Abstract
The emergence of extracellular vesicles (EVs), which are natural lipid bilayer membrane structures facilitating intercellular substance and information exchange, has sparked innovative approaches in drug development and carrier enhancement. Plant-derived EVs notably offer advantages including low preparation cost, low immunogenicity, flexible drug delivery, high stability, good tissue permeability, and high inherent medicinal value compared to their animal-derived counterparts. Despite these promising attributes, the research on plant-derived EVs remains fragmented and lacks comprehensive synthesis. This review aims to address this gap by summarizing the isolation methods, biological characteristics, and storage techniques of plant-derived EVs. Additionally, we explore the potential of plant-derived EVs as therapeutic agents and drug carriers for treating various diseases. Finally, we delineate the current impediments to plant-derived EV development and highlight future research directions. By providing a detailed overview, we hope to facilitate further research and application in this emerging field.
Keywords: Plant-derived extracellular vesicles, Therapeutic agents, Drug carriers, Diseases, Clinical applications
1. Introduction
In recent years, the investigation of plant-derived extracellular vesicles (EVs) has attracted considerable interest due to their potential in therapeutic applications and drug delivery. The initial observation of EVs in barley leaf cells affected by fungi was documented in 1967 using transmission electron microscopy [1]. However, comprehensive studies on EVs commenced with mammalian cells in the 1980s, focusing primarily on their role in cell communication and disease mechanisms [2]. Over the past few decades, research on mammalian-derived EVs has advanced significantly, leading to the recognition of their potential as drug carriers in the field of nanomedicine [3,4]. In the 2010s, interest in plant-derived EVs began to grow as researchers discovered their structural and compositional similarities to mammalian-derived EVs [5]. Moreover, plant-derived EVs have demonstrated specific advantages, including high biocompatibility, cost-effectiveness, and ease of production, which may complement or expand the applications of mammalian-derived EVs in drug delivery and therapeutic contexts [6,7].
Key milestones in the development of plant-derived EVs include the identification of their non-immunogenic nature, the discovery of their inherent medicinal properties, and the development of methods for their large-scale isolation and purification. Recently, plant-derived EVs have emerged as effective drug carriers because of their structural and compositional resemblances to mammalian-derived EVs [8]. Additionally, plant-derived EVs are regarded as nonimmunogenic and safe, as plants do not harbor zoonotic or human pathogens. Furthermore, plant-derived EVs retain various biological activities from their source plants [9]. For instance, mulberry (Morus alba L.) root bark is recognized for its anti-inflammatory effects. Edible EVs derived from mulberry bark are being investigated as novel agents for the prevention and treatment of intestinal-associated inflammatory diseases by activating COPS8 in intestinal epithelial cells [10]. Likewise, strawberries and their derived EVs, celebrated for their antioxidant properties, have demonstrated efficacy in reducing oxidative stress in human mesenchymal cells [11]. Importantly, less than 300 g of plant material is sufficient to yield 1 g of high-purity plant-derived EVs [12]. These high-yield, cost-effective natural drug carriers undoubtedly hold significant promise for clinical translation.
Although plant-derived EVs present numerous advantages, research in this field is still in its infancy and remains fragmented across various independent studies, lacking a comprehensive synthesis. To address this fragmentation, we conducted a systematic review of the literature, utilizing the PubMed, Embase, and Web of Science databases. The keywords employed for the search included ‘extracellular vesicles' and ‘plant’. The inclusion criteria consisted of: 1) studies focused on plant-derived vesicles; 2) original research published in English; and conference abstracts were excluded. This review seeks to bridge this gap by offering a thorough overview of the latest techniques for isolating plant-derived EVs, their biological properties, relevant characterization methods, and storage strategies. Additionally, we emphasize the utility of plant-derived EVs in diseases treatment and drug delivery. By consolidating existing knowledge and identifying key challenges, we aspire to pave the way for future research and clinical applications of plant-derived EVs.
2. Isolation of plant-derived EVs
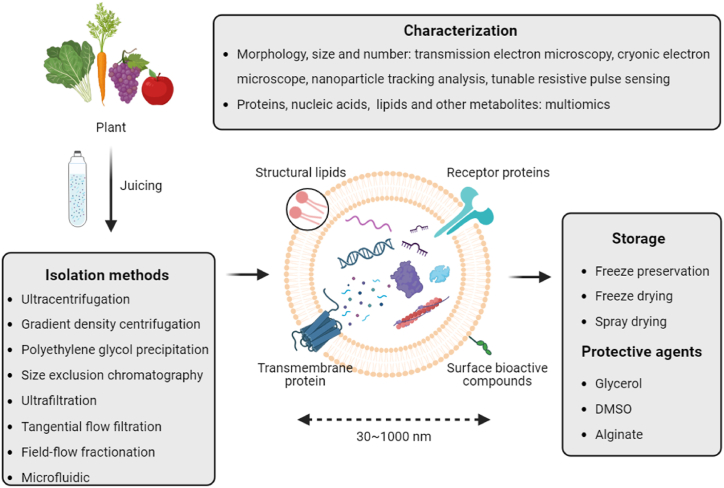
Effective isolation of plant-derived EVs is a prerequisite for their research and application. Current methods for isolating EVs from mammalian cells are relatively mature [13]. Given the structural similarities between plant-derived EVs and their mammalian counterparts, these methods serve as valuable references for isolating plant-derived EVs. Before extracting plant-derived EVs, the raw material should be pre-processed, i.e., the plant surface should be washed off with PBS or ultrapure water to eliminate contaminants such as soil. Plant juice is obtained using a juicer and then appropriately diluted, followed by differential centrifugation to remove fibrous debris and particles, yielding a supernatant containing plant-derived EVs and processed in the next step [14]. In this section, we will introduce several commonly used methods, including ultracentrifugation, gradient density centrifugation, polyethylene glycol precipitation, size exclusion chromatography, and tangential flow filtration (Fig. 1).
Fig. 1.
Isolation of plant-derived EVs and their characterization and storage methods. This figure illustrates the process of extracellular vesicles from plants. Initially, plant materials such as leafy greens, carrots, grapes, and tomatoes are juiced. The exosomes are then isolated using various methods including ultracentrifugation, gradient density centrifugation, polyethylene glycol precipitation, size exclusion chromatography, ultrafiltration, tangential flow filtration, field-flow fractionation, and microfluidics. The structure of exosomes, ranging from 30 to 1000 nm, is depicted with components such as structural lipids, receptor proteins, transmembrane proteins, and surface bioactive compounds. Characterization of exosomes is performed using transmission electron microscopy, cryo-electron microscopy, nanoparticle tracking analysis, tunable resistive pulse sensing, and multiomics for proteins, nucleic acids, lipids, and other metabolites. For storage, exosomes can be preserved through freeze preservation, freeze drying, and spray drying, using protective agents like glycerol, DMSO, and alginate.
2.1. Ultracentrifugation
Ultracentrifugation remains the predominant technique for extracting plant-derived EVs. This process begins with the low-speed centrifugation of the previously obtained supernatant at 1000–10,000×g for 20–60 min, effectively removing dead cells, cell debris, and large particulates. Subsequently, high-speed centrifugation at 100,000–150,000×g for 60–120 min is performed to pellet the EVs, which are then resuspended in phosphate-buffered saline (PBS) for further analysis [15]. Recent advancements in rotor design and centrifugation protocols have significantly enhanced EV yield and separation efficiency by minimizing protein contamination and improving purity [16]. Despite its widespread application, ultracentrifugation presents limitations, including the requirement for costly ultracentrifuges and potential for damage to EVs due to high shear forces, ultimately leading to a reduced yield of viable EVs [17].
2.2. Gradient density centrifugation
Gradient density centrifugation is employed to purify plant-derived EVs by leveraging the principle that particles will settle in distinct regions based on varying density gradients [18]. Initially, a sucrose solution is prepared with a concentration gradient of 8 %, 15 %, 30 %, 45 %, and 60 %. The supernatant obtained through differential centrifugation is then added to the sucrose gradient solution, followed by ultracentrifugation. Plant-derived EVs are primarily enriched within the sucrose layer ranging from 30 % to 45 % concentration [19,20]. Although effective, the high viscosity of sucrose solutions can pose challenges. Recent studies have proposed the use of iodixanol or potassium bromide gradients as alternatives, which may enhance separation efficiency and mitigate viscosity-related issues [21,22].
2.3. Polyethylene glycol (PEG) precipitation
PEG precipitation is conducted by modifying the solubility and dispersion of plant-derived EVs, resulting in their precipitation from solution [23,24]. Typically, a suitable amount of PEG6000 is added to the supernatant obtained through differential centrifugation, followed by incubation at 4 °C with shaking overnight. The following day, the treated solution is resuspended in PBS after low-speed centrifugation for 30 min to recover plant-derived EVs. While PEG precipitation is straightforward and facilitates large-scale EVs isolation, it necessitates further optimization due to its relatively low purification efficiency [25].
2.4. Size-exclusion chromatography (SEC)
The isolation of plant-derived EVs using SEC depends on the selectivity of the pore sizes in the resin beads of the column for particles of different radii [26]. As with the previous method, the supernatant obtained through differential centrifugation is pass through the column. Plant-derived EVs will preferentially elute from of the column, while smaller impurities remain within the column for an extended duration. SEC is straightforward to operate and relatively cost-effective, allowing the isolated EVs to maintain their structural integrity and biological activity. However, SEC may be time-consuming and less effective in removing large impurity particles [27,28].
2.5. Tangential flow filtration
Tangential flow filtration (TFF) represents an advanced method for the isolation of EVs, iespecially relevant for for clinical translation. TFF functions by allowing plant-derived EV-containing fluid to flow tangentially across the filter membrane's surface, enabling the separation of EVs based on size while preventing clogging and minimizing shear damage [29]. This method is highly scalable and efficiently process large volumes, making it suitable for both clinical and industrial applications [30]. For instance, Kim et al. demonstrated that TFF effectively isolates small extracellular vesicles from Aloe vera peels, yielding promising results for wound healing applications due to their antioxidant properties [31]. Additionally, Sukreet et al. emphasized the efficacy of TFF in isolating extracellular vesicles from cheesemaking byproducts, resulting in heterogeneous fractions of nanoparticles that may be beneficial for various biomedical applications [29].
2.6. Emerging techniques
In addition to traditional methods, several emerging techniques, including ultrafiltration and field-flow fractionation (AF4), are being utilized to isolate EVs from plants [32,33]. Microfluidic technology and nanoparticle-assisted isolation are also under investigation to improve the extraction of plant-derived EVs. Microfluidic devices facilitate high-efficiency, low-volume separation of EVs with high precise discrimination based on size and charge [34]. Nanoparticle-assisted methods enhance the purity and specificity of isolated EVs, resulting in improved yield and functionality, as evidenced by recent studies [35]. It is important to note that no single technique is flawless, and a combination of methods may be necessary [36]. In conclusion, the optimal method should be selected according to specific requirements. Integrating multiple techniques, such as combining differential centrifugation with ultrafiltration, can further enhance the purity and yield of plant-derived EVs.
3. Characterization and storage of plant-derived EVs
3.1. Characterization of plant-derived EVs
The characterization of plant-derived EVs, encompassing the identification of morphology, proteins, nucleic acids, and other small molecules, is essential to confirm their successful isolation and maintain their structural and functional integrity. Standardized characterization of plant-derived EVs is crucial for advancing further research and applications (Fig. 1).
3.1.1. Morphological characterization
Transmission electron microscopy (TEM) is the most widely employed technique for the morphological characterization of plant-derived EVs [37]. Typically, plant-derived EVs exhibit round and saucer-shaped morphologies [12]. In contrast, cryo-electron microscopy (Cryo-EM), which eliminates the need for sample fixation and staining, preserves the nearly native hydrated state of membrane vesicles [12,38]. Furthermore, Cryo-EM offers high-resolution imaging to examine the structure and biophysical properties of EVs, including size, shape, and membrane remodeling [39]. This facilitates a more accurate classification and understanding of the mechanisms underlying the formation of plant-derived EVs. In addition to direct microscopic observation, the morphological characteristics of plant-derived EVs can be assessed using nanoparticle tracking analysis (NTA) and tunable resistive pulse sensing (TRPS). NTA measures the size distribution and quantity of plant-derived EVs, while TRPS evaluates the size, zeta potential, and concentration [40,41]. Recent advancements in these imaging technologies have markedly improved the resolution and depth of morphological analysis, offering clearer insights into the structural integrity and functional capabilities of plant-derived EVs.
3.1.2. Protein characterization
The detection of proteins in mammalian EVs using immunoblotting assays is widely accepted [42]. In contrast, plant-derived EVs typically exhibit low protein abundance. Moreover, the protein composition may vary among different plant-derived EVs. As a result, a comprehensive protein database for the identification is currently lacking. Nonetheless, plant-derived EVs contain certain membrane proteins and intracellular proteins, including actin and proteolytic enzymes [43,44]. A previous study reported that 56.7 % of the proteins in lemon-derived EVs matched those found in mammalian EVs according to a comparison of the ExoCarta database, highlighting the potential for future identification of proteins in plant-derived EVs [45]. The advent of advanced mass spectrometry techniques has enabled researchers to identify novel proteins and lipids in plant-derived EVs that were previously undetectable [46]. This includes the discovery of unique surface markers that may be pivotal in targeted drug delivery and intercellular communication [47]. These findings are significant as they not only expand the known proteome and lipidome of plant-derived EVs but also indicate new functional roles and therapeutic potentials.
However, the characterization of plant-derived EVs remains in its infancy compared to that of mammalian EVs, primarily due to the absence of well-defined marker proteins. Recent studies have identified potential marker proteins for plant-derived EVs, including heat shock proteins, aquaporins, and certain glycoproteins, which can facilitate more precise characterization and isolation of these vesicles [6,44]. Moreover, advancements in proteomic and lipidomic technologies continue to reveal unique components within plant-derived EVs, enhancing our comprehensive understanding of their molecular composition and potential markers [48]. These developments are crucial for establishing standardized protocols for the characterization of plant EVs, akin to those currently available for mammalian EVs [49].
3.1.3. Nucleic acid characterization
Plant-derived EVs carry a substantial amount of nucleic acids. These genetic messages are delivered to the recipient cells via EVs, facilitating intercellular communication [50,51]. Notably, an increasing body of research indicates that miRNAs in plant-derived EVs may influence the progression of various human diseases [52,53]. For instance, miRNAs from buckwheat tartar-derived EVs can target functional genes in Escherichia coli and Lactobacillus rhamnosus (LGG), thereby enriching the diversity of the gut microbiome and enhancing intestinal health [54]. Similarly, mdo-miR7267-3p derived from ginger-derived EVs directly regulates the monooxygenase ycnE in LGG, promoting the production of IL-22 mediated by indole-3-carboxaldehyde (I3A). The expression of IL-22 has been shown to ameliorates colitis [55]. Collectively, the identification of nucleic acids in plant-derived EVs is of significant importance for their application, particularly as therapeutic agents and drug carriers.
3.1.4. Small molecule characterization
In addition to proteins and nucleic acids, plant-derived EVs encompass a diverse array of small molecules, including lipids and metabolites, that play crucial roles in their biological functions and therapeutic potential [56,57]. Recent studies underscore the importance of characterizing these small molecules to fully understand the capabilities of plant-derived EVs. For example, specific lipid profiles can significantly influence the biodistribution of EVs within the body. Plant-derived EVs enriched in phosphatidic acid (PA) preferentially localize in the intestine, presenting potential for targeted gastrointestinal therapies [56,57]. Conversely, those rich in phosphatidylcholine (PC) exhibit strong hepatic accumulation, indicating their suitability for liver-targeted drug delivery systems [20,55]. Furthermore, metabolites present in plant-derived EVs—including flavonoids, terpenoids, and alkaloids—contribute to their therapeutic effects [58]. The identification and quantification of these small molecules are essential for optimizing the application of plant-derived EVs in clinical settings. Understanding the small molecule composition of plant-derived EVs not only elucidates their functional mechanisms but also paves the way for their efficient application in medical practice.
3.2. Storage of plant-derived EVs
Drawing from experiences in storing mammalian-derived EVs, the preservation of plant-derived EVs primarily relies on freezing methods [59]. For short-term storage, plant-derived EVs can be kept at 4 °C or −20 °C, while −80 °C is recommended for long-term preservation. However, the activity and concentration of cryopreserved EVs decline with increasing storage time [60,61]. Protective agents, including glycerol, DMSO, and alginate, appear to mitigate this negative effect to some extent [62] (Table 1). Moreover, freeze-drying and spray-drying represent promising storage methods for plant-derived EVs [63,64] (Fig. 1). The integration of cryopreservation with protectants and optimized lyophilization technology may significantly enhance the viability and functionality of plant-derived EVs in the future.
Table 1.
Recommended storage conditions for plant-derived EVs.
| Storage Condition | Recommended Storage Time | Remarks |
|---|---|---|
| 4 °C | Short-term (days to weeks) | Suitable for short-term storage, activity may decrease over time [59]. |
| −20 °C | Short-term (weeks to months) | Better than 4 °C for slightly longer storage, still not ideal for long-term [59]. |
| −80 °C | Long-term (months to years) | Most suitable for long-term storage, but activity and concentration decrease with time. Addition of trehalose can improve stability [60,61]. |
| With protective agents (e.g., glycerol, DMSO, alginate) | Extended storage | Protective agents can help maintain activity and concentration during storage. Glycerol and DMSO effective for cryopreservation. Alginate for extended preservation [59,62]. |
| Freeze drying/Spray drying | Long-term | Potential methods for long-term storage, though optimization is needed. Lyophilization with stabilizers like trehalose shows promise. Spray drying still under research [63,64]. |
4. Application of plant-derived EVs as therapeutic agents and drug carriers
4.1. Therapeutic agents (Fig. 2, Table 2)
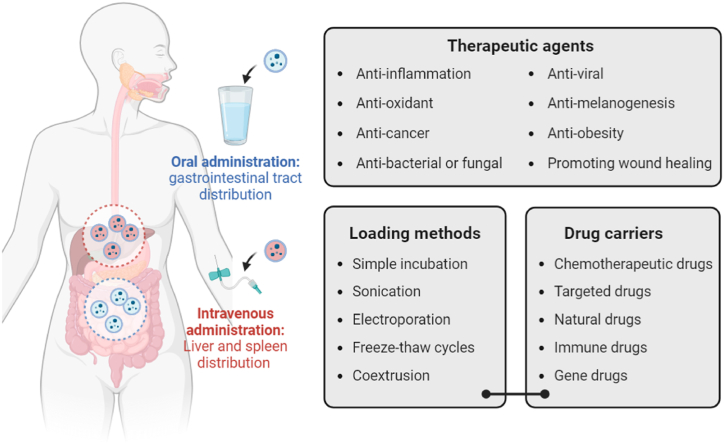
Fig. 2.
Plant-derived EVs as therapeutic agents and drug carriers. This figure illustrates the application of plant-derived EVs in therapeutic delivery through two main administration routes: oral and intravenous. Oral administration targets the gastrointestinal tract, while intravenous administration primarily distributes plant-derived EVs to the liver and spleen. Plant-derived EVs can carry various therapeutic agents, including anti-inflammatory, antioxidant, anti-cancer, anti-bacterial or antifungal, anti-viral, anti-melanogenesis, anti-obesity agents, and those promoting wound healing. Loading methods for plant-derived EVs include simple incubation, sonication, electroporation, freeze-thaw cycles, and coextrusion. They can be used as carriers for chemotherapeutic drugs, targeted drugs, natural drugs, immune drugs, and gene drugs.
Table 2.
Recent advances in the use of plant-derived EVs in disease treatment.
| Therapeutic Effect | Disease Type | Source of Plant EVs | Mechanism of Action | Related Research |
|---|---|---|---|---|
| Anti-inflammatory | Colitis | Grape | Induces Lgr5⁺ intestinal stem cells, enhances intestinal tissue renewal, targets intestinal stem cells through GELN lipids, and modulates β-catenin-mediated signaling pathways to protect against DSS-induced colitis. | Ju et al., 2013 [65] |
| Anti-inflammatory | Colitis | Grapefruit | Selectively taken up by intestinal macrophages, upregulates heme oxygenase-1 (HO-1), inhibits IL-1β and TNF-α production, and enhances therapeutic effects of Methotrexate (MTX) while reducing its toxicity. | Wang et al., 2014 [106] |
| Anti-inflammatory | Colitis | Ginger | Reduces pro-inflammatory cytokines (e.g., TNF-α, IL-6, and IL-1β), increases anti-inflammatory cytokines (e.g., IL-10 and IL-22) to restore immune balance in the gut, alleviates acute colitis symptoms, prevents chronic colitis and cancer development, and targets the colon to be efficiently absorbed by intestinal epithelial cells and macrophages, exerting significant local anti-inflammatory effects. | Zhang et al., 2016 [67] |
| Anti-inflammatory | Colitis | Broccoli | Activates AMP-activated protein kinase (AMPK) in dendritic cells (DCs), prevents DC activation, induces DC tolerance, and protects against DSS-induced colitis through sulforaphane (SFN)-mediated activation of AMPK. | Deng et al., 2017 [19] |
| Anti-inflammatory | Ulcerative Colitis | Ginger | Targets colon tissues specifically, reduces CD98 expression, and delivers siRNA efficiently with high biocompatibility, overcoming limitations of synthetic nanoparticles. | Zhang et al., 2017 [107] |
| Anti-inflammatory | Colitis | Mulberry bark | Activates AhR signaling through HSPA8, induces COPS8 expression in intestinal epithelial cells, and promotes the production of anti-microbial peptides, providing protection against DSS-induced colitis. | Sriwastva et al., 2022 [10] |
| Anti-inflammatory, Antioxidant | Intestinal Diseases | Ginger, Grapefruit, Carrot | Induces expression of heme oxygenase-1 and IL-10 in macrophages, promotes activation of nuclear factor (erythroid-derived 2), and activates Wnt signaling in intestinal crypts, leading to improved intestinal homeostasis. | Mu et al., 2014 [20] |
| Anti-inflammatory, Anti-cancer | Inflammatory Bowel Disease, Colitis-associated Colon Cancer | Tea Leaves | Mediates specific internalization by macrophages via galactose receptor-mediated endocytosis, reduces reactive oxygen species, inhibits pro-inflammatory cytokines, increases anti-inflammatory IL-10 secretion, restores colonic barriers, and enhances gut microbiota diversity and abundance. | Zu et al., 2021 [66] |
| Anti-inflammatory, Antiviral | COVID-19-induced Lung Inflammation | Ginger | Inhibits expression of Nsp12 and spike genes through miRNA aly-miR396a-5p and rlcv-miR-rL1-28–3p, reduces TNF-α, IL-6, and IL-1β secretion, and prevents apoptosis of lung epithelial cells by suppressing NF-κB activation. | Teng et al., 2021 [100] |
| Antioxidant | Alcohol-induced Liver Damage | Ginger | Activates Nrf2 pathway, induces expression of liver detoxifying and antioxidant genes, inhibits reactive oxygen species production, and mediates effects through shogaol in a TLR4/TRIF-dependent manner. | Zhuang et al., 2015 [76] |
| Antioxidant | Oxidative Stress | Strawberry | Contains anthocyanins, folic acid, flavonols, and vitamin C, prevents oxidative stress in human MSCs in a dose-dependent manner, and maintains cell viability through vitamin C-mediated antioxidative effects. | Perut et al., 2021 [11] |
| Antioxidant | Alzheimer's disease | Lemon | Crosses the blood-brain barrier (BBB), exhibits antioxidant activity comparable to ascorbic acid, and maintains over 80 % cell viability in SH-SY5Y cells, suggesting potential for combating oxidative stress in neurodegenerative diseases. | Dolma et al., 2024 [108] |
| Antioxidant, Pro-differentiative | Oxidative Stress, Bone Health | Lemon | Delivers and preserves micronutrients such as vitamin C and citrate, protects against oxidative stress, and promotes mesenchymal stromal cell differentiation towards the osteogenic lineage. | Baldini et al., 2018 [73] |
| Antioxidant, Anti-apoptotic | Myocardial Infarction, Parkinson's Disease | Carrot | Inhibits reactive oxygen species (ROS) generation and apoptosis, prevents reduction of antioxidative molecules (Nrf-2, HO-1, NQO-1), and shows low cytotoxicity in cardiomyoblasts and neuroblastoma cells. | Kim et al., 2021 [74] |
| Anticancer | Colitis-associated colorectal cancer (CAC) | Ginger | Inhibits the progression of CAC by targeting intestinal epithelial cells (IECs) and macrophages, promoting IEC survival and proliferation, repairing damaged tissues, modulating tumor-suppressive molecular targets, and preventing the transition from chronic inflammation to cancer. | Zhang et al., 2016 [67] |
| Anticancer | Melanoma | Ginseng (Panax ginseng C.A. Mey.) | Promotes M2-to-M1 macrophage polarization, increases ROS, induces melanoma cell apoptosis, and inhibits tumor growth via TLR4 and MyD88 signaling pathway activation. | Cao et al., 2019 [87] |
| Anticancer | Gastric Cancer | Lemon | Induces cell cycle S-phase arrest and apoptosis in gastric cancer cells, and mediates anticancer activities through the generation of ROS. | Yang et al., 2020 [83] |
| Anticancer | Colon Cancer | Ginger | Serves as a delivery platform for Doxorubicin (Dox), targets colon cancer cells efficiently, exhibits high biocompatibility, demonstrates superior pH-dependent drug-release profile, and enhances tumor inhibition through folic acid modification | Zhang et al., 2016 [109]; Zhang,.et al., 2024 [110] |
| Anticancer | Breast Cancer | Tea Flowers | Generates ROS, triggers mitochondrial damage, arrests cell cycle, and modulates gut microbiota, leading to inhibition of breast cancer growth and lung metastasis. | Chen et al., 2022 [84] |
| Intestinal Remodeling | Inflammatory Bowel Disease, Colorectal Cancer | Grape | Increases Lgr5 gene expression in intestinal stem cells, modulates stem cell microenvironment, and resists degradation in the gastrointestinal tract, enabling efficient oral delivery. | Rahimi et al., 2018 [111] |
| Antibacterial, Anti-pathogenic | Periodontitis | Ginger | Selectively taken up by Porphyromonas gingivalis via phosphatidic acid (PA) interactions with HBP35 protein, inhibits bacterial pathogenic mechanisms through PA and miRs cargo molecules, and reduces multiple pathogenic factors in P. gingivalis. | Sundaram et al., 2019 [94] |
| Wound Healing | Skin Wounds | Wheat (Triticum aestivum) | Promotes cell viability and migration of endothelial, epithelial, and dermal fibroblast cells, increases collagen type I mRNA expression, reduces apoptotic cell number, and enhances tube-like structure formation in endothelial cells. | Şahin et al., 2019 [102] |
| Anti-fibrotic | Liver Fibrosis | Tea Leaves | Inhibits hepatic stellate cell (HSC) activation, reduces collagen deposition and lipid droplets, lowers serum AST and ALT levels, and suppresses TGF-β1 signaling and miR-44 expression. | Gong et al., 2022 [112] |
4.1.1. Anti-inflammatory effects
Plant-derived EVs exhibit significant anti-inflammatory properties, positioning them as promising candidates for treatment of inflammation-related diseases. Recent research has deepened our understanding of how these EVs can modulate various biological pathways to elicit therapeutic outcomes. For instance, grape-derived EVs can modulate the Wnt/β-catenin pathway, alleviating intestinal inflammation in mice by activating Lgr5+ intestinal stem cells [65]. Phase I clinical trials have been conducted to evaluate oral grape-derived EVs for the treatment of oral mucositis induced by chemotherapy for neck and head cancers (www.clinicaltrials.gov/ct2/show/NCT01668849). Moreover, broccoli-derived EVs have been shown to significantly reduce dextran sodium sulfate (DSS)-induced colitis by maintaining intestinal immune homeostasis through AMPK induction in dendritic cells [19]. Similarly, Zu et al. demonstrated that the surface of green tea-derived EVs is enriched with galactose moieties, facilitating their entry into macrophages via the endocytic pathway [66]. Consequently, macrophages secreted substantial amounts of the anti-inflammatory factor IL-10, effectively preventing or inhibiting colitis and inflammation-associated colon cancer. Interestingly, green tea-derived EVs have also been shown to enhance the diversity of the intestinal microbiota and contribute to the restoration of the intestinal mucosal barrier [66].
Consistent with these findings, ginger-derived EVs not only directly induced the upregulation of anti-inflammatory factors in colon-26 and RAW264.7 cells but also reduced the secretion of pro-inflammatory factors, thereby alleviating DSS-induced colitis. Additionally, these EVs modulated the secretion of indole-3-carboxaldehyde (I3A) from Lactobacillus rhamnoides (LGG), further stimulating IL-22 production and enhancing the intestinal microflora [67]. Furthermore, exosome-like nanoparticles from mulberry bark have been shown to prevent DSS-induced colitis through the AhR/COPS8 pathway, underscoring another novel mechanism by which plant-derived nanoparticles can mitigate inflammation [10]. Exosome-like nanovesicles derived from pueraria lobata have been demonstrated to significantly ameliorate lung inflammation associated with DSS-induced colitis by modulating macrophage polarization, emphasizing their potential in treating both intestinal and related lung inflammation [68]. Nanovesicles derived from tomato fruit, enriched with curcumin, have shown significant anti-inflammatory effects [69]. Additionally, oral administration of exosome-like nanovesicles has proven effective in treating colitis in mice by exhibiting both anti-inflammatory and pro-resolving properties. These nanovesicles primarily alleviate symptoms of colitis by inhibiting the NF-κB pathway, which regulates the production of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β. Moreover, they enhance the expression of the antioxidant gene HO-1, thereby contributing to their therapeutic effect [70]. This underscores the substantial potential of plant-derived EVs in the treatment of inflammatory-related diseases.
4.1.2. Antioxidant effects
Numerous studies have investigated the antioxidant effects of specific components derived from fruits and vegetables [71,72]. Plant-derived EVs, which can effectively protect these antioxidants, may be utilized in the prevention and treatment of various oxidative stress-related diseases. Lemon-derived EVs are rich in ascorbic acid (vitamin C) and citrate, which protect mesenchymal stem cells (MSCs) from oxidative stress [73], suggesting that these natural EVs may contribute to tissue regeneration and positively influence bone development and repair. Similarly, strawberry-derived EVs, which are naturally rich in anthocyanins, folic acid, flavonols, and vitamin C, have demonstrated significant antioxidant capacity in MSCs [11]. Carrot-derived exosome-like nanoparticles, referred to as Carex, exhibited significant antioxidant activity in models of myocardial infarction and Parkinson's disease [74]. Carex induces the expression of several antioxidant proteins, including Nrf-2, HO-1 and NQO-1, thereby inhibiting excessive reactive oxygen species (ROS) production in H9C2 cardiomyocytes and SH-SY5Y neuroblastoma cells [74]. Extracellular vesicles derived from Citrus reticulata Blanco cv. ‘Dahongpao’ have demonstrated significant antioxidant activity and potential for drug delivery [75]. Moreover, ginger-derived EVs may effectively protect against alcohol-induced liver injury by reducing ROS. Mechanistically, shogaol present in ginger-derived EVs activates the key redox-related molecule Nrf2 by regulating the TLR4-TRIF axis, thereby promoting the transcription of various hepatic detoxification and antioxidant genes [76].
4.1.3. Anticancer effects
Despite the remarkable efficacy of many newly developed antineoplastic agents, their associated side effects—including allergic reactions, immune system dysfunction, and impaired hepatic function—pose significant clinical challenges [[77], [78], [79]]. Recent intensified research on plant-derived EVs has progressively unveiled their potent antitumor activity. These EVs, exhibit minimal side effects and low immunogenicity, thereby offering promising prospects for antitumor applications [[80], [81], [82]]. Yang and colleagues demonstrated that lemon-derived EVs significantly induced cell cycle arrest and apoptosis in gastric cancer cells through the accumulation of ROS [83]. Similarly, lemon-derived EVs have been shown to induce apoptosis in chronic myeloid leukemia cells by activating TRAIL-mediated pathway [45]. Moreover, recent studys have demonstrated the efficacy of tea flower-derived EVs in treating metastatic breast cancer. Specifically, tea flower-derived EVs induced oxidative stress in breast cancer cells, leading to mitochondrial damage and subsequent cell cycle arrest. Further in vivo experiments revealed that the accumulation of tea flower-derived EVs at primary tumor and lung metastases significantly inhibited breast cancer growth and metastasis. Interestingly, these EVs also found to modulate and improve gut microbiota composition [84]. Ginger-derived EVs have been shown to effectively prevent and treat colitis-associated colorectal cancer. In a mouse model of colorectal carcinogenesis induced by azoxymethane and DSS, ginger-derived EVs inhibited the proliferation of intestinal epithelial cells by downregulating the expression of cyclin D1 and various cytokines, thereby suppressing the development and progression of colorectal cancer [67]. Additionally, ginger-derived EVs upregulated the expression of PKG and transferrin, both of which are associated with a favorable prognosis in colitis-associated colorectal cancer [85,86].
Notably, ginseng-derived EVs have shown significant efficacy in enhancing melanoma by modulating macrophage polarization. Mechanistically, ginseng-derived EVs activated the TLR4/MyD88 axis, promoting macrophage polarization from an M2 to M1 phenotype and inducing oxidative stress-mediated apoptosis in murine melanoma cells. Furthermore, ceramide lipids and proteins within ginseng-derived EVs may play a pivotal role in driving macrophage polarization [87]. EVs from the leaves and stems of Dendropanax morbifera serve as effective anti-melanogenic agents, inhibiting melanogenesis-related proteins such as MITF, TYR, TRP-1, and TRP-2, in both murine melanoma and human epidermal models [88]. Notably, these EVs exhibited superior anti-melanogenic effects compared to arbutin, without exhibiting any overt cytotoxicity [88]. In a murine lung cancer model, artemisinin-derived nanovesicles (ADNVs) suppressed tumor growth and enhanced anti-tumor immunity by remodeling the tumor microenvironment and reprogramming tumor-associated macrophages (TAMs) [89]. Mitochondrial DNA from artemisinin can be internalized into TAMs via these vesicles, subsequently activating the cGAS-STING pathway. This activation shifts tumor macrophages from an immune-tolerant to a pro-inflammatory phenotype, thereby significantly enhancing the antitumor efficacy of PD-L1 inhibitors in murine models [89]. Moreover, EVs derived from other plants, such as Asparagus cochinchinensis and grapefruit, have also exhibited antitumor activity [80,90,91].
4.1.4. Antibacterial and antifungal effects
As previously discussed, the antibacterial and antifungal properties of plant-derived EVs have been demonstrated in various cancer studies, primarily through their modulatory effects on the intestinal microbiota. Nucleic acids within plant-derived EVs can be internalized by bacteria or fungi, thereby modulating their gene expression [52,92,93]. For instance, ginger-derived EVs can prevent or treat chronic periodontitis by inhibiting the growth of the oral pathogen Porphyromonas gingivalis(P. gingivalis). Mechanistically, phosphatidic acid (34:2) present in ginger-derived EVs interacts with HBP35 on the surface of P. gingivalis, facilitating its selective uptake. MiRNAs encapsulated in ginger-derived EVs inhibit the expression of T9SS family genes in P. gingivalis, thereby preventing its attachment to and invasion of oral epithelial cells [94]. Additionally, coconut water-derived EVs can regulate the growth of Escherichia coli K-12 MG1655 by modulating gene expression [95]. This regulatory effect underscores the versatility of plant-derived EVs in targeting diverse bacterial species, thereby broadening their potential application in antibacterial therapies.
4.1.5. Antiviral effects
The ongoing COVID-19 pandemic continues to pose a significant threat to both human health and economic development [96,97]. Plant-derived EVs, abundant in small RNAs (sRNAs), have shown potential for the treatment and prevention of novel coronavirus infections [98,99]. Teng and colleagues reported that ginger-derived EVs significantly alleviated pneumonia mediated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Mechanistically, rlcv-miR-rL1-28–3p present in ginger-derived EVs suppresses the expression of spike gene. Additionally, ally-miR396a-5p derived from ginger-derived EVs prevents lung macrophage apoptosis by downregulating NF-κB-mediated expression of inflammatory cytokines [100]. Similarly, miRNAs targeting SARS-CoV-2 genomic sequences were identified in grapefruit-derived EVs through predictions using RNA hybridization software [101].
4.1.6. Other therapeutic effects
Plant-derived EVs exhibit a diverse range of biological effects. Wheat-derived EVs have been shown to accelerate wound healing. One study demonstrated that wheat-derived EVs promote the proliferation and migration of endothelial cells, epithelial cells, and dermal fibroblasts. Consistently, the expression of type I collagen was transcriptionally upregulated in cells treated with wheat-derived EVs [102]. Grape-derived nanovesicles protect against LPS/D-GalN-induced acute liver failure, exhibiting pronounced hepatoprotective effects [103]. Garlic-derived exosome-like nanovesicles significantly alleviate acute liver failure by inhibiting CCR2/CCR5 signaling and reducing inflammation, highlighting their potential as hepatoprotective agents [104]. Moreover, ginger-derived EVs have been found to improve glucose tolerance and insulin response, suggesting promising potential for the treatment of obesity. In a high-fat diet-induced insulin resistance animal model, Kumar et al. demonstrated that ginger-derived EVs inhibited AhR by inducing miR-375 and VAMP7 expression [105]. Although a clinical trial investigating ginger-derived EVs for treating insulin resistance and chronic inflammation in patients with polycystic ovary syndrome (POS) was withdrawn due to non-recruitment (www.clinicaltrials.gov/ct2/show/NCT03493984), these findings underscore the potential of plant-derived EVs as therapeutic agents. The therapeutic applications of plant-derived EVs extend beyond the aforementioned effects, demonstrating their versatility across various medical fields.
4.2. Drug carriers (Fig. 2)
4.2.1. Methods for drug loading
Plant-derived EVs serve as exceptional drug delivery carriers due to their lipid bilayer structure, enabling the encapsulate both hydrophobic and hydrophilic drugs [113]. Several drug-loading techniques have been developed, each presenting distinct advantages and limitations (Table 3). These methods include passive loading, such as simple incubation, and active loading techniques, including sonication, electroporation, freeze-thaw cycles, and coextrusion [69,[114], [115], [116], [117], [118], [119]].
Table 3.
Advantages and disadvantages of drug loading methods for plant-derived EVs.
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Passive loading | |||
| Simple Incubation |
|
|
Kooijmans et al., 2013 [121]; Farheen et al., 2024 [122]; Ishida et al., 2023 [123]; Mammadova al., 2023 [69]; Kürtösi et al., 2024 [17]. |
| Active loading | |||
| Sonication |
|
|
Li et al., 2022 [115]; Chen et al., 2022 [116]; Mammadova al., 2023 [69]. |
| Electroporation |
|
|
Kooijmans et al., 2013 [121]; Zuppone et al., 2024 [117]. |
| Freeze-Thaw |
|
|
Nemidkanam et al., 2023 [118]. |
| Coextrusion |
|
|
Chen et al., 2022 [116]; Mammadova al., 2023 [69]; Fernandes al., 2020 [119]. |
Simple Incubation: This method involves directly incubating the drug with plant-derived EVs at a controlled temperature. This approach is straightforward and relatively effective for drug loading. For instance, Xiao et al. encapsulated doxorubicin into lemon-derived EVs via coincubation, which preserved the drug's antitumor activity while significantly reducing its side effects [120]. However, as drug encapsulation in this method relies solely on passive diffusion, the loading efficiency of coincubation tends to be low [[121], [122], [123]].
Sonication: Sonication rapidly alters the lipid bilayer structure of plant-derived EVs, facilitating drug penetration [69]. This method utilizes ultrasonic waves to induce cavitation in the liquid medium, temporarily disrupting the vesicle membrane to permit drug entry. Sonication is one of the most widely used approaches for drug loading due to its relative simplicity and high efficiency [115,116]. Studies have shown that plant-derived EVs retain their properties post-sonication with minimal alterations to their lipid and protein contents [6]. Furthermore, sonication has been shown to enhance the encapsulation efficiency of hydrophobic drugs; however, prolonged exposure to ultrasonic waves may lead to degradation of both vesicles and drug molecules [115,116]. In contrast, mammalian cell-derived EVs exhibit distinct behavior. Post-sonication, mammalian EVs often fail to fully recover their original properties and may experience more pronounced structural and functional impairments [124]. Therefore, the recovery and stability of EVs post-sonication can differ significantly between plant-derived and mammalian cell-derived EVs, underscoring the necessity of optimizing sonication parameters for each EV source.
Electroporation: Electroporation employs electric fields to generate transient pores in the vesicle membrane, enabling the entry of drug molecules. This technique is particularly effective for loading large or charged molecules. However, it may induce drug precipitation and compromise the vesicle's structural integrity and function [117,121].
Freeze-Thaw Cycles: This method involves freezing a mixture of EVs and the drug, followed by thawing, which induces pore in the vesicle membranes to facilitate drug entry. The freeze-thaw cycles are typically repeated multiple times to enhance the loading efficiency. However, this process may lead to vesicle aggregation and induce structural alterations in the EVs [118].
Coextrusion: Coextrusion is another effective method for encapsulating drugs into plant-derived EVs. In this method, the drug is passed through a filter with a defined pore size along with larger plant-derived EVs, resulting in successful encapsulation [125]. However, coextrusion requires specialized equipment and technical expertise, and there is a risk of EVs loss during the extrusion process [69,116,119].
In summary, each drug loading method presents distinct advantages and limitations, and thus the selection of an appropriate method should be guided by the specific objectives and requirements of the study. In certain cases, combining multiple methods may be necessary to achieve optimal loading efficiency.
4.2.2. Distribution and targeting
Plant-derived EVs encapsulated with therapeutic agents exhibit multiorgan distribution upon systemic administration, primarily targeting the gastrointestinal tissues, liver, and spleen [20,76,87]. The distribution is influenced by several factors, including the route of administration and surface composition of the EVs. From a drug administration perspective, plant-derived EVs administered orally primarily accumulate in the gastrointestinal tissues. In contrast, plant-derived EVs administered via intraperitoneal or intravenous routes are predominantly enriched in the liver and spleen. As drug carriers, plant-derived EVs should ideally avoid accumulation in non-target tissues or organs, making this a crucial consideration for researchers. Notably, surface proteins and polysaccharides of plant-derived EVs facilitate cellular entry by binding to specific receptors on target cells [17,126,127]. One study identified over 100 homologous proteins potentially involved in the vesicle transport process [44].
4.2.3. Applications in drug delivery (Table 4)
Table 4.
Application of plant-derived EVs as drug carriers.
| Type of Drug Carrier | Disease Type | Source of Plant EVs | Application Example | Related Research |
|---|---|---|---|---|
| Methotrexate | Inflammation | Grapefruit | Methotrexate in grapefruit-derived EVs improved anti-inflammatory effects and reduced toxicity | Wang et al., 2014 [106] |
| Paclitaxel | Colon Cancer | Grapefruit | Paclitaxel in grapefruit-derived EVs inhibited tumor growth and reduced chemotherapy-induced toxicity | Wang et al., 2015 [129] |
| Stat3 Inhibitor | Glioma | Grapefruit | Stat3 inhibitor JSI-124 in grapefruit-derived EVs crossed the blood-brain barrier and inhibited glioma tumor growth | Wang et al., 2015 [129] |
| Doxorubicin | Colorectal Cancer | Cabbage/Red cabbage | Doxorubicin in cabbage-derived EVs showed stronger antitumor activity in colorectal cancer | You et al., 2021 [130] |
| Sorafenib | Various Cancers | Kiwi | Kiwi-derived EVs significantly improved oral bioavailability and therapeutic efficacy of sorafenib | Fang et al., 2023 [131] |
| Curcumin | Colon Cancer | Various plants | Clinical trials investigating curcumin-loaded plant-derived EVs for modulating immune system and glucose metabolism in colon cancer patients | ClinicalTrials.gov |
Enhanced Drug Properties: Plant-derived EVs have the capability to encapsulate a broad range of small molecule drugs, enhancing their bioavailability, stability, and solubility while mitigating side effects. For instance, encapsulating methotrexate within grapefruit-derived EVs significantly enhanced its anti-inflammatory efficacy and reduced associated toxicity [106]. Grapefruit-derived EVs can also be used to encapsulate antitumor drugs. In a tumor xenograft model using mouse CT26 and human SW620 colon cancer cells, paclitaxel encapsulated within grapefruit-derived EVs significantly inhibited tumor growth and alleviated chemotherapy-induced toxic side effects [128]. Similarly, in a GL26 cell-derived murine glioma model, grapefruit-derived EVs encapsulating the Stat3 inhibitor JSI-124 successfully crossed the blood‒brain barrier and suppressed tumor growth, markedly extending the survival of mice. Notably, grapefruit-derived EVs do not cross the placental barrier, as evidenced by the lack of a fluorescent signal in the placenta after administration of fluorescently labeled EVs to pregnant mice [128]. This finding suggests that grapefruit-derived EVs may serve as promising drug carriers for therapeutic applications in pregnant women.
Advanced Delivery Systems: Researchers have further enhanced grapefruit-derived EVs by incorporating activated leukocyte plasma membranes to facilitate the targeted delivery of therapeutic agents to inflamed tumor sites [129]. Cabbage and red cabbage-derived EVs have been employed to deliver doxorubicin for colorectal cancer treatment, demonstrating enhanced antitumor activity compared to doxorubicin alone [130]. Another significant advancement is the use of kiwi-derived EVs for the oral delivery of sorafenib, which markedly increased its oral bioavailability, thereby enhancing therapeutic efficacy and reducing systemic toxicity [131]. Excitingly, clinical trials are currently recruiting participants to evaluate the use of plant-derived EVs as drug carriers (www.clinicaltrials.gov/ct2/show/NCT01294072). In this clinical study, curcumin—a natural chemopreventive agent for colon cancer—will be encapsulated into plant-derived EVs to modulate immune function and glucose metabolism in postoperative colon cancer patients [132].
4.2.4. Gene therapy (Table 5)
Table 5.
Application of plant-derived EVs in gene therapy.
| Gene Therapy Approach | Source of Plant EVs | Application Example | Related Research |
|---|---|---|---|
| miRNA Delivery | Broccoli | Broccoli-derived EVs loaded with miRNAs inhibited tumor cell viability in colorectal cancer | Del Pozo-Acebo et al., 2022 [136] |
| siRNA Delivery | Grapefruit | Grapefruit-derived EVs used for targeted gene silencing in human immortalized epidermal cells | Itakura et al., 2023 [137] |
| siRNA Delivery | Ginger | Ginger-derived EVs loaded with siRNA-CD98 reduced CD98 expression associated with colitis and colitis-associated cancers | Zhang et al., 2017 [107] |
| siRNA Delivery | Ginger | Ginger-derived EVs encapsulated with siRNA-BIRC5 prevented tumor progression and demonstrated safety in treated mice | Li et al., 2018 [138] |
Gene-based therapeutics are usually less stable and often exhibit high toxicity and immunogenicity [133,134]. Plant-derived EVs serve as ideal carriers to protect various gene therapeutics, such as miRNAs and siRNAs, from degradation [113,135]. A recent study revealed that broccoli-derived EVs can be efficiently loaded with exogenous miRNAs. These therapeutic miRNAs, when encapsulated within broccoli-derived EVs, exhibited enhanced cellular uptake and were protected from RNase degradation and gastrointestinal digestion. When broccoli-derived EVs loaded with ath-miR159a, ath-miR159b-3p, ath-miR166b-3p, and ath-miR403–3p were incubated with colorectal cancer Caco-2 cells, tumor cell viability was significantly inhibited [136]. However, whether these miRNA-loaded EVs affect the viability of normal cells remains to be further elucidated. Grapefruit-derived EVs have been engineered for targeted delivery via surface functionalization. These EVs effectively silenced genes in human immortalized epidermal cells using a microfluidic device [137]. Ginger-derived EVs can be loaded with therapeutic siRNAs for the treatment of ulcerative colitis. In colon-26 and RAW 264.7 cells, ginger-derived EVs loaded with siRNA-CD98 significantly reduced the expression levels of CD98, which are strongly associated with colitis and colitis-associated cancers. Notably, ginger-derived EVs alone did not induce apoptosis in RAW 264.7 cells [107]. Additionally, ginger-derived EVs encapsulated with siRNA-BIRC5 (survivin) inhibited tumor progression. No significant changes in body weight were observed in treated mice, indicating the safety of this drug delivery system [138].
5. Conclusion and future perspectives
Due to their lack of mobility, plants have evolved sophisticated intercellular communication mechanisms to maintain homeostasis under challenging environmental conditions. Plant-derived EVs, which encapsulate diverse proteins, lipids, and genetic material, serve as pivotal mediators of intercellular communication [139,140]. Given the historical importance of natural products in drug development, plant-derived EVs, enriched with the essence of plant constituents have garnered substantial attention. Extensive research has highlighted several advantages of these vesicles, including enhanced stability, ease of accessibility, low immunogenicity, and diverse bioactivities, making them promising candidates for disease treatment and drug delivery [141,142]. However, significant challenges remain in comprehensively understanding the therapeutic potential of plant-derived EVs and successfully translating these findings into clinical applications.
The limited understanding of the biological properties of plant-derived EVs currently hampers their broader therapeutic applications. Plant-derived EVs, which originate from various plant species, may carry a wide range of natural bioactive components. Therefore, the use of plant-derived EVs as therapeutic agents or drug carriers should be approached cautiously until their key bioactive components are thoroughly characterized. For instance, using plant-derived EVs with known wound-healing properties in cancer treatment, or as vehicles for delivering anticancer drugs to tumors, could lead to unintended and counterproductive outcomes. Recent advancements in multiomics technologies and bioinformatics have enabled the rapid and comprehensive characterization of plant-derived EVs, providing valuable insights for effective drug-loading strategies and synergistic therapeutic approaches [143,144].
The targeting mechanisms of plant-derived EVs remain incompletely understood. However, it is evident that membrane proteins or other bioactive components present on these EVs play pivotal roles in their targeting capabilities. Thus, modifying the membranes of these natural EVs, such as by incorporating folic acid, provides a viable strategy to overcome poor selectivity caused by interspecies differences [145,146]. Additionally, employing membrane fusion technology can endow plant-derived EVs with diverse properties, thereby partially mitigating this issue. It's also noteworthy that membrane fusion technology can enhance the cargo-loading capacity of plant-derived EVs [147].
In addition, the potential adverse effects of plant-derived EVs on homeostasis or disease progression should be considered. Although plant-derived EVs hold significant therapeutic promise, high-dose applications or specific conditions could lead to adverse effects. Studies on other types of extracellular vesicles, such as milk-derived EVs, have demonstrated that high-dose food-derived EVs may cause adverse effects, underscoring the importance of careful dose management and a comprehensive understanding of their molecular effectors [148,149].
The primary objective of researching plant-derived EVs is to facilitate their practical application. Although methods such as ultracentrifugation and density gradient centrifugation are commonly employed for isolation, they are suitable only for laboratory research and fail to meet the requirements for commercial or therapeutic translation [11]. Notably, the lack of standardized management and regulatory protocols for the clinical use of plant-derived EVs remains an unresolved issue [150]. This presents a significant barrier to their successful clinical translation.
Future research on plant-derived EVs should prioritize several key areas of investigation. Firstly, in-depth studies on the biogenesis and release mechanisms of plant-derived EVs are essential to optimize large-scale production strategies [139,140,151]. Efforts in this direction can leverage insights from the scalable and reproducible EV isolation protocols that have been successfully developed for MSCs. Pioneering work by Bernd Giebel and colleagues have demonstrated the successful large-scale production of MSC-derived EVs, offering a roadmap for analogous advancements in the large-scale production of plant-derived EVs [[152], [153], [154]].
Secondly, exploring the therapeutic potential of plant-derived EVs in treating neurodegenerative diseases, autoimmune disorders, and metabolic conditions could unveil new therapeutic avenues [155,156]. Additionally, enhancing the targeting and delivery efficiency of plant-derived EVs through bioengineering techniques, such as surface modification with ligands or antibodies and the application of nanotechnology, could significantly improve their specificity and therapeutic efficacy [157]. Integrating plant-derived EVs with other therapeutic modalities, such as chemotherapy, immunotherapy, and gene therapy, could yield synergistic effects and enhance patient outcomes [158].
Furthermore, critical considerations for developing potency assays and ensuring the clinical translation of human-derived EVs, discussed by researchers such as Warnecke A [159] and Mario Gimona [160], highlight the necessity of rigorous characterization and stringent manufacturing standards. These considerations are equally applicable to plant-derived EVs and can inform the development of standardized protocols for their isolation, characterization, and clinical application. Collaboration among researchers, industry stakeholders, and regulatory bodies is crucial for achieving these goals. By prioritizing these directions, the field of plant-derived EVs will continue to expand and make substantial contributions to medicine and healthcare.
This review provides an overview of the isolation methods for plant-derived EVs and explores their unique biological characteristics. Additionally, it summarizes and discusses the current state of research on the use of plant-derived EVs as therapeutic agents or drug delivery vehicles. Our aim is to engage biomedical researchers with diverse interests and provide foundational insights into this rapidly evolving field.
CRediT authorship contribution statement
Li Lv: Writing – original draft, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Zhenkun Li: Data curation, Conceptualization. Xin Liu: Software, Formal analysis, Data curation. Wenhui Zhang: Resources, Methodology. Yi Zhang: Methodology, Investigation, Formal analysis, Data curation. Ying Liang: Software, Resources, Project administration, Methodology. Zhixian Zhang: Visualization, Validation. Yueqiao Li: Visualization, Validation. Mingxia Ding: Writing – review & editing, Supervision, Conceptualization. Rongqing Li: Writing – original draft, Supervision, Funding acquisition, Conceptualization. Jie Lin: Writing – review & editing, Visualization, Validation, Supervision, Funding acquisition, Conceptualization.
Data availability statement
The data associated with this study are fully presented and referenced within the article. The research data is publicly available in the Zenodo repository and can be accessed through the following link: DOI 10.5281/zenodo.13269863.
Ethics statement
This review article does not involve any experimental studies with human participants or animals conducted by the authors. Therefore, no ethics approval or informed consent was required.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Rongqing Li, Jie Lin reports financial support was provided by National Natural Science Foundation of China (Project Nos. 81960423, 82160453, 81860415). Li Lv, Zhixian Zhang reports financial support was provided by Yunnan Provincial Science and Technology Planning Project (Project Nos. 202001AT070027, 202101AY070001-143, 202101AY070001-139). Li Lv, Jie Lin reports financial support was provided by 2023 Talent Training Project (RCTDHB-202306, RCTDXK-202304). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (Grant Nos. 81960423, 82160453, 81860415) and the Yunnan Provincial Science and Technology Planning Project (Grant Nos. 202001AT070027, 202101AY070001-143, 202101AY070001-139); and the 2023 Talent Training Project of the Second Affiliated Hospital of Kunming Medical University (RCTDHB-202306, RCTDXK-202304).
Contributor Information
Mingxia Ding, Email: dmx7166@126.com.
Rongqing Li, Email: lrqmxl@126.com.
Jie Lin, Email: linjie@kmmu.edu.cn.
References
- 1.Pan B.T., Johnstone R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone R.M., et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987;262(19):9412–9420. [PubMed] [Google Scholar]
- 3.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Andaloussi S., et al. Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug Deliv. Rev. 2013;65(3):391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Raimondo S., et al. Extracellular vesicles as biological shuttles for targeted therapies. Int. J. Mol. Sci. 2019;20(8):1848. doi: 10.3390/ijms20081848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garaeva L., et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021;11(1):6489. doi: 10.1038/s41598-021-85833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim M., et al. Therapeutic applications of plant-derived extracellular vesicles as antioxidants for oxidative stress-related diseases. Antioxidants. 2023;12(6):1286. doi: 10.3390/antiox12061286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dad H.A., et al. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 2021;29(1):13–31. doi: 10.1016/j.ymthe.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rome S. Biological properties of plant-derived extracellular vesicles. Food Funct. 2019;10(2):529–538. doi: 10.1039/c8fo02295j. [DOI] [PubMed] [Google Scholar]
- 10.Sriwastva M.K., et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022;23(3) doi: 10.15252/embr.202153365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perut F., et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;11(1):87. doi: 10.3390/biom11010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y., et al. Characterization of and isolation methods for plant leaf nanovesicles and small extracellular vesicles. Nanomedicine. 2020;29 doi: 10.1016/j.nano.2020.102271. [DOI] [PubMed] [Google Scholar]
- 13.Veerman R.E., et al. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J. Extracell. Vesicles. 2021;10(9) doi: 10.1002/jev2.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y., et al. Effective methods for isolation and purification of extracellular vesicles from plants. J. Integr. Plant Biol. 2021;63(12):2020–2030. doi: 10.1111/jipb.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai C., et al. Research status and challenges of plant-derived exosome-like nanoparticles. Biomed. Pharmacother. 2024;174 doi: 10.1016/j.biopha.2024.116543. [DOI] [PubMed] [Google Scholar]
- 16.Welsh J.A., et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J. Extracell. Vesicles. 2024;13(2) doi: 10.1002/jev2.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kürtösi B., Kazsoki A., Zelkó R. A systematic review on plant-derived extracellular vesicles as drug delivery systems. Int. J. Mol. Sci. 2024;25(14):7559. doi: 10.3390/ijms25147559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen A., He B., Jin H. Isolation of extracellular vesicles from arabidopsis. Curr Protoc. 2022;2(1):e352. doi: 10.1002/cpz1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng Z., et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017;25(7):1641–1654. doi: 10.1016/j.ymthe.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mu J., et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014;58(7):1561–1573. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutter B.D., Rutter K.L., Innes R.W. Isolation and quantification of plant extracellular vesicles. Bio Protoc. 2017;7(17):e2533. doi: 10.21769/BioProtoc.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gámez-Valero A., et al. Urinary extracellular vesicles as source of biomarkers in kidney diseases. Front. Immunol. 2015;6:6. doi: 10.3389/fimmu.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M., Park J.H. Isolation of Aloe saponaria-derived extracellular vesicles and investigation of their potential for chronic wound healing. Pharmaceutics. 2022;14(9):1905. doi: 10.3390/pharmaceutics14091905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deregibus M.C., et al. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016;38(5):1359–1366. doi: 10.3892/ijmm.2016.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor D.D., Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Ceron D., et al. Size-exclusion chromatography allows the isolation of EVs from the filamentous fungal plant pathogen Fusarium oxysporum f. sp. vasinfectum (Fov) Proteomics. 2021;21(13–14) doi: 10.1002/pmic.202000240. [DOI] [PubMed] [Google Scholar]
- 27.Monguió-Tortajada M., et al. Extracellular vesicle isolation methods: rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019;76(12):2369–2382. doi: 10.1007/s00018-019-03071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo J., et al. Establishment of a simplified dichotomic size-exclusion chromatography for isolating extracellular vesicles toward clinical applications. J. Extracell. Vesicles. 2021;10(11) doi: 10.1002/jev2.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sukreet S., et al. Isolation of extracellular vesicles from byproducts of cheesemaking by tangential flow filtration yields heterogeneous fractions of nanoparticles. J. Dairy Sci. 2021;104(9):9478–9493. doi: 10.3168/jds.2021-20300. [DOI] [PubMed] [Google Scholar]
- 30.Kim W.S., et al. Immunological effects of Aster yomena callus-derived extracellular vesicles as potential therapeutic agents against allergic asthma. Cells. 2022;11(18):2805. doi: 10.3390/cells11182805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M.K., et al. The antioxidant effect of small extracellular vesicles derived from Aloe vera peels for wound healing. Tissue Eng Regen Med. 2021;18(4):561–571. doi: 10.1007/s13770-021-00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H., Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019;14(4):1027–1053. doi: 10.1038/s41596-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordin J.Z., et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11(4):879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., et al. A review on separation and application of plant-derived exosome-like nanoparticles. J. Separ. Sci. 2024;47(8) doi: 10.1002/jssc.202300669. [DOI] [PubMed] [Google Scholar]
- 35.Guo X., et al. Magnetic nanoparticle-based microfluidic platform for automated enrichment of high-purity extracellular vesicles. Anal. Chem. 2024;96(18):7212–7219. doi: 10.1021/acs.analchem.4c00795. [DOI] [PubMed] [Google Scholar]
- 36.Visan K.S., et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles. J. Extracell. Vesicles. 2022;11(9) doi: 10.1002/jev2.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble J.M., et al. Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. J. Struct. Biol. 2020;210(1) doi: 10.1016/j.jsb.2020.107474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatischeff I., et al. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J. Extracell. Vesicles. 2012;(21):1. doi: 10.3402/jev.v1i0.19179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aissaoui N., et al. Free-standing DNA origami superlattice to facilitate cryo-EM visualization of membrane vesicles. J. Am. Chem. Soc. 2024;146(19):12925–12932. doi: 10.1021/jacs.3c07328. [DOI] [PubMed] [Google Scholar]
- 40.Coumans F.A., et al. Reproducible extracellular vesicle size and concentration determination with tunable resistive pulse sensing. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auger C., et al. Extracellular vesicle measurements with nanoparticle tracking analysis: a different appreciation of up and down secretion. Int. J. Mol. Sci. 2022;23(4):2310. doi: 10.3390/ijms23042310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowal E.J.K., et al. Extracellular vesicle isolation and analysis by Western blotting. Methods Mol. Biol. 2017;1660:143–152. doi: 10.1007/978-1-4939-7253-1_12. [DOI] [PubMed] [Google Scholar]
- 43.Pocsfalvi G., et al. Physiochemical and protein datasets related to citrus juice sac cells-derived nanovesicles and microvesicles. Data Brief. 2019;22:251–254. doi: 10.1016/j.dib.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pocsfalvi G., et al. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018;229:111–121. doi: 10.1016/j.jplph.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Raimondo S., et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6(23):19514–19527. doi: 10.18632/oncotarget.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kırbaş O.K., et al. Unveiling the potential: extracellular vesicles from plant cell suspension cultures as a promising source. Biofactors. 2024 doi: 10.1002/biof.2090. Jul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Z., et al. Plant-derived extracellular vesicles (PDEVs) in nanomedicine for human disease and therapeutic modalities. J. Nanobiotechnol. 2023;21(1):114. doi: 10.1186/s12951-023-01858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gianazza E., et al. Proteomics and Lipidomics to unveil the contribution of PCSK9 beyond cholesterol lowering: a narrative review. Front. Cardiovasc. Med. 2023;10 doi: 10.3389/fcvm.2023.1191303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J., et al. An update on isolation methods for proteomic studies of extracellular vesicles in biofluids. Molecules. 2019;24(19):3516. doi: 10.3390/molecules24193516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urzì O., et al. Plant-RNA in extracellular vesicles: the secret of cross-kingdom communication. Membranes. 2022;12(4):352. doi: 10.3390/membranes12040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janda M., Robatzek S. Extracellular vesicles from phytobacteria: properties, functions and uses. Biotechnol. Adv. 2022;58 doi: 10.1016/j.biotechadv.2022.107934. [DOI] [PubMed] [Google Scholar]
- 52.Rutter B.D., Innes R.W. Extracellular vesicles as key mediators of plant-microbe interactions. Curr. Opin. Plant Biol. 2018;44:16–22. doi: 10.1016/j.pbi.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Middleton H., et al. Rhizospheric plant-microbe interactions: miRNAs as a key mediator. Trends Plant Sci. 2021;26(2):132–141. doi: 10.1016/j.tplants.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y., et al. In vitro effects of tartary buckwheat-derived nanovesicles on gut microbiota. J. Agric. Food Chem. 2022;70(8):2616–2629. doi: 10.1021/acs.jafc.1c07658. [DOI] [PubMed] [Google Scholar]
- 55.Teng Y., et al. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(5):637–652.e8. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu N.J., et al. Lipidomic analysis reveals the importance of GIPCs in arabidopsis leaf extracellular vesicles. Mol. Plant. 2020;13(10):1523–1532. doi: 10.1016/j.molp.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 57.Woith E., et al. Plant extracellular vesicles and nanovesicles: focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int. J. Mol. Sci. 2021;22(7):3719. doi: 10.3390/ijms22073719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López de Las Hazas M.C., et al. Therapeutic potential of plant-derived extracellular vesicles as nanocarriers for exogenous miRNAs. Pharmacol. Res. 2023;198 doi: 10.1016/j.phrs.2023.106999. [DOI] [PubMed] [Google Scholar]
- 59.Görgens A., et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles. 2022;11(6) doi: 10.1002/jev2.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan F., Li Y.M., Wang Z. Preserving extracellular vesicles for biomedical applications: consideration of storage stability before and after isolation. Drug Deliv. 2021;28(1):1501–1509. doi: 10.1080/10717544.2021.1951896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu J.Y., et al. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: a comparative evaluation of storage conditions. Drug Deliv. 2021;28(1):162–170. doi: 10.1080/10717544.2020.1869866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merivaara A., et al. Preservation of biomaterials and cells by freeze-drying: change of paradigm. J. Contr. Release. 2021;336:480–498. doi: 10.1016/j.jconrel.2021.06.042. [DOI] [PubMed] [Google Scholar]
- 63.Trenkenschuh E., et al. Enhancing the stabilization potential of lyophilization for extracellular vesicles. Adv. Healthcare Mater. 2022;11(5) doi: 10.1002/adhm.202100538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kusuma G.D., et al. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front. Pharmacol. 2018;9:1199. doi: 10.3389/fphar.2018.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ju S., et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013;21(7):1345–1357. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zu M., et al. 'Green' nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279 doi: 10.1016/j.biomaterials.2021.121178. [DOI] [PubMed] [Google Scholar]
- 67.Zhang M., et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi: 10.1016/j.biomaterials.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu Y., et al. Edible pueraria lobata-derived exosome-like nanovesicles ameliorate dextran sulfate sodium-induced colitis associated lung inflammation through modulating macrophage polarization. Biomed. Pharmacother. 2024;170 doi: 10.1016/j.biopha.2023.116098. [DOI] [PubMed] [Google Scholar]
- 69.Mammadova R., et al. Protein biocargo and anti-inflammatory effect of tomato fruit-derived nanovesicles separated by density gradient ultracentrifugation and loaded with curcumin. Pharmaceutics. 2023;15(2):333. doi: 10.3390/pharmaceutics15020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu C., et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnol. 2022;20(1):206. doi: 10.1186/s12951-022-01421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nayak B., Liu R.H., Tang J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains--a review. Crit. Rev. Food Sci. Nutr. 2015;55(7):887–919. doi: 10.1080/10408398.2011.654142. [DOI] [PubMed] [Google Scholar]
- 72.Baby B., Antony P., Vijayan R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018;58(15):2491–2507. doi: 10.1080/10408398.2017.1329198. [DOI] [PubMed] [Google Scholar]
- 73.Baldini N., et al. Exosome-like nanovesicles isolated from citrus limon L. Exert antioxidative effect. Curr. Pharmaceut. Biotechnol. 2018;19(11):877–885. doi: 10.2174/1389201019666181017115755. [DOI] [PubMed] [Google Scholar]
- 74.Kim D.K., Rhee W.J. Antioxidative effects of carrot-derived nanovesicles in cardiomyoblast and neuroblastoma cells. Pharmaceutics. 2021;13(8):1203. doi: 10.3390/pharmaceutics13081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li S., et al. Evaluation of antioxidant activity and drug delivery potential of cell-derived extracellular vesicles from citrus reticulata Blanco cv. 'Dahongpao'. Antioxidants. 2023;12(9):1706. doi: 10.3390/antiox12091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhuang X., et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles. 2015;4 doi: 10.3402/jev.v4.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martins F., et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019;16(9):563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 78.LaFargue C.J., et al. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15–e28. doi: 10.1016/S1470-2045(18)30786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Curigliano G., et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA A Cancer J. Clin. 2016;66(4):309–325. doi: 10.3322/caac.21341. [DOI] [PubMed] [Google Scholar]
- 80.Kim K., et al. Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J. Funct. Biomater. 2020;11(2):22. doi: 10.3390/jfb11020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim K., et al. Anti-metastatic effects of plant sap-derived extracellular vesicles in a 3D microfluidic cancer metastasis model. J. Funct. Biomater. 2020;11(3):49. doi: 10.3390/jfb11030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boccia E., et al. Plant hairy roots for the production of extracellular vesicles with antitumor bioactivity. Commun. Biol. 2022;5(1):848. doi: 10.1038/s42003-022-03781-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang M., et al. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020;18(1):100. doi: 10.1186/s12951-020-00656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Q., et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B. 2022;12(2):907–923. doi: 10.1016/j.apsb.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Browning D.D., Kwon I.K., Wang R. cGMP-dependent protein kinases as potential targets for colon cancer prevention and treatment. Future Med. Chem. 2010;2(1):65–80. doi: 10.4155/fmc.09.142. [DOI] [PubMed] [Google Scholar]
- 86.Seril D.N., et al. High-iron diet: foe or feat in ulcerative colitis and ulcerative colitis-associated carcinogenesis. J. Clin. Gastroenterol. 2006;40(5):391–397. doi: 10.1097/00004836-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 87.Cao M., et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7(1):326. doi: 10.1186/s40425-019-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee R., et al. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles. 2020;9(1) doi: 10.1080/20013078.2019.1703480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu J., et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J. Nanobiotechnol. 2023;21(1):78. doi: 10.1186/s12951-023-01835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L., et al. Engineering exosome-like nanovesicles derived from Asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int. J. Nanomed. 2021;16:1575–1586. doi: 10.2147/IJN.S293067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stanly C., et al. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the A375 human melanoma cell line. Cells. 2020;9(12):2722. doi: 10.3390/cells9122722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Regente M., et al. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017;68(20):5485–5495. doi: 10.1093/jxb/erx355. [DOI] [PubMed] [Google Scholar]
- 93.Palma M d., et al. Plant roots release small extracellular vesicles with antifungal activity. Plants. 2020;9(12):1777. doi: 10.3390/plants9121777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sundaram K., et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of Porphyromonas gingivalis. iScience. 2019;21:308–327. doi: 10.1016/j.isci.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu S., et al. Characterization of three different types of extracellular vesicles and their impact on bacterial growth. Food Chem. 2019;272:372–378. doi: 10.1016/j.foodchem.2018.08.059. [DOI] [PubMed] [Google Scholar]
- 96.Wiersinga W.J., et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 97.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pillalamarri N., et al. Exploring the utility of extracellular vesicles in ameliorating viral infection-associated inflammation, cytokine storm and tissue damage. Transl. Oncol. 2021;14(7) doi: 10.1016/j.tranon.2021.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El-Nabi S.H., Elhiti M., El-Sheekh M. A new approach for COVID-19 treatment by micro-RNA. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teng Y., et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021;29(8):2424–2440. doi: 10.1016/j.ymthe.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kalarikkal S.P., Sundaram G.M. Edible plant-derived exosomal microRNAs: exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol. Appl. Pharmacol. 2021;414 doi: 10.1016/j.taap.2021.115425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Şahin F., et al. In vitro wound healing activity of wheat-derived nanovesicles. Appl. Biochem. Biotechnol. 2019;188(2):381–394. doi: 10.1007/s12010-018-2913-1. [DOI] [PubMed] [Google Scholar]
- 103.Zhao X., et al. Oral administration of grape-derived nanovesicles for protection against LPS/D-GalN-induced acute liver failure. Int. J. Pharm. 2024;652 doi: 10.1016/j.ijpharm.2024.123812. [DOI] [PubMed] [Google Scholar]
- 104.Zhao X., et al. Garlic-derived exosome-like nanovesicles as a hepatoprotective agent alleviating acute liver failure by inhibiting CCR2/CCR5 signaling and inflammation. Biomater. Adv. 2023;154 doi: 10.1016/j.bioadv.2023.213592. [DOI] [PubMed] [Google Scholar]
- 105.Kumar A., et al. miR-375 prevents high-fat diet-induced insulin resistance and obesity by targeting the aryl hydrocarbon receptor and bacterial tryptophanase (tnaA) gene. Theranostics. 2021;11(9):4061–4077. doi: 10.7150/thno.52558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang B., et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014;22(3):522–534. doi: 10.1038/mt.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang M., et al. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12(16):1927–1943. doi: 10.2217/nnm-2017-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dolma L., et al. Exosomes isolated from citrus lemon: a promising candidate for the treatment of Alzheimer's disease. Ther. Deliv. 2024:1–13. doi: 10.1080/20415990.2024.2354119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang M., et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol. Ther. 2016;24(10):1783–1796. doi: 10.1038/mt.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang S., et al. Gut‐liver axis: potential mechanisms of action of food‐derived extracellular vesicles. J. Extracell. Vesicles. 2024;13(6) doi: 10.1002/jev2.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rahimi Ghiasi M., et al. Leucine-rich repeat-containing G-protein coupled receptor 5 gene overexpression of the rat small intestinal progenitor cells in response to orally administered grape exosome-like nanovesicles. Adv. Biomed. Res. 2018;7:125. doi: 10.4103/abr.abr_114_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gong Q., et al. Anti-fibrotic effect of extracellular vesicles derived from tea leaves in hepatic stellate cells and liver fibrosis mice. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karamanidou T., Tsouknidas A. Plant-derived extracellular vesicles as therapeutic nanocarriers. Int. J. Mol. Sci. 2021;23(1):191. doi: 10.3390/ijms23010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nasiri Kenari A., Cheng L., Hill A.F. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods. 2020;177:103–113. doi: 10.1016/j.ymeth.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 115.Li C., et al. Preparation, characterization, and in vitro anticancer activity evaluation of broccoli-derived extracellular vesicle-coated astaxanthin nanoparticles. Molecules. 2022;27(12):3955. doi: 10.3390/molecules27123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen C., et al. Active cargo loading into extracellular vesicles: highlights the heterogeneous encapsulation behaviour. J. Extracell. Vesicles. 2021;10(13) doi: 10.1002/jev2.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zuppone S., et al. Novel loading protocol combines highly efficient encapsulation of exogenous therapeutic toxin with preservation of extracellular vesicles properties, uptake and cargo activity. Discov Nano. 2024;19(1):76. doi: 10.1186/s11671-024-04022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nemidkanam V., Chaichanawongsaroj N. Characterizing Kaempferia parviflora extracellular vesicles, a nanomedicine candidate. PLoS One. 2022;17(1) doi: 10.1371/journal.pone.0262884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fernandes M., et al. Exosome-like nanoparticles: a new type of nanocarrier. Curr. Med. Chem. 2020;27(23):3888–3905. doi: 10.2174/0929867326666190129142604. [DOI] [PubMed] [Google Scholar]
- 120.Xiao Q., et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. 2022;9(20) doi: 10.1002/advs.202105274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kooijmans S.A.A., et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Contr. Release. 2013;172(1):229–238. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 122.Farheen J., et al. Vitis vinifera Kyoho-derived exosome-like nanoparticles-based drug delivery and therapeutic modalities for breast cancer therapy. J. Drug Deliv. Sci. Technol. 2024;92 [Google Scholar]
- 123.Ishida T., et al. Exosome-like nanoparticles derived from Allium tuberosum prevent neuroinflammation in microglia-like cells. J. Pharm. Pharmacol. 2023;75(10):1322–1331. doi: 10.1093/jpp/rgad062. [DOI] [PubMed] [Google Scholar]
- 124.Nizamudeen Z.A., et al. Low-power sonication can alter extracellular vesicle size and properties. Cells. 2021;10(9):2413. doi: 10.3390/cells10092413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu J.Y., et al. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16(9):5895–5901. doi: 10.1021/acs.nanolett.6b02786. [DOI] [PubMed] [Google Scholar]
- 126.Maas S.L.N., Breakefield X.O., Weaver A.M. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.French K.C., Antonyak M.A., Cerione R.A. Extracellular vesicle docking at the cellular port: extracellular vesicle binding and uptake. Semin. Cell Dev. Biol. 2017;67:48–55. doi: 10.1016/j.semcdb.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Q., et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013;4:1867. doi: 10.1038/ncomms2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Q., et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75(12):2520–2529. doi: 10.1158/0008-5472.CAN-14-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.You J.Y., Kang S.J., Rhee W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021;6(12):4321–4332. doi: 10.1016/j.bioactmat.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fang Z., et al. Kiwi-derived extracellular vesicles for oral delivery of sorafenib. Eur. J. Pharmaceut. Sci. 2023;191 doi: 10.1016/j.ejps.2023.106604. [DOI] [PubMed] [Google Scholar]
- 132.Wu K., et al. Extracellular vesicles as emerging targets in cancer: recent development from bench to bedside. Biochim. Biophys. Acta Rev. Canc. 2017;1868(2):538–563. doi: 10.1016/j.bbcan.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kaufmann K.B., et al. Gene therapy on the move. EMBO Mol. Med. 2013;5(11):1642–1661. doi: 10.1002/emmm.201202287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.High K.A., Roncarolo M.G. Gene therapy. N. Engl. J. Med. 2019;381(5):455–464. doi: 10.1056/NEJMra1706910. [DOI] [PubMed] [Google Scholar]
- 135.Jiang L., Vader P., Schiffelers R.M. Extracellular vesicles for nucleic acid delivery: progress and prospects for safe RNA-based gene therapy. Gene Ther. 2017;24(3):157–166. doi: 10.1038/gt.2017.8. [DOI] [PubMed] [Google Scholar]
- 136.Del Pozo-Acebo L., et al. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol. Res. 2022;185 doi: 10.1016/j.phrs.2022.106472. [DOI] [PubMed] [Google Scholar]
- 137.Itakura S., et al. Gene knockdown in HaCaT cells by small interfering RNAs entrapped in grapefruit-derived extracellular vesicles using a microfluidic device. Sci. Rep. 2023;13(1):3102. doi: 10.1038/s41598-023-30180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Z., et al. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-32953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Woith E., Fuhrmann G., Melzig M.F. Extracellular vesicles-connecting kingdoms. Int. J. Mol. Sci. 2019;20(22):5695. doi: 10.3390/ijms20225695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rutter B.D., Innes R.W. Growing pains: addressing the pitfalls of plant extracellular vesicle research. New Phytol. 2020;228(5):1505–1510. doi: 10.1111/nph.16725. [DOI] [PubMed] [Google Scholar]
- 141.Urzì O., Raimondo S., Alessandro R. Extracellular vesicles from plants: current knowledge and open questions. Int. J. Mol. Sci. 2021;22(10):5366. doi: 10.3390/ijms22105366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pinedo M., de la Canal L., de Marcos Lousa C. A call for Rigor and standardization in plant extracellular vesicle research. 2021;10(6) doi: 10.1002/jev2.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shaba E., et al. Multi-omics integrative approach of extracellular vesicles: a future challenging milestone. Proteomes. 2022;10(2):12. doi: 10.3390/proteomes10020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Keerthikumar S., et al. Bioinformatics tools for extracellular vesicles research. Methods Mol. Biol. 2017;1545:189–196. doi: 10.1007/978-1-4939-6728-5_13. [DOI] [PubMed] [Google Scholar]
- 145.García-Manrique P., et al. Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J. Extracell. Vesicles. 2018;7(1) doi: 10.1080/20013078.2017.1422676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Armstrong J.P., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11(1):69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Geng T., et al. Recent advancement and technical challenges in developing small extracellular vesicles for cancer drug delivery. Pharm. Res. (N. Y.) 2021;38(2):179–197. doi: 10.1007/s11095-021-02988-z. [DOI] [PubMed] [Google Scholar]
- 148.Samuel M., et al. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 2021;12(1):3950. doi: 10.1038/s41467-021-24273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tong L., et al. Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis. Sci. Adv. 2023;9(15):eade5041. doi: 10.1126/sciadv.ade5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lener T., et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles. 2015;4 doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kang T., Atukorala I., Mathivanan S. Biogenesis of extracellular vesicles. Subcell. Biochem. 2021;97:19–43. doi: 10.1007/978-3-030-67171-6_2. [DOI] [PubMed] [Google Scholar]
- 152.Börger V., et al. Scaled isolation of mesenchymal stem/stromal cell‐derived extracellular vesicles. Curr. Protoc. Stem Cell Biol. 2020;55(1) doi: 10.1002/cpsc.128. [DOI] [PubMed] [Google Scholar]
- 153.Rohde E., Pachler K., Gimona M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy. 2019;21(6):581–592. doi: 10.1016/j.jcyt.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 154.Gimona M., et al. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol. Sci. 2017;18(6):1190. doi: 10.3390/ijms18061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Upadhya R., et al. Astrocyte-derived extracellular vesicles: neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J. Contr. Release. 2020;323:225–239. doi: 10.1016/j.jconrel.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kahmini F.R., Shahgaldi S. Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles as novel cell-free therapy for treatment of autoimmune disorders. Exp. Mol. Pathol. 2021;118 doi: 10.1016/j.yexmp.2020.104566. [DOI] [PubMed] [Google Scholar]
- 157.Ivanova A., et al. Creating designer engineered extracellular vesicles for diverse ligand display, target recognition, and controlled protein loading and delivery. Adv. Sci. 2023;10(34) doi: 10.1002/advs.202304389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Turano E., et al. Extracellular vesicles from mesenchymal stem cells: towards novel therapeutic strategies for neurodegenerative diseases. Int. J. Mol. Sci. 2023;24(3) doi: 10.3390/ijms24032917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Warnecke A., et al. First-in-human intracochlear application of human stromal cell-derived extracellular vesicles. J. Extracell. Vesicles. 2021;10(8) doi: 10.1002/jev2.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gimona M., et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy. 2021;23(5):373–380. doi: 10.1016/j.jcyt.2021.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with this study are fully presented and referenced within the article. The research data is publicly available in the Zenodo repository and can be accessed through the following link: DOI 10.5281/zenodo.13269863.