Abstract
Small interfering RNAs (siRNA) technology has emerged as a promising therapeutic tool for human health conditions like cancer due to its ability to regulate gene silencing. Despite FDA-approved, their delivery remains localized and limiting their systemic use. This study used single-walled carbon nanotubes (SWNTs) functionalized with polyethylene glycolated (PEGylated) phospholipids (PL-PEG) derivatives for systemic siRNA delivery. We developed an siRNA systemic delivery vehicle (SWNT-siRNA) by conjugating SWNT functionalized with PL-PEG containing either amine (PA) or maleimide (MA). The functionalized SWNT with a lower molecular weight of PA produced the SWNT-siRNA conjugate system with the highest stability and high siRNA loading quantity. The system delivered siRNA to a panel of tumour cell lines of different organs (i.e. HeLa, H1299 and MCF-7) and a non-cancerous human embryonic kidney 293 cells (HEK293T) with high biocompatibility and low toxicity. The cellular uptake of SWNT-siRNA conjugates by epithelial cells was found to be energy dependent. Importantly, the presence of P-glycoprotein, a marker for drug resistance, did not inhibit SWNT-mediated siRNA delivery. Mouse xenograft model further confirmed the potential of SWNT-siRNA conjugates with a significant gene knock-down without signs of acute toxicity. These findings pave the way for potential gene therapy applications using SWNTs as delivery vehicles.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80646-1.
Keywords: Single-walled carbon nanotubes, siRNA delivery, PEGylated phospholipid, Gene silencing
Subject terms: Biotechnology, Chemical biology, Drug delivery
Introduction
The potential of small interfering RNAs (siRNA) technology as a promising therapeutic intervention for diseases like cancer is undeniable. The Food and Drug Administration (FDA) approved the first siRNA drug, patisiran in August 2018 1; givosiran in November 2019 2; and lumasiran in November 2020. At present, seven siRNA drugs, namely vutrisiran, nedosiran, inclisiran, fitusiran, teprasiran, cosdosiran, and tivanisiran, are being evaluated in Phase III clinical trials3–5. While these agents showed promising results, their application is primarily limited to localized delivery to a certain target organ. This limitation makes these agents less useful for systemic diseases such as cancers that can metastasise to other organs, systemic autoimmune disorders, systemic infections, or genetic diseases that affect multiple organ systems. As such, it is pivotal to break through this challenge to design an effective siRNA agent for the treatment of systemic diseases.
Systemic delivery for siRNA therapeutics remained a significant challenge due to a variety of biological and technical factors. One of the key obstacles is maintaining the stability of siRNA molecules in the bloodstream, as they are prone to quick degradation by serum nucleases6. Additionally, these molecules need to efficiently penetrate cell membranes and escape endosomes to enact their gene-silencing effects—a feat that is challenging to achieve6,7. The kidneys can swiftly remove siRNA molecules from circulation, reducing their time to interact with target cells, which hampers the therapeutic efficacy6,7. Further, siRNA may inadvertently interfere with non-target genes, leading to unwanted off-target effects8. They can also trigger undesirable immune responses, diminishing therapy effectiveness and potentially posing harm8. The need for precise delivery to specific cells where the gene of interest remains active has added another layer of complexity to the design. Despite these challenges, ongoing research is looking into chemical modifications of siRNA molecules, innovative delivery vectors like nanoparticles, and the use of target-specific ligands to boost selective delivery6–12. RNA delivery techniques are currently under studied and some have even reached clinical trials10. While RNA therapies show great promise, challenges like immunogenicity, delivery issues, off-target effects, and toxicity remain10. Non-viral nanoparticle platforms have made progress, but they still struggle with low efficiency and lack of precise targeting, highlighting the need for further improvements10.
To overcome these constraints, researchers focus on developing diverse range of synthetic and biodegradable nanocarriers, which can enhance the effectiveness and safety profiles of siRNA systemic delivery. Engineered nanostructures, such as carbon nanotubes (CNTs) have been studied as an alternative to viral systems for gene delivery and designed to be conjugated to siRNA to silence specific genes of interest13–18. Advanced nanocarrier delivery could also overcome endosomal/lysosomal barriers to promote delivery using nanocarrier which has better cellular uptake and intracellular transport mechanisms14. In additional, the use of three-dimensional (3D) printing along with modern additive manufacturing (AM) technology further revolutionises the delivery system to achieve better precision medicine15. Such added advantages could be incorporated into CNTs, in which they have needle-like structures with unique high aspect ratios, high specific surface area, biocompatibility, and efficient cellular uptake, making it a better carrier for systemic siRNA delivery13–15.
In this study, single-walled carbon nanotubes (SWNTs) were functionalized with two derivatives of polyethylene glycolated (PEGylated) phospholipids, either with the terminal amine group, PL-PEG-NH2 (PA), or the maleimide group, PL-PEG-maleimide (MA), to increase their solubility in aqueous condition. Polyethylene glycol (PEG) and PEGylated phospholipids (PL-PEG) are known for their biocompatibility in an aqueous solution; therefore, they are efficient surface enhancers for bioconjugation of CNT19,20. Our team has successfully optimised the functionalization of SWNTs using different molecular weights of the PL-PEG reactive group (PA or MA), followed by a series of investigation of the efficiency of siRNA loading on these SWNT-PL-PEGylated carriers, including their knock-down efficiency and the toxicity of the SWNT-siRNA conjugates on various cancer cell lines along with its cellular uptake mechanisms. We also demonstrated the efficient gene knock-down in mouse xenograft model verifying the success of this siRNA system for systemic gene delivery. Our work demonstrates the potential of the SWNT-PL-PEGylated vehicle as a promising delivery system of siRNA in gene therapy applications.
Results
Functionalization of SWNT with PL-PEG-NH2 and PL-PEG-MA
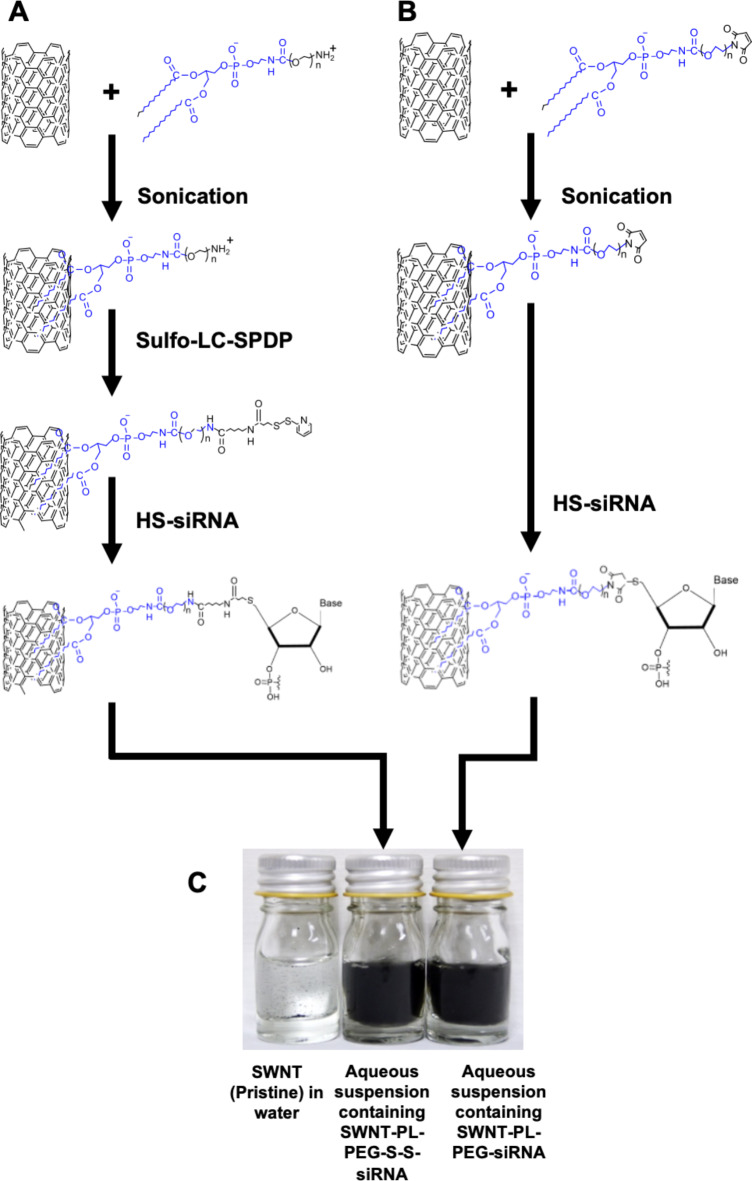
In this study, commercially available single-walled carbon nanotubes (SWNT) were functionalized with phospholipid-polyethylene glycol (PL-PEG) with a terminal amine (PL-PEG-NH2) or a maleimide group (PL-PEG-MA). Cleavable disulphide SWNT-PL-PEG-S-S-siRNA (Fig. 1A) and non-disulphide SWNT-PL-PEG-siRNA (Fig. 1B) via 5’ thiolated siRNA linkage were prepared. The stable suspensions of functionalized SWNTs were then loaded with siRNA (Fig. 1C).
Fig. 1.
Schematic drawing processing flow of functionalization of single-walled carbon nanotubes (SWNT) with PL-PEG-NH2 and PL-PEG-maleimide. (A) Preparation of SWNT-PL-PEG-S-S-siRNA. (B) Preparation of SWNT-PL-PEG-siRNA. (C) Aqueous suspensions of SWNT (pristine), SWNT-PL-PEG-S-S-siRNA and SWNT-PL-PEG-siRNA. (Picture taken by authors with a Canon EOS 5 Fujichrome Velvia).
Table 1 summarises the yield of functionalized SWNT and their subsequent conjugation with siRNA. Despite a relatively low yield of functionalized SWNT from this process, ranging from 1.77 ± 0.86% to 3.99 ± 3.74%, these functionalized SWNT were able to be conjugated with 5’-thiolated siRNA to produce SWNT-PL-PEG-S-S-siRNA and SWNT-PL-PEG-siRNA. It was found that the siRNA loading capacity into these functionalized SWNT ranged from 35 to 44% for the PA functional group and 29–45% for MA functional group; with a decrease in siRNA loading capacity as the molecular size of the PEG functional group increased. Furthermore, zeta potential measurements revealed that the SWNT-siRNA conjugates were stable in aqueous solutions with values of -38.24 ± 5.28 mV for SWNT-PL-PEG-S-S-siRNA and − 32.78 ± 3.76 mV for SWNT-PL-PEG-siRNA. Particle size analyses showed average sizes of 617 ± 115 nm and 703 ± 210 nm for the respective SWNT-siRNA conjugates, slightly larger than the functionalized SWNT but within the appropriate range for cellular infiltration, which is a critical factor for a successful transfection. These findings demonstrate an efficient loading of siRNA using functionalized SWNT.
Table 1.
Summary of SWNTs functionalized with various molecular weights (MW) of PL-PEG-PA or PL-PEG-MA and subsequent conjugation with siRNA. The yield of SWNTs functionalized with various molecular weights (MW) of PL-PEG-PA or PL-PEG-MA and conjugation with siRNA. Data represent mean ± SD (n = 3).
| Functionalized SWNTs | MW of PEG | Functionalized SWNT Yield (%) | SWNT-siRNA Conjugates | siRNA Loading (%) |
|---|---|---|---|---|
| SWNT-PL-PEG-PA | 2000 | 2.15 ± 0.89 | SWNT-PL-PEG-S-S-siRNA | 43.9 ± 5.3 |
| 3400 | 3.99 ± 3.74 | 43.4 ± 5.9 | ||
| 5000 | 3.68 ± 2.36 | 34.7 ± 5.2 | ||
| SWNT-PL-PEG-MA | 2000 | 2.11 ± 1.25 | SWNT-PL-PEG-siRNA | 45.2 ± 6.3 |
| 3400 | 1.77 ± 0.86 | 34.4 ± 5.4 | ||
| 5000 | 2.33 ± 0.82 | 28.9 ± 1.9 |
Gene silencing efficiency of the SWNT-siRNA conjugates
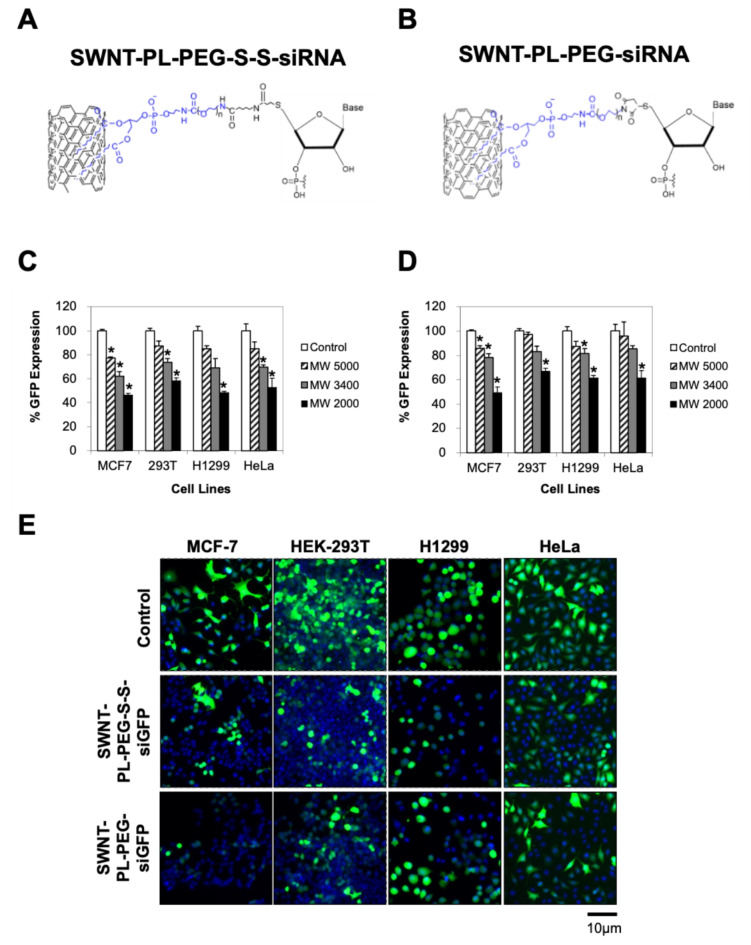
Next, we investigated the gene silencing efficiency of SWNT-PL-PEG-S-S-siGFP and SWNT-PL-PEG-siGFP conjugates using various molecular weight (MW 2000, MW 3400, and MW 5000) of PEG chains in four cells lines (i.e. MCF7, HEK-293T, H1299 and HeLa). These cell lines stably expressed GFP. The results demonstrated a significant silencing of GFP expression upon treatment with SWNT-PL-PEG-S-S-siGFP and SWNT-PL-PEG-siGFP conjugates with MW 2000 and MW 3400 at a concentration of 100 nM siRNA (Fig. 2). Conversely, no substantial silencing effect was observed when cells were treated with either SWNT-PL-PEG-S-S-siGFP or SWNT-PL-PEG-siGFP conjugates with MW 5000. These findings suggest that siRNA conjugated to the lower molecular weights (MW 2000 and MW 3400) of the SWNT-PL-PEG were more efficient in gene silencing compared to those with a higher molecular weight (MW 5000). Hence, further investigations were conducted using SWNT-PL-PEG-S-S-siRNA and SWNT-PL-PEG-siRNA conjugates with MW 2000.
Fig. 2.
Significant gene silencing efficiency using SWNT-siRNA conjugates. (A) Graphical representation of SWNT-PL-PEG-S-S-siRNA. (B) Graphical representation of SWNT-PL-PEG-siRNA. (C, D and E) Evaluation of gene silencing effect by SWNT-PL-PEG-S-S-siGFP and SWNT-PL-PEG-siGFP conjugates in MCF-7, HEK-293T, H1299 and HeLa cells. Cells expressing stable green fluorescent protein (GFP) were treated with SWNT-PL-PEG-S-S-siGFP or SWNT-PL-PEG-siGFP conjugates at a concentration of 100 nM siGFP. The cell nuclei were stained with DAPI (blue). Microscopic observation was captured under 100x magnification. Bars represent mean ± SD of at least 3 independent experiments. * represents statistical significance as compared to vehicle controls (Student’s t-test, p < 0.01).
To ensure that the observed reduction of GFP signals was not due to the toxic effects of SWNT-PL-PEG-S-S-siGFP or SWNT-PL-PEG-siGFP conjugates, the cytotoxic effects of SWNT-PL-PEG-S-S-siGFP or SWNT-PL-PEG-siGFP conjugates on these cell lines were determined using the LDH release assay at 72 h after treatment. As shown in Supplement Figure S1, neither the SWNT-PL-PEG-S-S-siGFP nor SWNT-PL-PEG-siGFP conjugates significantly affected cell viability, implying the non-toxic nature of SWNT-siRNA conjugates.
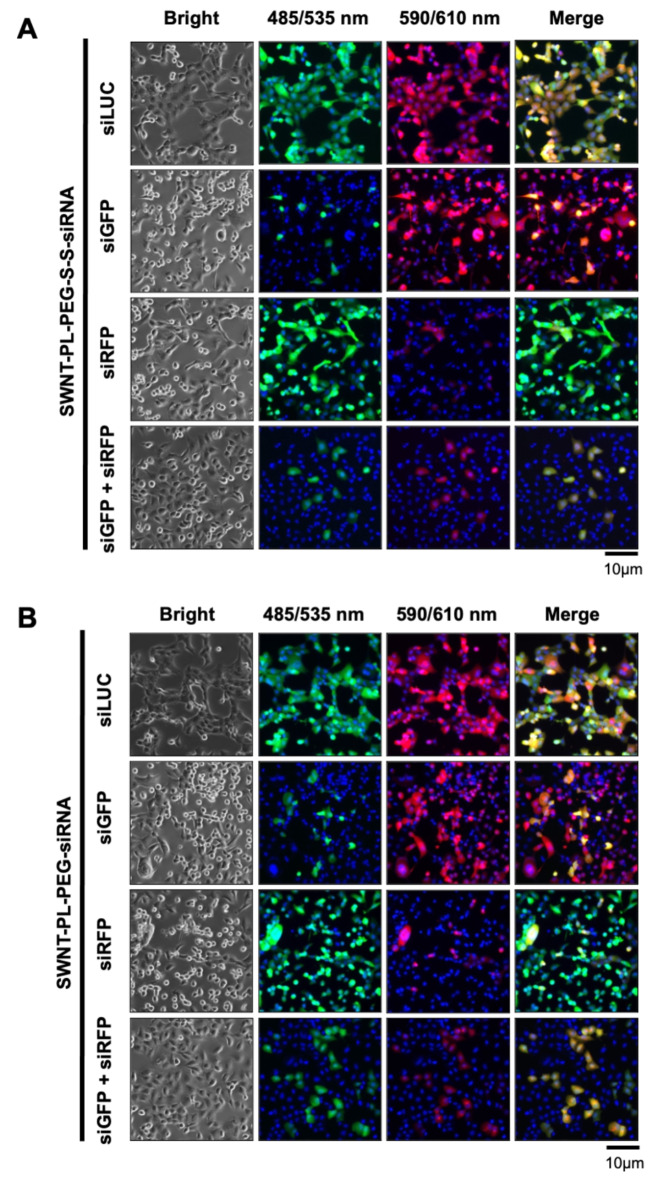
After confirming the efficacy of the SWNT-siRNA conjugates in silencing gene of interest, we further investigated the possibility of the conjugates to silence two genes of interest simultaneously by conjugating two different siRNA to the SWNT. The same amount of siRNA to GFP (siGFP) and RFP (siRFP) to the SWNT-PL-PEG-S-S- and SWNT-PL-PEG- Co-expressions of GFP and RFP in H1299 were established in house. When the H1299 cells were treated with SWNT-PL-PEG-S-S-siGFP/siRFP (Fig. 3A) or SWNT-PL-PEG-siGFP/siRFP (Fig. 3B), both GFP and RFP expressions were suppressed simultaneously. The co-suppression of GFP and RFP was accomplished by conjugating two different siRNAs to SWNT which achieved a dual targeting outcome. This proof of concept of gene co-silencing achieved by SWNT which deliver two different siRNA simultaneously marks an important milestone for future clinical application. Also, the co-silencing demonstrated that there is no difference of the binding affinity across different types of siRNA to the SWNT inducating that no specific siGFP nor siRFP was particularly effective in the gene silencing delivered by the functionalised SWNT.
Fig. 3.
Dual-silencing efficacy of siRNA-conjugated SWNT delivery system. Fluorescence microscopic images of co-expressed GFP and RFP in H1299 cells incubated with 100 nM of (A) SWNT-PL-PEG-S-S-siRNA or (B) SWNT-PL-PEG-siRNA conjugates targeting GFP (siGFP) or RFP (siRFP) or LUC (siLUC; negative control) alone, or both (dual targeting; siGFP + siRFP) for 72 h. The cell nuclei were stained with DAPI. The furthest right panel was the combined images. Microscopic observation was captured under 100x magnification.
Energy dependent cellular uptake of the functionalized SWNT-siRNA conjugates
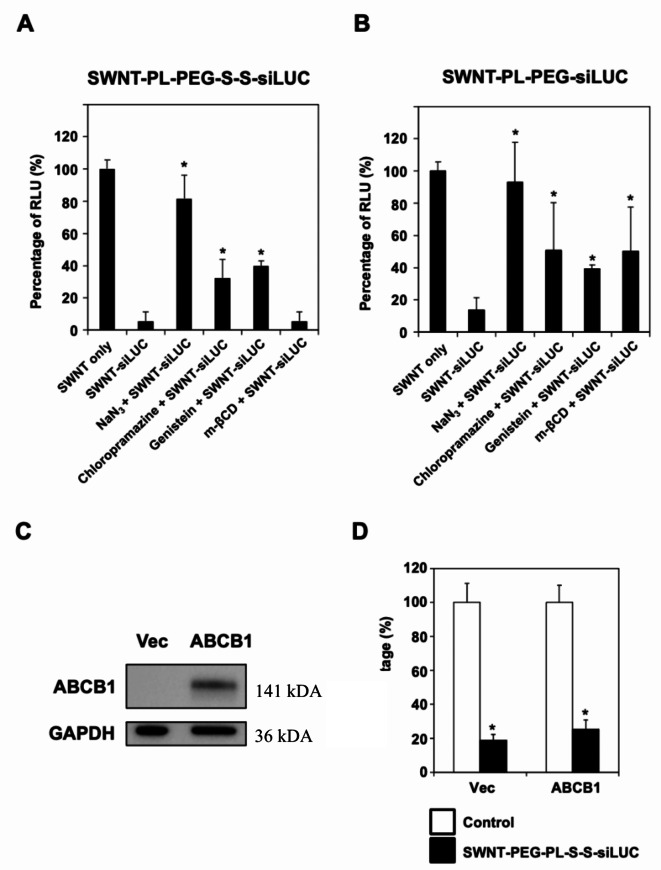
We aimed to elucidate the cellular uptake mechanisms of functionalized SWNT conjugated with siRNA. Luciferase-expressing H1299 cells were treated with SWNT-PL-PEG-S-S-siLUC or SWNT-PL-PEG-siLUC, which target the luciferase enzyme, in combination with various endocytosis inhibitors (Fig. 4A). The results suggested that the uptake of these SWNT-siRNA conjugates is energy-dependent, as confirmed by completely negating the silencing effects through co-treatment with sodium azide. Additionally, partial reductions in the siRNA silencing effects were observed when cells were co-treated with chlorpromazine or genistein, implicating partial involvement of clathrin-mediated and caveolae-mediated endocytosis pathways in the uptake of SWNT-siRNA conjugates. Notably, methyl-β-cyclodextrin (m-βCD) reduced the luciferase silencing effects of SWNT-PL-PEG-siLUC, but not SWNT-PL-PEG-S-S-siLUC, indicating that disulphide-bond presence might significantly affect the route of cellular uptake (Fig. 4B). Furthermore, we observed substantial gene knock-down efficiencies in various cell lines with both biocleavable SWNT-PL-PEG-S-S-siRNA and non-biocleavable SWNT-PL-PEG-siRNA conjugates indicating biocleavable conjugates demonstrated superior performance compared to the non-biocleavable ones. In summary, our findings suggested that the cellular uptake of SWNT-siRNA conjugates by the cancer cells and the normal human embryonic kidney HEK293 cells is mainly mediated through energy-dependent endocytosis, but the exact mechanism requires further investigation.
Fig. 4.
Energy dependent cellular internalization mechanisms of SWNT-siRNA conjugates independent of ABCB1. Graph shows the mean relative luminescent units (RLU) of (A and B) luciferase expressing H1299 cells co-treated with SWNT only (without siLUC), SWNT-PL-PEG-S-S-siLUC or SWNT-PL-PEG-siLUC with or without sub-lethal concentrations of various endocytosis inhibitors. NaN3, chlorpromazine, genistein and m-βCD are inhibitors for energy-dependent cellular uptake, clathrin-mediated endocytosis, caveolae-dependent endocytosis and macro-pinocytosis, respectively. Bars represent mean ± SD of at least 3 independent experiments. * represents statistical significance as compared to SWNT-siLUC (Student’s t-test, p < 0.01). (C) Western blot of ABCB1 and loading control GAPDH, cropped blots. Ectopic expression of ABCB1 (multiple drug resistance) protein in H1299-LUC cells was confirmed by the immunoblotting with increased expression of ABCB1 at 141 kDA, while GAPDH at 36 kDA served as the loading control. Full blots available in Supplementary Figure S2-S3. (D) Expression of ABCB1 did not affect the delivery of siRNA by the SWNT. Bars represent mean ± SD of at least 3 independent experiments. * represents statistical significance as compared to controls (Student’s t-test, p < 0.01).
Impact of P-glycoprotein on siRNA delivery by SWNT
The impact of P-glycoprotein (such as ABCB1) on the efficiency of siRNA delivery by SWNT was investigated. The ABCB1 gene and luciferase were co-expressed in H1299 cells, followed by SWNT-PL-PEG-S-S-siLUC treatment. Successful ABCB1 gene transduction was confirmed by immunoblotting, where the protein product of the ABCB1 gene was observed at a 141 kDa band, while GAPDH serving as a loading control (Fig. 4C). The overexpression of ABCB1 is related to drug efflux and multiple drug resistance, its expression had no detrimental effect were on siRNA delivery by the SWNT (Fig. 4D). The findings suggest that the cellular uptake of SWNT-siRNA is not impacted by ABCB1-mediated efflux, indicating that SWNTs could potentially serve as effective carriers for gene therapy in multiple drug-resistant tumours.
Significant knock-down efficacy using SWNT-PL-PEG-S-S-siRNA conjugate in a mouse xenograft model
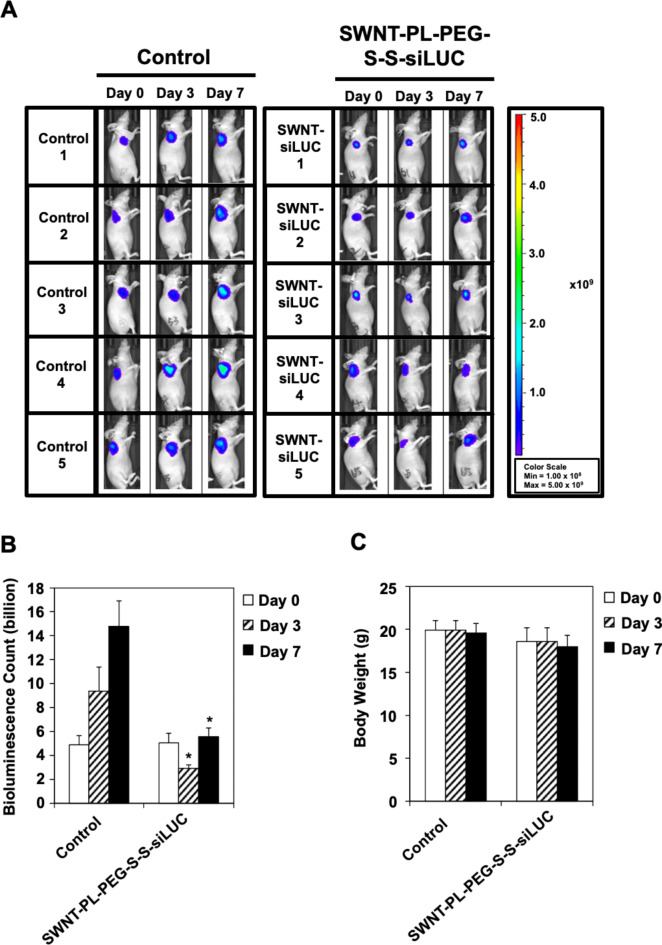
Understanding the limitation of translating in vitro findings to animal study21, we further confirmed the gene knock-down efficiency of the SWNT-siRNA conjugates using a mouse xenograft model. Luciferase-overexpressing HCT-116 cells were subcutaneously xenografted into BALB/c nude mice, followed by tail vein administration of 0.8 mg/kg of SWNT-PL-PEG-S-S-siLUC. The bioluminescence imaging revealed a significant reduction in luciferase expression in the group treated with SWNT-PL-PEG-S-S-siLUC, compared to the control group receiving saline PBS (Fig. 5A and B). Specifically, there was a 68.9% reduction in bioluminescence counts on day 3, followed by a 62.4% reduction on day 7 in the SWNT-PL-PEG-S-S-siLUC treated group. Additionally, no significant weight loss difference was observed in the animals receiving the saline and SWNT-PL-PEG-S-S-siLUC conjugates (Fig. 5C), suggesting that the SWNT-PL-PEG-S-S-siLUC conjugates did not induce acute toxicity leading to weight loss. Overall, these results indicate that SWNT-siRNA conjugates can induce efficient gene knock-down in both in vitro and in vivo models, warranting further research into their potential for gene therapy applications.
Fig. 5.
Significant knock-down efficacy using SWNT-PL-PEG-S-S-siRNA conjugate in mouse xenograft model. (A) In vivo bioluminescence imaging of luciferase expressing HCT-116 xenograft in BALB/C nude mice. The images show intensity of luminescence as ‘heat’ maps in which red is the maximum intensity. The scale shows the number of photons detected. (Left) Mice were treated with saline PBS as negative controls while (right) mice were injected with single dose of SWNT-PL-PEG-S-S-siLUC at 0.8 mg/kg through tail-vein. (B) Significant gene knock-down by SWNT-PL-PEG-S-S-siLUC in mouse xenograft model. Quantitation of bioluminescence was determined as depicted in Methods. Bars represent mean ± SD (n = 5). * indicates statistical significance as compared to vehicle control (Student’s t-test, p < 0.05). (C) No significant body weight loss was observed following administration of SWNT-PL-PEG-S-S-siLUC to the HCT-116 xenograft in BALB/C nude mice. Bars represent mean ± SD (n = 5).
Discussion
Systemic delivery of siRNA therapeutics presents several challenges. These include the rapid degradation of siRNA molecules by serum nucleases and the inadequate cellular uptake of these molecules. Furthermore, kidneys rapidly eliminate siRNA molecules from the body, reducing their interaction with the target cells, leading to treatment failure. Off-target effect, whereby siRNA molecules unintentionally interfere with non-target gene expression or unwanted immune response, is also a significant concern. Therefore, the ongoing research is to strengthen the systemic delivery of a selective and effective siRNA therapeutic8,22–25.
Carbon nanotubes (CNTs) have garnered significant attention in the field of nanomedicine due to their unique physical and chemical properties, positioning them as a promising nanocarrier for therapeutic and diagnostic applications17,26. Their high surface area, tuneable surface chemistry, and inherent electrical conductivity make them an attractive candidate for the delivery of bioactive molecules, including drugs, proteins, and nucleic acids. Additionally, their hollow cylindrical structure offers a potential internal cargo space, enabling dual external and internal functionalization. However, despite the promising features of CNT, there are substantial challenges that must be addressed before they can be adopted as therapeutic carrier27,28. A primary concern is the possible cytotoxic effects from CNTs when they accumulate in an organ. Furthermore, the inconsistency during CNT production often results in a mix of different lengths and chirality, which may affect the consistency in drug delivery29–34. While the promise of CNTs as nanocarriers is undeniable, rigorous research and optimization are required to overcome these limitations.
The utilisation of single-walled carbon nanotubes (SWNTs) for siRNA systemic delivery has garnered significant attention. An appropriate functionalization is paramount to ensure SWNT will be effective in the biological systems13–18. The functionalization of SWNTs with phospholipid-polyethylene glycol (PL-PEG) offers a compelling rationale. Firstly, phospholipids are amphipathic in nature, ensuring a compatible interface between the hydrophobic SWNTs and the aqueous biological environment13. The polyethylene glycol (PEG) component, on the other hand, is renowned for its biocompatibility and ability to enhance solubility in physiological fluids. Its hydrophilic nature promotes stable dispersion of SWNTs, preventing undesirable aggregation in biological media. Furthermore, PEGylation reduces the potential immunogenicity of nanoparticles, facilitating longer circulation times and reducing premature clearance from the bloodstream35,36. This ensures that a higher proportion of the siRNA cargo reaches its intended target. Additionally, the functionalized SWNTs can safeguard siRNA from enzymatic degradation in the bloodstream, while the modifiability of the PL-PEG allows for further attachments, such as targeting ligands, to improve the specificity of siRNA delivery35,37. In essence, PL-PEG functionalization aligns the physicochemical properties of SWNTs with the requirements of an effective systemic delivery system, ensuring that the therapeutic potential of siRNA is fully utilized. Our findings demonstrated a low yield of SWNT functionalization, this maybe due to the dispersion of SWNT which can be improved further by optimizing sonication parameters, utilizing surfactants, and mildly oxidizing of the SWNT in the future studies. Additionally, we could further explore improving the recovery by redispersing the functionalized SWNT in a suitable solvent if necessary, minimizing losses during purification and handling.
Our study reports several promising findings when functionalization of pristine SWNTs with phospholipid-polyethylene glycol (PL-PEG), which makes these hydrophobic entities suitable for siRNA delivery in biological applications14,16–18. The hydrophobic chains of PL-PEG with either an amine group or maleimide side chain, were observed to bind strongly to SWNTs, with the hydrophilic PEG chain rendering the overall complex soluble and biocompatible. Interestingly, the efficiency of gene knock-down was higher with lower molecular weight PL-PEG, suggesting that excessive PEGylation or longer PEG chains may hinder cellular uptake10,38.
Additionally, we observed significant gene knock-down efficiencies across various cell lines with both biocleavable SWNT-PL-PEG-S-S-siRNA and non-biocleavable SWNT-PL-PEG-siRNA conjugates. It was interesting to note that the biocleavable conjugates outperformed the non-biocleavable ones, consistent with existing literature highlighting the role of disulphide bond reduction in high transfection efficiency39–41. It has been reported that disulfide bonds are relatively stable in circulation42, and cleavage of the disulfide bond in the reducing environment of the endosome may enhance the release of siRNA from its carrier43,44. The SWNT-siRNA conjugates used in this study target luciferase (LUC) and are labeled as SWNT-PL-PEG-S-S-siLUC. Furthermore, its non-toxic nature and dual-targeting capabilities of SWNT-siRNA conjugates enable them as a viable delivery system for diseases with multiple gene mutations and dysregulation, such as cancer45,46. These conjugates could potentially silence multiple pathogenic genes simultaneously, strengthen the therapeutic outcomes without inducing significant toxicity or other safety concern. However, we acknowledge that the current study did not investigate whether the cleavage was completed. It is known that tumours microenvironment may exhibit mechanisms that impede cleavage45–52. Future mechanistic studies using fluorescent or radiolabelled tracking as well as targeted cleavage mechanisms, are warrant.
Cellular uptake of SWNT-siRNA conjugates is one of the bottle-neck issues for their therapeutic efficacy. Our studies indicate the SWNT-siRNA conjugates primarily enter the cells via energy-dependent endocytosis, a process that utilizes cellular energy to engulf and to internalise external molecules53. Clathrin-mediated endocytosis is another predominant cellular uptake mechanisms, whereby the SWNT-siRNA complexes are taken up by cells through clathrin-coated pits, forming vesicles that later fuse with endosomes. Another significant cellular uptake route is the caveolae-mediated endocytosis, which involves flask-shaped invaginations called caveolae. These structures are rich in proteins and lipids and facilitate the transport of the conjugates directly to the endoplasmic reticulum, bypassing the lysosomal degradation pathway54. Additionally, macropinocytosis, a process by which cells gulp in large volumes of the extracellular fluid, can also contribute to the internalisation of these complexes. This mechanism results in the formation of large vesicles, allowing the simultaneous uptake of multiple SWNT-siRNA units55. Consistent with the previous findings, our study reinforces the notion that the internalisation of SWNT-PL-PEG-S-S-siRNA and SWNT-PL-PEG-siRNA involves a variety of energy-dependent cellular uptake mechanisms, primarily through endocytosis9,56–58. Interestingly, as compared with the commercial siRNA delivery kit, LipoRNAiMax reagent, the uptake of SWNT-PL-PEG-S-S-siRNA and SWNT-PL-PEG-siRNA was not affected by the presence of P-glycoprotein, a membrane protein often associated with drug resistance. This observation suggests that SWNTs could be applied in delivering agents that could be beneficial in treating drug-resistant tumours, addressing a significant challenge in cancer treatment.
Finally, we also demonstrated the potential of SWNT-siRNA conjugates as a vehicle for systemic siRNA delivery with significant gene silencing effects in vivo. This observation indicates that not only were the SWNT-siRNA conjugates successful in entering the cells and releasing the siRNA into the cytoplasm, but the delivered siRNA was also functionally effective in knocking down the expression of its target gene. This drug delivery system will open up new horizon in effectively and safely delivering siRNA together with chemotherapy agents, immunotherapy agents, bioactive natural compounds or new chemical entities which may have beneficial effects against human disorders59–63. However, there is a limitation in this study whereby organ histopathology and blood chemistry assessments are indeed valuable but not conducted. This study focused on short-term outcomes and chronic toxicity were not within the scope of this initial study. As part of our ongoing research, we plan to conduct more detailed toxicological studies, including organ histopathology, blood chemistry, and chronic toxicity assessments, in future work to ensure the comprehensive safety profile of these materials. Also, direct comparison between SWNT-based siRNA delivery and commercially available kits has been investigated in vitro, with a focus on in vivo delivery efficiency, toxicity, and therapeutic efficacy could also be further explored. This will provide a more comprehensive understanding of the potential benefits of SWNTs as siRNA carriers in human cancer therapy. Nevertheless, future study will expand the validation on other mRNA targets, so to further validate the efficiency of this system. With the advance in nanotechnology64,65, it is certainly warranted to investigate whether co-delivery with other synthetic compound or biological molecule may further improve its efficacy.
In conclusion, the research findings highlight the promising potential of SWNT-siRNA conjugates as gene-silencing tools. Their capability to silence multiple genes simultaneously, their non-toxic nature, and their effectiveness in drug-resistant conditions are promising aspects for cancer treatment. The transition from in vitro to in vivo efficiency is a crucial step in drug development, and the success of SWNT-siRNA conjugates in animal models provides a promising lead for future investigations.
Materials and methods
Functionalization of single-walled carbon nanotubes
Cylindrical structure SWNT were functionalized as previously described66. Briefly, 1 mg HiPCo® SWNT with a diameter of approximately 1.0 nm (ranging between 0.8 nm and 1.2 nm) and individual SWNT length between 100 nm and 1000 nm, with high aspect ratio (length to radius) is typically ranging between 8,333 and 12,500; and > 95% purity (Unidym Inc., Sunnyvale, CA, USA) and 5 mg PEG derivatives, either PL-PEG-NH2 (PA) or PL-PEG-maleimide (MA) (NOF America Corporation, Japan), were dissolved in 5 mL diethylpyrocarbonate (DEPC)-treated water (nuclease-free). This mixture was sonicated for 60 min in a water bath at room temperature (approximately 22 °C). PEG derivatives and PEG chains of varying molecular weights (2000, 3400, and 5000) were employed. Insoluble contaminants and bundled SWNT were eliminated through centrifugation using a Beckman Coulter Optima L-90 K Ultracentrifuge (Beckman Coulter, Inc., California, USA) at 24,000 × g for 6 h at room temperature. Subsequently, the supernatant was collected and further centrifuged for 10 min at 4,000 × g at room temperature using an Amicon centrifugal filter with a cut-off size of 100 kDa (Millipore, USA). The residues underwent five rounds of washing using water to ensure a thorough removal of excess PL-PEG from the SWNT solution. After the final wash, the SWNT concentration was determined using an ultraviolet-visible spectrometer (Perkin Elmer, Massachusetts, USA) at 808 nm.
Preparation of cleavable disulphide SWNT-PL-PEG-S-S-siRNA
The resulting non-covalently functionalized SWNT was subsequently conjugated with a 5’-thiolated siRNA targeting green fluorescent protein (GFP) (target sequence: 5’-CGUGAUCUUCACCGACAAGAUTT-3’), red fluorescent protein (RFP) (target sequence: 5’-GUGGGAGCGCGUGAUGAACTT-3’), or luciferase mRNA (LUC) (target sequence: 5’-CUUACGCUGAGUACUUCGATT-3’). Briefly, 0.5 mg sulfosuccinimidyl 6-(3’-[2-pyridyldithio]-propionamido) hexanoate (sulfo-LC-SPDP) was added to 25 µg SWNT-PL-PEG-NH2 functionalized SWNT, combined with 50 µL 10X PBS. The mixture was incubated for 2 h at room temperature. The siRNA was then conjugated with SWNT by combining 15 µL of 100 µM siRNA with 1.5 µL 1,4-dithiothreitol (DTT) solution, followed by 1.5 h incubation at room temperature. This mixture was allowed to react for an additional hour at room temperature. Subsequently, the conjugates were centrifuged through a centrifugal filter with a cut-off of 100 kDa (Millipore, USA) twice for 5 min each to remove excess sulfo-LC-SPDP cross-linkers. Concurrently, as the sulfo-LC-SPDP was being removed from the SWNT solution, the DTT-treated siRNA was purified using a NAP-5 column (GE Healthcare, UK) as per the manufacturer’s protocol. siRNA was eluted with 500 µL DNase/RNase-free 1X PBS. Conjugation proceeded by mixing the activated SWNT with 500 µL purified siRNA and incubated for 24 h at 4 ºC. Following this incubation, the SWNT-siRNA solution was centrifuged using a filter with a 100 kDa molecular weight cut-off (Millipore, USA) twice for 5 min to remove any excess, unconjugated siRNA (Fig. 1A).
Preparation of non-disulphide SWNT-PL-PEG-siRNA
The SWNT-PL-PEG-maleimide (MA) was prepared similarly to the cleavable disulfide SWNT-PL-PEG-S-S-siRNA, but the PL-PEG-NH2 (PA) was replaced by PL-PEG-MA. The functionalized SWNT-PL-PEG-MA was mixed with 5’-thiolated siRNA in a phosphate buffer (pH 8.0) for 4 h at room temperature. The resulting SWNT-PL-PEG-siRNA conjugates, which contain no disulfide bonds, were then purified (Fig. 1B).
Dynamic light scattering (DLS) and zeta potential
Both the SWNT-PL-PEG-S-S-siRNA and SWNT-PL-PEG-siRNA conjugates were prepared as described above, and transferred into plastic optical cuvettes. Dynamic light scattering (DLS) and zeta potential measurements were performed using Malvern Zen Zetasizer Nano (Malvern Instrument, UK). Hydrodynamic dimensions of SWNT in suspension were determined by automatic software analysis of dynamic light scattering autocorrelation curves. Zeta potentials were measured in Malvern Instruments Folded Capillary Cells with embedded electrodes. Each measurement consisted of up to 100 runs from which the average zeta potential value was calculated.
Cell lines and cell culture
The human breast cancer cell line MCF-7, human non-small cell lung carcinoma line H1299, and human cervical cancer cells HeLa were procured from the American Type Culture Collection (ATCC; Manassas, VA, USA). They were cultured in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA) fortified with 10% fetal bovine serum (FBS, i-DNA Biotechnology, Singapore) and 1% penicillin-streptomycin (Biowest, Nuaille, France). The HEK-293T (CRL-11268, American Type Culture Collection) was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) enriched with 10% fetal bovine serum and 1% penicillin-streptomycin. All cell lines were kept in logarithmic growth inside a 37 °C humidified incubator with an atmosphere of 5% CO2.
Generation of GFP, RFP, luciferase and ABCB1 expressing cells
Stable expressions of green fluorescent protein (GFP), and / or red fluorescent protein (RFP), luciferase mRNA (LUC) and ABCB1 were established in MCF-7, H1299, HEK-293T and HeLa using lentiviral transduction. Lentiviral expression plasmids were purchased from OriGene (Rockville, MD, USA). Lentiviral stocks were produced by co-transfection with 5 µg of packaging plasmids psPAX2 (Addgene; plasmid 12260) and 5 µg envelope plasmids pMD2.G (Addgene; plasmid 12259) into HEK-293T cells as described previously using CalPhosTM Mammalian Transfection Kit (Clontech, Palo Alto, USA) [22–25].
Gene silencing efficiency of the SWNT-siRNA conjugates
The MCF-7, H1299, HEK-293T, and HeLa cells expressing GFP, RFP, and/or LUC were seeded overnight at a density of 3 × 105 cells per 60 mm culture dish, or 5 × 103 cells per well on a 96-well culture plate. Subsequently, 100 nM of siRNA conjugated to SWNT-PL-PEG-S-S-siRNA or SWNT-PL-PEG-siRNA was introduced to the cells expressing GFP, RFP, and/or LUC. The siRNA concentration was calculated based on an absorbance at 260 nm, where one absorbance unit equals 44 µg/mL. The expression levels of GFP, RFP, and/or LUC post-transfection were assessed at 72 h using the Eclipse Ti-U inverted fluorescent microscope (Nikon, NY, USA). Quantitative measurements were conducted using the Infinite F200 microplate reader (Tecan Group, Ltd., CH, Männedorf, Switzerland). Statistical significance was assessed using Student’s t-tests. Values of P < 0.01 were considered statistically significant (*). All data are presented as mean ± standard deviation (SD).
Cell viability
Cell viability assay was performed using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, Wisconsin, USA) as according to manufacturer’s protocol and previous studies [24,26]. Quantitative measurements were conducted using a SpectraMax Plus 384 Microplate Reader (Molecular Devices, San Francisco, CA, USA). Statistical significance was assessed using Student’s t-tests. Values of P < 0.01 were considered statistically significant (*). All data are presented as mean ± standard deviation (SD).
Cellular uptake
To determine the cellular update efficiency, H1299 cells expressing luciferase (H1299-LUC) were pre-treated with sublethal concentration of sodium azide, NaN3 (25 mM), chlorpromazine (2 µg/mL), genistein (20 mM), or methyl-b-cyclodextrin (m-βCD; 5 mM) 30 min prior to incubation with SWNT-siLUC conjugates. NaN3 may inhibit energy-dependent cellular uptake [27], while chlorpromazine may block clathrin-mediated endocytosis [28]. Genistein and m-βCD may inhibit caveolae-dependent endocytosis and macro-pinocytosis, respectively [27]. The inhibitory effects of each pathway were verified by assessing the silencing efficiency of firefly luciferase expression by the SWNT-PL-PEG-S-S-siLUC or SWNT-PL-PEG-siLUC conjugates after 72 h incubation. PL-PEG of MW 2000 were used. Luciferase expression and cell viability were measured using the ONE-Glo™ + Tox Luciferase Reporter and Cell Viability Assay (Promega, Madison, Wisconsin, USA) simultaneously. Luminescence signals were read using the SpectraMax Plus 384 Microplate Reader (Molecular Devices, San Francisco, CA, USA) and normalised against signals from untreated cells. Statistical significance was assessed using Student’s t-tests. Values of P < 0.01 were considered statistically significant (*). All data are presented as mean ± standard deviation (SD).
Protein isolation and Western blot analysis
Cell lysates were prepared using an ice-cold lysis buffer containing 1% NP-40, 1 mM DTT, protease inhibitors (Roche, Basel, Switzerland), and Phosphatase Inhibitor Cocktails I and II (Sigma-Aldrich, St. Louis, MO, USA) in PBS, as outlined in the earlier studies52,67–69. A total 50 µg protein was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting as described in previous studies52,67–69. Primary and secondary antibodies targeting ABCB1 and GAPDH (as the loading control) were sourced from Santa Cruz Biotechnology, CA, USA. The results were viewed using ChemiDoc™ XRS + molecular imager (Bio-Rad Laboratories, USA).
In vivo study
All animal studies were conducted in accordance with the ARRIVE Guidelines and National Institute of Health Guide for the Care and Use of Laboratory Animals. The animal studies were also approved and monitored by the Animal Ethics Committee of the International Medical University (Approval No.: IMU R073/11). Briefly, male BALB/c nude mice (6–8 weeks old) were housed (n = 10) in filter-topped cages, maintained on a standard rodent diet with ad libitum access to water, and subjected to a 12-hour light/dark cycle. The mice were grouped using a randomised block design based on body weights. Animal with sign of distress or unhealthy before the experiment were included. Each mouse was subcutaneously inoculated on the right flank with 5 × 106 luciferase-expressing HCT-116 (LUC2) cells. Seven days post-inoculation, when the tumour volume reached about 60 mm3, they were split into two groups of 5 mice each: (1) saline (control) group and (2) SWNT-PL-PEG-S-S-siLUC (PL-PEG of MW 2000) treatment group. The treatment group was administered a single dose (0.8 mg/kg) of SWNT-PL-PEG-S-S-siLUC conjugates intravenously, whereas the control group received an equivalent saline volume. The treatment was performed by a staff whom was the only person aware of the grouping. Daily monitoring involved any tumour growth, any change in body weight, any immobility, any variations in food or water intake, and other clinical signs was conducted by other investigators whom didn’t perform the treatment. Any deaths or clinical signs were logged according to the animal subsets. If animals displayed continual deterioration or had tumours larger than 2,000 mm3, they were euthanized pre-emptively. Upon formation of a palpable tumour, in vivo bioluminescence assessments were carried out daily for a week. Tumour dimensions were logged bi-weekly using a calliper, and volumes were calculated as: V = 0.5 a × b2 (where ‘a’ and ‘b’ represent the tumour’s long and short diameters, respectively). For imaging, mice were given 150 mg/kg luciferin intraperitoneally. After 13 min of administration, the mice were anesthetised using the mixture of oxygen and isoflurane. Once the mice were fully anesthetized, they were placed in an imaging chamber for bioluminescence measurements using IVIS® Lumina II optical imaging system (Caliper Life Sciences, USA). Bioluminescence images from the whole body, inclusive of primary and metastatic tumours, were taken. The primary study endpoint was tumour growth rate. Data are depicted as mean ± S.D. (n = 5) and statistically significant differences were concluded using independent t-test when p value less than 0.05.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded by Ministry of Higher Education, Malaysia (Grant No. FRGS/1/2023/SKK10/UCSI/02/1, FRGS/1/2020/TK0/UNIM/03/3, FRGS/1/2019/SKK15/IMU/01/1), the Hubert Curien Partnership – Hibiscus (PHC-HIBISCUS) France-Malaysia (Grant no. MyPAIR/1/2019/SKK08/IMU/1), the Malaysia Ministry of Science, Technology and Innovation Nanotechnology Research Grant (NND/NA/1/TD11-002) and UCSI University Research Excellence and Innovation Grant (REIG-FPS-2023/038).
Author contributions
Y.T. performed the experiments, analyzed the results and wrote the first draft of the manuscript. L.H., and W.L., performed the experiments, and analyzed the results. S.C., and C.L. provided intellectual input and troubleshooting, provided supervision, secured fundings and revised the manuscript. M.Y. and C. M. conceptualized the study, secured fundings, provided intellectual inputs and troubleshooting, analyzed the results, and revised the manuscript.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maxine Swee-Li Yee, Email: Maxine.Yee@nottingham.edu.my.
Chun-Wai Mai, Email: maicw@ucsiuniversity.edu.my.
References
- 1.Wood, H. FDA approves patisiran to treat hereditary transthyretin amyloidosis. Nat. Rev. Neurol.14, 570–570 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Scott, L. J. Givosiran: first approval. Drugs80, 335–339 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Narasipura, E. A., VanKeulen-Miller, R., Ma, Y. & Fenton, O. S. Ongoing clinical trials of nonviral siRNA therapeutics. Bioconjug. Chem.34(7), 1177–1197 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Hattab, D., Gazzali, A. M. & Bakhtiar, A. Clinical advances of siRNA-based nanotherapeutics for cancer treatment. Pharmaceutics13, 1009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh, S., Firdous, S. M. & Nath, A. siRNA could be a potential therapy for COVID-19. EXCLI J.19, 528 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tieu, T., Wei, Y., Cifuentes-Rius, A. & Voelcker, N. H. Overcoming barriers: clinical translation of siRNA nanomedicines. Adv. Ther.4, 2100108 (2021). [Google Scholar]
- 7.Sajid, M. I., Moazzam, M., Kato, S., Yeseom Cho, K. & Tiwari, R. K. Overcoming barriers for siRNA therapeutics: from bench to bedside. Pharmaceuticals13, 294 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subhan, M. A. & Torchilin, V. siRNA based drug design, quality, delivery and clinical translation. Nanomedicine29, 102239 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Li, Z., de Barros, A. L. B., Soares, D. C. F., Moss, S. N. & Alisaraie, L. Functionalized single-walled carbon nanotubes: cellular uptake, biodistribution and applications in drug delivery. Int. J. Pharm.524, 41–54 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Wang, T., Tang, Y., Tao, Y., Zhou, H. & Ding, D. Nucleic acid drug and delivery techniques for disease therapy: Present situation and future prospect. Interdiscip Med.2, e20230041 (2024). [Google Scholar]
- 11.Yan, C. et al. Design, strategies, and therapeutics in nanoparticle-based siRNA delivery systems for breast cancer. J. Mater. Chem. B. 11, 8096–8116 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Jain, D., Prajapati, S. K., Jain, A. & Singhal, R. Nano-formulated siRNA-based therapeutic approaches for cancer therapy. Nano Trends. 1, 100006 (2023). [Google Scholar]
- 13.Ijaz, H. et al. Review on carbon nanotubes (CNTs) and their chemical and physical characteristics, with particular emphasis on potential applications in biomedicine. Inorg. Chem. Commun.155, 111020 (2023). [Google Scholar]
- 14.Qiu, C. et al. Advanced strategies for overcoming endosomal/lysosomal barrier in nanodrug delivery. Res6, 0148 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman, M. H. et al. Additive manufacturing in nano drug delivery systems. Pharm. Sci. Adv.2, 100036 (2024). [Google Scholar]
- 16.Tang, L. et al. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotech. 19, 1–28 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubey, R., Dutta, D., Sarkar, A. & Chattopadhyay, P. Functionalized carbon nanotubes: synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv.3, 5722–5744 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chadar, R., Afzal, O., Alqahtani, S. M. & Kesharwani, P. Carbon nanotubes as an emerging nanocarrier for the delivery of doxorubicin for improved chemotherapy. Colloids Surf. B Biointerfaces. 208, 112044 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Skwarecki, A. S., Nowak, M. G. & Milewska, M. J. Synthetic strategies in construction of organic macromolecular carrier-drug conjugates. Org. Biomol. Chem.18, 5764–5783 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Ravelli, D. et al. PEGylated carbon nanotubes: preparation, properties and applications. RSC Adv.3, 13569–13582 (2013). [Google Scholar]
- 21.Mai, C. W. et al. Modeling prostate cancer: what does it take to build an ideal tumor model? Cancer Lett.543, 215794 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Saunders, N. R. M. et al. A nanoprimer to improve the systemic delivery of siRNA and mRNA. Nano Lett.20, 4264–4269 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Karlsson, J. et al. Engineered nanoparticles for systemic siRNA delivery to malignant brain tumours. Nanoscale11, 20045–20057 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song, F. et al. Design of a novel PEGylated liposomal vector for systemic delivery of siRNA to solid tumors. Biol. Pharm. Bull.42, 996–1003 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Chung, S. L. et al. Advances in nanomaterials used in co-delivery of siRNA and small molecule drugs for cancer treatment. Nanomater1110.3390/nano11102467 (2021). [DOI] [PMC free article] [PubMed]
- 26.Jampilek, J. & Kralova, K. Advances in drug delivery nanosystems using graphene-based materials and carbon nanotubes. Materials14, 1059 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jha, R., Singh, A., Sharma, P. & Fuloria, N. K. Smart carbon nanotubes for drug delivery system: a comprehensive study. J. Drug Deliv Sci. Technol.58, 101811 (2020). [Google Scholar]
- 28.Kiran, A. R., Kumari, G. K. & Krishnamurthy, P. T. Carbon nanotubes in drug delivery: focus on anticancer therapies. J. Drug Deliv Sci. Technol.59, 101892 (2020). [Google Scholar]
- 29.Ong, L. C., Chung, F. F., Tan, Y. F. & Leong, C. O. Toxicity of single-walled carbon nanotubes. Arch. Toxicol.90, 103–118. 10.1007/s00204-014-1376-6 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Ong, L. C. et al. Single-walled carbon nanotubes (SWCNTs) inhibit heat shock protein 90 (HSP90) signaling in human lung fibroblasts and keratinocytes. Toxicol. Appl. Pharmacol.329, 347–357 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Prajapati, S. K., Malaiya, A., Kesharwani, P., Soni, D. & Jain, A. Biomedical applications and toxicities of carbon nanotubes. Drug Chem. Toxicol.45, 435–450 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Mohanta, D., Patnaik, S., Sood, S. & Das, N. Carbon nanotubes: evaluation of toxicity at biointerfaces. J. Pharm. Anal.9, 293–300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan, H. et al. Toxicity of carbon nanotubes as anti-tumor drug carriers. Int. J. Nanomed.14, 10179–10194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleemi, M. A. et al. Toxicity of carbon nanotubes: molecular mechanisms, signaling cascades, and remedies in biomedical applications. Chem. Res. Toxicol.34, 24–46 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Hussain, Z. et al. PEGylation: a promising strategy to overcome challenges to cancer-targeted nanomedicines: a review of challenges to clinical transition and promising resolution. Drug Deliv Transl Res.9, 721–734 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Guo, C. et al. The interplay between PEGylated nanoparticles and blood immune system. Adv. Drug Deliv Rev.11504410.1016/j.addr.2023.115044 (2023). [DOI] [PubMed]
- 37.Yadav, D. & Dewangan, H. K. Peglyation: an important approach for novel drug delivery system. J. Biomater. Sci. Polym. Ed.32, 266–280 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Nair, J. K. et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc.136, 16958–16961 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Lin, C. & Engbersen, J. F. The role of the disulfide group in disulfide-based polymeric gene carriers. Expert Opin. Drug Deliv. 6, 421–439 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Saito, G., Swanson, J. A. & Lee, K. D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv. Drug Deliv Rev.55, 199–215 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Son, S., Namgung, R., Kim, J., Singha, K. & Kim, W. J. Bioreducible polymers for gene silencing and delivery. Acc. Chem. Res.45, 1100–1112 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Perez, H. L. et al. Antibody-drug conjugates: current status and future directions. Drug Discov Today. 19, 869–881 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Ou, M. et al. Novel biodegradable poly(disulfide amine)s for gene delivery with high efficiency and low cytotoxicity. Bioconjug. Chem.19, 626–633 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim, T. I., Ou, M., Lee, M. & Kim, S. W. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials30, 658–664 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sim, W., Lim, W. M., Hii, L. W., Leong, C. O. & Mai, C. W. Targeting pancreatic cancer immune evasion by inhibiting histone deacetylases. World J. Gastroenterol.28, 1934–1945 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Looi, C. K. et al. Targeting the crosstalk of epigenetic modifications and immune evasion in nasopharyngeal cancer. Cell. Biol. Toxicol.39, 2501–2526 (2023). [DOI] [PubMed] [Google Scholar]
- 47.Looi, C. K. et al. Roles of inflammasomes in Epstein-Barr virus-associated nasopharyngeal cancer. Cancers1310.3390/cancers13081786 (2021). [DOI] [PMC free article] [PubMed]
- 48.Mai, C. W., Chung, F. F. & Leong, C. O. Targeting legumain as a novel therapeutic strategy in cancers. Curr. Drug Targets. 18, 1259–1268 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Chung, F. F., Mai, C. W., Ng, P. Y. & Leong, C. O. Cytochrome P450 2W1 (CYP2W1) in colorectal cancers. Curr. Cancer Drug Targets. 16, 71–78 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Gan, L. L., Hii, L. W., Wong, S. F., Leong, C. O. & Mai, C. W. Molecular mechanisms and potential therapeutic reversal of pancreatic cancer-induced immune evasion. Cancers1210.3390/cancers12071872 (2020). [DOI] [PMC free article] [PubMed]
- 51.Looi, C. K., Hii, L. W., Ngai, S. C., Leong, C. O. & Mai, C. W. The Role of Ras-Associated Protein 1 (Rap1) in cancer: Bad actor or good player? Biomedicines 8, doi: 10.3390/biomedicines8090334 (2020). [DOI] [PMC free article] [PubMed]
- 52.Liew, K. et al. Parallel genome-wide RNAi screens identify lymphocyte-specific protein tyrosine kinase (LCK) as a targetable vulnerability of cell proliferation and chemoresistance in nasopharyngeal carcinoma. Cancer Lett.504, 81–90 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Augustine, R. et al. Cellular uptake and retention of nanoparticles: insights on particle properties and interaction with cellular components. Mater. Today Comm.25, 101692 (2020). [Google Scholar]
- 54.Donahue, N. D., Acar, H. & Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv Rev.143, 68–96 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Zhang, W. et al. Nano-structural effects on gene transfection: large, botryoid-shaped nanoparticles enhance DNA delivery via macropinocytosis and effective dissociation. Theranostics9, 1580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raffa, V., Ciofani, G., Vittorio, O., Riggio, C. & Cuschieri, A. Physicochemical properties affecting cellular uptake of carbon nanotubes. Nanomedicine5, 89–97 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Caoduro, C. et al. Carbon nanotubes as gene carriers: focus on internalization pathways related to functionalization and properties. Acta Biomater.49, 36–44 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Ma, N. et al. Influence of nanoparticle shape, size, and surface functionalization on cellular uptake. J. Nanosci. Nanotechnol. 13, 6485–6498 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Tuval, A., Strandgren, C., Heldin, A., Palomar-Siles, M. & Wiman, K. G. Pharmacological reactivation of p53 in the era of precision anticancer medicine. Nat. Rev. Clin. Oncol.21(2), 106–120 (2024). [DOI] [PubMed] [Google Scholar]
- 60.Keng, J. W. et al. Cassia alata and its phytochemiaals: A promising natural strategy in wound recovery. Sci6, 34. 10.1016/j.mtcomm.2020.101692 (2024). [Google Scholar]
- 61.Pang, K. L., Mai, C. W. & Chin, K. Y. Molecular mechanism of tocotrienol-mediated anticancer properties: a systematic review of the involvement of endoplasmic reticulum stress and unfolded protein response. Nutrients15, 1854. 10.3390/nu15081854 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ekeuku, S. O., Etim, E. P., Pang, K. L., Chin, K. Y. & Mai, C. W. Vitamin E in the management of pancreatic cancer: a scoping review. World J. Gastrointest. Oncol.15, 943–958. 10.4251/wjgo.v15.i6.943 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen, Y. et al. Targeting Xkr8 via nanoparticle-mediated in situ co-delivery of siRNA and chemotherapy drugs for cancer immunochemotherapy. Nat. Nanotechnol. 18, 193–204 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maniam, G., Mai, C. W., Zulkefeli, M. & Fu, J. Y. Co-encapsulation of gemcitabine and tocotrienols in nanovesicles enhanced efficacy in pancreatic cancer. Nanomedicine16, 373–389 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Maniam, G. et al. Challenges and opportunities of nanotechnology as delivery platform for tocotrienols in cancer therapy. Front. Pharmacol.9, 1358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu, Z., Tabakman, S. M., Chen, Z. & Dai, H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat. Protoc.4, 1372–1382 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan, H. H. et al. Effects of amyloid precursor protein overexpression on NF-kappaB, Rho-GTPase and pro-apoptosis Bcl-2 pathways in neuronal cells. Rep. Biochem. Mol. Biol.9, 417–425 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nalairndran, G. et al. Phosphoinositide-dependent kinase-1 (PDPK1) regulates serum/glucocorticoid-regulated kinase 3 (SGK3) for prostate cancer cell survival. J. Cell. Mol. Med.24, 12188–12198 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hii, L. W. et al. Sphingosine kinase 1 regulates the survival of breast cancer stem cells and non-stem breast cancer cells by suppression of STAT1. Cells 9, doi: 10.3390/cells9040886 (2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).