Abstract
Chagas disease following infection with Trypanosoma cruzi is a major public health issue, with the disease spreading beyond endemic regions and becoming more global due to the migration of infected individuals. The currently available anti-parasitic drugs, nifurtimox and benznidazole, remain insufficiently evaluated for their efficacy in adult patients. A key challenge is the lack of markers for parasitological cure, which also precludes the development of new treatments. Consequently, there is a critical need for a practical method to assess drug performance within a short timeframe. In this retrospective analysis of the phase 2 randomized controlled BENDITA trial (ClinicalTrials.gov: NCT03378661), we report the potential of a serological multiplex method (MultiCruzi), combined with advanced statistical analytical methods, to measure the response to anti-parasitic treatment of adult Chagas patients. Applying this approach to serum samples from adult patients in the indeterminate chronic stage of Chagas disease, treated with different benznidazole regimens and combinations, we predict treatment efficacy after just 6 months of follow-up, in sharp contrast to data obtained with conventional and recombinant T. cruzi ELISA tests. The obtained results are also compared with the PCR data. We propose integrating MultiCruzi as a serological method endpoint in proof-of-concept clinical trials for Chagas disease.
Subject terms: Parasitic infection, Diagnostic markers, Phase II trials, ELISA
Here, using samples from a randomized controlled trial, the authors show that a multiplex immunoassay (MultiCruzi), paired with statistical analysis, can predict early treatment efficacy in adult Chagas patients, suggesting that MultiCruzi could serve as an endpoint in future clinical trials.
Introduction
Chagas disease, or American trypanosomiasis, is a chronic, life-threatening disease caused by the protozoan parasite Trypanosoma cruzi (T. cruzi) and is considered a neglected tropical disease by the World Health Organization (WHO)1,2. Endemic in 21 Latin American countries, it has spread globally due to migration, affecting an estimated 6 to 7 million people worldwide2–4. The disease has acute and chronic phases. The acute phase usually presents mild symptoms or is asymptomatic, with the parasite replicating, and circulating in the bloodstream. This phase transitions into the chronic indeterminate form, where the parasite becomes hardly detectable and tightly controlled by the immune system. Around 30% of individuals develop severe complications, such as cardiomyopathy and digestive disorders, which can lead to sudden death5–7.
Current treatments are limited to nifurtimox (NF) and benznidazole (BZN)8, which have high toxicity and severe side effects, especially in adults9–12. To circumvent these tolerability issues, alternative drug regimens and combinations are under investigation, including fosravuconazole (E1224) alone or in combination with benznidazole13,14. One major challenge in drug development for Chagas disease is the lack of reliable markers to assess parasitological cure in a timely manner15–17. Polymerase chain reaction (PCR), often used in Phase II trials, has variable sensitivity and specificity due to the low and cyclic parasitemia during the indeterminate form of chronic Chagas disease18,19. The United States Food and Drug Administration (FDA) recommends serological testing for blood donors and the use of two different serological tests in paediatric trials to determine effectiveness of a new trypanocide therapy20,21. Seroreversion, the disappearance of antibodies/Immunoglobulins G against T. cruzi, is the only marker of parasitological cure accepted by health authorities as waning antibodies is associated with parasite clearance8,22–24, and this endpoint was used to register both benznidazole and nifurtimox for the treatment of children25,26. However, it takes decades in adults for antibodies to fully disappear from the blood stream27–29, making current serological tests not feasible for establishing parasite clearance and not adequate for drug development.
Great progress has been made in epitope and serological biomarkers research30. Moreover, high-performance serology tests were developed with overall high sensitivity and specificity, though field performance varies29. Therefore, the WHO recommends using two serological tests to diagnose chronic infection8, which can lead to underdiagnosis due to discordant results27. There is an urgent need for serology tests and other markers or combinations thereof that can be used to assess drug efficacy in a timely manner. This would allow for appropriate development timelines of new drugs, the follow-up and interpretation of clinical trial data, and the improved care / counselling of patients through the assessment of cure soon after therapy22.
The MultiCruzi assay, a multiplex antibody serology test, has proven effective in confirming Chagas disease and predicting parasitological cure earlier than conventional tests in infants and children with acute or early chronic Chagas disease31–33. This study aims to apply the MultiCruzi method to adults with chronic Chagas disease, using serial dilutions and advanced statistical analytical methods to identify response to treatment early during follow-up13.
Results
Samples tested
Samples from the phase II double-blind randomized BENDITA trial were assessed in this study13. Patients with sera samples at the three timepoints: baseline (before treatment), 6 months, and 12 months post-treatment, were included in this analysis and assayed using MultiCruzi. Following curation of the database, eight patients were excluded in the analysis as they either discontinued the study early or withdrew their consent; samples from 201 patients were included corresponding to 6 treatment arms and placebo: (1) 150 mg of Benznidazole daily for 4 weeks, (2) 300 mg of Benznidazole daily for 2 weeks, (3) 300 mg of Benznidazole daily for 4 weeks, (4) 300 mg of Benznidazole daily for 8 weeks, (5) 150 mg of Benznidazole daily for 4 weeks plus fosravuconazole, (6) 150 mg of Benznidazole daily for 8 weeks plus fosravuconazole and (7) Placebo. See Supplementary Fig. 1 for more details.
Dilution Method with MultiCruzi allows Antibody Quantification
The MultiCruzi assay was initially developed to confirm infection by measuring the T. cruzi antibody levels in serum samples. It is a multiplex composed of fifteen T. cruzi antigens printed onto 96-well plates, that generates an overall signature of all the antigens detected32,33. The assay was originally optimized to yield robust signals with sera from infected subjects, while producing negligible or no background signals with sera from uninfected subjects. This design inherently limits the ability to detect the waning or declining of antibodies over time due to signal saturation with seropositive samples. To effectively monitor and quantify a decrease in antibody titers, and ultimately seroreversion, in patients’ samples over time, we employed a previously established dilution method. This method ensures that at least one antibody measurement falls within the quantifiable range34. The relationship between the colorimetric intensity for each biomarker and the dilution factor is characteristically sigmoidal, displaying plateaus at both low and high signal intensities. Quantification is most reliable within the linear portion of these curves. At the extremes of the signal spectrum, both at low and high ranges, minor shifts in signal intensity are unfit for accurately estimating changes in antibody levels. This imprecision is largely due to the possibility that small fluctuations may stem from measurement noise rather than genuine changes in antibody concentrations.
The dilution factors were carefully selected to ensure overlap in the linear ranges for all antigens, to enable quantification at maximum concentrations and to minimize the number of dilutions necessary. This process was previously described in more detail34. Dilutions of 1/50, 1/400, and 1/3200 were found to be optimal for all fifteen antibodies assessed with the MultiCruzi assay.
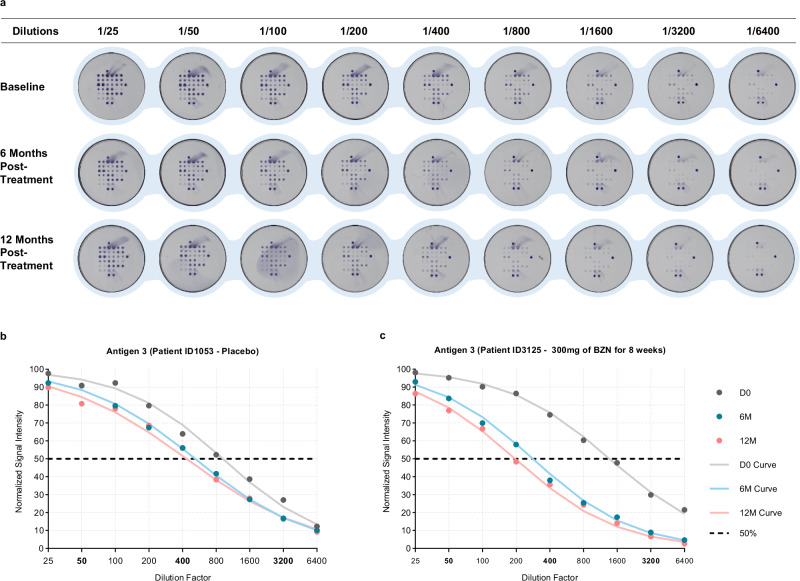
Following sample dilution (Fig. 1a), antibody reactivities were converted into an arbitrary dilution factor (DF), a surrogate for the antibody concentration. A DF50 score corresponds to the dilution at which a signal intensity value of 50% of the maximal signal would be observed. This value summarizes the 3 dilution sequence antibody intensities (Supplementary Note 1). Although the unit of DF50 is arbitrary, its relationship with concentration is linear. The scores were calculated for each timepoint (Baseline, 6 months, and 12 months of follow-up post-treatment). The assumption is that a treatment effect will lead to a shift in the sigmoidal dose-response curve of the dilution series to lower dilution factors. Figure 1 shows an example for Antigen 3 reactivity in patients treated with either (b) placebo or (c) benznidazole (BZN). This means that the DF50-value decreases over time when antibodies start waning (Supplementary Fig. 2a,b). Another example shows the effect of treatment on the reactivity of antigen 10 using the dilution method (Supplementary Fig. 3a, b).
Fig. 1. Dilution method showing the effect of treatment on the reactivity of antigen.
a Well images of serum samples diluted at 1/25, 1/50, 1/100, 1/200, 1/400, 1/800, 1/1600, 1/3200, 1/6400. These samples were collected at Baseline, 6 months and 12 months after the start of treatment with 300 mg of Benznidazole for 8 Weeks (patient ID3125). The curves showing the effect of treatment of the reactivity of Antigen 3 for (b) patient ID1053 and (c) patient ID3125. D0, Day 0; 6 M, 6 months following treatment; 12 M, 12 months following treatment; DF50, Dilution Factor 50 at which 50% of the original reactivity remains. Bold numbers indicate the 3 selected dilution factors: 50, 400 and 3200. Each plate is imaged and analyzed using a colorimetric reader. An integrated software calculates the pixel intensity for each spot. To establish the net intensity for each antigen, the mean value of the duplicated spots is considered. Net signals correspond to the signal measured from each spot from which the background is subtracted.
This DF50 factor was then used in the analysis to study the variability or decrease in each of the treatment groups (Supplementary Note 2) using a linear mixed model (LMM) (Supplementary Note 3).
Identifying antibody decline over time using a random intercept linear mixed model
Following dilution of the clinical samples, a random intercepts linear mixed model analysis was performed for each individual antigen present on MultiCruzi to determine the antibodies’ reactivity over time for each treatment regimen. We then plotted the linear predictor versus time for the seven treatment arms obtained (See Supplementary Fig. 4 for Antigen 11 as an example). Taking Antigen 11 as an example, the slopes of most treatment arms for this specific antigen (except for 300 mg BZN for 4 weeks, 300 mg BZN + E1224 for 8 weeks, and placebo) are significantly different from zero (p < 0.05) (Supplementary Table 1). All slopes are negative, indicating that the sigmoidal curve shifts to lower dilution factors (DF50) over time, representative of a decline in antibody titer over time compared to baseline. In this case, the decline rate or slope is significantly lower than zero (p ≤ 0.05) for Antigen 11 after treatment with 150 mg BZN for 4 weeks (−0.1617, p = 0.0045), 300 mg BZN for 2 weeks (−0.2584, p < 0.0001), 300 mg BZN for 8 weeks (−0.1678, p = 0.0044), and 150 mg BZN + E1224 for 4 weeks (−0.1971, p = 0.0010). On the other hand, the slope in the placebo group is only slightly negative (−0.00518) meaning that there is also a slight decrease in antibody against Antigen 11 over time, although this is not significantly different from zero (p = 0.9271) (Supplementary Note 3).
The slopes of all treatment groups were then compared to the slope of the placebo group (arm 7). Arms 2 (BZN—300 mg for 2 Weeks) (p = 0.0018), 4 (BZN—300 mg for 8 Weeks) (p = 0.0464) and 5 (BZN—150 mg + E1224 for 4 Weeks) (p = 0.0200) showed significant differences in their decline rate (p < 0.05) as compared to the placebo (Supplementary Table 2).
Random intercept linear mixed models (LMM) with nested antigens
The variability (the decrease, in this study) in log2(DF50) in each of the 7 treatment arms was studied with a nested LMM, using time, treatment and the interaction of time with treatment as covariables, considering about 30 patients in each treatment group, 3 timepoints (D0, 6 M, 12 M) per patient, and 15 antigens for each patient and timepoint. The advantage of a nested model is that it keeps the connection between the patient and the antigens, and allows one analysis for the combined antigens, thus increasing the sample size and consequently the power to detect possible treatment effects.
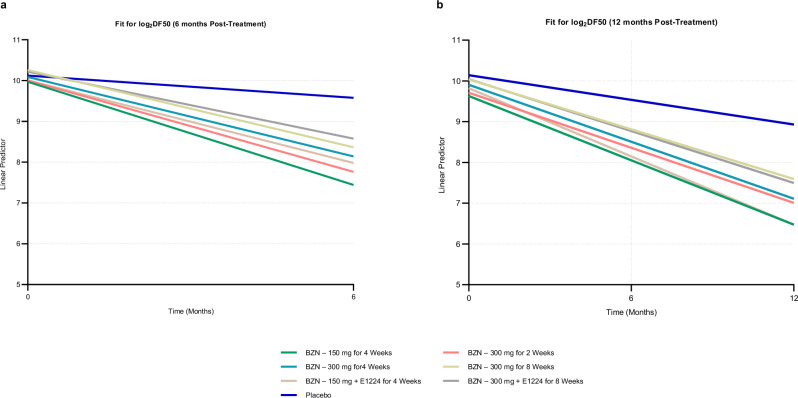
The linear predictor (log2DF50) versus time, for the 7 treatments arms, was obtained at 6 months (Fig. 2a) and 12 months (Fig. 2b) following the start of treatment.
Fig. 2. Linear predictor for the fit of the Log2-based DF50.
The Log2DF50 was modelled with a longitudinal random intercept nested Linear Mixed Model, against time, treatment and the interaction of time and treatment, taking into account 2 (baseline and 6 months) or 3 (baseline, 6 and 12 months) timepoints, nesting 15 antigens per patient, and 30 patients in each of 7 treatment subgroups. Nested models keep the connection between the patient and the antigens. a At 6 months, and (b) At 12 months after the start of treatment. Log2 Binary logarithm, DF50 Dilution Factor 50 at which 50% of the original reactivity remains, BZN Benznidazole.
At 6 months of follow-up, the slopes of all treatment groups, except for placebo, were significantly different from 0 with a p < 0.0001 (Fig. 2a and Supplementary Table 3) and the slopes of all the treatment groups were significantly different from the slope of the placebo group, with p ranging between <0.0001 and 0.0007.
At 12 months post follow-up, the slopes of all treatment groups were clearly negative and significantly different from zero (p < 0.0001), including the slope for the placebo group (−0.02766, p = 0.0221) (Fig. 2b and Supplementary Table 4). Slopes of all the treatment groups were significantly more negative than the slope of the placebo group (p < 0.0001), indicating that the antibodies decline more rapidly in treated patients than in patients administered the placebo.
Slopes of all treatment groups were then compared to the slope of the placebo group (arm 7) at 6 and 12 months after start of treatment. At both timepoints, all treatment regimens showed significant differences expressing significant decline rates (p < 0.05) as compared with the placebo (Supplementary Table 5a and Supplementary Table 5b). In addition, non-overlapping 95% Confidence Intervals (CI) of the treatment slopes with the Placebo’s slope at 6 (Supplementary Fig. 5a) and 12 months after treatment (Supplementary Fig. 5b) confirm that all treatment groups have slopes significantly different from those of the Placebo group. However, treatment groups do not have slopes different from each other.
Interpretation algorithms and optimal threshold for the slope
To identify a response to treatment, an individual should show a decline in antibodies (slope < 0) but preferably faster than in the placebo group. Therefore, a specific threshold can be defined by conducting a receiver operating characteristic (ROC) analysis comparing the slopes of the treatment groups versus the slopes in the placebo group.
For each of the 15 antigens (AG), a ROC analysis on the DF50 slopes was performed using the treated versus placebo arms to obtain the best threshold for the slope, in terms of sensitivity (S) and specificity (Sp), based on the optimal Youden index S + Sp – 1 (Supplementary Note 4, Supplementary Table 6).
Alternatively, the slopes of the antigens can be considered collectively. We propose setting an overall threshold for all antigen slopes, requiring that 50% of the antigens reactive at baseline meet this threshold. We adhered to this 50% requirement, but this algorithm can be adjusted to be more lenient or stringent based on the percentage of initially reactive antigens that must decrease by a predefined amount or percentage change and the threshold for this change.
To determine the optimal threshold for distinguishing between treatment and placebo patients, we performed a ROC analysis comparing the slope of the placebo group to the slopes of the combined six treatment regimens (Fig. 3). The analysis yielded an area under the curve (AUC) of 0.737 and identified a cut-off of −0.025, corresponding to the optimal Youden index (maximum of sensitivity + specificity − 1). Moreover, as the cut-off slope is −0.025 over the 12 month period, then:
| 1 |
Fig. 3. The ROC curve for the placebo versus treated groups analysis.
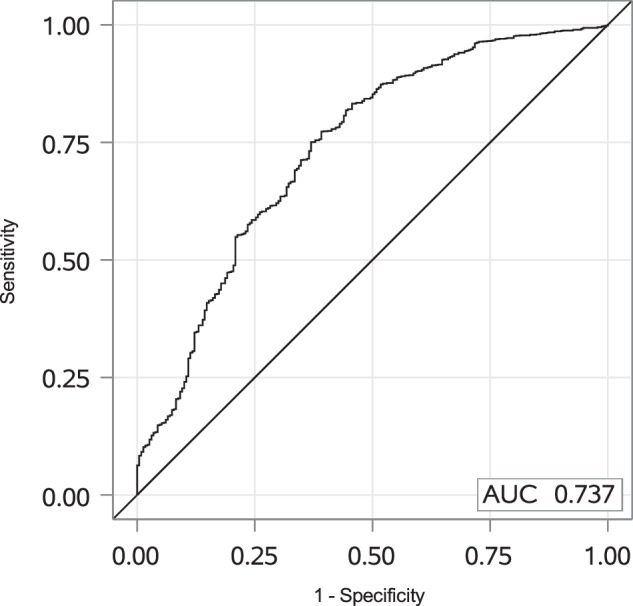
The slopes for log2DF50 against time (D0, 6 M, 12 M) were calculated for each patient and each antigen and pooled for all treatment groups. The ROC analysis was performed on the slopes from the placebo group versus the treated groups. ROC Receiver Operating Characteristic.
Thus, a change of −0.3 between baseline and 12 month of log2DF50 could be used to define the response to treatment for each originally reactive antigen.Therefore, in order to make a clinical decision for each patient, the number of reactive antigens (N) at baseline was calculated, then, the DF50 value of each of the 15 antigens was calculated, at each timepoint: baseline, 6 months and 12 months. The evolution of antibody load at each of the follow-up timepoints was then compared to the baseline by calculating the (t is equal to 6 or 12 months) value. The number of antigens (Nt) having a change superior to −0.3 at 12 months was calculated and compared to N. Then the following set of rules were proposed as individual interpretation algorithm for the 12 months follow-up:
If is equal or higher than 0.5, the result is “Response to Treatment”. The patient shows a response to the anti-parasitic treatment.
If is equal to 0.3 or between 0.3 and 0.5, the result is “Inconclusive”. The patient should be rechecked at a further timepoint to give a definitive result.
If is <0.3, the result is “No Response to Treatment”. The patient did not respond to the treatment according to the test.
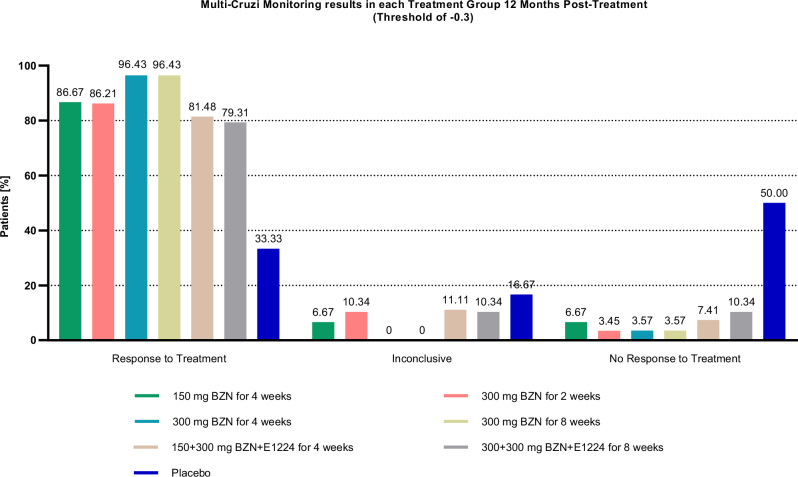
Using this analysis, the percentage of patients with “Response to Treatment” (Table 1), “Inconclusive” and “No Response to Treatment” were calculated in each treatment regimen (Fig. 4). The number of patients per treatment arm does not allow the comparison between treatments. After 12 months of follow-up, 150/171 (87.21%) patients treated with benznidazole ± E1224 were identified to respond to treatment by MultiCruzi as compared to 10/30 (33.33%) patients in the placebo group (p < 0.0001).
Table 1.
Proportions of patients found to have a ‘Response to Treatment’ per treatment group using a threshold of −0.3
| Dosing regimen | Total Number of patients | Patients with “Response to Treatment” |
|---|---|---|
| BZN—150 mg for 4 Weeks | 30 | 26 (86.67%) |
| BZN—300 mg for 2 Weeks | 29 | 25 (86.21%) |
| BZN—300 mg for 4 Weeks | 28 | 27 (96.43%) |
| BZN—300 mg for 8 Weeks | 28 | 27 (96.43%) |
| BZN—150 mg + E1224 for 4 Weeks | 27 | 22 (81.48%) |
| BZN—300 mg + E1224 for 8 Weeks | 29 | 23 (79.31%) |
| Placebo | 30 | 10 (33.33%) |
BZN Benznidazole; (1) BZN—150 mg for 4 Weeks: samples collected from individuals treated with 150 mg of Benznidazole daily for 4 weeks; (2) BZN—300 mg for 2 Weeks: samples collected from individuals treated with 300 mg of Benznidazole daily for 2 weeks; (3) BZN—300 mg for 4 Weeks: samples collected from individuals treated with 300 mg of Benznidazole daily for 4 weeks; (4) BZN—300 mg for 8 Weeks: samples collected from individuals treated with 300 mg of Benznidazole daily for 8 weeks; (5) BZN—150 mg + E1224 for 4 Weeks: samples collected from individuals treated with 150 mg of Benznidazole daily for 4 weeks plus fosravuconazole; (6) BZN—300 mg + E1224 for 8 Weeks: samples collected from individuals treated with 150 mg of Benznidazole daily for 8 weeks plus fosravuconazole and (7) Placebo: samples collected from individuals treated with Placebo. The proportions of patients were calculated from the slope of −0.025 times the 12 month period, at a log ratio threshold of the DF50 of −0.3.
Fig. 4. The MultiCruzi monitoring results in each treatment Group, 12 Months after the start of treatment.
The result per patient was generated according to the defined algorithm and log2DF50 ratio threshold of −0.3, the percentage of each patients’ result (“Response to Treatment”, “Inconclusive” and “No Response to Treatment”) was then calculated per treatment arm. BZN Benznidazole.
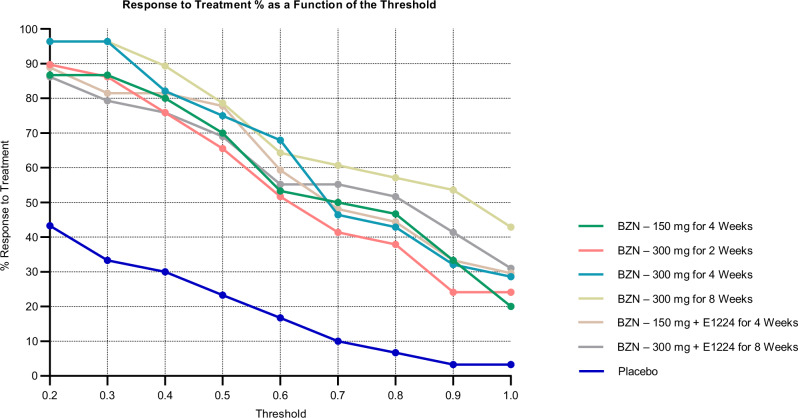
The same analysis was performed with the different thresholds for log2DF50 change between baseline and 12 month (Supplementary Table 7). The fixed threshold has an impact on the outcome, notably the proportion of patients with ‘response to treatment’. However, the difference between the treatment groups and the placebo group remains large across all cut-offs (Fig. 5). Moreover, considering these proportions, using a threshold for the slope of −0.3 (Supplementary Fig. 6a) and of −0.7 (Supplementary Fig. 6b), non-overlapping 95% CIs of the treatment slopes with the Placebo’s slope at 12 months after treatment indicate that all treatment groups have slopes significantly different from the slope of the Placebo group. However, treatment groups do not have slopes different from each other.
Fig. 5. The percentage of patients responsive to treatment in function of the threshold for log2DF50 decrease between baseline and 12 months.
At all thresholds, the level of patients responsive to treatment in the Placebo group is significantly lower than in the treatment groups. The threshold is calculated according to the following formula: . For each patient, the number of antigens (Nt) with a change superior to the fixed threshold at 12 months was calculated and compared to the number of reactive antigens (N) at baseline. The results are set according to the following conditions: if , ; if , “Inconclusive”; if , “No Response to Treatment”. BZN Benznidazole.
Results with conventional and recombinant ELISA
The trend of anti-T. cruzi antibodies was measured at two timepoints (baseline and 12 months after start of treatment) using enzyme-linked immunosorbent assays (ELISA) serology tests.
For both conventional and recombinant ELISAs, the optical densities remain above the threshold defined according to the manufacturer’s instructions, with no significant decrease in the mean reactivity of samples 12 months later in all treatment groups, including placebo, with conventional ELISA (Supplementary Fig. 7). With the recombinant ELISA, there was an insignificant decrease of the anti-T. cruzi antibody levels 12 months after the start of treatment in all but two groups: patients treated with either placebo or 300 mg BZN + 300 mg E1224 (Supplementary Fig. 8).
As previously described for children, a threshold for declaring patients to be cured was set to at least 20% reduction in mean optical density measured in the two conventional ELISA tests at 12 months compared with baseline27.
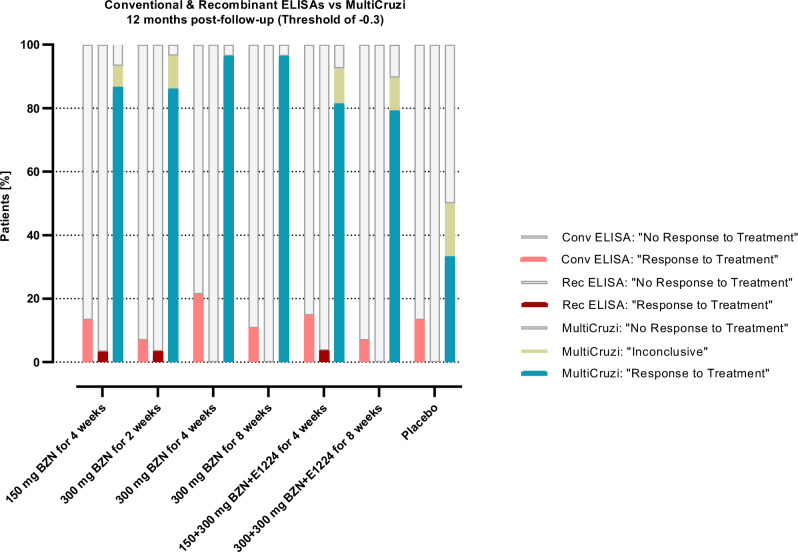
The results obtained with MultiCruzi at a threshold of −0.3 were then compared to those obtained with conventional and recombinant commercial ELISA tests after applying the threshold of at least 20% reduction in mean optical density. At 12 months compared to baseline, MultiCruzi shows the highest percentage of “Response to Treatment” in treated patients as compared to the placebo group with the lowest number of patients responding to their assigned treatment (Fig. 6, Supplementary Table 8).
Fig. 6. Percentage of patients with “Response to Treatment”, “Inconclusive” and “No Response to Treatment” results revealed by conventional, recombinant and MultiCruzi ELISA tests at 12 months following treatment.
For the conventional and recombinant ELISA, the percentage was calculated by setting a threshold of minimum 20% in seroreduction at 12 months post-treatment. For MultiCruzi, the threshold for the decrease of the log2DF50 is −0.3. Conv Conventional, Rec Recombinant, BZN Benznidazole.
The nested linear mixed model shows the average decline in antibodies with time per treatment group, keeping the connection between the patient and the antigens. The model shows a significantly faster decline in antibodies in the treatment groups compared to the placebo group. Similarly, a linear mixed model for the conventional ELISAs was calculated. No significant difference in slopes between the treated groups and the placebo group was found.
In summary, the nested LMM with MultiCruzi data shows significant differences in slopes between treatment and placebo groups after just 6 months, and this is confirmed and sustained after 12 months in sharp contrast to traditional ELISA tests that do not show any such significant decrease.
Comparison of the MultiCruzi results with the PCR data
Despite the difference in the outcomes measured by MultiCruzi and PCR (MultiCruzi measures a decline in antibodies as a sign of treatment response while PCR determines treatment failure by measuring the presence of T. cruzi DNA in the blood), we made an attempt to assess the concordance between both methods across the different thresholds for
| 2 |
In this context, the “Response to Treatment Agreement” was defined as the number of patients with “Response to Treatment” obtained from the algorithm at the mentioned threshold as well as “Parasitological Clearance” according to PCR across all treatment groups and placebo, divided by the number of patients with PCR indicating “Parasitological Clearance” (PCR < 0; n = 144):
| 3 |
The “No Response to Treatment Agreement” was defined as the number of patients with “No Response to treatment” by MultiCruzi at the defined threshold as well as “No Parasitological Clearance” by PCR, divided by the number of patients with PCR indicating “No Parasitological Clearance” (PCR > 0; n = 57):
| 4 |
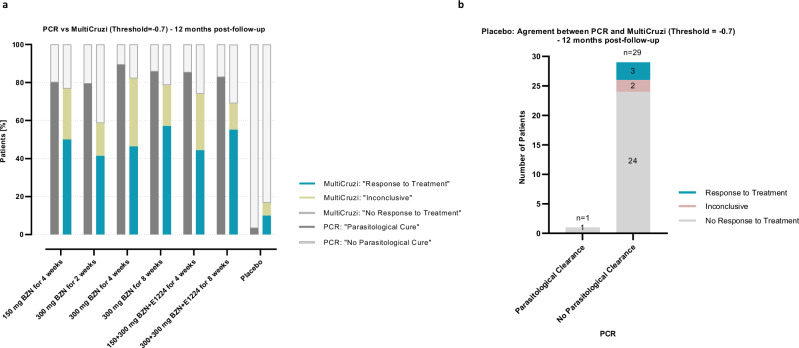
(Supplementary Table 9a). Looking more specifically at the placebo group, the “No Response to Treatment Agreement” was defined as the number of patients with “No Response to Treatment” observed by multiplex and a PCR indicating “No Parasitological Clearance”, divided by the total number of patients with PCR indicating “No parasitological Clearance” (PCR > 0; n = 29) (Supplementary Table 9b). At a slope threshold of −0.7, the agreement between both methods reaches 82.8%. At 12 months compared to baseline, MultiCruzi shows a lower percentage of “Response to Treatment” in treated patients compared to PCR (Fig. 7a, Table 2, Supplementary Table 10). Meanwhile, the placebo group shows the lowest number of patients responding to their assigned treatment, albeit a higher number is detected to respond to cure (n = 3) than PCR (n = 1) (Fig. 7b).
Fig. 7. Agreement between MultiCruzi outcome and PCR results at a threshold for log2DF50 change of −0.7, 12 month post-treatment.
a Percentage of patients with « Response to Treatment », « Inconclusive » and « No Response to Treatment » results revealed by PCR and MultiCruzi ELISA tests at 12 months following treatment. b Classification of Placebo patients according to PCR and MultiCruzi. For PCR, patients having sustained negative PCR results after 12 months post-treatment are concluded to have « Parasitological clearance ». BZN Benznidazole.
Table 2.
Proportions of patients found to have a ‘Response to Treatment’ with MultiCruzi using a threshold of −0.7 and with PCR per treatment group
| Dosing regimen | Total Number of patients | Patients with “Response to Treatment” | Patients with PCR “Parasitological Clearance” |
|---|---|---|---|
| BZN—150 mg for 4 Weeks | 30 | 15 (50.00%) | 24 (80.00%) |
| BZN—300 mg for 2 Weeks | 29 | 12 (41.38%) | 23 (79.31%) |
| BZN—300 mg for 4 Weeks | 28 | 13 (46.43%) | 25 (89.29%) |
| BZN—300 mg for 8 Weeks | 28 | 16 (57.14%) | 24 (85.71%) |
| BZN—150 mg + E1224 for 4 Weeks | 27 | 12 (44.44%) | 23 (85.19%) |
| BZN—300 mg + E1224 for 8 Weeks | 29 | 16 (55.17%) | 24 (82.76%) |
| Placebo | 30 | 3 (10.00%) | 1 (3.33%) |
BZN Benznidazole; (1) BZN—150 mg for 4 Weeks: samples collected from individuals treated with 150 mg of Benznidazole daily for 4 weeks; (2) BZN—300 mg for 2 Weeks: samples collected from individuals treated with 300 mg of Benznidazole daily for 2 weeks; (3) BZN—300 mg for 4 Weeks: samples collected from individuals treated with 300 mg of Benznidazole daily for 4 weeks; (4) BZN—300 mg for 8 Weeks: samples collected from individuals treated with 300 mg of Benznidazole daily for 8 weeks; (5) BZN—150 mg + E1224 for 4 Weeks: samples collected from individuals treated with 150 mg of Benznidazole daily for 4 weeks plus fosravuconazole; (6) BZN—300 mg + E1224 for 8 Weeks: samples collected from individuals treated with 150 mg of Benznidazole daily for 8 weeks plus fosravuconazole and (7) Placebo: samples collected from individuals treated with Placebo. The proportions of patients were calculated at a log ratio threshold of the DF50 of −0.7.
Discussion
The objective of this study was to quantify the degree of seroreduction, and thus predict future seroreversion, as a surrogate marker of parasitological cure for adult patients with chronic Chagas disease. We report the use of a multiplex test (MultiCruzi) and the use of antigen reactivities at specific timepoints for predicting seroreversion. We examined serological changes in 15 different T. cruzi antigens during the follow-up period of patients who had received one of 7 different treatment regimens. We were able to demonstrate that predictions based on the experimental MultiCruzi results within 6–12 months of follow-up were sufficient to forecast future seroreversion, assuming that the decline in antibodies’ titers are predictive of future full seroreversion11.
We have previously shown that seroreversion can be assessed more rapidly by MultiCruzi than by standard serology in samples from children treated with benznidazole31. While this was possible without using a dilution approach in a paediatric population where patients seroconvert faster and seroreversion can be observed within years following treatment, in adults, seroreversion takes decades27–29, making it impossible to determine waning of antibodies in a reasonable timeframe using the conventional serological tests and methods. We demonstrate that this issue can be addressed by carefully monitoring the dynamic of selected specific antibodies in a multiplexed serology immunoassay in serially diluted samples and applying statistical methods. Using this method, it becomes feasible to show, in benznidazole treated adult Chagas patients, a decline in antibody signature / antibodies’ titers as early as 6 months post-treatment that can be associated with a future seroreversion therefore much earlier than using conventional serology where seroreversion takes decades.
We observe an inconsistency of the outcomes obtained with the two commercial ELISA techniques used in this study. This is a core issue in the evaluation of treatment effectiveness for Chagas disease. In fact, ELISA tests vary from one assay to another, depending on antigen compositions, giving results that do not match, making it difficult to draw conclusions about treatment effectiveness. Moreover, due to the low number of patients per treatment group, between-group analysis was not performed, as the power of the test would have been insufficient.
While we showed a significantly more pronounced decrease in the antibody levels in each treatment group compared to the placebo group, we wanted to further analyze the data at individual patient level. The challenge in reaching this objective is related to the small number of patients per group, especially in the placebo group and the absence of a consensus related to the setting of a threshold for serology tests. Using the MultiCruzi, we showed that across the different thresholds for the log2DF50 reduction between baseline and 12 months assessed, the level of patients responsive to treatment in the Placebo group is always lower than in the treatment groups. However, when fixing this threshold for log2DF50 decrease between baseline and 12 months at −0.3, which corresponds to the maximum Youden index on the ROC curve, one third of the patients treated with placebo were predicted to be responsive to the treatment. It is now well recognized that patients with initial infection can show complete spontaneous seroreversion35,36, reaching in some cases up to 30% of seropositive people as described in a study of 1423 blood donors from the Chaco region of Argentina (this study showed that about 30% of participants had low antibody level responses with different commercial immunoassays and this was correlated with the spontaneous clearance of T. cruzi infection37). Additional data with larger groups of placebo-treated patients will allow to define the appropriate threshold. Indeed, the application of this method at individual patient level requires the definition of thresholds; the current ones are based on a small number of patients (only 30 placebo treated patients in our study) or on the literature (e.g., for ELISAs), which may result in inaccurate estimations of sensitivity and specificity.
We then attempted to compare the results obtained with MultiCruzi with the PCR data from the original clinical study. In this case, we observe that the agreement between both methods also depends on the fixed threshold for log2DF50 decrease. When this threshold is fixed at −0.7, the correspondence is optimal between MultiCruzi and PCR with a “Response to Treatment Agreement” of 51.4% and a “No Response to Treatment Agreement” of 57.9% in the entire population. Our findings are in line with a recent analysis of qPCR data, which shows that Ct values remain stable over time in most placebo-treated patients, unlike those receiving other treatments in the BENDITA trial. Furthermore, all treatment groups differ significantly from the placebo group, as demonstrated by the non-overlapping 95% Confidence Intervals for the estimated probability of cure in the Ct-based model for each treatment regimen compared to the placebo38. Looking more specifically into the ratio of patients with the different outcomes in each group, PCR data show sustained parasitological clearance in more patients than MultiCruzi. This could be explained by the limitations of the PCR method as the level of parasites in the bloodstream does not reflect the level of parasites in the tissues; the very low and sporadic parasitemia during the chronic phase of the disease makes direct detection of the parasite intrinsically difficult39. In fact, only around 30–70% of individuals with chronic Chagas disease show a positive blood PCR test result before treatment40,41. Moreover, a series of negative PCR results does not necessarily prove the absence of the parasite. Some longer follow-up studies for example have shown positive PCR in patients after 4 or 5 years of follow-up while during the first years a sustained negative PCR was observed42.
In addition, variability in PCR outcomes with the current standard benznidazole treatment (300 mg of BZN for 8 weeks) is evident when comparing similar clinical trials. In fact, using the same qPCR method43 to measure sustained parasite clearance from the blood over 1 year, 83% of patients in the BENDITA trial13 showed sustained clearance, whereas only 54% of patients in the MULTIBENZ trial40 achieved the same result.
On another note, the higher number of patients responding to treatment with MultiCruzi compared to the PCR method in the placebo group could be due to several reasons related to both techniques. It may be a consequence of the fixed threshold for the dilution method as previously discussed on one hand and the arbitrary and variable Cycle thresholds (Ct) among triplicates of the quantifiable positive PCR samples on the other hand. False positive or variation in the Limit of Quantification (LOQ) and/or the Limit of Detection (LOD) cannot be ruled out either. In fact, the performance of the PCR method shows higher variability of measurements at low parasite loads close to the LOD43 knowing that 99.45% of PCR positive samples included in this analysis are below the LOQ. Moreover, as these patients are present in an endemic area throughout the study, the presence of the Trypanosoma rangeli which can be found in the same vectors and vertebrate hosts cannot be eliminated44,45. While studies have shown that human infection with this nonpathogenic protozoan does not affect the serodiagnosis of Chagas disease44,46, it was shown to induce false positive PCR signals when present at certain levels in the serum sample43. The presence of DNA from dead T. cruzi parasites cannot be ruled out either.
In summary, both methods are measuring different outcomes: the MultiCruzi is measuring antibodies decline as a surrogate of future seroreversion/treatment efficacy (seroreversion is accepted by the regulatory authorities8,20,21); PCR is looking at treatment failure measuring the presence of T. cruzi DNA in blood (sustained PCR negativity during 1 year following treatment) and is not accepted by the health authorities as a valid endpoint. We therefore believe that our results open new avenues for performing clinical trials with novel drugs and therapies, as the MultiCruzi test assesses the therapeutic response—a previously unmet medical need of adult patients with Chagas disease47,48.
There are several limitations to this study. The data used were obtained from the BENDITA trial13,49, which was not designed for the aim of our ‘retrospective’ analysis (the low number of patients per treatment group preventing comparison of efficacy between treatment regimens). Moreover, the possible spontaneous cure of some placebo-treated patients over time makes them indistinguishable from patients that show the decline due to medication. These factors could probably explain the low power to detect possible differences between antibodies for treatment and placebo groups. However, even with the small sample size available, we were able to show significantly faster decline in antibodies in the treatment groups compared to the placebo group, with the MultiCruzi test combined with the serial dilution technique.
Due to the absence of validated biomarkers and/or tests, and to the fluctuating levels of parasitemia, some seropositive patients were excluded from the study because of negative PCR results. Moreover, all patients were from Bolivia, where DTU V T cruzi is the dominant parasite strain; additional studies on patients with T. cruzi from other DTUs are needed. The prediction model will need to be assessed and adjusted for additional study cohorts, such as adults from other regions, because the duration and timing of the infection affect the serological course post-treatment. While the antigens in MultiCruzi were selected for their high diagnostic performances and ability to cover various DTUs33, further testing in patients from different regions who are followed over time will help assess how antibody levels evolve across Latin America and identify the specific antigens out of the 15 present in the test that contribute to evaluating treatment response. Adult’s antibody dynamics are known to be slow, taking decades and since patients were only followed-up for 1 year, correlation with full seroreversion cannot be determined, as the necessary data will only be available after an extended period (decades) due to slow seroreversion after cure of Chagas disease spontaneously or following treatment.
In conclusion, we propose a method that shows the antibody signatures and their dynamic decline in reactivity for each treated patient already 6 months after treatment with benznidazole, anticipating the future seroreversion. We highly recommend integrating this new methodology as an endpoint into future Chagas disease clinical trials.
Methods
Clinical trial samples
Serum samples drawn during the BENDITA (BEnznidazole New Doses Improved Treatment and Associations) phase II double-blind randomized trial13,49 were stored at −80 °C following written consent of patients for further use for research purposes. Serum samples corresponding to the timepoints at baseline (screening for inclusion before treatment start), 6 months, and 12 months after treatment were sent to InfYnity Biomarkers for evaluation with the MultiCruzi assay31,32.
The results obtained with the conventional serological tests were compared with those obtained with MultiCruzi at the tested timepoints.
Serology
Conventional and recombinant ELISA serology assays
Serology was assessed to detect peripheral anti-T. cruzi antibodies using two serological tests: the conventional ELISA kit (CHAGATEK ELISA, Laboratorio Lemos SRL, Argentina) containing purified T. cruzi antigens in serum and plasma and a recombinant ELISA kit I (Chagatest ELISA recombinante, V3.0, Wiener Lab, Argentina) made of six very conserved recombinant antigens (1, 2, 13, 30, 36 and SAPA). Both tests were conducted on samples collected at baseline and 12 months after treatment following the manufacturers’ instructions13.
Samples were stored at −80 °C with the written consent of patients and the serum samples drawn at screening, 6 months and 12 months after treatment were then evaluated with MultiCruzi to monitor serological signatures and evaluate its usefulness as a predictive tool for parasitological cure as previously described in mice and in children31,32,50.
Multiplex immunoassay: MultiCruzi
The MultiCruzi immunoassay is a multiplex ELISA test incorporating 15 different T. cruzi antigens31–33. The latter were printed (arrayed) in duplicate in each well of 96-well microtiter plates (Supplementary Fig. 8). The printed antigens were identified from 15 protein immunodominant regions of T. cruzi and were designed and synthesized according to reviewed and published nonredundant sequences from UniProt (Supplementary Table 11). Among the printed 15 antigens, three are derived from discrete typing unit (DTU) specific antigens from TcI, TcII, and TcVI protein regions of the T cruzi DTU. The twelve remaining antigen sequences are highly conserved across T. cruzi strains and DTUs. In addition, positive control spots are printed in quadruplicate to verify that all test reagents are functional and are sequentially added in the correct order: serum samples, enzymatic-conjugate, and then substrate. These control spots also define a spatial orientation of the array for the reader’s camera. Moreover, cutoff control and medium control spots were added in duplicate.
The MultiCruzi assay was carried out as previously described33 at three different sample dilutions (see “Serial dilutions” below). In summary, microplates were incubated with sera from patients, diluted at 1:50, 1:400, and 1:3200, for 1 h at room temperature, then washed three times with phosphate-buffered saline with Tween buffer (PBST). Horseradish Peroxidase-conjugated goat anti-human IgG antibodies (SouthernBiotech, Ref 2040-05) diluted at 1:2000 were added, incubated for another hour, and washed three times. TMB solution (SDT GmbH, Baesweiler, Germany) was then added and incubated in the dark for 20 min at room temperature. Finally, the TMB solution was removed, and the plates were dried at 37 °C for 10 min. This test detects specific IgG antibodies against 15 T. cruzi antigens, specific differing informative antibody profiles for each sample. Every well on the plate was imaged, and the spot signals were analyzed using a colorimetric reader. Mean spot intensities were normalized by dividing the obtained value for each antigen by the average positive control intensity, thus obtaining the maximum attainable intensity in the assay defined as 100%. This method aimed at reducing assay variability and allowing the fixation of a common pixel intensity range of [0–100] for all tested antigens. The data were incorporated into a statistical analysis package and analyzed accordingly.
Each batch of printed MultiCruzi plates was validated after testing six T. cruzi positive sera or plasma samples with different levels of reactivity to the 15 antigens and two negative samples coming from healthy blood donors.
For the determination of intra-assay precision, Coefficients of variation (standard deviation/mean x 100), CV% were calculated for each antigen by testing in triplicates 8 Trypanosoma cruzi positive human serum sample with different levels of reactivities (Supplementary Table 12).
Database
The raw data obtained from the image analysis reader were validated using the internal controls embedded in each test. The mean pixel intensity of the reaction was corrected for background noise, expressed as a value ranging between 0 and 130, and used without preprocessing in the analysis. Inconsistent or aberrant signal measures or antigen patterns were retested. Patients who withdrew from the study or who discontinued early were eliminated from the analysis. Validated data were incorporated into a Microsoft Excel 2016 database for further analysis.
Serial dilutions
When plotting the colorimetric intensity of each biomarker against the dilution factor, the resultant curve has a sigmoidal shape with one plateau at high signal to no signal after multiple dilutions of the same sample (Supplementary Note 1, Supplementary Fig. 1a, b). We hypothesized that after successful treatment, the sigmoidal curve shifts to lower dilution factors, and since this ‘shift’ can be measured, the treatment effect can be quantified. Quantification is better assessed within the linear range of the curve. At extreme signal values (low and high), a minor change in signal intensity does not lead to an accurate estimation of the change in antibody levels—at the usual dilution factor for routine diagnostic testing with MultiCruzi (1/50), signals are high and remain high a year after infection, while the sigmoidal curve shifts from day 0.
To ensure reading each antigen within the linear range, samples were serially diluted as has previously been described34. The dilution factors were defined such that overlap of linear ranges for all antigens was assured, maximal concentrations could be quantified, and the number of dilutions was minimized (to reduce operational costs). See more details in Supplementary Note 1. Finally, we selected 3 dilutions (1/50, 1/400 and 1/3200) that were best suited for all 15 antigens.
Information on the shift of the sigmoidal curve is given by the DF50 value, the estimated dilution factor corresponding to a signal of 50% (between top and bottom). The DF50 value was obtained for each serial dilution. For each patient, in each treatment group, three timepoints (baseline, 6 months and 12 months) were used to calculate three DF50-values. The sigmoidal curve was expected to shift to lower dilution factors after effective treatment, meaning that DF50-values shift to lower values. The log2(DF50) was, therefore, considered the dependent variable of interest.
Linear mixed modelling
A random intercept linear mixed model (LMM) was applied to explain the variability in log2(DF50) with time, treatment, and the interaction between time and treatment as explaining variables. The intercept and slope were obtained, and the slopes were compared between the treatment and placebo groups. This approach was applied to (i) each antigen separately, which allows for the evaluation of the response to treatment for each MultiCruzi antigen separately and (ii) using a nested LMM, keeping the connection between the patient and the antigens. This allows the evaluation of the response to treatment for all 15 MultiCruzi antigens together.
Some antigens were not reactive at baseline, or the reactivity was very low, resulting in DF50-values which were very low (much lower than the initial dilution factor). DF50-values that were estimated as very low, were set to 0.1. On the other side of the dilution factor range, it was possible that DF50-values would be extrapolated beyond the highest dilution factor of 3200. We allowed one dilution factor outside the range 50–3200, but when DF50 was estimated >6400, the DF50-value was set to 6400.
The effect of the presence of DF50-values of 0.1 was evaluated by performing the analysis with and without the presence of such DF50-values.
The LMM analysis was performed at three time-points (baseline, 6 months, and 12 months) but also on the more restricted timescale of 6 months (baseline + 6 months) to evaluate the possibility of using the analysis to reveal treatment response within the first 6 months after treatment start. See “Supplementary Information” for the detailed mathematical and statistical procedures.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
I.S. and E.C. wish to thank colleagues at DNDi for their valuable support and scientific input during this project. This work was partially funded by DNDi. For these activities, DNDi received funding from UK Aid, UK; and for its overall mission, from Médecins Sans Frontières International.
Author contributions
U.S., I.S., E.C., and M.Z. conceived the idea for the project. U.S., M.Z., H.P., and J.dB. developed or designed the methodology and assays / models used in these studies. U.S. performed the assays, measurements and data visualization. M.Z., E.C. and I.S. were responsible for the administration of the project. M.Z., E.C. and I.S. supervised the studies. M.Z., E.C. and I.S. were involved in funding acquisition. U.S., M.Z. and H.P. wrote the original draft. U.S., M.Z., H.P., J.dB., E.C., I.S. and J.A. reviewed and edited the original draft. J.A. contributed to the administrative and logistics aspects of the study. All authors read, revised and authorized the manuscript before submission.
Peer review
Peer review information
Nature Communications thanks James Watson, who co-reviewed with Cintia Cruz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data generated in this study are provided in the “Supplementary Software 1” zip file. The data underlying the results presented in this study are available upon request because they contain potentially sensitive personal information, which must be deidentified at the individual level. Interested researchers may contact the Drugs for Neglected Diseases initiative (DNDi), commissioner of this study, for data access requests via email at CTdata@dndi.org. Researchers may also request data by completing the form available at https://www.dndi.org/category/clinical-trials/. In this, they confirm that they will share data and results with DNDi and will publish any results open access.
Code availability
A Visual Basic for Applications (VBA 7.1) User Defined Function from Microsoft Excel 2016 was programmed to calculate the DF50-value from the DFs and biomarker intensities (Y-values). Linear Mixed Model analysis was performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The code is available in the “Supplementary Software 1” file.
Competing interests
U.S. and M.Z. are employed by InfYnity Biomarkers. All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eric Chatelain, Email: echatelain@dndi.org.
Maan Zrein, Email: mzrein@infynity-biomarkers.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-54910-x.
References
- 1.Pérez-Molina, J. A. & Molina, I. Chagas disease. Lancet391, 82–94 (2018). [DOI] [PubMed] [Google Scholar]
- 2.WHO. Chagas Disease.https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (2023).
- 3.Rassi, A., Rassi, A. & Marcondes de Rezende, J. American trypanosomiasis (Chagas Disease). Infectious Disease Clinics of North America26, 275–291 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Edwards, M. S. & Montgomery, S. P. Congenital Chagas disease: progress toward implementation of pregnancy-based screening. Curr Opin Infect Dis34, 538–545 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes, M. C. P. et al. Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American heart association. Circulation138, e169–e209 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Echeverria, L. E. & Morillo, C. A. American trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am.33, 119–134 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro, A. L. P., Marcolino, M. S., Prineas, R. J. & Lima-Costa, M. F. Electrocardiographic abnormalities in elderly Chagas disease patients: 10 year follow-up of the bambui cohort study of aging. J Am. Heart Assoc.3, e000632 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan American Health Organization. Guidelines for the Diagnosis and Treatment of Chagas Disease.https://iris.paho.org/handle/10665.2/49653 (2019).
- 9.Jackson, Y., Wyssa, B. & Chappuis, F. Tolerance to nifurtimox and benznidazole in adult patients with chronic Chagas’ disease. J. Antimicrob. Chemother.75, 690–696 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altcheh, J., Moscatelli, G., Moroni, S., Garcia-Bournissen, F. & Freilij, H. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics127, e212–e218 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Sguassero, Y. et al. Course of chronic trypanosoma cruzi infection after treatment based on parasitological and serological tests: a systematic review of follow-up studies. PLoS One10, e0139363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasslocher-Moreno, A. M. et al. Safety of benznidazole use in the treatment of chronic Chagas’ disease. J. Antimicrob. Chemother.67, 1261–1266 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Torrico, F. et al. New regimens of benznidazole monotherapy and in combination with fosravuconazole for treatment of Chagas disease (BENDITA): a phase 2, double-blind, randomised trial. Lancet Infect. Dis.21, 1129–1140 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Bustamante, J. M., Craft, J. M., Crowe, B. D., Ketchie, S. A. & Tarleton, R. L. New, combined, and reduced dosing treatment protocols cure trypanosoma cruzi infection in mice. J. Infect. Dis.209, 150–162 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatelain, E. & Konar, N. Translational challenges of animal models in Chagas disease drug development: a review. DDDT9, 4807–4823 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bustamante, J. M. & Tarleton, R. L. Methodological advances in drug discovery for Chagas disease. Expert Opin. Drug Discov.6, 653–661 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kratz, J. M. Drug discovery for chagas disease: a viewpoint. Acta. Tropica.198, 105107 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Schijman, A. G. et al. International study to evaluate PCR methods for detection of trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl. Tropica. Dis.5, e931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes, M. C. & Andrews, N. W. Host cell invasion by trypanosoma cruzi: a unique strategy that promotes persistence. FEMS Microbiol Rev36, 734–747 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossmann, U. & Rodriguez, M.-L. Chagas disease treatment efficacy markers: experiences from a phase III study with nifurtimox in children. Front. Parasitol.10.3389/fpara.2023.1229467 (2023).
- 21.Use of Serological Tests to Reduce the Risk of Transmission of Trypanosoma cruzi Infection in Blood and Blood Components.https://www.fda.gov/regulatory-information (2017).
- 22.Alonso-Padilla, J. et al. Target product profile for a test for the early assessment of treatment efficacy in Chagas disease patients: an expert consensus. PLOS Neglected Trop. Dis.14, e0008035 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viotti, R. et al. Impact of aetiological treatment on conventional and multiplex serology in Chronic Chagas disease. PLoS Negl. Trop. Dis.5, e1314 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viotti, R. et al. Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrob. Agents Chemother.58, 635–639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traynor, K. Benznidazole approved for Chagas disease in children. Am. J. Health Syst. Pharmacy74, 1519 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Altcheh, J. et al. Prospective, historically controlled study to evaluate the efficacy and safety of a new paediatric formulation of nifurtimox in children aged 0 – 17 years with Chagas disease one year after treatment (CHICO). PLoS Negl. Trop. Dis.15, e0008912 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balouz, V., Agüero, F. & Buscaglia, C. A. Chagas disease diagnostic applications: present knowledge and future steps. Adv Parasitol97, 1–45 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buss, L. F. et al. Declining antibody levels to Trypanosoma cruzi correlate with polymerase chain reaction positivity and electrocardiographic changes in a retrospective cohort of untreated Brazilian blood donors. PLoS Neglected Trop. Dis.14, e0008787 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afonso, A. M., Ebell, M. H. & Tarleton, R. L. A systematic review of high quality diagnostic tests for Chagas disease. PLoS Negl Trop Dis6, e1881 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricci, A. D. et al. The trypanosoma cruzi antigen and epitope atlas: antibody specificities in Chagas disease patients across the Americas. Nat. Commun.14, 1850 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina, L. J. et al. Prediction of parasitological cure in children infected with trypanosoma cruzi using a novel multiplex serological approach: an observational, retrospective cohort study. Lancet Infect. Dis.21, 1141–1150 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Zrein, M. et al. A novel antibody surrogate biomarker to monitor parasite persistence in Trypanosoma cruzi-infected patients. PLoS Neglected Trop. Dis.12, e0006226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granjon, E. et al. Development of a novel multiplex immunoassay multi-cruzi for the serological confirmation of Chagas disease. PLoS Neglected Trop. Dis.10, e0004596 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boer et al. A novel assessment method for COVID-19 humoral immunity duration using serial measurements in naturally infected and vaccinated subjects. PLoS One17, e0274553 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dias, J. C. P. et al. Further evidence of spontaneous cure in human Chagas disease. Rev. Soc. Bras. Med. Trop.41, 505–506 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Francolino, S. S. et al. New evidence of spontaneous cure in human Chagas’ disease. Rev. Soc. Bras. Med. Trop.36, 103–107 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Remesar, M. et al. Bimodal distribution of Trypanosoma cruzi antibody levels in blood donors from a highly endemic area of Argentina: what is the significance of low-reactive samples? Transfusion55, 2499–2504 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Watson, J. A. et al. Quantifying anti-trypanosomal treatment effects in chronic indeterminate Chagas disease: an individual patient data meta-analysis of two proof of concept trials. medRxiv10.1101/2024.07.14.24310398 (2024).
- 39.Alonso-Padilla, J., Gallego, M., Schijman, A. G. & Gascon, J. Molecular diagnostics for Chagas disease: up to date and novel methodologies. Expert Rev. Mol. Diagn.17, 699–710 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Molina-Morant, D. et al. Efficacy and safety assessment of different dosage of benznidazol for the treatment of Chagas disease in chronic phase in adults (MULTIBENZ study): study protocol for a multicenter randomized phase II superiority clinical trial. Trials21, 328 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molina, I. et al. Randomized trial of posaconazole and benznidazole for Chronic Chagas’ disease. N. Engl. J. Med.370, 1899–1908 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Sulleiro, E. et al. Usefulness of real-time PCR during follow-up of patients treated with Benznidazole for chronic Chagas disease: Experience in two referral centers in Barcelona. PLoS Negl. Trop. Dis.14, e0008067 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duffy, T. et al. Analytical performance of a multiplex real-time PCR assay using TaqMan probes for quantification of trypanosoma cruzi satellite DNA in blood samples. PLoS Negl. Trop. Dis.7, e2000 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saldaña, A. et al. Predominance of Trypanosoma rangeli infection in children from a Chagas disease endemic area in the west-shore of the Panama canal. Mem. Inst. Oswaldo Cruz.100, 729–731 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Ramirez, L. E. et al. High prevalence of trypanosoma rangeli and Trypanosoma cruzi in opossums and triatomids in a formerly-endemic area of Chagas disease in Southeast Brazil. Acta. Trop.84, 189–198 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Saldaña, A. & Sousa, O. E. Trypanosoma rangeli: epimastigote immunogenicity and cross-reaction with Trypanosoma cruzi. J. Parasitol.82, 363–366 (1996). [PubMed] [Google Scholar]
- 47.Zrein, M. & Chatelain, E. The unmet medical need for Trypanosoma cruzi-infected patients: monitoring the disease status. Biochim. Biophys. Acta Mol. Basis Dis.1866, 165628 (2020). [DOI] [PubMed] [Google Scholar]
- 48.PLOS Neglected Tropical Diseases. Development of Diagnostics for Chagas Disease: Where Should We Put Our Limited Resources?https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0005148 (2017). [DOI] [PMC free article] [PubMed]
- 49. Drugs for Neglected Diseases. Phase 2 Randomized, Multicenter, Safety and Efficacy Trial to Evaluate Different Oral Benznidazole Monotherapy and Benznidazole/E1224 Combination Regimens for the Treatment of Adult Patients With Chronic Indeterminate Chagas Disease.https://clinicaltrials.gov/study/NCT03378661 (2017).
- 50.Francisco, A. F. et al. Comparing in vivo bioluminescence imaging and the multi-cruzi immunoassay platform to develop improved Chagas disease diagnostic procedures and biomarkers for monitoring parasitological cure. PLoS Negl. Trop. Dis.16, e0010827 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data generated in this study are provided in the “Supplementary Software 1” zip file. The data underlying the results presented in this study are available upon request because they contain potentially sensitive personal information, which must be deidentified at the individual level. Interested researchers may contact the Drugs for Neglected Diseases initiative (DNDi), commissioner of this study, for data access requests via email at CTdata@dndi.org. Researchers may also request data by completing the form available at https://www.dndi.org/category/clinical-trials/. In this, they confirm that they will share data and results with DNDi and will publish any results open access.
A Visual Basic for Applications (VBA 7.1) User Defined Function from Microsoft Excel 2016 was programmed to calculate the DF50-value from the DFs and biomarker intensities (Y-values). Linear Mixed Model analysis was performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The code is available in the “Supplementary Software 1” file.