Abstract
We describe a biophysical mechanism for animal magnetoreception, orientation and navigation in the geomagnetic field (GMF), based on the ion forced oscillation (IFO) mechanism in animal cell membrane voltage-gated ion channels (VGICs) (IFO-VGIC mechanism). We review previously suggested hypotheses. We describe the structure and function of VGICs and argue that they are the most sensitive electromagnetic sensors in all animals. We consider the magnetic force exerted by the GMF on a mobile ion within a VGIC of an animal with periodic velocity variation. We apply this force in the IFO equation resulting in solution connecting the GMF intensity with the velocity variation rate. We show that animals with periodic velocity variations, receive oscillating forces on their mobile ions within VGICs, which are forced to oscillate exerting forces on the voltage sensors of the channels, similar or greater to the forces from membrane voltage changes that normally induce gating. Thus, the GMF in combination with the varying animal velocity can gate VGICs and alter cell homeostasis in a degree depending, for a given velocity and velocity variation rate, on GMF intensity (unique in each latitude) and the angle between velocity and GMF axis, which determine animal position and orientation.
Keywords: Geomagnetic field, Animal magnetoreception, Orientation, Navigation, Electromagnetic fields, Voltage-gated ion channels, Ion forced-oscillation mechanism
Subject terms: Biophysics, Systems biology, Environmental sciences, Planetary science, Physics
Introduction
Geomagnetic field and navigation—animal migration
It is widely accepted that animals have a sense of orientation on Earth which allows them to migrate toward specific directions, travel long distances and find specific locations, with most migratory animals (including birds) migrating at night1–4. The biophysical explanation of this phenomenon has been a great challenge in science and, especially after the early 1970s, one of the hottest topics in bioelectromagnetics/electromagnetic biology with hundreds of scientific publications trying to explore and resolve this mystery1,2,5,6. It was reasonable to assume that such an ability is related to the sensing of the geomagnetic field (GMF), which is mainly static with specific orientation and a magnetic axis forming an angle of a few degrees with the Earth’s geographic north–south axis and reversed polarity, i.e. the geomagnetic north pole is close to the geographic south pole and vice-versa. The intensity of the GMF is maximal at the poles and minimal at the equator having an average value at intermediate latitudes of ~ 0.5 G or ~ 50 μT5–7. Magnetic compasses, which are thin magnets rotating around a vertical axis in their center, orient parallel to the GMF lines indicating the geomagnetic south which is very close to the geographic north pole. This is how people have been navigating for centuries by use of magnetic compasses since they were discovered in the ancient times.
During the past fifty years, several hypotheses for mechanisms of animal magnetoreception have been proposed, related mainly to a) biogenic magnetite found in living organisms, b) light-sensitive chemical magnetoreception, and c) electromagnetic induction. There are certain weaknesses in those hypotheses which we believe is one reason why the phenomenon of animal magnetoreception and navigation has remained unexplained to this day1,3,4,8–11. According to1, “determining how animals orient themselves using Earth’s magnetic field can be even more difficult than finding a needle in a haystack. It is like finding a needle in a stack of needles.”
Suggested hypotheses for a mechanism of animal magnetoreception
The first hypothesis is based on the existence of magnetite nanoparticles (Fe3O4) in living organisms12,13. Such particles were first found in denticle caps of chitons and in magnetotactic bacteria14–18. Later it was reported that they were also detected in certain other species including humans, birds and bees1,9,11,13. It was suggested that chains of magnetite crystals called magnetosomes found in the aforementioned organisms, reoriented by the GMF could act as compass needles subserving their magneto-sensing ability. It was claimed that magnetosome chains anchored to nerve cell membranes near mechanically-gated ion channels were present in fish10,19,20, and was hypothesized that these chains aligned under the action of the GMF they could possibly cause gating (opening/closing) of such ion channels1,3,4,9–13,21–24. Yet, despite the many publications on magnetite-based magnetoreception, no numerical calculations of the force/torque exerted by the GMF on magnetosomes were presented or shown to produce the pressure required to open/close mechanically-gated ion channels. Neither was shown how exactly this pressure is exerted on such ion channels by reorientation of magnetosomes supposedly anchored in the plasma membrane. Thus, suggestions on magnetite-based magnetoreception remain largely hypothetical, and in our view, complicated and unlikely. Ernst and Lohmann25 observed orientation changes (lasting at least 10 h after exposure) in Caribbean spiny lobster (Panulirus argus) in response to magnetic pulses that could theoretically cause magnetite rotation. But the effect could also be due to the fact that electromagnetic pulses affect cells in various ways and initiate a plethora of biological effects26–29. Thus, the observed effect may be compatible with, but does not actually confirm the magnetite hypothesis. In a series of experiments Malkemper et al.30 found no evidence of magnetite-based magnetoreceptor in the pigeon ear and suggested that any possible inner ear magnetoreceptor would likely be activated by electromagnetic induction. This is in line with Treiber et al.31, and Edelman et al.32 who found that structures previously suggested as strong candidates for magnetite-based magnetoreceptors in pigeons were macrophages or non-magnetite iron accumulations. Moreover, Edelman et al.32 correctly pointed out that in order for magnetic particles to be magnetoreception sensors they should be found inside cells at exactly the same location and be associated with nerve tissue in many individuals (in fact in all) of a certain species, which is definitely not the case with the animals in which magnetosomes have been found. Thus, even though Kirschvink et al.10 claimed that magnetite biomineralization is common in all living organisms including all animals and is the key for magnetoreception, this is apparently not the case, apart perhaps from the magnetotactic bacteria, in which magnetosome chains of similar length to the bacteria were clearly detected, and may indeed orient like magnetic needles in the GMF, dragging and orienting in this way the entire organism.
The second hypothesis suggests that a light stimulus on a cryptochrome protein, a photoreceptive molecule in the retina of bird eyes, induces the formation of pairs of free radicals (atoms/molecules each one having an unpaired electron) which, in the presence of a magnetic field, align their spin momentums parallel or antiparallel to the field, and, in this case, the GMF may change a radical pair from a singlet (parallel spins) to a triplet (antiparallel spins) state and vice-versa. Then the corresponding signal related to changes in the singlet/triplet yield is transferred to some hypothetical specialized sensory cell type that recognizes the GMF intensity and direction4,10,11,33–38. This possibility has been specifically examined for birds and is considered the most prevailing hypothesis, supposedly supported by chemical, physical, and biological facts4,39,40. But it has never been explained how exactly the singlet/triplet yield in a sensory cell could provide the animal with the sense of the GMF intensity and direction. Moreover, all free radicals are extremely bioactive because of their unpaired electron and have an extremely short lifetime (usually ~10–11-10–7s)41–44. How do they provide sufficient time for a complicated biological event to take place? Certain authors refer to “long-lived” radical pairs4 but they do not report their molecular identity or how much longer are their lifetimes than those of ordinary free radicals. Most importantly, how can light which is non-ionizing, apart from vacuum ultraviolet which gets absorbed in the upper atmosphere, induce the formation of radical pairs which is ionization? The only known physical effect that induces the formation of radical pairs with non-ionizing radiation is the Electron Spin Resonance (ESR) effect, in which the formation of free radical pairs with opposite electronic spins is induced by Radio Frequency (RF)/microwave (300 kHz-300 GHz) radiation (usually 9–36 GHz) in the presence of a very strong and spatially inhomogeneous magnetic field with corresponding intensities ranging between 100 μT and 1.3 T (already significantly stronger than the GMF which actually is locally homogenous). The equation connecting the frequency (ν) with the magnetic field intensity (B) in the ESR effect in the case of free electrons, is:
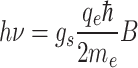 |
(gs is the spectroscopic splitting factor, = 9.27 × 10–24 J/T the Bohr’s magnetone with qe, me the electronic charge and mass respectively and ħ = h/2π, h the Planck’s constant)45. If a radical pair is to be formed by visible light, this would require about four orders of magnitude even stronger magnetic field (than 100 μT—1.3 T), which is at least 20,000 times stronger than the GMF. The above facts make extremely unlikely the formation of radical pairs by light in the presence of the GMF, if not impossible. Furthermore, if light is necessary for animals to be able to orient and navigate, why most migratory animals (including birds) migrate at night? The argument provided by Mouritsen4that light-dependent magnetoreception could (hypothetically) be activated by just a few photons and such a quantity of light is always present, is not convincing. Even if birds migrating during the night receive a few photons in darkest nights, what about migrating fish at the bottom of oceans or animals moving underground with literally no photons present? And why should an important sense be based on a trigger that is limitedly available or not available at all? In our view, this hypothesis is also completely conjectural and unrealistically complicated. It is unlikely that nature would work via complicated and thus, energy-consuming mechanisms, when it could work in simpler and more economic terms (Occam’s razor or law of parsimony)46. We searched for the facts supporting the hypothesis that magnetoreception requires light4,33,34. We did find evidence that bird orientation is affected by light37, which is reasonable, as birds definitely use visual landmarks as complementary cues for their navigation, but we found no evidence that bird or other animal orientation requires light and that it cannot occur without it. Therefore, it is mystifying to us that dozens of publications insist on a hypothesis that combines magnetoreception with light sensitivity. An experimental study that showed birds losing their orientation in the presence of “electromagnetic noise”47and was reported3to support the radical-pair hypothesis states that “the effects might be explained if hyperfine interactions in light-induced radical pairs or large clusters of iron-containing particles are involved”. Thus47, provided neither proof nor support of the radical-pair or the magnetite hypotheses, but simply speculated that perhaps the recorded effect could be explained by one of these two hypotheses. Another study found that an Extremely Low Frequency (ELF) magnetic field (300 μT, 50 Hz) shortened the 24-h circadian period in fruit flies and that this effect did not occur in mutated flies that could not produce cryptochrome48. This result was also interpreted as supporting the role of cryptochrome in magnetically sensitive behaviour (and, thus, the radical pair magnetoreception hypothesis) which is again not the case, as the circadian period in all forms of life on Earth is controlled by the 24-h light periodicity, the sensing of which involves cryptochrome/photoreceptive proteins. If the authors studied magnetic field effects not related to circadian rhythms and light they would find no dependence on cryptochrome, as is the case with the vast majority of the recorded ELF magnetic field effects on living organisms6,7,26–29,49,50.
The third hypothesis is based on the Faraday-Henry law of electromagnetic induction51 which states that when the magnetic flow through a surface S enclosed by a closed conductive line l changes with time, a voltage (and consequently an electric field) is induced along the closed line. The law implies that any accelerating animal movement in a static and locally homogenous magnetic field such as the GMF will induce an electric field in its body. In this way, the magnetic field would be converted into an electric signal detectable by putative voltage-sensing cells/organs named electroreceptors, such as the ampullae of Lorenzini in elasmobranches (e.g. sharks, rays, etc.)1,52–55, or the inner ear, or the olfactory epithelium tissue of birds23,56. This scenario has not been investigated in invertebrate species, because no “identified” electroreceptors have been attributed to these animals57. Electromagnetic induction has been studied in elasmobranches58,59and was recently proposed to underlie the navigation of pigeons30,60, as suggested earlier56. It is considered that for animals to be able to sense the GMF via electromagnetic induction, large internal ring-shaped structures filled with conductive liquid would be needed which are more likely to exist in aquatic animals, and, thus, for terrestrial animals another mechanism should be sought4. But in all living organisms, including terrestrial mammals, including humans, there are endogenous weak electric currents consisting of ion flows through cells, cell membranes, and whole tissues/organs, controlling practically all cellular/tissue functions. These endogenous physiological currents flow through the extracellular and intracellular aqueous solutions and through ion channels in the plasma membranes61–63. Therefore, while electromagnetic induction is an apparent physical effect, we do not see why specific tissue structures are needed for electromagnetic induction to take place in any animal.
The possibility of electromagnetic induction in the GMF as a mechanism for animal orientation/navigation has not been considered so far in accordance with the voltage-gated ion channels (VGICs) and the ion forced-oscillation (IFO)-VGIC mechanism, which is an accepted biophysical mechanism for electromagnetic field (EMF)-bioeffects44,64–67. By contrast, it was considered in relation to some hypothetical unknown organ or cell type (“electroreceptor”) presumably located in the eyes34or in the olfactory epithelium23. But the eyes or any sensory tissues are connected to neurons that transport the electromagnetic signals to the brain, and nerve cells have higher percentages of VGICs in their plasma membranes than other types of cells49,68,69.
Apart from the various hypotheses for a mechanism to explain animal magnetoreception, orientation and navigation with the weaknesses discussed above, the experimental work in the field is extensive, sophisticated, and important by itself1–4,9–11,15–25,30–32,34–40.
Animal sensors for magnetoreception
The existence of specific organs to detect EMFs seems to be a common assumption among many researchers who study animal electro/magnetoreception4. Threshold electric field values that can be detected by various animals/organisms have been reported in papers to be as low as 10–5-10–4 V/m and even lower for certain species such as the elasmobranches (~10–6 V/m)1,70.
But why should there be specific organs (receptors) to detect EMFs when all animals and plants/trees are equipped with the most sensitive EMF-sensors, in large numbers in all membranes of each of their trillions of cells, which are no other that the VGICs, the most abundant class of transmembrane proteins forming ion channels in the cell membranes in all cells of all animals68,69? It seems that this fact has escaped researchers’ attention even though certain studies had long ago indicated the importance of VGICs as potential EMF sensors71–73. Also, it seems that our published theoretical model has escaped attention, even though it has demonstrated, by equations and accurate numerical calculations based on molecular data on the structure and function of VGICs, that polarized and coherent applied oscillating EMFs, especially in the lower frequency bands, namely Ultra Low Frequency (ULF: 0–3 Hz), ELF (3–3000 Hz), and Very Low Frequency (VLF: 3–30 kHz), can induce gating (opening/closing) in VGICs at very low threshold intensities (down to ~10–5 V/m)44,64–66. Instead, complicated scenarios involving specific hypothetical organs supposedly serving as “electroreceptors” or “magnetoreceptors” have been considered and developed. While several studies have considered the involvement of ion channels in the final steps of eliciting a cellular effect in combination with the various suggested hypothetical mechanisms3,10, only a few recent studies have suggested a critical role of the VGICs, mostly in combination with electromagnetic induction-based magnetoreception58–60, and even those few studies, did not consider our detailed theoretical model.
The suggestion we have introduced that VGICs serve as the sensitive EMF detectors in animals44,64–66does not contradict the existence of specific structures in certain animals such as the ampullae of Lorenzini in elasmobranches1,52,53which may act as signal amplifiers in those animals, collecting and amplifying signals from many VGICs and many cells. The existence of amplifying mechanisms in certain species may explain their enhanced electromagnetic sensitivity compared to other animals and to the VGIC sensitivity that we have theoretically calculated1,44,66.
VGICs: The most sensitive electro/magnetoreceptors. Structure and function. Purpose of the study
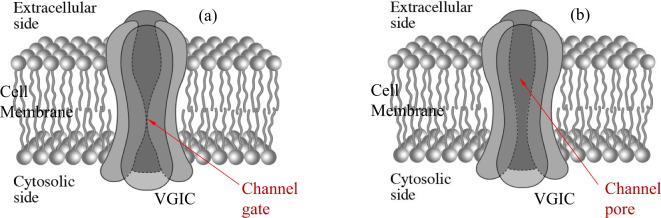
It is known that VGICs convert from open to closed states and vice-versa by membrane voltage changes dV ≥ 30 mV which exert forces on their voltage-sensors (more specifically VGICs respond to changes between ~30 and ~100 mV). The voltage sensors of the VGICs are four symmetrically arranged, transmembrane, positively charged α-helices (subunits), each one named S4. They occupy the 4th position in a group of 6 parallel α-helices (S1-S6) (Fig. 1). They are the closest helices to the pore apart from the S5-S6 helices which form the pore walls. The channel consists of four identical such groups (units I-IV) in symmetrical positions around the pore of the channel (Fig. 1, 2a, b). More specifically, the sensors are positive Lys and Arg amino-acids in the S4 helices. The effective (net) charge on each S4 sensor has been calculated to be q = 1.7qe, where qeis the elementary charge. The positive charges of the S4 sensors are paired with negative charges from adjacent helices so that the net charge in the pore of the channel is zero. The ions pass dehydrated and in single file through the channel pore. The narrowest part of the pore is called the pore gate. At least four dehydrated mobile ions are very close any moment to the S4 sensors at a distance of the order of 1 nm, as – except for the ion(s) that may be passing through the pore gate any moment or is just outside the pore gate ready to pass – at least three more are bound at specific ion-binding sites very close to the gate67,69,74–78. While VGICs are normally gated by ~30–100 mV voltage changes in the very strong transmembrane field, in other words respond to field changes between 3 × 106 and 107 V/m, they may also respond to very weak polarized, coherent, and varying EMFs down to ~10–5 V/m via the forced-oscillation such EMFs induce on mobile ions in close proximity (< 1 nm) to the sensors. This happens because the force exerted on the S4 sensors by oscillating ions in close proximity, depending upon the inverse third power of the distance between charges (see Eq. 2 below), is much greater than a direct force from an externally applied EMF44. The aforementioned (at least) four ions close to the pore gate, once forced to oscillate in phase, exert constructive forces on the S4 sensors able to gate the channel, and this is a critical point for understanding the described IFO-VGIC mechanism (Fig. 3). The ion transit time through an open VGIC (e.g. a VGIC sodium channel) is calculated to be ~0.4 × 10-7s64,65,67, and the time needed for the channel to open/close is ~2.5 × 10-5s69. This gives enough time to any polarized EMF oscillating with a frequency up to ~100 kHz to interact with the channel sensors during just one semi period and cause gating (opening/closing) with subsequent alterations in intracellular ionic concentrations and cell redox state and homeostasis (Fig. 4).
Fig. 1.
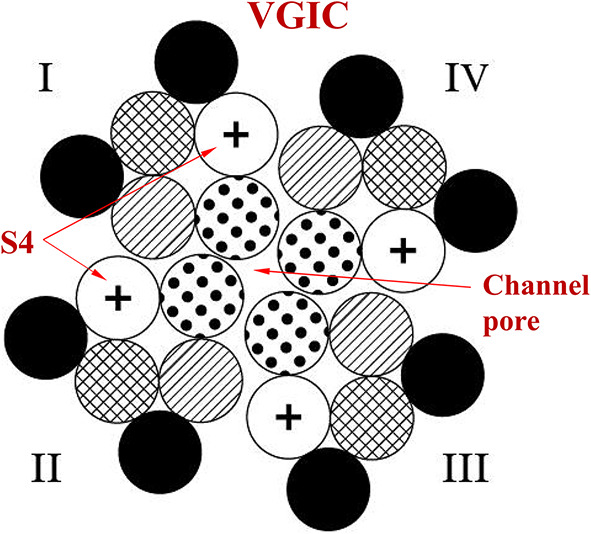
Schematic cross-section of a VGIC with the four units (I-IV) in symmetrical positions around the channel pore. Each unit consists of six parallel α-helices/subunits (S1-S6). The voltage-sensors of the VGIC are the four S4 positively charged helices ( +) one in each unit. The S4 are the closest to the pore-forming S5-S6 helices having less than 1 nm distance from the pore (modified from74).
Fig. 2.
Schematic representation of a VGIC (a) closed; (b) opened.
Fig. 3.
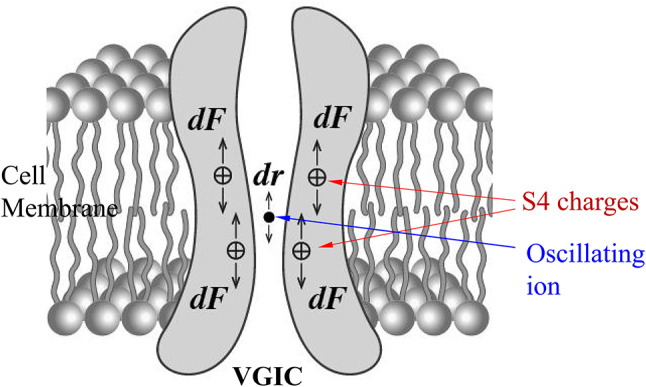
Schematic representation of the forces dF exerted on the S4 positive charges in a VGIC due to displacement dr of an oscillating ion in the channel pore.
Fig. 4.
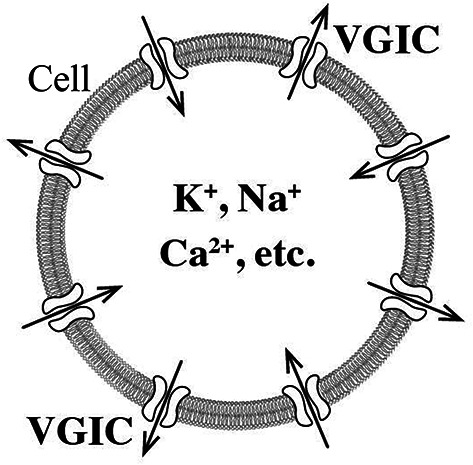
A cell with inward and outward ion flows through VGICs in its plasma membrane and consequent alterations in intracellular concentrations of critical ions (K+, Na+, Ca2+, etc.).
We have shown in the IFO-VGIC mechanism by precise numerical calculations that VGICs respond to changes of polarized and coherent electric fields down to ~10–5-10–4V/m44,65–67, which is in impressive agreement with the reported threshold intensities for man-made EMFs to be sensed by various animals and cell/tissue cultures1,70,79,80, and very close to the increased sensitivity of elasmobranches. VGICs exist in great numbers in all cell membranes of all living cells. Moreover, as mentioned already, their numbers/percentages are significantly greater in neurons (nerve cells) as the Na+ and K+ VGICs are necessary for the transmission of the nerve impulses, and especially in brain neurons49,68,69.
The purpose of this theoretical study is to test whether the IFO-VGIC mechanism can explain the sensing of the GMF by moving animals, and specifically, whether it can explain the sensing of their direction and position in relation to the GMF, which are both necessary for orientation and navigation.
Biophysical mechanism of animal orientation and navigation
Applying the IFO-VGIC theory to the problem
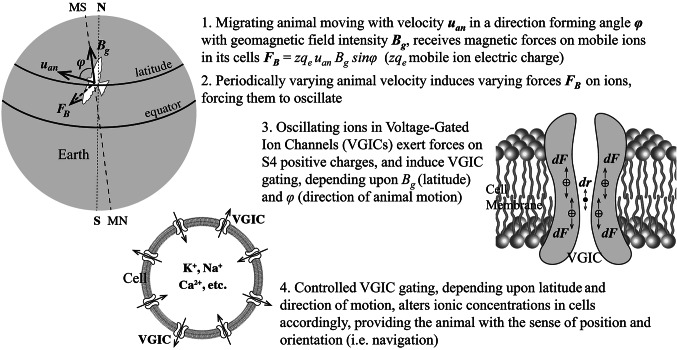
Consider an animal, e.g. a bird, moving in any direction parallel to the Earth’s surface with a drift velocity uan ~ 20 m/s (Fig. 5). Every charged particle in its body, and specifically a mobile ion of charge zqe (z the ion valence, and qe the elementary charge) within a VGIC and close to the S4 sensors (at a reasonable distance of ~1 nm) in a plasma membrane in any cell of this animal, is subjected to a magnetic force by the GMF of magnitude,
| 1 |
Fig. 5.
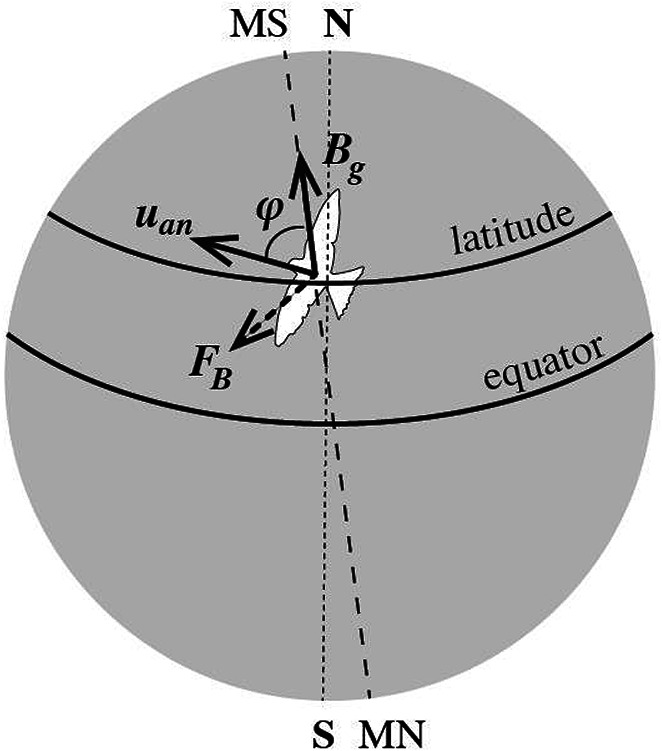
Magnetic force FB on electrically charged particles (mobile ions) in the cells of a migrating bird on Earth flying with velocity uan in a direction forming angle φ with the geomagnetic field intensity Bg which is unique at every latitude. For any given velocity magnitude and velocity variation rate, the magnetic force depends upon φ and Bg (direction of motion and latitude), providing the migrating animal with the sense of its direction and position on Earth. (N: geographic north pole. S: geographic south pole. MN: magnetic north pole. MS: magnetic south pole).
(φ is the angle between the animal velocity uan and the GMF intensity Bg).
This magnetic force is vertical to the animal velocity and would result in a curved motion of the ion within the channel protein. The radius of this curve (r = miuan/zqeBg) depending upon ion mass (mi), charge (zqe), velocity (uan), and Bg is calculated to be of the order of 0.1 m. This is too large compared to the available space for an ion within a channel protein (a few Å), and thus we can reasonably accept that the ion’s displacement dr within the channel due to the magnetic force is practically linear.
Such magnetic forces will be exerted on any mobile ion in the animal’s body and specifically within any VGIC, causing a displacement dr from its “initial” position which in turn exerts an additional Coulomb force dF on the S4 voltage-sensors of the VGIC which may result in the opening/closing (gating) of the channel64,65,73 (Fig. 3). This additional Coulomb force on each S4 sensor due to the ion displacement dr is described by the equation:
| 2 |
with q = 1.7 qe the effective (net) charge of the S4 sensor, zqe the mobile ion charge, εo = 8.854⋅10–12 N-1 m-2C2 the vacuum permittivity, ε ~4 the relative permittivity of the ion channel, and r= 1 nm the “initial” distance between the two charges75,81.
The minimum required force on the S4 charge, perpendicular to the membrane, generated by a change of 30 mV in the transmembrane voltage required to gate the channel normally, is calculated to be44,64–66:
| 3 |
This force, according to Eq. 2, corresponds to a minimum required displacement of one z-valence ion within the channel along the direction of the channel axis44,64–67:
| 4 |
According to the IFO-VGIC mechanism and the standard oscillation mechanics, the equation that describes the motion of this mobile ion in an animal moving with velocity uan forming an angle φ with the GMF intensity Bg, with the forces exerted on it, is written as:
| 5 |
where mi is the ion mass (e.g. mi ~ 3.8 × 10–26 kg for sodium ions), r the ion position during its displacement due to the applied magnetic field, z the ion valence, qe = 1.6 × C the elementary charge, β the damping coefficient (found to be within channels, β = ≅ 6.4z × kg/s, with Em ~ 107 V/m the transmembrane electric field, and uo = 0.25 m/s the maximum ion velocity through an open channel), and ωo = 2πνo (νothe ion’s oscillation self-frequency)44,64,65,67.
The right part of Eq. 5 is the magnetic force on the ion. The first term of the left part (mi ) is the resultant force, the second term (β ) is a damping force, and the third term (miωo2r) a restoring force exerted by the medium44,64–66. These are the standard forces in a forced-oscillation equation, with their parameters depending on the specific system.[Note: The damping force is always opposite to the velocity of the particle. The restoring force is always directed toward the equilibrium position and it is a function only of the position of the particle. Any particle within a material medium displaced from a considered position will receive a restoring force toward its initial position.]
In order for an applied polarized and coherent electric or magnetic field to be able to gate (open/close) a VGIC it has to be oscillating/varying in time. Invariable static fields such as the very strong cell membrane field or the geomagnetic and the geoelectric field (GEF) do not normally cause VGIC gating and subsequent initiation of biological/health effects. Those fields only induce biological/health effects during 20–30% changes in their intensities taking place physiologically during membrane depolarizations, or during magnetic storms respectively6,7,82. In the case of an oscillating/varying magnetic force, Eq. 5 describes a forced-oscillation of the ion (IFO). In the simplest case of a harmonically oscillating magnetic force FB = FBo sinωt with amplitude FBo and circular frequency ω = 2πν (ν the frequency) due to a harmonically varying animal velocity,
| 6 |
in a direction forming an angle φ with the GMF Bg, the solution of Eq. 5 describing the forced-oscillation of the mobile ion is44,64,65,67:
| 7 |
Since the animal velocity varies but is always in one direction, sinωt > 0, or 0 < ωt < π. This does not affect Eq. 7. In this case uano is the average velocity (~ 20 m/s) which varies with animal movement, e.g. in birds with winging.
The first term of the solution (Eq. 7) is an harmonically oscillating term with an amplitude
| 8 |
and the second is a constant term equal to this amplitude A. The constant term displaces the whole oscillation at a distance equal to A. Thus, the moment when the variable force is applied (i.e. when the animal acceleration, e.g. winging, starts) there is a sudden-instant push of the whole oscillation at a distance equal to A due to this constant term (Fig. 6). For pulsing fields with on/off pulses this instant push takes place repeatedly at the onset of every next pulse, making the pulsing fields considerably more bioactive than corresponding continuous-wave fields. The maximum displacement of the ion in one direction during the forced-oscillation is always 2A (Fig. 6).
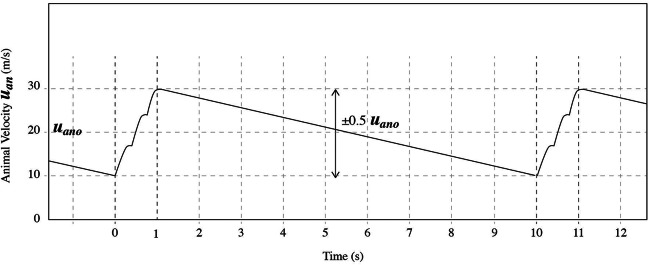
Fig. 6.
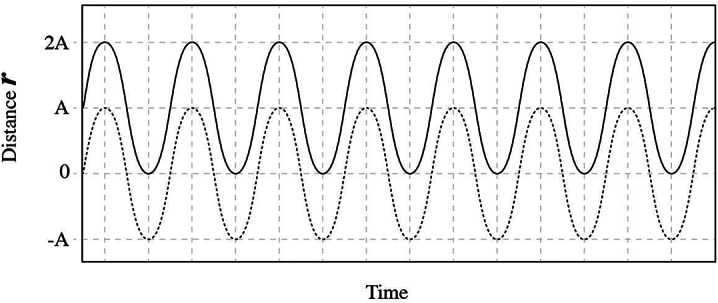
Representation of the ion oscillation (displacement r from initial position with respect to time) due to a harmonically varying applied electric or magnetic force (solid line) (as described by Eq. 7 in the case of the GMF). The same oscillation without the constant term in Eq. 7 is represented by the dashed line. In both cases the maximum ion displacement in one direction during the oscillation is 2A (A the oscillation amplitude). The moment the force is applied there is an instant displacement of the whole oscillation at a distance equal to A.
When the temporal variation in the velocity is not harmonic but periodic in general, the result is still the same, with the time dependence of the varying term not harmonic but simply periodic.
Therefore, in order for an applied oscillating EMF to be able to induce biological effects via opening/closing of VGICs it must be able to displace a z-valence mobile ion at a distance equal or greater to the distance given by Eq. 4, in other words it must induce an IFO that satisfies the condition
| 9 |
Hence, the potential of an EMF to induce VGIC gating is proportional to the IFO amplitude, and thus, proportional to its intensity and inversely proportional to its frequency (Eq. 8).[Note: In previous publications we wrote A ≥ 4 × 10–12 m for a single-valence ion and considered a double amplitude (2A) only for pulsed fields. Actually, the max displacement is always 2A whether the field is continuous-wave or pulsed.]
Let us accept that uan varies by ± 50% from the average velocity uano due to the bird’s winging (which is a reasonable variation for a moving animal), with the bird winging a few times within 1 s (accelerating), and then continuing without winging (decelerating) for another 9 s which is a usual case for a flying bird/moving animal, and that this mode of winging is repeated during its flight (Fig. 7). In such a realistic case the basic frequency of velocity variation is 0.1 Hz.
Fig. 7.
Periodic variation in the velocity uan of a migrating bird with respect to time: Increase in the velocity from 0 to 1 s by three flutters, slowing down for the next 9 s with no fluttering, acceleration by another three flutters between 10th and 11th s, and so on.
What is necessary for an animal/human to navigate is a coordinate system with a reference axis (which in this case is the GMF axis) and a zero point on it. Then, in cyclic coordinates in two dimensions, the location and orientation at each point of the journey is determined by a) the distance from the zero point, and b) the angle between animal velocity and the reference axis. We may reasonably accept that the zero point is the starting point, usually the home or birthplace of the animal.
In the following, instead of the resultant GMF intensity Bg, we shall consider separately the effects of its horizontal (Bg//) and vertical (Bg⊥) components which have unique values at every specific location along the GMF axis (i.e. at every different latitude).
Sensing the direction of motion (angle between velocity and Bg//)
The intensity of the horizontal component of the GMF Bg// at a specific location required to gate a VGIC oriented parallel to the magnetic force via one z-valence ion moving with a periodically varying animal velocity of average value uano = 20 m/s in a direction forming an angle φ with Bg//, must satisfy Condition 9:
| 10 |
Replacing uano by 0.5 uano to incorporate the velocity variation, and substituting the values of the various parameters, we get:
| 11 |
or
| 12 |
For four double-valence ions acting simultaneously on the S4 sensors, the right part is divided by 8 and the condition becomes:
| 13 |
Substituting ν by 0.1 Hz, we finally get, approximately:
| 14 |
which is the final condition for the magnitude of the horizontal component Bg// of the GMF at a specific location to be sensed by an animal moving horizontally with an average velocity uan ~ 20 m/s forming an angle φ with Bg//. For sinφ = 1, this final condition is well satisfied (by ~ 25–58 times), since Bg// ~ 15–35 μT at North African and European latitudes83.
In this case the animal velocity is vertical to Bg//. Thus, when the animal moves vertically to Bg//, i.e. approximately in the east–west direction, its motion in the GMF causes a maximum effect on VGIC gating, which may be sensed by the animal as a type of mild cellular stress. The effect on VGIC gating and the corresponding cellular stress will diminish as the angle between the animal velocity and the Bg// decreases from ± π/2 to zero depending on sinφ, and thus, the sensing will be different for every different direction of the animal motion, and the effect will be zero when the animal moves along Bg//. Because Cond 14 for sinφ = 1 or φ = ± π/2 is satisfied by ~ 25–58 times, it comes that for 0 < φ < π/2 it is still satisfied enough to provide the animal with a direction sensitivity of ~1.5–3.6 degrees which is reasonable and adequate for migration.
Thus, it seems that a migrating animal senses zero stress and has a most relaxed journey when it moves along the north–south direction of the GMF (along Bg//). The migrating animal receives a mild stress when it moves in any other direction, with this mild stress increasing as φ changes from 0 to ± π/2 and becoming maximum with φ = ± π/2 when the animal moves vertically to Bg//. This automatically provides the animal with the sense of direction in its motion. Whether the direction is left or right with regard to Bg// we may assume it is known to a migrating animal, as all animals and humans have the sense of right and left at any moment.
Therefore, we have shown that animal acceleration parallel to the earth surface with any angle other than 0 (or 180) degrees from the horizontal component of the GMF induces a magnetic force on mobile ions in VGICs in their brains/bodies capable of altering VGIC state, and, consequently, cell homeostasis. This magnetic force depends upon the horizontal component of the GMF (Bg//) which has a unique value at every different latitude, the animal velocity (uan), the angle between these two vectors, and the magnitude and rate of velocity variation. For any given values of Bg//, velocity, and velocity variation, it depends only upon sinφ as shown by Cond. 14, providing the animal with the sense of direction (i.e. orientation).
Sensing the latitude (position along Bg//)
Sensing the declination of its velocity from the GMF north–south axis alone is not enough for a migrating animal to navigate. For this, the additional sensing of its position along the north–south axis (latitude) is required. Here comes the role of the vertical component of the GMF Bg⊥: Let us consider a drifting animal (e.g. bird, mammal, fish, insect, etc.) accelerating in any direction parallel to the Earth surface and thus vertically to the vertical component of the GMF Bg⊥. In this case sinφ = 1, and the requirement for a static magnetic field and an animal average velocity uano = 20 m/s varying periodically by ± 50% in order to be able to cause VGIC gating (opening/closing) is (as Cond 13):
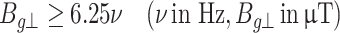 |
15 |
and again, for the accepted basic rate of 0.1 Hz in the ± 50% variation of the average velocity, the condition becomes approximately
| 16 |
The final Condition is well satisfied (by ~ 50–83 times), since Bg⊥~ 30–50 μT (for North Africa – Europe, or USA – Canada latitudes)83, and thus, the animal can sense the vertical component of the GMF during its motion. This effect is independent of the direction of animal motion and only depends upon the magnitude of the GMF vertical component Bg⊥, which is (alike Bg//) unique for every different location along the north–south direction (latitude). Thus, the animal can sense the latitude of each location along its motion. As the animal moves from the meridian toward the poles, the magnitude of the vertical component of the GMF Bg⊥ increases and vice-versa, and this provides a unique sense of its position along the GMF north–south axis.
We note that, according to our calculations, the effect of sensing the latitude is about twice stronger than the maximum effect of sensing the direction of motion (Cond. 16 is satisfied by 50–83 times, while Cond. 14 is satisfied by 25–58 times when sinφ = 1).
Electromagnetic induction and its complementary role in sensing the latitude
As we showed, the GMF can directly induce a biological response related to the declination of animal velocity from the GMF north–south axis and the latitude of the location. A similar effect in sensing the latitude can be elicited indirectly by the magnetically induced electric field Eind in the accelerating animal body according to the Faraday-Henry law of electromagnetic induction (Maxwell’s third equation of EMF):
| 17 |
(, , the magnetic and the induced electric field intensities respectively, an incremental length along a closed conductive path l of induced electric field circulation in the animal body enclosing a surface S, and the unit vector vertical to S).
The left part of Eq. 17 represents the circulation of the induced electric field along the closed line l, and the right part is the temporal change of the magnetic flow through the enclosed surface S.
Let us consider S parallel to the Earth surface. In this case the horizontal component of the GMF Bg// has no effect as the second part of Eq. 17 is zero. Since Bg⊥ is vertical to and independent of S and can be considered invariable in time at the specific location, and assuming Eind parallel to and independent of l, Eq. 17 becomes:
| 18 |
Assuming for simplicity that l is by average a circular path of radius r including the surface S, and active S swept by the magnetic flow (considering S as an ellipse the one half axis of which r΄ is in the direction of motion with its length varying in time proportionally to the animal’s acceleration a, thus dr΄/dt = aκ, and S = πrr΄, with r the other half axis) depending on animal acceleration*, we get
| 19 |
or
| 20 |
(Eind in V/m, Bg⊥ in T, r in m, a in m/s2, and κ a proportionality coefficient).*[Note: For a > 0, r΄ > r; for a < 0, r΄ < r; and for a = 0, r΄ = r. Since acceleration/deceleration interchange during motion, we may accept that average r΄equals r.]
Interestingly, we see that the induced electric field in the animal body is independent of r, the radius of the circular path, and thus independent of the animal’s size, depending only upon Bg⊥ and a.
By replacing in Eq. 20, a = 20 m/s2 the magnitude of bird acceleration during its flight that we have accepted (2 × 0.5 × 20 m/s2), and κ = 1 s for simplicity, we get:
Eind (in μV/m) = 10Bg⊥ (in μT), and for Bg⊥ ~ 30–50 μT, we get Eind ~ 300–500 μV/m, or
| 21 |
This is the induced electric field intensity along a closed conductive path in the body/brain of a flying bird/moving animal.
According to the IFO-VGIC mechanism, in order to be able to cause VGIC gating, the induced electric field Eind with circular frequency ω = 2πν (ν the frequency) and in the case of four z-valence ions oscillating in parallel and in phase, must satisfy the condition44,64–67:
| 22 |
where qe = 1.6 × C the elementary charge, and β the damping coefficient (being within an ion channel β ≅ 6.4 z × kg/s). For double-valence ions we get:
| 23 |
Substituting ν = 0.1 Hz, the variation rate in animal velocity, the condition finally becomes:
| 24 |
The final condition provides the minimum induced electric field required to elicit a biological effect via VGIC gating and, as we can see, the calculated Eind (Eq. 21) is greater by ~ 25–42 times.
Therefore, it comes that the effect of the magnetically induced electric field Eind due to electromagnetic induction is about 50% smaller than the direct effect of the vertical component of the GMF Bg⊥ (which satisfies the corresponding Cond. 16 by ~ 50–83 times), and thus, Εind contributes complementarily to the sensing of the latitude by the moving animal.
Altogether, it comes that the sensing of latitude by a migrating animal, via both the direct effect of Bg⊥ and the indirect effect of Eind, is about 3 times stronger than the maximum sensing of its direction of motion via Bg//.
The case of stationary or slow-moving animals that quickly move their heads/bodies
The above numerical results refer to fast moving animals that accelerate towards the direction of their journeys (such as birds, fishes, wild mammals, hunting dogs, etc.). Yet, the described mechanism can also work for animals that move slowly or they are even confined/tethered but quickly move their heads/bodies perpendicularly to their body axis, i.e. left–right or up-down with respect to their body axis. In such a case we can assume similar movement rates as in Fig. 7, i.e. an animal that moves its body perpendicularly to its body axis three times left–right per second and then remains still for the next few seconds and all this is repeated every 10 s. We may assume a reasonable average left–right (or up-down) velocity of its head/body of approximately 1 m/s and a corresponding acceleration of approximately 12 m/s2. We have 20 times smaller velocity than in the previous calculations (for Cond. 14, 16, and Eq. 21) but we now have 100% variation in the velocity (it varies from –uano to uano) , and applying Cond. 10 we get approximately ten times weaker effect. Specifically we get Bg// sinφ ≥ 500ν /z (ν in Hz, Bg// in μT) instead of Cond 12, which for four double-valence ions, it becomes Bg// sinφ ≥ 62.5ν, and for ν = 0.1 Hz, we finally get approximately Bg// sinφ ≥ 6 μT which is satisfied by ~ 2.5–5.8 times for sinφ = 1 (which is now the case when the animal body axis is parallel to the GMF north–south axis). This shows that even a confined or tethered animal with zero velocity forward can still sense its orientation with respect to the GMF north–south axis by quickly moving its head/body perpendicularly to its body axis. Similarly, we can get a corresponding condition to Cond 16 (with approximately ten times weaker effect) for the sensing of the latitude (vertical GMF component) Bg⊥ ≥ 6 μT, (satisfied by ~ 5–8.3 times), but only when the animal moves its head/body left–right (sinφ = 1) and not up-down, as in the latter case sinφ = 0 and there is no effect. Finally we can get a corresponding equation to Eq. 20 for the induced electric field in its head/body, in this case due to the horizontal GMF component Eind = (Bg// /2) aκ with ~ 1.7 times weaker effect due to the smaller acceleration (20/12). This gives Eind ~ (18–29) × 10–5 V/m (instead of Eq. 21) which satisfies Cond 24 by ~ 15–24 times. In this case the induced electric field complements the sensing of the horizontal GMF component (orientation).
Thus, even stationary (confined/tethered) animals that quickly move their heads/bodies can sense their position and orientation in a lesser but still significant degree than the fast-travelling animals. In fact, all animals in some degree move their heads/bodies perpendicularly to their body axis during their journeys, increasing in this way their sensitivity of navigation.
Figure 8 shows a graphical summary of the mechanism described above for animal magnetoreception, orientation, and navigation.
Fig. 8.
Graphical Summary of the mechanism of animal magnetoreception, orientation, and navigation.
Discussion
Review articles have underscored the inability of previously suggested biophysical mechanisms to explain animal magnetoreception, orientation and navigation on Earth1,3,4,10, and more specifically, the “lack of biophysical mechanism through which the weak GMF might lead to the controlled depolarization of a sensory nerve membrane”10. In the present study we have described such a biophysical mechanism, even though we did not consider membrane depolarization specifically, but VGIC gating, which is a closely connected event, as VGICs are normally gated by membrane voltage-changes (including depolarizations) and vice-versa68,69,77. Specifically, we have described a biophysical mechanism of controlled VGIC-gating in the animal cells, due to magnetic (or magnetically induced) forces depending on animal position and direction of motion, and consequent alterations of intracellular concentrations of critical ions (K+, Na+, Ca2+, etc.). These controlled alterations in cell homeostasis provide the animal with the sense of orientation and position on Earth and, thus, with the ability of navigation.
We have shown that the angle between animal velocity and GMF axis (direction of motion) as well as the position along the GMF axis (latitude) can be sensed by moving and periodically accelerating animals on Earth. The sensing is accomplished via the change elicited by the horizontal and vertical components of the GMF intensity and the induced electric field in the electrochemical balance of the plasma membranes in the animal cells, which in turn determines redox state and homeostasis in all cells, including neural. Hunting dogs have been observed to run a fast curse of 20–30 m before taking the direction to return home50,84. By doing this, they seem to activate their biological compass and their sense of orientation and position as the present study describes. Since nerve cells, especially in the brain, have higher percentages of VGICs than other types of cells/tissues, they are reasonably expected to be more affected in their electrochemical balance/homeostasis by EMFs including the GMF.
As repeatedly reported in the EMF-bioeffects literature, living organisms are not particularly affected by static electric or magnetic fields but mostly by oscillating/varying (and polarized) ones. This is of particular importance for understanding the IFO-VGIC mechanism, and the essence of the biological effects of EMFs, as the VGICs are not gated by the normal voltage/electric field across the cell membrane, but only by membrane voltage changes of the order of 30% in this voltage/field that cause membrane depolarization. In other words, VGICs do not respond simply to the presence of an invariable (static) electric field. The same holds for the static magnetic fields. This is the reason why the GMF and the GEF are not particularly bioactive under normal conditions but they become bioactive when ~20% changes in their normal intensities occur during magnetic storms6. Biological effects from static electric or magnetic fields have been reported in the literature and they may be due to either very strong intensities that can directly interfere with the endogenous electric currents in cells and tissues, or to the onset/removal of the static field exposure, or to various other fields in the form of noise that most of the times coexist with the static fields and are due to the electric/electronic circuits that are used to generate the static fields. This should be investigated by those groups who have reported biological effects of static electric or magnetic fields.
As most people have observed, birds wing only sporadically during flight. They usually wing a few times and then fly a long distance for several seconds without winging. Similarly, fishes and other aquatic animals move their bodies/tails left–right or up-down a few times and then drift for several seconds while their bodies are still. As the present study shows, this is obviously done not only for energy saving reasons, but also for activating their sensing of orientation and position. We showed that according to the IFO-VGIC mechanism, the lower the winging (or body movement) rate (frequency of velocity variation) the stronger the induced effect and the corresponding sensing of their orientation and position. This comes from the IFO-VGIC theory44,64–67 which shows that the bioactivity of an applied EMF, represented by the amplitude of the ion forced oscillation, is inversely proportional to its frequency (Eq. 8). The physical explanation of this result is that, due to inertia, the higher the frequency of the applied force, the smaller the amplitude of the forced oscillation, as the ion cannot follow the fast changes of the force, except if its oscillation amplitude becomes smaller.
The effect in a moving animal’s brain/body due to periodic acceleration within the GMF, as estimated in the present study, varies according to the animal’s velocity, and acceleration, with velocity variation rates in the ULF (0–3 Hz) range. The dependence on velocity, velocity variation, and frequency (variation rate) is shown in Eqs. 1, 5, 20, and Conditions 10, 13, 15. The forces exerted on the VGIC S4 voltage sensors are slow-varying at ULF rates, arising from the variations in the animal velocity. The slow-varying forces are in a specific direction related to the direction of motion, and thus are polarized which is a condition for the application of the IFO-VGIC mechanism66,67. These polarized slow-varying electromagnetic forces arising whenever the animal accelerates (e.g. when a bird flutters) or decelerates, are applied on the VGIC S4 sensors in the plasma membranes of various cells at different angles with respect to the channel axes depending on the orientation of each VGIC. Those channels that their axes are oriented parallel to the forces (vertically to the GMF intensity) will receive forces on their voltage-sensors most effective in altering their channel state from opened to closed and vice-versa.
Our present study shows that animals/living organisms do not need specific organs/cells for sensing polarized EMFs (including the GMF), as they are all equipped with VGICs in great numbers in all their cell membranes, especially in neurons/brain cells, which according to the IFO-VGIC mechanism can sense ELF/ULF electric fields down to ~10–5 V/m44,64–67. This explains all known EMF-bioeffects including magnetoreception. Yet, this does not contradict the role of specific organs in certain electrosensitive animals that move slower than e.g. birds such as the ampullae of Lorenzini in elesmobranches which may further amplify VGIC signals.
We demonstrated by accurate calculations that migrating animals can sense; a) their direction of motion with respect to the GMF north–south axis through the effect exerted on them by the horizontal component of the GMF (Bg//), and b) their latitude through the direct effect exerted on them by the vertical component of the GMF (Bg⊥), and complementarily through the indirect effect of the corresponding induced electric field. These effects on animals take place through the variations in the force exerted on their VGIC sensors due to corresponding variations in their velocity.
Because the GMF varies by less than 1 μT over distances of several km along the GMF north–south direction, and we have shown (Cond. 14) than the weakest magnetic field a moving animal can detect is ~0.6 μT, migrating animals may not sense differences within less than ~10 km along the axis, and therefore they use additional senses such as vision, smell, etc., and environmental signals, such as differences in humidity, temperature, altitude/atmospheric pressure, etc. to find exact locations2,4,6. Perhaps this difficulty in sensing the latitude by a migrating animal due to the small differences in GMF intensity per km is the reason why the sensitivity in the latitude (resulting from the combined effect of Bg⊥ and Eind) is found to be about 3 times higher than the max sensitivity in the direction of motion.
It should be noted that in the solution (Eq. 7) of the differential equation (Eq. 5) for the ion forced-oscillation due to the oscillating force exerted by the externally applied EMF (in this case the GMF), the constant term does not actually increase the ion maximum displacement which is always twice the amplitude of the forced oscillation (Cond. 9) but it doubles the ion displacement only during the first semi-period, and displaces the whole oscillation at a distance equal to its amplitude. With a pulsing field emitting on/off pulses, the displacement of the whole oscillation is repeated in a unique direction with the onset of every successive pulse. Most importantly, the pulse repetition frequency is always significantly smaller than the carrier frequency within the pulses, making the pulsed EMF exposure significantly more bioactive, as bioactivity is inversely proportional to the frequency according to the IFO-VGIC theory (Eq. 8, 10). These are the reasons why pulsing EMFs are found in many studies to be more bioactive than uninterrupted (continuous-wave) EMFs of the same intensity and carrier frequency. For a review of comparison studies between pulsing and corresponding continuous-wave manmade EMFs with respect to their bioactivity, see85.
Even though for standing animals/humans on Earth, it has been shown that a ULF/ELF electric field may be more bioactive than a magnetic field of the same frequency and waveform in inducing VGIC gating26,44,64,65,67, the magnetic field becomes increasingly bioactive for moving animals, especially fast moving ones such as birds, or humans in fast moving vehicles, as the magnetic force on the ions close to VGIC sensors is proportional to the animal/human velocity (Eq. 1). This increases the bioactivity of magnetic fields in the case of moving animals/humans than in the case of stationary ones. Indeed, for a still stationary animal, the maximum ion velocity is that through an open ion channel (0.25 m/s)64,67, while in the case of a moving animal with u = 20 m/s the velocity of the ions and the corresponding GMF force on them is 80 times greater. In cases of stationary or very slowly moving animals the effect of the magnetic field (including the GMF) can again become significant when the animal quickly moves its head/body left–right or up-down with respect to its body axis.
Certainly, anthropogenic ELF/ULF magnetic fields of similar magnitude to the GMF, e.g. from electric power transmission lines, wireless communication base antennas/towers, etc., can interfere locally with the GMF distorting its intensity and/or direction, and thus disrupting the ability of animals to orient and navigate25,47,55,86.
We showed that the IFO-VGIC mechanism can explain the orientation and navigation ability of the moving and accelerating animals on Earth. This may contribute significantly to resolving the problem of animal magnetoreception, which has been a mystery for centuries and has remained as such until today, despite the systematic research on this during the past 50–60 years (see1–4,10).
We showed that the IFO-VGIC mechanism plausibly explains animal magnetoreception, orientation and navigation according to established physical laws, molecular/biological data, and accurate mathematical operations. Additional confirmation on the validity of this mechanism is: a) Already in Panagopoulos et al. (2002)65 (Fig. 3) it was shown that the intensities and frequencies used in many experiments that had shown ELF EMF bioeffects in cells and animals were within the bioactive values predicted by this theory. Since then, it has been shown that this theory explains most (if not all) recorded bioeffects including oxidative stress and genetic damage induced by anthropogenic ELF EMFs44,67. b) The same mechanism has explained the sensing of upcoming thunderstorms by sensitive individuals87, and the sensing of upcoming earthquakes by animals88through the action of the partially polarized natural ULF/ELF EMFs associated with these phenomena. c) It is experimentally confirmed that ELF EMFs can gate VGICs in cell membranes of various cell types and alter intracellular ionic concentrations at very low field intensities49,89–92. d) It is experimentally confirmed that ELF EMFs induce biological effects in isolated tissues at very low threshold intensities (~ 10–3V/m)79,80 as those predicted by this theory. e) It is experimentally confirmed that ELF EMFs are sensed by animals at very low threshold intensities (~ 10–5-10–4V/m) coinciding with those predicted by this theory1,70. Such evidence does not exist for the previously published hypotheses.
For further and more specific experimental confirmation of the IFO-VGIC mechanism in animal orientation and navigation, an experimental setup could possibly consist of a) a proper biological system (e.g. cell cultures or isolated cells/neurons); b) similar conditions of motion as those described in our numerical calculations (e.g. as in Fig. 7 and/or for a stationary animal that quickly moves its head/body perpendicularly to its body axis); c) assessment of intracellular concentrations of critical ions (e.g. Ca2+, Na+, K+ etc.) and/or measurement of VGIC ion currents before and after motion, without and with use of channel blockers; d) testing of different motion orientations with respect to the north–south GMF axis.
In contrast to the previously suggested hypotheses to explain animal magnetoreception which restricted in qualitative descriptions on how these mechanisms might work at cellular level, our proposed mechanism results in accurate numerical predictions and a complete description of the VGIC-gating effect induced in the cells of a moving and periodically accelerating animal on Earth, depending on the specific values of GMF intensity, animal velocity, acceleration, and acceleration rate, which in turn determine animal orientation and navigation. While previously suggested hypotheses were unrealistically complicated, none of them had specifically shown how exactly migrating animals determine their direction and position and they only restricted in searching how the GMF could elicit a cellular/biological effect in general, without finding that either.
Kirschvink et al.10 stated that over the past years “a plethora of biophysical transduction hypotheses have been winnowed down to two or three that have experimental support: some form of electrical induction, specialized receptor cells involving biogenic magnetite …and that of weak-field magnetic effects on photochemically generated radical pairs in the eye”. But they did not consider our proposed mechanism which was already published64,65, as well as other previous publications focusing on the VGICs for the explanation of the effects of magnetic fields on living cells/organisms71–73. Moreover, they and others did not consider the vast and undeniable experimental evidence that environmentally existing anthropogenic polarized EMFs affect VGICs and consequently alter the cell redox status and homeostasis initiating a plethora of biological/health effects (see reviews in44,49,90,93).
The scientific literature contains already thousands of studies during the past 60 years presenting a panorama of biological/health effects of various types of man-made EMFs on a wide variety of animals (including humans) and cell/tissue types, and in addition, a plausible biophysical mechanism explaining all those recorded effects is published for more than 20 years (having already been referenced in more than a thousand other publications altogether). Yet, the research focusing on the explanation of animal magnetoreception did not seem to be affected by the above scientific progress, and insisted on specific hypothetical mechanisms namely the magnetite and the radical pair hypotheses, even though it was admitted that there was no progress with resolving the problem in this way3,4,10,11,36,38.
Here, we have presented a plausible biophysical mechanism for animal magnetoreception, orientation and navigation, based on molecular data for the structure and function of VGICs in all animals, and combined with accurate mathematical calculations predicting that the GMF is indeed able to affect cell homeostasis and thus be sensed by migrating animals.
Acknowledgements
The study was supported by the Special Account for Research Grants of the National and Kapodistrian University of Athens. We thank graphic designer Angela Dounas for precious help with the figures.
Abbreviations
- ELF
Extremely Low Frequency
- EMF
Electromagnetic field
- ESR
Electron Spin Resonance
- GEF
Geoelectric field
- GMF
Geomagnetic field
- IFO
Ion forced oscillation
- RF
Radio Frequency
- ULF
Ultra Low Frequency
- VGIC
Voltage-gated ion channel
- VLF
Very Low Frequency
Author contributions
D.J.P. designed the study, wrote equations, preformed calculations, and wrote the main manuscript. A.K. checked all equations and calculations, reviewed and evaluated all data. G.P.C. reviewed and evaluated all data.
Data availability
All data generated or analyzed during this study are included in this published article and its references.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johnsen, S. & Lohmann, K. J. Magnetoreception in animals. Phys. Today61(3), 29–35 (2008). [Google Scholar]
- 2.Komolkin, A. V. et al. Theoretically possible spatial accuracy of geomagnetic maps used by migrating animals. J. R. Soc. Interface14, 20161002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann, G. C., Hochstoeger, T. & Keays, D. A. Magnetoreception - A sense without a receptor. PLoS Biol15(10), e2003234. 10.1371/journal.pbio.2003234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mouritsen, H. Long-distance navigation and magnetoreception in migratory animals. Nature558, 50–59 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Presman, A. S. Electromagnetic Fields and Life (Plenum Press, 1977). [Google Scholar]
- 6.Dubrov, A. P. The Geomagnetic Field and Life (Plenum Press, 1978). [Google Scholar]
- 7.Panagopoulos D. J. Electromagnetic Interaction between Environmental Fields and Living Systems determines Health and Well-Being, In “Electromagnetic Fields: Principles, Engineering Applications and Biophysical Effects”, Nova Science Publishers, New York. (2013).
- 8.Creasey WA and Goldberg RB, (2001), A new twist on an old mechanism for EMF bioeffects?, EMF Health Report, 9 (2), 1–11. [Available in: https://www.emfsa.co.za/wp-content/uploads/2021/06/Creasey-WA-Goldberg-RB-2001-A-new-twist-on-an-old-mechanism-for-EMF-bioeffects.pdf]
- 9.Johnsen, S. & Lohmann, K. J. The physics and neurobiology of magnetoreception. Nat Rev Neurosci6, 703–712. 10.1038/nrn1745 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Kirschvink JL, Winklhofer M, Walker MM, (2010). Biophysics of magnetic orientation: strengthening the interface between theory and experimental design. J R Soc Interface. (Suppl 2):S179–91. [DOI] [PMC free article] [PubMed]
- 11.Shaw J, Boyd A, House M, et al, (2015). Magnetic particle-mediated magnetoreception. J. R. Soc. Interface, 20150499. 10.1098/rsif.2015.0499 [DOI] [PMC free article] [PubMed]
- 12.Kirschvink, J. L. Magnetite biomineralization and geomagnetic sensitivity in higher animals: an update and recommendations for future study. Bioelectromagnetics.10(3), 239–259 (1989). [DOI] [PubMed] [Google Scholar]
- 13.Kirschvink, J. L., Kobayashi-Kirschvink, A. & Woodford, B. J. Magnetite biomineralization in the human brain. Proc Natl Acad Sci U S A89, 7683–7687 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowenstam, H. A. Magnetite in denticle capping in recent chitons (Polyplacophora). Geol. Soc. Am. Bull.73(4), 435–438 (1962). [Google Scholar]
- 15.Bellini, S. Su di un particolare comportamento di batteri d’acqua dolce (On a unique behavior of freshwater bacteria) (University of Pavia, Italy, 1963). [Google Scholar]
- 16.Bellini, S. Ulteriori studi sui “batteri magnetosensibili” (Further studies on magnetosensitive bacteria) (University of Pavia, Italy, 1963). [Google Scholar]
- 17.Blakemore, R. P. Magnetotactic bacteria. Science190, 377–379 (1975). [DOI] [PubMed] [Google Scholar]
- 18.Blakemore, R. P. Magnetotactic bacteria. Annu. Rev. Microbiol.36, 217–238 (1982). [DOI] [PubMed] [Google Scholar]
- 19.Walker, M. M. et al. Structure and function of the vertebrate magnetic sense. Nature390, 371–376 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Diebel, C. E., Proksch, R., Green, C. R., Neilson, P. & Walker, M. M. Magnetite defines a vertebrate magnetoreceptor. Nature406, 299–302 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Cadiou, H. & McNaughton, P. A. Avian magnetite-based magnetoreception: a physiologist’s perspective. JFR Soc. Interface7(suppl 2), 193–205. 10.1098/rsif.2009.0423.focus (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winklhofer, M. & Kirschvink, J. L. A quantitative assessment of torque-transducer models for magnetoreception. J. R. Soc. Interface7, 273–289. 10.1098/rsif.2009.0435.focus (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eder, S. H. K. et al. Magnetic characterization of isolated candidate vertebrate magnetoreceptor cells. Proc. Natl. Acad. Sci.109(30), 12022–12027 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavokin, K. V. Compass in the ear: can animals sense magnetic fields with hair cells?. Eur. Phys. J. Spec. Top.232, 261–268. 10.1140/epjs/s11734-022-00654-y (2023). [Google Scholar]
- 25.Ernst, D. A. & Lohmann, K. Effect of magnetic pulses on Caribbean spiny lobsters: implications for magnetoreception. J. Exp. Biol.219(Pt 12), 1827–1832. 10.1242/jeb.136036 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Goodman, E. M., Greenebaum, B. & Marron, M. T. Effects of Electro- magnetic Fields on Molecules and Cells, International Rev. Cytol.158, 279–338 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Huber, R. et al. Electromagnetic fields, such as those from mobile phones, alter regional cerebral blood flow and sleep and waking EEG. J Sleep Res.11(4), 289–295 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Santini, M. T., Ferrante, A., Rainaldi, G., Indovina, P. & Indovina, P. L. Extremely low frequency (ELF) magnetic fields and apoptosis: a review. Int J Radiat Biol.81(1), 1–11 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Panagopoulos, D. J. (ed.) Electromagnetic Fields of Wireless Communications: Biological and Health Effects 1st edition. (CRC Press, 2023). [Google Scholar]
- 30.Malkemper, E. P. et al. No evidence for a magnetite-based magnetoreceptor in the lagena of pigeons. Current Biology29(1), R14–R15 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Treiber, C. D. et al. Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons. Nature484, 367–370 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Edelman, N. B. et al. No evidence for intracellular magnetite in putative vertebrate magnetoreceptors identifed by magnetic screening. Proc. Natl Acad. Sci. USA112, 262–267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulten, K., Swenberg, C. E. & Weller, A. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z. Für Phys. Chem.111(1), 1–5 (1978). [Google Scholar]
- 34.Ritz, T., Adem, S. & Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J.78(2), 707–718 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritz, T., Thalau, P., Phillips, J. B., Wiltschko, R. & Wiltschko, W. Resonance effects indicate a radicalpair mechanism for avian magnetic compass. Nature429, 177–180 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Ritz, T., Ahmad, M., Mouritsen, H., Wiltschko, R. & Wiltschko, W. Photoreceptor-based magnetoreception: optimal design of receptor molecules, cells, and neuronal processing. J. R. Soc. Interface7, S135–S146 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiltschko, W. & Wiltschko, R. Light-dependent magnetoreception in birds: the behaviour of European robins, Erithacus rubecula, under monochromatic light of various wavelengths and intensities. J Exp Biol204, 3295–3302 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Hore, P. J. & Mouritsen, H. The Radical-Pair Mechanism of Magnetoreception. Annu Rev Biophys45, 299–344 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Worster, S., Mouritsen, H. & Hore, P. J. A light-dependent magnetoreception mechanism insensitive to light intensity and polarization. J. R. Soc. Interface14, 20170405. 10.1098/rsif.2017.0405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiltschko, R. & Wiltschko, W. Magnetoreception in birds. J. R. Soc. Interface16, 20190295. 10.1098/rsif.2019.0295 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casarett, A. P. Radiation Biology (Prentice-Hall Inc, 1968). [Google Scholar]
- 42.Coggle, J. E. Biological Effects of Radiation (Taylor & Francis, 1983). [Google Scholar]
- 43.Hall, E. J. & Giaccia, A. J. Radiobiology for the Radiologist 6th edn. (Lippincott Williams & Wilkins, 2006). [Google Scholar]
- 44.Panagopoulos, D. J., Karabarbounis, A., Yakymenko, I. & Chrousos, G. P. Human-made electromagnetic fields: Ion forced-oscillation and voltage-gated ion channel dysfunction, oxidative stress and DNA damage. International Journal of Oncology59, 92 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swartz, H. M. et al. (eds) Biological Applications of ESR (John Wiley and sons, 1972). [Google Scholar]
- 46.Sober, E. The Principle of Parsimony. Brit. J. Phil. Sci.32, 145–156 (1981). [Google Scholar]
- 47.Engels, S. et al. Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature509, 353–356 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Fedele, G. et al. Genetic analysis of circadian responses to low frequency electromagnetic fields in Drosophila melanogaster. PLoS Genet10, e1004804. 10.1371/journal.pgen.1004804 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertagna, F., Lewis, R., Silva, S. R. P., McFadden, J. & Jeevaratnam, K. Effects of electromagnetic fields on neuronal ion channels: a systematic review. Ann N Y Acad Sci.1499(1), 82–103 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Balmori A, (2022). Effects of Man-made and Especially Wireless Communication Electromagnetic Fields on Wildlife. In: DJ Panagopoulos (Editor), “Electromagnetic Fields of Wireless Communications: Biological and Health Effects”, CRC Press, Taylor and Francis, Boca Raton.
- 51.Alonso M and Finn EJ, (1967): Fundamental University Physics, Vol. 2: Fields and Waves, Addison-Wesley, USA.
- 52.Kalmijn, A. J. The electric sense of sharks and rays. J. Exp. Biol.55, 371–383 (1971). [DOI] [PubMed] [Google Scholar]
- 53.Kalmijn AJ, (1973). Electro-orientation in sharks and rays: Theory and experimental evidence. Office of Naval Research, National Technical Information Service, US Department of Commerce.
- 54.Kalmijn, A. J. Electric and magnetic field detection in elasmobranch fishes. Science218, 916–918 (1982). [DOI] [PubMed] [Google Scholar]
- 55.Meyer, C. G., Holland, K. N. & Papastamatiou, Y. P. Sharks can detect changes in the geomagnetic field. J R Soc Interface2, 129–130 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jungerman, R. L. & Rosenblum, B. Magnetic induction for the sensing of magnetic fields by animals–an analysis. J. Theor. Biol.87, 25–32 (1980). [DOI] [PubMed] [Google Scholar]
- 57.Albert L, Deschamps F, Jolivet A, Olivier F, Chauvaud L, et al., (2020): A current synthesis on the effects of electric and magnetic fields emitted by submarine power cables on invertebrates. Marine Environmental Research, 159, pp.104958.ff10.1016/j.marenvres. 2020.104958ff. ffhal-02559660. [DOI] [PubMed]
- 58.Bellono NW, Leitch DB, (2017). Julius D. Molecular basis of ancestral vertebrate electroreception. Nature. 543(7645):391–96. [DOI] [PMC free article] [PubMed]
- 59.Bellono, N. W., Leitch, D. B. & Julius, D. Molecular tuning of electroreception in sharks and skates. Nature558, 122–126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nimpf, S. et al. A Putative Mechanism for Magnetoreception by Electromagnetic Induction in the Pigeon Inner Ear. Current Biology29, 4052–4059 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Nuccitelli R, (2011), Measuring Endogenous Electric Fields. In: C.E. Pullar (Ed), The Physiology of Bioelectricity in Development, Tissue Regeneration and Cancer, CRC Press, Taylor and Francis, Boca Raton.
- 62.Levin M. Endogenous Bioelectric Signals as Morphogenetic Controls of Development, Regeneration, and Neoplasm. In: C.E. Pullar (Ed), The Physiology of Bioelectricity in Development, Tissue Regeneration and Cancer, CRC Press, Taylor and Francis, Boca Raton. (2011).
- 63.Titushkin I, Sun S, Rao V, and Cho M. Stem Cell Physiological Responses to Noninvasive Electrical Stimulation. In: C.E. Pullar (Ed), The Physiology of Bioelectricity in Development, Tissue Regeneration and Cancer, CRC Press, Taylor and Francis, Boca Raton (2011).
- 64.Panagopoulos, D. J., Messini, N., Karabarbounis, A., Filippetis, A. L. & Margaritis, L. H. A Mechanism for Action of Oscillating Electric Fields on Cells. Biochemical and Biophysical Research Communications272(3), 634–640 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Panagopoulos, D. J., Karabarbounis, A. & Margaritis, L. H. Mechanism for Action of Electromagnetic Fields on Cells. Biochemical and Biophysical Research Communications298(1), 95–102 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Panagopoulos DJ, Johansson O, Carlo GL, (2015): Polarization: A Key Difference between Man-Made and Natural Electromagnetic Fields, in regard to Biological Activity, Scientific Reports 5, 14914; 10.1038/srep14914. [DOI] [PMC free article] [PubMed]
- 67.Panagopoulos DJ, (2022): Mechanism of Ion Forced-Oscillation and Voltage-Gated Ion Channel Dysfunction by Polarized and Coherent Electromagnetic Fields. In: DJ Panagopoulos (Editor), “Electromagnetic Fields of Wireless Communications: Biological and Health Effects”, CRC Press, Boca Raton.
- 68.Alberts, B. et al. Molecular Biology of the Cell (Garland Publishing Inc, 1994). [Google Scholar]
- 69.Stryer L. (1995), Biochemistry, 4th ed., W.H. Freeman and Co, New York.
- 70.England, S. J. & Robert, D. The ecology of electricity and electroreception. Biological Reviews97, 383–413 (2022). [DOI] [PubMed] [Google Scholar]
- 71.Cain, C. A. Biological effects of oscillating electric fields: role of voltage-sensitive ion channels. Bioelectromagnetics2(1), 23–32 (1981). [DOI] [PubMed] [Google Scholar]
- 72.McLeod, B. R., Liboff, A. R. & Smith, S. D. Electromagnetic gating in ion channels. J Theor Biol.158(1), 15–31 (1992). [DOI] [PubMed] [Google Scholar]
- 73.Balcavage, W. X. et al. A mechanism for action of extremely low frequency electromagnetic fields on biological systems. Biochem. Biophys. Res. Commun.222, 374–378 (1996). [DOI] [PubMed] [Google Scholar]
- 74.Noda, M. et al. Existence of distinct sodium channel messenger RNAs in rat brain. Nature320, 188–192 (1986). [DOI] [PubMed] [Google Scholar]
- 75.Liman, E. R., Hess, P., Weaver, F. & Koren, G. Voltage sensing residues in the S4 region of a mammalian K+ channel. Nature353, 752–756 (1991). [DOI] [PubMed] [Google Scholar]
- 76.Miller C, (2000). An overview of the potassium channel family. Genome Biol 1(4). [DOI] [PMC free article] [PubMed]
- 77.Sandipan C, and Baron C. (2015): Basic mechanisms of voltage sensing, In: Zheng J, and Trudeau MC (Eds), Handbook of Ion Channels, CRC Press, Boca Raton.
- 78.Zhang, X. C., Yang, H., Liu, Z. & Sun, F. Thermodynamics of voltage-gated ion channels. Biophys Rep4(6), 300–319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McLeod, K. J., Lee, R. C. & Ehrlich, H. P. Frequency dependence of electric field modulation of fibroblast protein synthesis. Science236(4807), 1465–1469 (1987). [DOI] [PubMed] [Google Scholar]
- 80.Cleary, S. F., Liu, L. M., Graham, R. & Diegelmann, R. F. Modulation of tendon fibroplasia by exogenous electric currents. Bioelectromagnetics9, 183–194 (1988). [DOI] [PubMed] [Google Scholar]
- 81.Honig, B. H., Hubbell, W. L. & Flewelling, R. F. Electrostatic Interactions in Membranes and Proteins. Ann. Rev. Biophys. Biophys. Chem15, 163–193 (1986). [DOI] [PubMed] [Google Scholar]
- 82.Panagopoulos, D. J. Comparing DNA Damage Induced by Mobile Telephony and Other Types of Man-Made Electromagnetic Fields. Mutation Research Reviews781, 53–62 (2019). [DOI] [PubMed] [Google Scholar]
- 83.NOAA (US National Oceanic and Atmospheric Administration) (2023): Magnetic Field Estimated Values (https://www.ngdc.noaa.gov/geomag/calculators/magcalc.shtml# igrfwmm)
- 84.Benediktová, K. et al. Magnetic alignment enhances homing efficiency of hunting dogs. Elife9, e55080 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Panagopoulos DJ, Karabarbounis A, Lioliousis C. Defining Wireless Communication (WC) Electromagnetic Fields (EMFs): A. Polarization is a principal property of all man-made EMFs; B. Modulation, pulsation, and variability are inherent parameters of WC EMFs; C. Most man-made EMF exposures are non-thermal; D. Measuring incident EMFs is more relevant than Specific Absorption Rate (SAR); E. All man-made EMFs emit continuous waves, not photons; F. Differences from natural EMFs. Interaction with matter. In: DJ Panagopoulos (Editor), “Electromagnetic Fields of Wireless Communications: Biological and Health Effects”, CRC Press, Boca Raton (2022).
- 86.Hutchison, Z. L., Gill, A. B., Sigray, P., He, H. & King, J. W. Anthropogenic electromagnetic fields (EMF) influence the behaviour of bottom-dwelling marine species. Sci Rep.10(1), 4219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panagopoulos, D. J. & Balmori, A. On the biophysical mechanism of sensing atmospheric discharges by living organisms. Science of the Total Environment599–600(2017), 2026–2034 (2017). [DOI] [PubMed] [Google Scholar]
- 88.Panagopoulos, D. J., Balmori, A. & Chrousos, G. P. (2020): On the biophysical mechanism of sensing upcoming earthquakes by animals. Sci Total Environ.717, 136989 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Piacentini, R., Ripoli, C., Mezzogori, D., Azzena, G. B. & Grassi, C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Cav1-channel activity. J. Cell. Physiol.215, 129–139 (2008). [DOI] [PubMed] [Google Scholar]
- 90.Pall, M. L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med17, 958–965 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cecchetto C, Maschietto M, Boccaccio P, Vassanelli S, (2020): Electromagnetic field affects the voltage-dependent potassium channel Kv1.3. Electromagn Biol Med. 39(4):316–322. [DOI] [PubMed]
- 92.Zheng, Y., Xia, P., Dong, L., Tian, L. & Xiong, C. Effects of modulation on sodium and potassium channel currents by extremely low frequency electromagnetic fields stimulation on hippocampal CA1 pyramidal cells. Electromagn Biol Med.17, 1–12 (2021). [DOI] [PubMed] [Google Scholar]
- 93.Panagopoulos DJ, Yakymenko I, and Chrousos GP. Electromagnetic field-induced dysfunction of voltage-gated ion channels, oxidative stress, DNA damage, and related pathologies. In: DJ Panagopoulos (Editor), “Electromagnetic Fields of Wireless Communications: Biological and Health Effects”, CRC Press, Boca Raton (2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its references.