7.86 |
| > 99 | 7.9 | 2.63–23.75 | ||
| Male | < 13 | 3.07 | 1.6–5.87 | |
| > 98.9 | 4.14 | 1.41–11.52 | ||
| Female | < 9 | 3.59 | 2.06–6.23 | |
| > 97 | 2.17 | 0.77–6.09 | ||
| 34–36 + 6 | Total | < 8 | 5.56 | 3.9–7.91 |
| > 98 | 4.03 | 1.75–9.3 | ||
| Male | < 8 | 5.98 | 3.91–9.14 | |
| > 96 | 1.43 | 0.69–2.96 | ||
| Female | < 10 | 4.89 | 3.16–7.57 | |
| > 95 | 1.13 | 0.41–3.09 | ||
| > 37 | Total | < 7 | 3.75 | 3.07–4.59 |
| > 100 | 3.69 | 2.09–6.51 | ||
| Male | < 6 | 5.32 | 4.14–6.82 | |
| > 89.9 | 0.78 | 0.57–1.08 | ||
| Female | < 11 | 3.28 | 2.55–4.22 | |
| > 100 | 4.19 | 2.15–8.16 | ||
Discussion
We developed a prescriptive BW chart by using the data of a large population of Taiwanese infants from low-risk pregnancy women, and we calculated the optimal BW percentiles for predicting infant mortality in various GA groups. According to our review of the literature, this is the first such prescriptive BW chart based on a large cohort of Asian newborns.
Numerous BW charts have been developed worldwide. Experts have attempted to develop BW charts that are applicable to all infants and can predict mortality and morbidity. Nevertheless, considerable debate exists regarding the inclusion criteria for infants in a specific population and whether specific groups of infants should be evaluated using specific charts. Previous studies have demonstrated that prescriptive BW charts can be used to accurately predict clinically significant adverse outcomes in infants with SGA23,24. For example, Hoftiezer et al. developed a prescriptive BW chart by using the data of singleton infants in the Netherlands (2000–2014). However, previous studies have not developed prescriptive BW charts for Asian infants. To address this gap, we developed our prescriptive BW chart by using the data of a large cohort of Asian infants.
Our BW chart differs considerably from a previously developed BW chart for Taiwanese infants in a previous study20. For example, our chart was based on data obtained from a larger population (2,956,475 vs 1,308,558) and over a longer assessment period when compared with the previously developed BW chart (2004–2019 vs 1998–2002). Furthermore, the male infants in our study population had lower BW values in the 10th, 50th, and 90th percentiles at a GA of 40 weeks than did the infants in the previous study population (2900, 3320, and 3790 g vs 2914, 3374, and 3890 g, respectively); the female infants in our study population also had lower BW values in the 10th, 50th, and 90th percentiles at 40 weeks of gestation than did those in the previous study population (2800, 3200, and 3660 g vs 2816, 3250, and 3747 g, respectively). Previous studies conducted in other countries have also reported trends of decreased BW25,26. For example, a study conducted in Korea revealed that mean BW has decreased with a gradual increase in advanced maternal age (≥ 35 years old) since 199327. A study conducted in Taiwan analyzed 66,135 women who gave birth between 2011 and 2016 and demonstrated that the prevalence of low BW increased from 5.3 to 7% with increased maternal age and incidence of inadequate gestational weight gain28. The male-to-female ratio in our original study population (1.085) is similar to that in the population used in the previous study (range: 1.088–1.096). After excluding high-risk patients, we observed that the male-to-female ratio in our study population decreased to 1.064. Research has demonstrated that male infants have higher morbidity than do female infants29,30, which may explain why we excluded more male infants than female infants from our study population.
The INTERGROWTH-21st project introduced an international BW standard in 2014, which was established using the data of low-risk newborns from 8 urban populations across different locations31. This project demonstrated that when the nutritional and health needs of mothers are fulfilled and environmental factors that may inhibit fetal growth are minimized, fetal growth and newborn length exhibit similarities across different geographical locations32. Although the INTERGROWTH-21st BW chart, the Netherlands BW chart, and our BW were all developed using data from populations of low-risk infants, these charts differ. For example, our BW chart provides similar values to those of the Netherlands BW chart before a GA of 37 weeks, but our chart provides lower values after a GA of 37 weeks. In addition, our BW chart provides slightly higher values than those of the INTERGROWTH-21st chart before a GA of 38 weeks, but our chart provides lower values after that. A possible reason for the difference in BW values between our chart and the INTERGROWTH-21st chart is the difference in racial characters between the study populations. A previous study observed substantial natural variations in fetal growth and BW between countries, even after accounting for differences in the duration of pregnancy33. In 2018, Yao et al.18 proposed a new BW chart based on a population of newborns from southern China (Guangdong Province) and compared their chart with the INTERGROWTH-21st chart. When developing their chart—a conventional chart—they did not use the data of all newborns, and they did not calculate specific BW percentiles. Nevertheless, they reported that the BW values provided by their chart are lower than those provided by the INTERGROWTH-21st chart and that the BW values in Guangdong are even lower than the national average in China18. Our study population has similar racial characteristics to the population in Guangdong. This may explain why our BW values are smaller than those in the INTERGROWTH-21st and Netherlands charts at term.
Unlike the study of Hoftiezer et al., we did not exclude infants whose mothers underwent provider-initiated deliveries. This is because we assumed that most of the iatrogenic preterm births were associated with other risk factors for abnormal fetal growth, which had been excluded for other reasons. Regarding term pregnancy, a recent study verified that adverse outcomes did not increase significantly for newborns delivered after elective induction of labor at term34.
We observed that the curves of infant mortality rates calculated according to the BW percentiles for each GA group exhibited a “reversed-J” curve. In the term group, we noted that infants whose BWs were in or below the 7th percentile had a higher risk of infant mortality. But infants with larger BW did not associate with increased infant mortality. Previous studies have also reported significantly higher infant mortality in term SGA infants (< 10th percentile) than in term AGA infants (25th to 74th percentiles)35,36. A Swedish study that included term and postern nonmalformed singleton infants found that infants whose BWs were below the 3rd percentile and those whose BWs were between the 3rd and 10th percentiles had 2- and 1.5-fold higher adjusted risks of infant mortality, respectively, than did AGA infants36. However, after adjusting for mother disease and birth history, the mentioned study reported that risk of infant mortality in infants whose BWs were between the 90th and 97th percentiles or above the 97th percentile did not increase when compared with that in AGA infants. These findings agree with our results.
Regarding mortality rates in preterm-born infants, our results reveal that in extremely preterm infants, BW below the 22nd percentile or above the 96th percentile is associated with a high rate of infant mortality; in very-preterm infants, BW below the 11th percentile or above the 98th percentile is associated with a high rate of infant mortality; in moderately preterm infants, BW below the 9th percentile or above the 99th percentile is associated with a high rate of infant mortality; and in late-preterm infants, BW below the 8th percentile or above the 98th percentile is associated with a high rate of infant mortality. This trend indicates that in extremely preterm and early preterm infants, infant health may be more severely affected by immaturity and its consequences than by low BW for GA. Previous studies have shown that preterm SGA infants have a considerably higher risk of early neonatal and postneonatal mortality than do preterm AGA infants in low- to middle-income countries and in low– to high–Human Development Index (HDI) countries37,38. However, the findings of research from some countries with very high HDI scores revealed that the infant mortality rate did not differ significantly between preterm SGA infants and preterm AGA infants35. Our data from Taiwan, a high-HDI country with a high rate of in utero transport to level III maternity units, are similar to those of studies from other high-HDI countries. Even if immediate, high-quality neonatal care is available, the negative impact of immaturity and its associated effects on infant health may be more severe than that of low BW for GA.
Study strengths and limitations
One strength of our study is that we used a large sample, enabling reliable estimates of percentiles, even for the extreme values of BW and GA. By using data from the NBRD and NHIRD, which contain information on up to 99.99% of Taiwan’s population, we could obtain population-level data to provide real-world evidence that can support clinical decision-making39. We excluded numerous potential risk factors for abnormal fetal growth, which may help us effectively distinguish between normal and abnormal BWs. Moreover, our BW chart is the first such prescriptive chart based on a large Asian population. This chart can enable clinicians to compare the BWs of Asian infants with those of infants from different populations in order to effectively provide clinical care for specific individuals. The cutoff point for detecting infant mortality in various GA groups can also help clinicians and parents effectively make decisions regarding the health of their babies. However, Infant mortality is multifactorial although we adjusted mother age, delivery method and baby gender, the result still require further validation. Furthermore, while this chart provides valuable insights for Taiwanese infants, additional research is necessary to validate its applicability in other Asian populations before generalizing the findings more broadly.
Despite its strengths, this study has some limitations. First, in our BW chart, we did not account for parity or maternal conditions such as previous birth history, body mass index, weight gain, or smoking status, which may affect neonatal outcomes. However, according to a report from the Adult Smoking Behavior Surveillance System, the smoking rate in women aged 18 years or older in Taiwan was 2.3–5.1% from 2004 to 2020, and half of women quit smoking when they become pregnant39. Hence, our findings may not have been affected by smoking status owing to the low-rate smoking in women in Taiwan. Second, we did not investigate the effects of paternal age and health status. Several studies have demonstrated that both maternal and paternal preconception health conditions and behaviors affect infant birth outcomes40,41. However, we observed that most relevant studies have not included paternal data. Third, we did not exclude newborns conceived from assisted reproductive technology (ART) pregnancies. Concerns about poor perinatal outcomes in newborns from ART pregnancies may be due to patient selection or maternal complications, such as preeclampsia or placenta problems, which were not accounted for in our study. Moreover, the effects of ART itself on BW are unknown. Therefore, further research is warranted to compare ART-conceived newborns with those conceived through traditional methods.
Conclusion
We developed a prescriptive BW chart by employing the data of a large population of Taiwanese infants without risk factors for SGA or LGA; this is the first such chart based a large population of Asian infants. This chart can help clinicians identify infants who are at risk of negative clinical outcomes. We also estimated BW percentiles for predicting infant mortality in different GA groups, which can enable clinicians make more precise estimates of neonatal survival on the basis of infant BW and GA. Our chart and findings could aid obstetricians, neonatologists, and parents in making informed decisions, particularly in cases where preterm delivery is expected. However, while our chart provides valuable insights for Taiwanese infants, further research is necessary to validate the findings in other Asian populations before generalizing this prescriptive birth weight chart to a broader Asian context.
Author contributions
J.-C.C., Y.-J.C. and C.-H.L. wrote the main manuscript text. I.-C.C. and C.-T.L. prepared figures and tables. Y.-C.L. and Y.-M.C. collect the data. W.-S.L. do the statistics. All authors reviewed the manuscript.
Funding
This study was funded by a grant from Taichung Veterans General Hospital Research Fund (Registration number TCVGH-1127303C, TCVGH-112G211, and TCVGH-YM1120103, TCVGH-113G211, TCVGH-1137315C, TCVGH-YM1130105, TCVGH-NHRI11301, TCVGH-NCHU1137618).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Institutional Review Board, TCVGH, No. CE23059C Date of approval: February 27, 2023.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilcox, A. J. On the importance-and the unimportance of birthweight. Int. J. Epidemiol.30, 1233–1241. 10.1093/ije/30.6.1233 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Graz, M. B., Tolsa, J. F. & Fumeaux, C. J. Being small for gestational age: does it matter for the neurodevelopment of premature infants? A cohort study. PLoS ONE10, e0125769. 10.1371/journal.pone.0125769 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nobile, S., Marchionni, P. & Carnielli, V. P. Neonatal outcome of small for gestational age preterm infants. Eur. J. Pediatr.176(8), 1083–1088. 10.1007/s00431-017-2957-1 (2017). [DOI] [PubMed] [Google Scholar]
- 4.McIntire, D. D., Bloom, S. L., Casey, B. M. & Leveno, K. J. Birth weight in relation to morbidity and mortality among newborn infants. N. Engl. J. Med.340(16), 1234–1238. 10.1056/NEJM199904223401603 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Iughetti, L., Lucaccioni, L. & Ferrari, F. Challenges in the development and growth of small for gestational age newborns. Expert Rev. Endocrinol. Metab.12(4), 253–260. 10.1080/17446651.2017.1338137 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Bérard, J. et al. Fetal macrosomia: Risk factors and outcome. A study of the outcome concerning 100 cases >4500 g. Eur. J. Obstet. Gynecol. Reprod. Biol.77(1), 51–59. 10.1016/s0301-2115(97)00242-x (1998). [DOI] [PubMed] [Google Scholar]
- 7.Weissmann-Brenner, A. et al. Maternal and neonatal outcomes of large for gestational age pregnancies. Acta Obstet. Gynecol. Scand.91(7), 844–849. 10.1111/j.1600-0412.2012.01412.x (2012). [DOI] [PubMed] [Google Scholar]
- 8.Harder, T., Rodekamp, E., Schellong, K., Dudenhausen, J. W. & Plagemann, A. Birth weight and subsequent risk of type 2 diabetes: A meta-analysis. Am. J. Epidemiol.165(8), 849–857. 10.1093/aje/kwk071 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Yu, Z. B. et al. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obes. Rev.12(7), 525–542. 10.1111/j.1467-789X.2011.00867.x (2011). [DOI] [PubMed] [Google Scholar]
- 10.Hales, C. N. et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ303(6809), 1019–1022. 10.1136/bmj.303.6809.1019 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker, D. J., Winter, P. D., Osmond, C., Margetts, B. & Simmonds, S. J. Weight in infancy and death from ischaemic heart disease. Lancet2(8663), 577–580. 10.1016/s0140-6736(89)90710-1 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Barker, D. J., Osmond, C., Golding, J., Kuh, D. & Wadsworth, M. E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ298(6673), 564–567. 10.1136/bmj.298.6673.564 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulet, S. L., Alexander, G. R., Salihu, H. M., Kirby, R. S. & Carlo, W. A. Fetal growth risk curves: Defining levels of fetal growth restriction by neonatal death risk. Am. J. Obstet. Gynecol.195(6), 1571–1577. 10.1016/j.ajog.2006.03.069 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Wilcox, A. J., Cortese, M., McConnaughey, D. R., Moster, D. & Basso, O. The limits of small-for-gestational-age as a high-risk category. Eur. J. Epidemiol. (2021). [DOI] [PMC free article] [PubMed]
- 15.Ananth, C. V., Brandt, J. S. & Vintzileos, A. M. Standard vs population reference curves in obstetrics: Which one should we use?. Am. J. Obstet. Gynecol.220(4), 293–296. 10.1016/j.ajog.2019.02.060 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Ferdynus, C. et al. Can birth weight standards based on healthy populations improve the identification of small-for-gestational-age newborns at risk of adverse neonatal outcomes?. Pediatrics123(2), 723–730. 10.1542/peds.2007-2564 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Hoftiezer, L. et al. From population reference to national standard: New and improved birthweight charts. Am. J. Obstet. Gynecol.220(4), 383.e1-383.e17 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Yao, F., Miao, H., Li, B., Wu, Y. & Zhao, Q. New birthweight percentiles by sex and gestational age in Southern China and its comparison with the INTERGROWTH-21st Standard. Sci. Rep.8(1), 7567. 10.1038/s41598-018-25744-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duryea, E. L., Hawkins, J. S., McIntire, D. D., Casey, B. M. & Leveno, K. J. A revised birth weight reference for the United States. Obstet. Gynecol.124(1), 16–22. 10.1097/AOG.0000000000000345 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Hsieh, W. S. et al. Nationwide singleton birth weight percentiles by gestational age in Taiwan, 1998–2002. Acta Paediatr. Taiwan47(1), 25–33 (2006). [PubMed] [Google Scholar]
- 21.Youden, W. J. Index for rating diagnostic tests. Cancer3(1), 32–35. 10.1002/1097-0142(1950)3:1%3c32::aid-cncr2820030106%3e3.0.co;2-3 (1950). [DOI] [PubMed] [Google Scholar]
- 22.Greiner, M., Pfeiffer, D. & Smith, R. D. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med.45(1–2), 23–41. 10.1016/s0167-5877(00)00115-x (2000). [DOI] [PubMed] [Google Scholar]
- 23.Hoftiezer, L., Hukkelhoven, C. W., Hogeveen, M., Straatman, H. M. & van Lingen, R. A. Defining small-for-gestational-age: Prescriptive versus descriptive birthweight standards. Eur. J. Pediatr.175(8), 1047–1057. 10.1007/s00431-016-2740-8 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Hoftiezer, L., Snijders, R. G., Hukkelhoven, C. W. P. M., van Lingen, R. A. & Hogeveen, M. Prescriptive birthweight charts can improve the prediction of adverse outcomes in very preterm infants who are small for gestational age. Acta Paediatr.107(6), 981–989. 10.1111/apa.14243 (2018). [DOI] [PubMed] [Google Scholar]
- 25.OECD. Health at a Glance 2017: OECD Indicators (OECD Publishing, 2017). 10.1787/health_glance-2017-en. [Google Scholar]
- 26.Martin, J. A., Hamilton, B. E., Osterman, M. J., Driscoll, A. K. & Mathews, T. J. Births: Final data for 2015. Natl. Vital Stat. Rep.66(1), 1 (2017). [PubMed] [Google Scholar]
- 27.Kim, H. E., Song, I. G., Chung, S. H., Choi, Y. S. & Bae, C. W. Trends in birth weight and the incidence of low birth weight and advanced maternal age in Korea between 1993 and 2016. J. Korean Med. Sci.34(4), e34. 10.3346/jkms.2019.34.e34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waits, A., Guo, C. Y. & Chien, L. Y. Inadequate gestational weight gain contributes to increasing rates of low birth weight in Taiwan: 2011–2016 nationwide surveys. Taiwan J. Obstet. Gynecol.60(5), 857–862. 10.1016/j.tjog.2021.07.013 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Shim, S. Y., Cho, S. J., Kong, K. A. & Park, E. A. Gestational age-specific sex difference in mortality and morbidities of preterm infants: A nationwide study. Sci. Rep.7(1), 6161. 10.1038/s41598-017-06490-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu, S. T. et al. Nationwide birth weight and gestational age-specific neonatal mortality rate in Taiwan. Pediatr. Neonatol.56(3), 149–158. 10.1016/j.pedneo.2014.07.006 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Villar, J. et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet384(9946), 857–868. 10.1016/S0140-6736(14)60932-6 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Villar, J. et al. The likeness of fetal growth and newborn size across non-isolated populations in the INTERGROWTH-21st Project: The Fetal Growth Longitudinal Study and Newborn Cross-Sectional Study. Lancet Diabetes Endocrinol.2(10), 781–92. 10.1016/S2213-8587(14)70121-4 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Kiserud, T., Piaggio, G., Carroli, G., Widmer, M., Carvalho, J., Neerup Jensen, L., Giordano, D., Cecatti, J. G., Abdel Aleem, H., Talegawkar, S. A., Benachi, A., Diemert, A., Tshefu Kitoto, A., Thinkhamrop, J., Lumbiganon, P., Tabor, A., Kriplani, A., Gonzalez Perez, R., Hecher, K., Hanson, M. A., Gülmezoglu, A. M. & Platt, L. D. The World Health Organization fetal growth charts: A multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med.14(1), e1002220 (2017). 10.1371/journal.pmed.1002220. Erratum in: PLoS Med. 2017 Mar 24;14 (3):e1002284. Erratum in: PLoS Med. 2017 Apr 20;14 (4):e1002301. Erratum in: PLoS Med. 2021 Jan 7;18(1):e1003526
- 34.Grobman, W. A. & Caughey, A. B. Elective induction of labor at 39 weeks compared with expectant management: A meta-analysis of cohort studies. Am. J. Obstet. Gynecol.221(4), 304–310. 10.1016/j.ajog.2019.02.046 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Premru-Srsen, T. et al. Infant mortality and causes of death by birth weight for gestational age in non-malformed singleton infants: A 2002–2012 population-based study. J. Perinat. Med.46(5), 547–553. 10.1515/jpm-2017-0103 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Altman, M., Edstedt Bonamy, A. K., Wikström, A. K. & Cnattingius, S. Cause-specific infant mortality in a population-based Swedish study of term and post-term births: The contribution of gestational age and birth weight. BMJ Open2(4), e001152. 10.1136/bmjopen-2012-001152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katz, J. et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: A pooled country analysis. Lancet382, 417–425 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ota, E. et al. Risk factors and adverse perinatal outcomes among term and preterm infants born small-for-gestational-age: Secondary analyses of the WHO Multi-Country Survey on Maternal and Newborn Health. PLoS ONE9, e105155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, P. C. Introduction to the National Health Insurance of Taiwan. In Digital Health Care in Taiwan (eds Lee, P. C. et al.) (Springer, 2022). [Google Scholar]
- 40.Moss, J. L. & Harris, K. M. Impact of maternal and paternal preconception health on birth outcomes using prospective couples’ data in Add Health. Arch. Gynecol. Obstet.291(2), 287–298. 10.1007/s00404-014-3521-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murugappan, G. et al. Association of preconception paternal health and adverse maternal outcomes among healthy mothers. Am. J. Obstet. Gynecol. MFM3(5), 100384. 10.1016/j.ajogmf.2021.100384 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.