Abstract
The EFSA Panel on Food Additives and Flavourings (FAF) was requested to evaluate the safety of hesperetin dihydrochalcone [FL‐no: 16.137] as a new flavouring substance, in accordance with Regulation (EC) No 1331/2008. The substance is structurally related to the group of flavonoids evaluated in FGE.32 and is the aglycone of neohesperidine dihydrochalcone. Based on the data provided for [FL‐no: 16.137], the Panel considered that a read‐across between hesperetin dihydrochalcone and the substances in FGE.32 is not needed. Nevertheless, the flavonoids evaluated in FGE.32 were considered in a cumulative exposure assessment. The information provided on the manufacturing process, the composition and the stability of [FL‐no: 16.137] was considered sufficient. The Panel concluded that there is no concern with respect to genotoxicity. No absorption, distribution, metabolism and excretion (ADME) studies on [FL‐no: 16.137] were provided, but studies investigating the ADME of neohesperidine dihydrochalcone were submitted. The Panel noted that [FL‐no: 16.137] has the same fate in the organism, as that of neohesperidine dihydrochalcone and considered that [FL‐no: 16.137] can be anticipated to be metabolised to innocuous products only. In a prenatal developmental toxicity study, no maternal or foetal toxicity was observed. In a 90‐day toxicity study, indications were obtained that the substance affects thyroid hormone levels at all doses tested (100–1000 mg/kg bw per day). Since these changes were not accompanied by apical findings indicative of hypothyroidism, the Panel considered these hormonal effects as not adverse. Using 1000 mg/kg bodyweight (bw) per day as reference point, adequate margins of exposure were calculated for adults and children, when considering the chronic added portions exposure technique (APET) dietary exposure estimates. Cumulative chronic exposure estimates to [FL‐no: 16.137] and the four structurally related substances evaluated in FGE.32 do not raise a safety concern. The use of [FL‐no: 16.137] as food flavouring, under the proposed conditions of use, does not raise a safety concern.
Keywords: FGE.32, FGE.420, Flavouring, FL‐no: 16.137, hesperetin dihydrochalcone
1. INTRODUCTION
The present scientific opinion, Flavouring Group Evaluation 420 (FGE.420), deals with the safety assessment of hesperetin dihydrochalcone (3‐(3‐hydroxy‐4‐methoxyphenyl)‐1‐(2,4,6‐trihydroxy‐phenyl)propan‐1‐one) [FL‐no: 16.137] proposed for use as a new flavouring substance in and on food.
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
In accordance with Article 10 of Regulation (EC) No 1334/2008 1 on food flavourings, only flavouring substances included in the Union list may be placed on the market as such and used in foods under the conditions of use specified therein.
On 22 November 2022, a new application has been introduced by the applicant “Symrise AG” for the authorisation of the flavouring substance 3‐(3‐hydroxy‐4‐methoxyphenyl)‐1‐(2,4,6‐trihydroxyphenyl)propan‐1‐one.
1.1.2. Terms of Reference
The European Commission requests the European Food Safety Authority to carry out the safety assessment and the assessment of possible confidentiality requests of the following food flavouring: 3‐(3‐hydroxy‐4‐methoxy‐phenyl)‐1‐(2,4,6‐trihydroxyphenyl)propan‐1‐one, in accordance with Regulation (EC) No 1331/2008 2 establishing a common authorisation procedure for food additives, food enzymes and food flavourings.
1.2. Existing authorisations and evaluations
Hesperetin dihydrochalcone has been considered by the Flavour and Extract Manufactures Association (FEMA) expert Panel as ‘generally regarded as safe’ (GRAS) (FEMA GRAS no 4872, GRAS list 28).
Hesperetin dihydrochalcone was evaluated at the 89th meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 2021, 2022) and specifications were set (JECFA no. 2262). 3 The flavouring substance was evaluated by the revised Procedure for the Safety Evaluation of Flavouring in the ‘Phenol and phenol derivatives’ category and assigned to structural class III. The Committee stated that ‘the NOAEL of 750 mg/kg bw per day for the structurally related substance neohesperidine dihydrochalcone in a 90‐day dietary study in male and female rats (..) provides an adequate MOE (15 000) relative to the SPET estimate of 3000 μg/day when it is used as a flavouring agent’. The Committee concluded that hesperetin dihydrochalcone would not give rise to safety concerns at the estimated dietary exposure based on single portion exposure technique (SPET).
The FAF Panel noted that hesperetin dihydrochalcone is not registered in the ECHA chemical database. 4
1.2.1. Existing evaluations of structurally related substance neohesperidine dihydrochalcone
Beside the evaluation of neohesperidine dihydrochalcone [FL‐no: 16.061] as a flavouring substance by the CEF Panel in FGE.32 (EFSA CEF Panel, 2010a), this substance has been evaluated as a food additive and as a feed additive. A brief description is reported below (for more details see EFSA FAF Panel, 2022a; EFSA FEEDAP Panel, 2011, 2014).
The food additive neohesperidine dihydrochalcone (E 959) was re‐evaluated by the Panel in 2022 for its use as a food additive (E 959) belonging to the functional class of sweeteners (EFSA FAF Panel, 2022a). The Panel derived an ADI of 20 mg/kg body weight (bw) per day based on a NOAEL of 4000 mg/kg bw per day from a 13‐week study in rats, applying the standard default factors of 100 for inter‐ and intraspecies differences and of 2 for extrapolation from subchronic to chronic exposure. The food additive was considered safe at the reported uses and use levels in foods (EFSA FAF Panel, 2022a).
The Panel noted that both JECFA and the FAF Panel considered the same 90‐day toxicity study on neohesperidine dihydrochalcone (Lina et al., 1990) from which they derived a different reference point. In this study, JECFA (2012) considered the mid‐dose of 760 5 mg/kg bw per day as a NOAEL, based on effects observed in the highest dose group: ‘In the high‐dose group, body weight gains were decreased in males throughout the study and in females in the first 2 weeks. Feed intake was only slightly decreased in high‐dose males in the first 2 weeks. Ophthalmoscopy and haematological examinations did not reveal treatment‐related effects. Plasma alkaline phosphatase activity (+20%) and bilirubin concentration (+225%) were increased in high‐dose females. Total plasma protein concentration was decreased (−6%) in high‐dose males. Urine analysis revealed a decrease (up to 10%) in urinary pH in both sexes in the high‐dose group. The macroscopic examinations at autopsy revealed no treatment‐related changes. Relative caecum weight was markedly increased in the high‐dose group in both sexes (up to 76% for caecum with content and 52% for caecum without content) (...). The relative weights of the brain and testicles were increased in males of the high‐dose group, although the absolute weights of these organs (data not shown) were comparable to those of the control group. The changes were therefore considered to be due to the decreased body weights in this group. Microscopic examination did not reveal any treatment‐related changes in the caecum or in other organs or tissues’. Regarding body weight gains observed in this study, the FAF Panel (2022a) reported: ‘Body weight changes in rats and mice in two of the three evaluated studies were within 10% of control values and were considered to be non‐adverse’. Overall, the FAF Panel considered the effects mentioned above as not toxicologically relevant and therefore considered the highest dose tested of 4000 mg/kg bw as the reference point (EFSA FAF Panel, 2022a).
Neohesperidine dihydrochalcone was also evaluated by the FEEDAP Panel (EFSA FEEDAP Panel, 2011) as an additive to feed and water for drinking for piglets (suckling and weaned), pigs for fattening, calves for rearing, calves for fattening, lambs for rearing, lambs for fattening, dairy sheep, ewes for reproduction, salmon and trout, and dogs. Regarding the safety for the consumer, the FEEDAP Panel concluded: ‘The exposure of consumers to NHDC 6 in food would not be significantly increased by its use as a feed additive for mammals and poultry. However, the lack of data on metabolism and residues in fish precludes an assessment of consumer exposure from this source’.
Based on additional data provided on the metabolism in fish of substances structurally related to neohesperidine dihydrochalcone, the FEEDAP Panel concluded that ‘NHDC will essentially undergo the same routes of metabolism in all species, including fish. Based on the rapid excretion of NHDC in mammals and the similar metabolism of flavonoids in fish, the FEEDAP Panel considers that NHDC would not accumulate in edible tissues of fish. Consequently, the exposure of consumers to NHDC would not be significantly increased by its use as a feed additive for fish’. (EFSA FEEDAP Panel, 2014).
2. DATA AND METHODOLOGIES
2.1. Data
The present evaluation is based on data on hesperetin dihydrochalcone [FL‐no: 16.137] provided by the applicant in a dossier (Documentation provided to EFSA no. 1) to support its safety evaluation as a food flavouring substance.
In accordance with Article 38 of the Regulation (EC) No 178/2002 7 and taking into account the protection of confidential information and of personal data in accordance with Articles 39 to 39e of the same Regulation and of the Decision of the EFSA's Executive Director laying down practical arrangements concerning transparency and confidentiality, 8 the non‐confidential version of the dossier is published on Open.EFSA. 9
According to Art. 32c(2) of Regulation (EC) No 178/2002 and to the Decision of EFSA's Executive Director laying down the practical arrangements on pre‐submission phase and public consultations, EFSA carried out a public consultation on the non‐confidential version of the application from 29 June to 20 July 2023. 10 Comments were received and published. 11 None of the comments submitted was deemed relevant to the scope of the public consultation and were not considered further.
Additional information was provided by the applicant on 8 February 2024 (Documentation provided to EFSA no. 2) and on 14 June 2024 (Documentation provided to EFSA no. 3) in response to a request from EFSA sent on 26 June 2023 and on 19 March 2024, respectively.
2.2. Methodologies
This opinion was prepared following the principles described in the EFSA Guidance of the Scientific Committee on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing Guidance documents from the EFSA Scientific Committee.
The application on hesperetin dihydrochalcone [FL‐no: 16.137] was submitted to EFSA before the publication of the latest EFSA guidance on data required for the risk assessment of flavourings to be used in or on foods (EFSA FAF Panel, 2022b). Therefore, the safety assessment of hesperetin dihydrochalcone [FL‐no: 16.137] was carried out in accordance with the procedure as outlined in the EFSA scientific opinion ‘Guidance on the data required for the risk assessment of flavourings to be used in or on foods’ (EFSA CEF Panel, 2010b) and the EFSA technical report ‘Proposed template to be used in drafting scientific opinions on flavouring substances (explanatory notes for guidance included)’ (EFSA, 2012).
3. ASSESSMENT
3.1. Technical data
3.1.1. Identity of the substance
Hesperetin dihydrochalcone (IUPAC name: 3‐(3‐hydroxy‐4‐methoxy‐phenyl)‐1‐(2,4,6‐trihydroxyphenyl)propan‐1‐one; SMILES code: COc1ccc(CCC(=O)c2c(O)cc(O)cc2O)cc1O) has been allocated the FLAVIS number [FL‐no: 16.137]. The trivial name of the flavouring substance, hesperetin dihydrochalcone, will be used hereafter. The chemical structure of the candidate substance hesperetin dihydrochalcone and the specification data provided by the applicant are shown in Table 1.
TABLE 1.
Specification data for hesperetin dihydrochalcone as proposed by the applicant (Documentation provided to EFSA no. 1, 2, 3).
| Common Chemical name IUPAC |
CAS no FL‐no JECFA no FEMA no EINECS no CoE no |
Chemical formula MW (Da) | Structural formula | Physical form | Solubility data | ID test | Purity a | Impurities | Boiling point Melting point Specific gravity Refractive index | Information on the configuration of the flavouring substance |
|---|---|---|---|---|---|---|---|---|---|---|
|
Hesperetin dihydrochalcone 3‐(3‐hydroxy‐4‐methoxyphenyl)‐1‐(2,4,6‐trihydroxyphenyl)propan‐1‐one |
35400–60‐3 16.137 2262 4872 – – |
C16H16O6 304.29 |
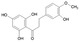
|
White to brown powder |
Water: Practically insoluble to insoluble Ethanol: Soluble DMSO: Soluble Propylene glycol: Soluble at a concentration of 10 wt% |
LC/MS IR NMR |
≥ 95% |
Hesperetin ≤ 0.5% a Phloretin ≤ 1.5% a Hesperetin dihydrochalcone glucoside ≤ 1.5% a Neohesperidine dihydrochalcone ≤ 0.05% a Isosakuranetin dihydrochalcone ≤ 1.5% a Nickel < 10 mg/kg Palladium < 10 mg/kg Arsenic < 1 mg/kg Lead < 0.5 mg/kg Cadmium < 0.1 mg/kg Mercury < 0.05 mg/kg Water content ≤ 6% Ash content ≤ 0.5% |
Decomposition > 120°C |
– b |
On a dry matter basis.
Hesperetin dihydrochalcone does not have geometrical or optical stereoisomers.
The applicant proposed infrared spectroscopy (IR), nuclear magnetic resonance (NMR) and liquid chromatography–mass spectrometry (LC/MS) for identifying hesperetin dihydrochalcone and provided relevant identification data (Documentation provided to EFSA no. 1).
According to the analytical data provided by the applicant for five batches, the content of hesperetin dihydrochalcone ranged from 95.0 to 98.1%. In two other batches, ■■■■■, the content of hesperetin dihydrochalcone amounted to 96.0% and 96.6%, respectively (Documentation provided to EFSA no. 1, 2).
For the five batches investigated by the applicant ■■■■■, quantitative data were also provided for the following impurities: hesperetin (below the reporting limit of 0.1%–0.3%), phloretin (0.1%–0.9%), hesperetin dihydrochalcone glucoside (0.2%–1.2% in four batches, not detected in two batches). For two of the batches, contents of neohesperidine dihydrochalcone (below the reporting limit of 0.01 and below the reporting limit of 0.05%, respectively) were reported. These impurities are all structurally related to the candidate substance and their structures are shown in Table 2 (Documentation provided to EFSA no. 1, 2).
TABLE 2.
Chemical structures of impurities structurally related to the candidate substance identified in commercial batches (Documentation provided to EFSA no. 1, 2, 3).
| Chemical name/(CAS no.)/[FL‐no:] | Chemical structure |
|---|---|
| Hesperetin dihydrochalcone glucoside/(21940‐36‐3) |
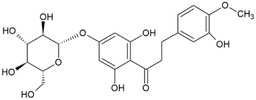
|
| Phloretin/(60‐82‐2)/[16.109] |
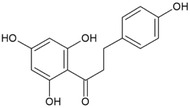
|
| Hesperetin/(520‐33‐2)/[16.097] |
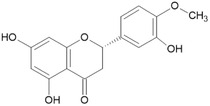
|
| Neohesperidine dihydrochalcone/(20702‐77‐6)/[16.061] |
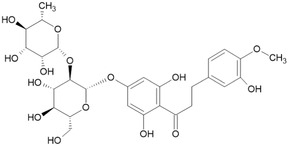
|
|
Isosakuranetin dihydrochalcone [1‐Propanone, 3‐(4‐methoxyphenyl)‐1‐(2,4,6‐trihydroxyphenyl)‐ (9CI, ACI)]/(76172‐68‐4) |
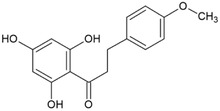
|
LC/MS investigations, using a corona detector, resulted in the identification of isosakuranetin dihydrochalcone (Table 2) in two of the five investigated batches (0.80% and 0.83%). An impurity detected in three of the batches (0.03%–1.84%) was tentatively assigned as the corresponding alcohol of dihydrochalcone glucoside (sum formula C26H28O8). Using the corona detector, four further structurally not assigned impurities were detected in some of the batches; according to the performed semi‐quantifications their concentrations ranged from 0.02% to 0.51% (Documentation provided to EFSA no. 1, 2).
In addition, the applicant provided analytical data on the content of water (2.0%–4.8% determined in seven batches), ash (0.01%–0.27% determined in five batches), chlorine (below the limit of detection (LOD) of 1–10 mg/kg determined in three batches) and pesticides (below specific reporting limits, which are defined by the applicant to be equal to or higher than the limits of quantification (LOQs), and ranging from 0.01 to 0.5 mg/kg in two determined batches). The applicant, also, provided analytical data on the presence of residual solvents i.e. methyl tert‐butyl ether, ethyl acetate, ethanol, 1‐butanol, methanol, acetone, n‐hexane (all below the reporting limit of 10 mg/kg determined in one batch) and toluene (below the LOQ of 1 mg/kg determined in one batch) and microbiological parameters, i.e. total aerobic count, moulds, yeasts (below the reporting limit of 10 cfu/g in one batch) and Escherichia coli (not detected in one determined batch) (Documentation provided to EFSA no. 1, 2, 3).
In terms of toxic elements, analytical data on the concentrations of arsenic, lead, cadmium, mercury, nickel and palladium were provided by the applicant. ■■■■■. In all cases (except for nickel (0.5 mg/kg in one batch)) the lowest levels determined were below the LODs (being 0.04 mg/kg for arsenic and cadmium, 0.02 mg/kg for lead and palladium and 0.002 mg/kg for mercury). The highest concentrations for all these elements were below the maximum specification levels as proposed by the applicant (see Table 1) (Documentation provided to EFSA no. 1, 2, 3).
3.1.2. Organoleptic characteristics
The applicant described the organoleptic characteristics of hesperetin dihydrochalcone as ‘flavour with taste modifying properties’ (Documentation provided to EFSA no. 1).
3.1.3. Manufacturing process
The starting substance for the manufacturing of hesperetin dihydrochalcone is hesperidin which is obtained by extraction from bitter oranges (Citrus aurantium L.).
■■■■■ which use the approaches outlined in Figure 1 to convert the isolated hesperidin to hesperetin dihydrochalcone, obtained as powder with a purity of ≥ 95% on dry basis. Hesperidin is subjected to acid hydrolysis, and the liberated hesperetin is subsequently hydrogenated to hesperetin dihydrochalcone. Alternatively, hesperidin is first hydrogenated and the formed neohesperidine dihydrochalcone is subjected to hydrolysis resulting in hesperetin dihydrochalcone (Documentation provided to EFSA no. 1, 2).
FIGURE 1.
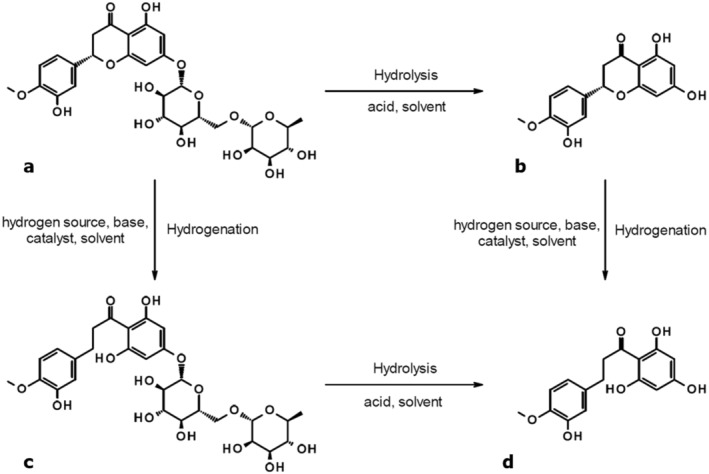
Processes employed to manufacture the flavouring substance starting from hesperidin (a: Hesperidin, b: Hesperetin, c: Neohesperidine dihydrochalcone, d: Hesperetin dihydrochalcone) (Documentation provided to EFSA no. 1).
The applicant provided information on the hydrolysis and hydrogenation conditions (Documentation provided to EFSA no. 1); no certificate on the source material and no information on the employed solvents was provided.
3.1.4. Genetically modified organisms
According to a statement by the applicant, hesperetin dihydrochalcone is not produced by or from genetically modified organisms (Documentation provided to EFSA no. 2).
3.1.5. Solubility and particle size
Solubility
The applicant attempted to determine the water solubility of hesperetin dihydrochalcone according to the elution column method described in OECD test guideline (TG) 105 (OECD, 1995). Several organic solvents (i.e. acetonitrile, methanol, ethanol, tert‐butyl methyl ether, tetrahydrofuran and dichloromethane) were tested to assess their suitability for loading the elution column. However, the testing was unsuccessful due to insufficient solubility of the flavouring substance in all tested solvent systems. Based on preliminary testing, the applicant estimated the water solubility of hesperetin dihydrochalcone to be in the range of 10–20 mg/L (Noack Laboratorien, 2022).
The applicant stated that the flavouring substance is intended to be added to food solely as a solution in 1,2‐propanediol (1,2‐propylene glycol) (max 20% w/w), resulting in a concentration of flavouring substance maximally 10 mg/kg in the final food (Documentation provided to EFSA no. 1, 2 and 3). The Panel noted that 1,2‐propanediol is authorised as a food additive (E 1520) in the European Union (EU) according to Annex II and Annex III of Regulation (EC) No 1333/2008 12 on food additives including carriers in food flavourings.
Based on visual inspection, the applicant concluded that the solubility of the substance in 1,2‐propanediol was ≥ 300 g/L (Documentation provided to EFSA no. 1).
The Panel considered that the method used in this study was insufficient to substantiate the reported solubility of hesperetin dihydrochalcone in 1,2‐propanediol.
After EFSΑ's request, the applicant provided a new study on the dissolution of hesperetin dihydrochalcone in 1,2‐propanediol. Four concentrations of hesperetin dihydrochalcone in 1,2‐propandiol, namely 5, 10, 15 and 20% w/w, were subjected to ultrafiltration employing a 10 kDa polyether sulfone membrane. Concentrations of hesperetin dihydrochalcone prior to and after ultrafiltration were compared. For all solutions the recovery rates were approximately 90%, and not dependent on the starting concentration of hesperetin dihydrochalcone (Documentation provided to EFSA no. 2, 3). Following these results, the Panel agreed with the applicant that the solubility of hesperetin dihydrochalcone in 1,2‐propanediol is at least 200 g/L.
Additionally, the applicant provided information on the partition coefficient (n‐octanol/water) of hesperetin dihydrochalcone (one batch analysed) determined using a high‐performance liquid chromatography–diode array detector (HPLC–DAD) method according to OECD test guideline (TG) 117 (OECD, 2004) to be 1.87 (40°C and pH‐neutral) (Symrise AG, 2021). The Panel noted that according to OECD (2009), the use of OECD TG 117 for substances consisting of nanoparticles (as described in the next paragraph) is considered inappropriate in most cases.
Particle size
Regarding particle size, the applicant provided a scanning electron microscopy (SEM) analysis coupled with energy‐dispersive X‐ray spectroscopy (EDX) of five batches of hesperetin dihydrochalcone. Based on the results, the applicant concluded that the material contains at least 50% of particles, by number, with one dimension smaller than 500 nm. Within this fraction more than 10% of particles (number based) have at least one dimension smaller than 250 nm (Documentation provided to EFSA no. 1). According to EFSA Guidance‐TR (EFSA Scientific Committee, 2021) this indicates that hesperetin dihydrochalone, as pristine material, contains a fraction of small particles, including nanoparticles.
The applicant stated that hesperetin dihydrochalcone will be added to food only as a solution in 1,2‐propanediol (up to 20% w/w), resulting in a maximum concentration of 10 mg/kg in the final product, with no other solvents intended for use (Documentation provided to EFSA no. 1, 2, 3). Therefore, considering the data provided on the solubility of hesperetin dihydrochalcone in 1,2‐propanediol (i.e. at least 200 g\L) the Panel considered that there is no concern with regard to the potential presence of small particles, including nanoparticles, in the flavouring substance under the proposed conditions of use and that hesperetin dihydrochalcone can be assessed following the conventional risk assessment, i.e. Guidance on risk assessment of Flavourings (EFSA CEF Panel, 2010b) should be applied.
3.1.6. Proposed specifications
The proposed specifications for hesperetin dihydrochalcone are shown in Table 1.
The Panel noted that no data have been provided by the applicant regarding the solubility of hesperetin dihydrochalcone in ethanol and dimethylsulfoxide (DMSO) supporting the proposed specifications which list the substance as ‘soluble’ in both solvents.
Additionally, the applicant specified the solubility of the candidate substance in 1,2‐propanediol as ‘soluble at 10% w/w’ (Table 1). However, the Panel noted that the data submitted by the applicant indicate a solubility of the proposed food flavouring in 1,2‐propanediol of at least 20% w/w (see Section 3.1.5). Furthermore, the Panel noted that hesperetin dihydrochalcone is intended to be added to food solely as a solution in 1,2‐propanediol (max 20% w/w) and therefore the solubility in 1,2‐propanediol up to 20% w/w should be captured in the specifications.
3.1.7. Stability and fate in food
The stability of hesperetin dihydrochalcone has been tested by the applicant in one batch at room temperature at three different timepoints (7, 18 and 33 months from the manufacturing date). The recovery determined by high‐performance liquid chromatography–ultraviolet (HPLC–UV) analysis was consistently higher than 95%. The concentrations of impurities such as hesperetin (0.1%–0.2%), phloretin (0.3%) and hesperetin dihydrochalcone glucoside (1.2%) also did not notably change over time in this stability test (Documentation provided to EFSA no. 1).
The applicant tested the stability of hesperetin dihydrochalcone (12 and 13 mg/kg) at different pH values (2, 3, 4) in model beverage applications with and without the addition of sucrose (0 and 5%), over 7, 28 and 84 days under accelerated storage conditions (40°C). In addition, the stability of the substance (10 mg/kg) in acidic model beverage applications at two different pH values (2.5 and 2.8) with the addition of different acidifiers (citric acid and phosphoric acid) and sweeteners (beet sugar, high fructose corn syrup, steviol glycosides) was investigated at two temperatures (5°C and 40°C) in the time period of 1 month. The concentration of the substance did not notably change under the various testing conditions (Documentation provided to EFSA no. 1).
After EFSA΄s request, the applicant provided accelerated stability studies of hesperetin dihydrochalcone in 1,2‐propanediol, since the flavouring is intended to be dissolved in this solvent prior to its addition to food (Documentation provided to EFSA no. 2, 3).
In the first study, hesperetin dihydrochalcone was dissolved in 1,2‐propanediol at 5, 10, 15 and 20% w/w and the solutions were stored in the dark at 40°C. The concentration of hesperetin dihydrochalcone was analysed after 2 and 4 weeks with HPLC–UV. The recovery was more than 89.9% for all samples analysed. According to the applicant΄s internal validation procedure, 4 weeks of this accelerated test corresponds to 4 months of shelf life at room temperature (Documentation provided to EFSA no. 2, 3).
In the second study, hesperetin dihydrochalcone was dissolved in 1,2‐propanediol at 20% w/w and the solution was stored at 40°C and 5 bar oxygen pressure. The concentration of hesperetin dihydrochalcone was analysed after 86 and 168 h with HPLC–UV. The recovery was more than 92.9% for all samples analysed. According to the applicant΄s internal validation procedure, this accelerated test corresponds to 12 months of shelf life at room temperature (Documentation provided to EFSA no. 2, 3).
Based on the provided data, the Panel considered that hesperetin dihydrochalcone is expected to be stable under the intended conditions of use.
3.1.8. Methods of analysis in food
An HPLC method has been proposed by the applicant to quantify hesperetin dihydrochalcone in beverages, food and rodent feed. The detectors used are UV, DAD and MS (Documentation provided to EFSA no. 1). The Panel noted that relevant methods of analysis are available in the literature (e.g. Montijano et al., 1997).
3.2. Structural/metabolic similarity to flavouring substances in existing FGE
Hesperetin dihydrochalcone [FL‐no: 16.137] is classified as structural Class III 13 (according to the classification by Cramer et al., 1978). This substance belongs to the group of flavonoids and is the aglycone of neohesperidine dihydrochalcone, which has been evaluated by the CEF Panel as flavouring substance [FL‐no: 16.061] in FGE.32 (EFSA CEF Panel, 2010a). Neohesperidine dihydrochalcone was also evaluated as feed additive by the FEEDAP Panel (EFSA FEEDAP Panel, 2011, 2014) and re‐evaluated by the FAF Panel (EFSA FAF Panel, 2022a) for its use as a food additive, belonging to the functional class of sweeteners (see Section 1.2).
The CEF Panel evaluated a group of seven flavonoids in FGE.32 (EFSA CEF Panel, 2010a). These seven flavonoids were all 1,3‐diphenylpropan‐1‐one derivatives with 3 or 4 aromatic hydroxy groups. Among these, the dihydrochalcones neohesperidine dihydrochalcone [FL‐no: 16.061], 3‐(4‐hydroxyphenyl)‐1‐(2,4,6‐trihydroxyphenyl)propan‐1‐one [FL‐no: 16.109], naringin dihydrochalcone [FL‐no: 16.110] and trilobatin [FL‐no: 16.112] were classified as structural Class III by the CEF Panel (EFSA CEF Panel, 2010a). The genotoxicity data available in FGE.32 did not prevent the evaluation of these substances through the Procedure (see Annex I in FGE.32).
In FGE.32, the CEF Panel considered that [FL‐no: 16.061, 16.109, 16.110 and 16.112] can be predicted to be metabolised to innocuous products, therefore the A‐side of the Procedure scheme was applied (EFSA CEF Panel, 2010a). For these four substances, the CEF Panel concluded that they ‘would present no safety concern based on the levels of intake estimated on the basis of the MSDI approach’.
Since hesperetin dihydrochalcone is a metabolite of neohesperidine dihydrochalcone [FL‐no: 16.061], the applicant considered that the closest analogous flavouring substances are those evaluated in FGE.32.
The applicant provided toxicity data because he anticipated that the flavouring substance might be evaluated via the B‐side of the Procedure. Accordingly, since the chronic dietary exposure estimates are above the threshold of toxicological concern (TTC) for Class III (90 μg/person per day), the applicant provided a 90‐day toxicity study and a developmental toxicity study for hesperetin dihydrochalcone (Documentation provided to EFSA no. 1).
With this information available, the Panel considered that a read‐across between hesperetin dihydrochalcone and the substances in FGE.32 is not needed. Nevertheless, the Panel considered that the flavonoids evaluated in FGE.32, mentioned above, should be considered in the assessment of cumulative exposure (see Section 3.3.5).
3.3. Exposure assessment
Hesperetin dihydrochalcone is intended to be added to food solely as a solution in 1,2‐propanediol (max 20% w/w) (Documentation provided to EFSA no. 1, 2 and 3). The use levels as proposed by the applicant (see Table B.1, Annex B) refer to the concentration of hesperetin dihydrochalcone in the final product (Documentation provided to EFSA no. 2).
3.3.1. Natural occurrence in food
A search in the online database of volatile compounds in food (VCF) did not give any results on natural occurrence of hesperetin dihydrochalcone in food (VCF, 2024).
Hesperetin dihydrochalcone has also not been reported to occur naturally in any food or food source at the time of compiling the dossier (September 2022) (Documentation provided to EFSA no. 1).
In the EFSA FEEDAP Panel opinion on the safety of neohesperidine dihydrochalcone used as a feed additive, the FEEDAP Panel reported that ‘the absorption of NHDC is associated with deglycosylation leading to the aglycone hesperetin dihydrochalcone, followed by the formation of glucuronate and sulphate conjugates, which are excreted as such with the urine and bile’ (EFSA FEEDAP Panel, 2011). The FEEDAP Panel considered that ‘Given the likely rapid metabolism and excretion of NHDC, it would not be expected to accumulate in edible tissues of mammals and poultry given this substance in feed. In consequence, the existing exposure of consumers to NHDC in food would not be significantly increased by its use as a feed additive for mammals and poultry’. In 2014, the FEEDAP Panel concluded the same for the consumption of fish, i.e. ‘the exposure of consumers to NHDC would not be significantly increased by its use as a feed additive for fish.’ (EFSA FEEDAP Panel, 2014, see Section 1.2). Therefore, the applicant did not estimate the exposure to hesperetin dihydrochalcone from food products of animal origin since these were anticipated not to contain residues of this flavouring substance. The FAF Panel agrees with this approach.
The Panel noted that hesperetin dihydrochalcone is not listed in the EFSA's Compendium of Botanicals. 14
3.3.2. Non‐food sources of exposure
Non‐food sources of exposure to hesperetin dihydrochalcone were not reported by the applicant (Documentation provided to EFSA no. 1). Hesperetin dihydrochalcone is also not listed in the ECHA chemical database. 15
However, the applicant provided some anecdotal reports in literature indicating the natural occurrence of hesperetin dihydrochalcone in plants traditionally used outside Europe as a remedy against several health conditions (Documentation provided to EFSA no. 1). Nevertheless, these plants grow outside Europe (Central and South America; Asia‐Oceania) and are currently not anticipated to be consumed in Europe.
3.3.3. Chronic dietary exposure
In accordance with the applicable guidance, the exposure assessment to be used in the Procedure for the safety evaluation of hesperetin dihydrochalcone is the chronic added portions exposure technique (APET) estimate (EFSA CEF Panel, 2010b). The chronic APET was calculated for adults and children (see Table 3). These values, expressed per kg body weight (bw) per day, will be used in the Procedure (see Appendices A and B). The chronic APET calculation is based on the proposed normal use levels and the standard portion sizes (see Table B.1, Appendix B) (Documentation provided to EFSA no. 1 and 2).
TABLE 3.
APET – Chronic Dietary Exposure to hesperetin dihydrochalcone as calculated by EFSA.
| Chronic APET | Added as flavouring substance a | Other dietary sources b | Combined c | |||
|---|---|---|---|---|---|---|
| μg/kg bw per day | μg/person per day | μg/kg bw per day | μg/person per day | μg/kg bw per day | μg/person per day | |
| Adults d | 14 | 850 f | 0 | 0 | 14 | 850 f |
| Children e | 36 | 540 | 0 | 0 | 36 | 540 |
Abbreviations: APET, added portions exposure technique; bw, body weight.
APET Added is calculated on the basis of the normal amount of flavouring added to a specific food category.
APET Other Dietary Sources is calculated based on the natural occurrence of the flavouring in a specified food category.
APET Combined is calculated based on the combined amount of added flavouring and naturally occurring flavouring in a specified food category.
For the adult APET calculation, a 60 kg person is considered representative.
For the child APET calculation, a 3‐year‐old child with a 15 kg bw is considered representative.
Mathematical discrepancies derive from the degree of approximation used in the calculations.
Based on the information provided by the applicant, the Panel considered that hesperetin dihydrochalcone is not intended to be used in food category 13.2 (foods for infants and young children).
3.3.4. Acute dietary exposure
The applicant did not provide an acute APET calculation for hesperetin dihydrochalcone. However, based on the applicable guidance (EFSA CEF Panel, 2010b), the Panel calculated the acute APET, based on the proposed maximum use levels and large portion sizes (i.e. three times standard portion sizes) (EFSA CEF Panel, 2010b). Acute exposure is reported in Table 4.
TABLE 4.
APET – Acute Dietary Exposure to hesperetin dihydrochalcone as calculated by EFSA.
| Acute APET | Added as flavouring substance a | Other dietary sources b | Combined c | |||
|---|---|---|---|---|---|---|
| μg/kg bw | μg/person | μg/kg bw | μg/person | μg/kg bw | μg/person | |
| Adults d | 150 | 9000 | 0 | 0 | 150 | 9000 |
| Children e | 378 | 5670 | 0 | 0 | 378 | 5670 |
Abbreviations: APET, added portions exposure technique; bw, body weight.
APET Added is calculated on the basis of the maximum amount of flavouring added to a specific food category considering large portion sizes.
APET Other dietary sources is calculated based on the natural occurrence of the flavouring in a specified food category.
APET Combined is calculated based on the combined amount of added flavouring and naturally occurring flavouring in a specified food category.
For the adult APET calculation, a 60‐kg person is considered representative.
For the child APET calculation, a 3‐year‐old child with a 15 kg bw is considered representative.
3.3.5. Cumulative dietary exposure
According to the applicable guidance (EFSA CEF Panel, 2010b), cumulative dietary exposure to flavouring substances that are structurally and metabolically related to the substance under study should be assessed.
Accordingly, the applicant considered five structurally related flavouring substances in the group of flavanones and dihydrochalcones with the highest annual production volumes, i.e. [FL‐no: 16.061, 16.097, 16.109, 16.110, 16.112]. These substances were previously evaluated in FGE.32 (EFSA CEF Panel, 2010a).
Average use levels for these five substances were submitted based on data from FGE.32 (EFSA CEF Panel, 2010a; see Table B.1, Appendix B). In FGE.32, data on uses and use levels have been reported according to Commission Regulation (EC) No 1565/2000. 16 In order to apply the APET calculation, the applicant adapted these data to the food subcategories and portion sizes reported in the EFSA Guidance on the data required for the risk assessment of flavourings to be used in or on foods (EFSA CEF Panel, 2010b). For each food subcategory, the same use levels as for the corresponding main category were used in the APET calculation. Based on these data, APET exposure estimates were calculated for the five substances [FL‐no: 16.061, 16.097, 16.109, 16.110, 16.112] (Documentation provided to EFSA no. 2).
To calculate the cumulative exposure, the APET exposure estimates for the structurally related substances of the same structural class (III) [FL‐no: 16.061, 16.109, 16.110, 16.112] were added to that of hesperetin dihydrochalcone, resulting in a cumulative exposure estimate of 2.5 mg/kg bw per day for adults and 6.0 mg/kg bw per day for children (Table 5). Since hesperetin [FL‐no: 16.097] is classified as structural class (II) (EFSA CEF Panel, 2010a), the Panel did not include this substance in the calculation of the cumulative exposure.
TABLE 5.
APET estimates for the candidate and the four structurally and metabolically related flavouring substances, in the group of dihydrochalcones, with the highest MSDI. The cumulative chronic APET is the sum of the APETs of the structural class III substances.
| FL‐no | Union list name | Structural formula | Structural class | APET adult (μg/kg bw per day) (μg/person per day) | APET children (μg/kg bw per day) (μg/person per day) | TTC a (μg/person per day) | MSDI b (μg/capita per day) |
|---|---|---|---|---|---|---|---|
| 16.137 | Hesperetin dihydrochalcone |
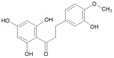
|
III |
14 850 |
36 540 |
90 | – |
| 16.061 | Neohesperidine dihydrochalcone |
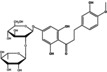
|
III |
20 1200 |
50 750 |
90 | 12 |
| 16.109 | Phloretin |
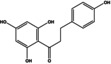
|
III |
333 20,000 |
588 8820 |
90 | 61 |
| 16.110 | Naringin dihydrochalcone |
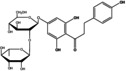
|
III |
750 45,000 |
1890 28,350 |
90 | 120 |
| 16.112 | Trilobatin |
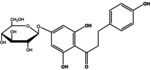
|
III |
1333 80,000 |
3360 50,400 |
90 | 1200 |
| Cumulative chronic APET |
2450 147,050 |
5924 88,860 |
90 | ||||
Considering the estimated exposure to hesperetin dihydrochalcone [FL‐no: 16.137] (14 and 36 μg/kg bw per day for adults and children, respectively, Table 3), the use of hesperetin dihydrochalcone as a flavouring substance is anticipated to have a minor impact, contributing only 0.6% to the overall cumulative dietary exposure to the five substances [FL‐no: 16.061, 16.109, 16.110, 16.112, 16.137].
3.3.6. Exposure from other food sources
The Panel noted that an additional source of exposure to hesperetin dihydrochalcone arises from its formation as a metabolite of neohesperidine dihydrochalcone, used as a sweetener (E 959).
Based on the EFSA European Food Consumption Database (Comprehensive Database) and use levels provided by industry, the exposure to neohesperidine dihydrochalcone (E 959) from its use as a sweetener was estimated according to the sweeteners exposure protocol (EFSA, 2020) following a consumers' only approach. These exposure estimates ranged at the mean from < 0.01 mg/kg bw per day for adults and the elderly to 0.09 mg/kg bw per day for toddlers, 17 respectively, and from 0.01 to 0.24 mg/kg bw per day for the same populations, at the high level (95th percentile) (EFSA FAF Panel, 2022a). These dietary exposure estimates were based on the refined brand‐loyal scenario and were considered to provide the most appropriate exposure estimates of the sweetener.
Since the exposure to this sweetener (EFSA FAF Panel, 2022a) was estimated using a different method than the one applied for estimating the exposure from its use as flavouring (APET method), the contribution to the exposure from the use of E 959 could not formally be included in the calculation of the cumulative exposure described above.
Even if the highest exposure estimate of neohesperidine dihydrochalcone (E 959) at 0.24 mg/kg bw per day were included in the cumulative calculation, it would contribute minimally compared to the highest cumulative exposure of 6 mg/kg bw per day for structurally related flavourings.
3.4. Biological and toxicological data
3.4.1. Absorption, distribution, metabolism and excretion (ADME)
Hesperetin dihydrochalcone [FL‐no: 16.137] is the aglycone resulting from the hydrolysis of neohesperidine dihydrochalcone [FL‐no: 16.061] evaluated – along with structurally related flavonoids ‐ by the CEF Panel in FGE.32 (see Section 3.2) and re‐evaluated by the FAF Panel for use as a food additive (EFSA FAF Panel, 2022a).
The applicant submitted information on the ADME of hesperetin dihydrochalcone available in the public domain. The Panel noted that the data were mainly from studies performed to investigate the ADME of the related substance neohesperidine dihydrochalcone (Borrego and Montijano, 2001; Braune et al., 2005).
These and other studies, reviewed before in more detail by EFSA (EFSA CEF Panel, 2010a; EFSA FEEDAP Panel, 2011, 2014) and in the re‐evaluation of neohesperidine dihydrochalcone for its use as a sweetener (EFSA FAF Panel, 2022a), are summarised as follows.
Absorption of the aglycone moiety of ingested flavanone and dihydrochalcone glycosides takes place mainly in the intestine colonised by bacteria (Braune et al., 2005; EFSA CEF Panel, 2010a).
Following ingestion, in a first step, neohesperidine dihydrochalcone is deglycosylated by the intestinal microbiota to the transient metabolite hesperetin dihydrochalcone 4′‐ß‐d‐glucoside and to hesperetin dihydrochalcone (aglycone) (Figure 2). Hesperetin dihydrochalcone is the major metabolite observed in in vivo studies (EFSA CEF Panel, 2010a; Zhang et al., 2021). In a second step, hesperetin dihydrochalcone can be degraded further by intestinal microbiota to 3‐(3‐hydroxy‐4‐methoxyphenyl)propionic acid, and most likely to phloroglucinol among several other break‐down products. Both hesperetin dihydrochalcone and the other break‐down products are absorbed and then undergo conjugation to glucuronides, sulfates and di‐conjugated forms which are mainly excreted in urine. Metabolites identified in urine and faeces of rats after ingestion of neohesperidine dihydrochalcone thus represent the combined result of the metabolic activity of mammalian enzymes and the intestinal microbiota (EFSA CEF Panel, 2010a; EFSA FAF Panel, 2022a; Zhang et al., 2021). Non‐absorbed parent compound and part of the degradation products are excreted with faeces (EFSA CEF Panel, 2010a).
FIGURE 2.
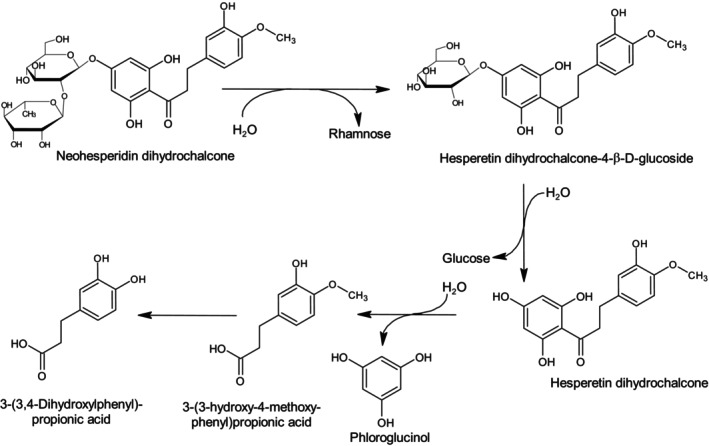
Proposed degradation pathway of neohesperidine dihydrochalcone by the human intestinal microbiota (Braune et al., 2005). For further details see FGE.32 (Annex III, EFSA CEF Panel, 2010a).
In line with in vivo data from rats, also in vitro incubation studies with human faecal suspensions and bacterial cultures confirm the hydrolysis of neohesperidine dihydrochalcone to its aglycone hesperetin dihydrochalcone and its degradation by common human intestinal bacteria (Braune et al., 2005).
The Panel noted that hesperetin dihydrochalcone shares a common pattern of excretion with neohesperidin dihydrochalcone and considered that hesperetin dihydrochalcone can be anticipated to be metabolised to innocuous products only and therefore it can be evaluated along the A‐side of the Procedure.
3.4.2. Genotoxicity
The applicant provided two studies on genotoxicity of hesperetin dihydrochalcone. The dataset consisted of the in vitro basic test battery, i.e. a bacterial reverse mutation test (LPT, 2016) and an in vitro micronucleus (MN) test (Gentronix, 2018).
In addition, the applicant provided three publicly available studies (Brown & Dietrich, 1979; MacGregor et al., 1983; MacGregor & Jurd, 1978). These studies were already considered in the CEF Panel opinion on FGE.32 (EFSA CEF Panel, 2010a) and recently considered in the opinion on the re‐evaluation of neohesperidine dihydrochalcone as sweetener (EFSA FAF Panel, 2022a). Negative results in bacterial reverse mutation assays were reported for hesperetin dihydrochalcone (Brown & Dietrich, 1979; MacGregor & Jurd, 1978). In an in vivo micronucleus assay in mice (MacGregor et al., 1983), the oral administration of hesperetin dihydrochalcone did not induce detectable increases in micronuclei compared to control mice. However, exposure of the bone marrow could not be confirmed. Therefore, the result of this study is considered to be inconclusive rather than negative (EFSA FAF Panel, 2022a; EFSA Scientific Committee, 2017).
The new in vitro studies provided by the applicant for hesperetin dihydrochalcone are described below.
Bacterial reverse gene mutation assay
Hesperetin dihydrochalcone (purity 96.3%) was tested for the induction of gene mutations in the Salmonella Typhimurium strains TA98, TA100, TA1535 and TA1537 and in the Escherichia coli strain WP2 uvrA [pKM101] in two separate experiments, each carried out with and without metabolic activation (S9 fraction obtained from Aroclor 1254‐induced rat liver) (LPT, 2016). The study was performed according to OECD Test Guideline (TG) 471 (OECD, 1997) and in compliance with GLP principles (Table C.1).
The first experiment was carried out as a plate incorporation test and the second as a pre‐incubation test. The test item was dissolved in DMSO for which a solubility of the flavouring substance of 30% was stated in the study report. The vehicle DMSO served as the negative control (Table D.1).
Based on a preliminary cytotoxicity test conducted in the strain TA100, 1000 μg/plate was chosen as top concentration for the main study. Six concentrations ranging from 3.16 to 1000 μg/plate were employed both in the plate incorporation test and in the pre‐incubation test, triplicate plating for each concentration. At the highest concentrations tested, cytotoxicity was observed with all bacterial strains (scarce background lawn and reduction in the number of revertant colonies by more than 50% compared with the DMSO negative control).
No increase in revertant colony numbers as compared with control counts was observed in any experimental condition.
The positive controls induced a significant increase in the number of revertants, confirming the sensitivity of the test system.
The Panel considered the study to be reliable without restrictions and its result of high relevance. The Panel considered that hesperetin dihydrochalcone did not induce gene mutations in bacteria under these test conditions.
Summary of the study is reported in Appendix C.
In vitro micronucleus assay
Hesperetin dihydrochalcone (purity > 95%) was tested in vitro for its potential to induce micronuclei in human peripheral lymphocytes from healthy non‐smoking donors, with and without metabolic activation (S9 fraction obtained from Aroclor 1254‐induced rat liver, S9‐mix) (Gentronix, 2018). The study was conducted according to OECD TG 487 (OECD, 2016) and in compliance with GLP principles.
Three treatment schedules were applied: a 3‐h treatment followed by 21‐h recovery period (3 + 21 h) both in the absence and presence of S9‐mix, and a 24‐h continuous treatment in the absence of S9‐mix.
In all treatments, the solvent used was DMSO for which a solubility of the flavouring substance up to the maximum concentration of 200 mg/mL was stated in the study report. Cells were cytokinesis blocked using cytochalasin B. To enable calculation of the Cytokinesis Block Proliferation Index (CBPI), at least 500 cells from appropriate cultures were scored. For the selected concentrations, the micronucleus frequency was determined manually from 2000 binucleated cells per concentration.
In the treatment of 3h + 21h in the presence of S9‐mix, the following concentrations were chosen for MN analysis: 260, 273 and 287 μg/mL (cytotoxicity of 10.2%, 18.6% and 50%, respectively).
In the treatment of 3 + 21 h in the absence of S9‐mix, the following concentrations were chosen for MN analysis: 214, 225 and 236 μg/mL (cytotoxicity of 9.3%, 46% and 56%, respectively).
In the continuous treatment of 24 h in the absence of S9‐mix, the following concentrations were chosen for MN analysis: 17, 26 and 39 μg/mL (cytotoxicity of 4.3%, 35.8% and 55%, respectively).
The Panel noted the narrow range of the concentrations tested, nevertheless this is considered acceptable because the cytotoxicity ranged from 4.3%–10.2% at the lowest concentrations to 50%–56% at the highest concentrations tested.
The positive controls induced statistically significant increases in the frequency of micronucleated cells with and without metabolic activation, demonstrating the sensitivity of the assay. For all treatments, the frequency of micronucleated cells was within the distribution of the historical negative control data (Poisson‐ based 95% control limits).
Hesperetin dihydrochalcone did not induce the formation of micronuclei in human lymphocytes in any experimental condition. The Panel considered the study to be reliable without restrictions and its results of high relevance. Summary of the study is reported in Appendix C.
Conclusions on genotoxicity
Hesperetin dihydrochalcone did not induce gene mutations in a bacterial reverse mutation assay and it was neither clastogenic nor aneugenic in an in vitro micronucleus test. Therefore, there was no requirement to test the candidate substance for genotoxicity in vivo (EFSA Scientific Committee, 2011). Overall, the Panel concluded that hesperetin dihydrochalcone does not raise a concern for genotoxicity.
3.4.3. Toxicological data
14‐day dose range‐finding study
A 14‐day dose range‐finding study (Covance, 2020a) was performed with hesperetin dihydrochalcone (purity 95%) to support dose selection for the subsequent 90‐day oral toxicity study.
Seven weeks old Crl: Sprague–Dawley® CD® BR rats (5/sex/group) were fed with a diet containing hesperetin dihydrochalcone at concentrations that corresponded to doses of 0, 300 and 1000 mg/kg bw per day (based on food consumption data, the actual doses were equal to 0, 287, and 931 and 0, 301, 1084 mg/kg bw per day for males and females, respectively) for 14 days.
No mortality occurred and no test substance‐related clinical observations or body weight changes were observed. A slight decrease in food consumption and body weight gain was observed in males at 1000 mg/kg bw per day. This was not observed in males at 300 mg/kg bw per day or females at both dose groups. Small and/or soft testes and epididymides were seen in two of five males in the highest dose group, unilateral in one and bilateral in the other. Generally, there were no test substance‐related effects for the other parameters measured: haematology, coagulation, clinical chemistry and organ weights.
Thyroid+parathyroid weight (absolute and relative to body weight) was reduced at the high dose in both males and females and at the mid dose in males (relative weight −26% compared to controls). Data on thyroid hormones were not collected.
It was concluded that treatment with hesperetin dihydrochalcone was well tolerated at doses up to 1000 mg/kg bw per day.
90‐day toxicity study
Hesperetin dihydrochalcone (purity 95%) was tested in a 90‐day repeated dose toxicity study in rats (Covance, 2020b) according to OECD TG 408 (OECD, 2018a) and in compliance with GLP. Seven to eight weeks old Sprague–Dawley CD® rats (10/sex/group) were fed a diet containing hesperetin dihydrochalcone at concentrations that corresponded to doses of 0, 100, 300 or 1000 mg/kg bw per day (based on food consumption data, the actual doses were equal to 0, 104, 314 and 1037 and 0, 103, 308, 1006 mg/kg bw per day for males and females, respectively). The feed was prepared fresh every week and stored refrigerated when not in use. Dietary feed concentrations were adjusted weekly to account for changes in body weights and feed consumption. The test substance had previously been shown to be stable for up to 10 days. Homogeneity in diet was also confirmed.
Parameters evaluated during the study were mortality, clinical observations, ophthalmological parameters, body weights, food and water consumption, neurobehavioral evaluations (functional observational battery and motor activity), thyroid hormone analysis, oestrous cycle evaluations, sperm analysis, clinical chemistry, urinalysis, organ weights, macroscopic observations and histopathology. In the 14‐day dose range‐finding study, small and/or soft testes and epididymides were observed in the highest dose group. No testicular effects were observed in the 90‐day toxicity study.
No mortality occurred and no test substance‐related clinical signs were observed. No effects on ophthalmological parameters, body weights, food and water consumption were observed. No neurobehavioral effects were observed at week 12. No test substance‐related effects on the oestrous cycle were seen at 13 weeks.
A dose‐related increase in urine volume was observed for males (40%–93%), but not for females, compared to controls. The differences reached statistical significance at the highest dose group. In absence of major changes in water consumption and lack of evidence for dehydration, the Panel considered this observation to be of marginal toxicological relevance.
A statistically significant increase (72%) in plasma alanine aminotransferase (ALT) activity for the high‐dose males was seen. A statistically significant decrease in plasma aspartate transaminase (AST) activity (14%–29%) and a statistically significant increase in plasma phosphate (22%–29%) were seen for all exposed female rats.
In male rats, a dose‐related increase in absolute (2%–12%) and relative liver weight (3%–16%) compared to controls was observed. The increase was only statistically significant for the relative liver weight for males in the highest dose group. No difference for absolute or relative liver weight was observed for female rats.
In one male and one female rat in the highest dose group, minimal necrosis (hepatocellular, focal) of the liver was observed, but histopathological changes in the liver were not observed in the other animals. For these two animals the plasma levels of ALT were not the highest in the groups. Taking this into account and considering the limited change in ALT activity, the change in AST which went in opposite direction and the limited change in liver weight, the Panel considered that these observations are not indicative of liver toxicity.
In male rats, there was a statistically significant increase in absolute (15%) and relative (24%) pituitary gland weight at the highest dose.
Thyroid+parathyroid weight (absolute and relative to body weight) was increased at the high dose in in males only (relative weight + 45% compared to controls). One of the high‐dose males had macroscopic enlargement of thyroid which was attributed to inflammatory cell infiltrates.
It is noted that thyroid weight was decreased at 1000 mg/kg in the 14‐day range‐finding study, but was increased at the same dose in this 90‐day study.
For serum thyroxine (T4), there was a dose‐related increase for both males (38%–106%) and females (58%–200%). The difference in T4 compared to controls was statistically significant for all dose groups for both sexes.
A dose‐related decrease in serum triiodothyronine (T3) (7%–35%) was observed for exposed male rats, reaching statistical significance for the mid‐ and high‐dose groups. However, for female rats, there was a dose‐dependent increase, instead of decrease, in T3 (20%–28%), reaching statistical significance for the mid‐ and high‐dose groups.
There was a statistically significant increase in serum thyroid stimulating hormone (TSH) level for males in the high‐dose group (50%) and all exposed female rats (43%–135%).
The Panel noted that the test substance caused changes in thyroid hormone levels. These changes were statistically significant and dose‐dependent for some of the parameters. The Panel considered that effects on thyroid hormone levels were treatment related.
To clarify the underlying mechanism for the sex‐specific differences of T3 hormone in rats, the Panel calculated the T4/T3 ratio which reflects the activity of deiodinase transforming T4 to T3 which is biologically the most metabolically active hormone, three to four times more active than T4. For the ratio T4/T3, a dose‐dependent increase was observed in both sexes which ranged from 46 for the control group to 145 in males and from 37 for the control group to 87 in females. An increase in T4/T3 indicates a reduced production of T3. However, the reduced production of T3 hormone was not accompanied by apical findings indicative of hypothyroidism, e.g. changes in thyroid histopathology, body weight, motor activity and neurobehavioral parameters. The Panel, therefore, considered the hormonal changes as not adverse.
Overall, the Panel considered that the highest tested dose level of 1000 mg/kg bw per day was the NOAEL in this study. Summary of the study is reported in Appendix D.
Prenatal developmental toxicity study
Hesperetin dihydrochalcone was tested in two prenatal developmental toxicity studies in rats (Covance, 2020c; Labcorp, 2022).
The first study was indicated as ‘preliminary’ and was performed according to OECD TG 414 but not in compliance with GLP (Covance, 2020c). In this preliminary prenatal developmental toxicity study, four groups of Crl:CD (Sprague–Dawley) rats (six pregnant females per group) were fed a diet containing hesperetin dihydrochalcone (purity > 95%) at concentrations that corresponded to nominal doses of 100, 300 and 1000 mg/kg bw per day or the vehicle control (basal diet only). The test substance or vehicle control was given in the diet (7 days/week) during gestation days (GD) 5–20. All animals survived until sacrifice at GD 21. Incidental clinical signs noted in dams included red vulvar discharge of 1/6 rats in the control group and 1/6 rats in the low‐dose group. No changes in body weight, body weight gain and food consumption were observed compared to control group. No effects were observed in uterine and reproductive parameters (including early and late resorptions). Seventy‐two foetuses from six litters from the control group, and 64, 65, 74 foetuses from six litters from low, mid and high‐dose groups, respectively, were evaluated for skeletal malformations and developmental variations. No visceral or skeletal teratogenic effects were observed.
The main study (Labcorp, 2022) was performed according to OECD TG 414 (OECD, 2018) and in compliance with GLP. In this prenatal developmental toxicity study, four groups of Crl:CD (Sprague–Dawley) rats (22 pregnant females per group) were fed with a diet containing hesperetin dihydrochalcone (purity > 95%) at concentrations that corresponded to nominal doses of 100 (Group 2), 300 (Group 3) and 1000 (Group 4) mg/kg bw per day or the vehicle control (basal diet only, Group 1). Based on food consumption, the actual doses received by the rats were slightly lower than the target doses i.e. 85, 245 and 821 mg/kg bw per day. The test substance or vehicle control was given in the diet (7 days/week) during GD 5–20. All dams survived until sacrifice at GD 21. Incidental clinical signs noted included thinning hair coat of 1/22 rats (low dose), 1/22 rats (mid dose) and 2/22 rats in the high‐dose group. A statistically significant decrease (− 44.4%) of body weight gain was observed in the dams of the high‐dose group from GD 5 to GD 8 compared to controls. This change was accompanied by a decrease in food consumption. Statistically significantly decreased food consumption was observed in the high‐dose group from GD 5 to GD 8 (− 13.6%) and GD 17 to GD 20 (− 12.5%), and from GD 8 to 11 in the mid‐dose group (−9%). According to the study's authors, these changes were due to palatability and therefore considered test‐item related, but not adverse. The study's authors also noted that the magnitudes of the changes were not large and there were no dose‐responses. The Panel agreed with the evaluation of the study authors. No treatment‐related macroscopic or microscopic observations were noted for any tissue/organ. No effects on maternal thyroid hormones (T3, T4 and TSH) were observed within the exposure duration in this study of 15 days. In the high‐dose group, there was a reduction of the absolute and relative (to bw) thyroid including parathyroid weight with 12% or 9% respectively, without statistical significance. In absence of microscopic changes, in the absence of changes in serum T3, T4 and TSH levels and taking into account the low degree of effects, the reduction in thyroid including parathyroid weight is considered to be non‐adverse.
No adverse effects were observed in uterine and reproductive parameters (including early and late fetal resorptions).
A total of, 132 foetuses (from 21 litters) from control group, 137 (from 22 litters), 136 (from 21 litters) and 138 (from 22 litters) foetuses from Group 2, 3 and 4, respectively were evaluated for visceral malformations, whereas for skeletal malformations 134 foetuses (from 22 litter) from control group, 137 (from 22 litters), 135 (from 21 litters), 134 (from 22 litters) foetuses from Group 2, 3 and 4, respectively were assessed. No visceral or skeletal malformations were observed in all foetuses. Anogenital distance was also measured and no effects were observed in male or female foetuses.
In line with the study authors, the Panel considered that the highest tested dose level of 821 mg/kg bw per day was the NOAEL in this study. Summary of the study is reported in Appendix D.
3.5. Application of the procedure
Since hesperetin dihydrochalcone [FL‐no: 16.137] does not raise a concern for genotoxicity, it is appropriate to evaluate the use of [FL‐no: 16.137] as a flavouring substance following the stepwise evaluation Procedure for individual substances as outlined in the ‘Guidance on the data required for the risk assessment of flavourings to be used in or on foods’ (EFSA CEF Panel, 2010b) and Appendix A.
Step 1
Hesperetin dihydrochalcone [FL‐no: 16.137] is allocated to structural class III. 18
Step 2
On the basis of the data available, the Panel anticipated that hesperetin dihydrochalcone is converted to innocuous metabolites (see Section 3.4.1). Hence, the substance can be evaluated via the left (A‐) side of the Procedure (see Appendix A, Figure A.1).
Step A3–A4
The conditions of use as flavouring substance result in chronic APET exposure estimates of 14 and 36 μg/kg bw per day (850 and 540 μg/person per day), for adults and children, respectively. These estimates are above the TTC for Cramer Class III (90 μg/person per day) and below 10‐fold this TTC (900 μg/person per day). Based on these chronic APET exposure estimates, the applicant provided a 90‐day toxicity study and a prenatal developmental toxicity study, because he anticipated that the flavouring substance might be evaluated via the B‐side of the Procedure.
In the prenatal developmental toxicity study, no toxic effects were observed. In the 90‐day toxicity study, dose‐related changes in thyroid hormones were observed and considered treatment‐related. However, in the absence of any apical findings indicative of hypothyroidism, these changes were considered not adverse (see Section 3.4.3).
The Panel decided to use as a reference point 1000 mg/kg bw per day, the highest dose tested and without adverse effects, from the rat 90‐day toxicity study. Using this reference point at step A4 of the Procedure, margins of exposure (MoE) of 71 × 103 and 28 × 103 could be calculated for adults and children, respectively, when considering the chronic dietary exposure to hesperetin dihydrochalcone based on APET.
3.6. Assessment of acute, combined and cumulative exposure
3.6.1. Safety evaluation of the acute exposure
The estimates for acute exposure to hesperetin dihydrochalcone are 150 and 378 μg/kg bw corresponding to 9000 and 5670 μg/person for adults and children, respectively. Although no acute toxicity studies are available for hesperetin dihydrochalcone, an acute toxicity study is available for the structurally related substance neohesperidine dihydrochalcone [FL‐no: 16.061] as reported in FGE.32 (EFSA CEF Panel, 2010a). The study was performed in rats (males and females) and resulted in an oral median lethal dose (LD50) higher than 5000 mg/kg bw (EFSA CEF Panel, 2010a). In addition, no signs of toxicity were observed with hesperetin dihydrochalcone in a short‐term range‐finding study and in a subchronic toxicity study with dosing up to 1000 mg/kg bw per day and in a developmental toxicity study with dose levels up to 821 mg/kg bw per day (actual dose level), (see Section 3.4.3). Since these dose levels are far above the potential acute exposure in humans, there is no concern for acute toxicity.
3.6.2. Safety evaluation of the combined exposure
Since exposure to hesperetin dihydrochalcone from food (as natural occurrence) and non‐food sources is not expected (see Section 3.3), the APET estimates for use as flavouring substance reflect the combined exposure estimates for both the chronic and acute scenario (see Tables 3 and 4). The combined exposure estimates will not be addressed as such.
3.6.3. Safety evaluation of the cumulative exposure
The estimates of cumulative exposure to hesperetin dihydrochalcone [FL‐no: 16.137] and the structurally related substances [FL‐no: 16.061, 16.109, 16.110 and 16.112] are above the TTC for structural class III (90 μg/person per day) for both adults (2.5 mg/kg bw per day) and children (6.0 mg/kg bw per day), respectively (see Table 5). Based on the reference point of 1000 mg/kg bw per day derived from the 90‐day toxicity study on hesperetin dihydrochalcone, the MoEs are 400 and 167 for adults and children, respectively.
The Panel noted that the contribution of [FL‐no: 16.137] to the cumulative exposure is minor (approximately 0.6%) and that approximately 55% of the cumulative exposure estimate comes from the APET estimate for trilobatin [FL‐no: 16.112]. Overall, the Panel concluded that the cumulative exposure to these five substances [FL‐no: 16.061, 16.109, 16.110, 16.112 and 16.137] does not raise a safety concern.
4. DISCUSSION
The European Commission requested the European Food Safety Authority (EFSA) to carry out the safety assessment of the substance hesperetin dihydrochalcone (3‐(3‐hydroxy‐4‐methoxy‐phenyl)‐1‐(2,4,6‐trihydroxyphenyl)propan‐1‐one) (CAS no. 35400‐60‐3) as a new flavouring substance in accordance with Regulation (EC) No 1331/2008. EFSA allocated hesperetin dihydrochalcone [FL‐no: 16.137] to Flavouring Group Evaluation 420 (FGE.420) and used the procedure as referred to in Regulation (EC) No 1334/2008 for the safety assessment.
Hesperetin dihydrochalcone has structural similarity to flavouring substances evaluated by the EFSA CEF Panel in FGE.32 (EFSA CEF Panel, 2010a).
It is manufactured by acid hydrolysis and hydrogenation of the starting material hesperidin, isolated from bitter oranges. It has not been reported to occur naturally in food and is not produced by or from genetically modified organisms.
Hesperetin dihydrochalcone does not possess chiral centres and does not have geometrical isomers. The information provided on the manufacturing process, the composition and the stability of the flavouring substance was considered sufficient.
Based on the available data, the Panel considered that hesperetin dihydrochalcone is expected to be stable under the intended conditions of use.
In accordance with Regulation (EC) No 1334/2008, the purity of hesperetin dihydrochalcone is at least 95%. In addition, maximum limits for potential impurities were provided by the applicant.
Regarding particle size, data submitted showed that hesperetin dihydrochalcone, as pristine material, contains a fraction of small particles, including nanoparticles, according to EFSA Guidance‐TR (EFSA Scientific Committee, 2021). The applicant declared that hesperetin dihydrochalcone is intended to be added to food solely as a solution in 1,2‐propanediol (max 20% w/w), resulting in a concentration of the flavouring substance of maximally 10 mg/kg in the final food. The Panel noted that 1,2‐propanediol (E 1520) is authorised to be used in food according to Regulation (EC) No 1333/2008 on food additives. The applicant provided results of the dissolution of hesperetin dihydrochalcone in 1,2‐propanediol up to 20% w/w demonstrating that it is soluble in 1,2‐propanediol up to at least 200 g/L.
Taking into account the provided analytical data on the solubility of hesperetin dihydrochalcone in 1,2‐propanediol, as well as the proposed conditions of use, the Panel considered that there is no concern with regard to the presence of small particles, including nanoparticles, in the flavouring substance under the proposed conditions of use and that hesperetin dihydrochalcone can be assessed following the conventional risk assessment, i.e. by applying the guidance on risk assessment of flavourings (EFSA CEF Panel, 2010b). This conclusion only applies when the flavouring substance is dissolved in 1,2‐propanediol up to 20% w/w prior to its addition to food, and this condition of use should be reflected in the specifications.
Regarding the proposed specifications, based on the available data and the proposed conditions of use, the entry solubility in 1,2‐propanediol of ‘soluble at 10 % w/w’, should be changed to ‘soluble at 20 % w/w’. The Panel also noted that the applicant did not provide data regarding the solubility of hesperetin dihydrochalcone in ethanol and DMSO to support the proposed specifications which list the substance as ‘soluble’ in both solvents. Therefore, the Panel considered that these entries should not be included in the specifications of hesperetin dihydrochalcone.
The chronic and acute dietary exposure to hesperetin dihydrochalcone were estimated using the APET method. The chronic APET exposure estimates were 14 and 36 μg/kg bw per day (850 and 540 μg/person per day) for adults (60 kg bw) and children (15‐kg bw; 3‐years‐old), respectively. The acute APET exposure estimates were 150 and 378 μg/kg bw (9000 and 5670 μg/person) for adults and children, respectively.
The applicant submitted adequate studies to investigate the genotoxic potential of hesperetin dihydrochalcone. From the available data, the Panel concluded that there is no concern with respect to genotoxicity.
The applicant reported information on the ADME of hesperetin dihydrochalcone available in the public domain. The Panel noted that the data were mainly from studies on ADME of the related substance neohesperidine dihydrochalcone.
Following ingestion, in a first step, neohesperidine dihydrochalcone is deglycosylated by the intestinal microbiota to the transient metabolite hesperetin dihydrochalcone 4′‐ß‐d‐glucoside and to hesperetin dihydrochalcone (aglycone). Hesperetin dihydrochalcone is the major metabolite observed in in vivo studies (EFSA CEF Panel, 2010a; Zhang et al., 2021). In a second step, hesperetin dihydrochalcone can be degraded further by intestinal microbiota to 3‐(3‐hydroxy‐4‐methoxyphenyl)propionic acid, and most likely to phloroglucinol among several other break‐down products. Both hesperetin dihydrochalcone and the other break‐down products are absorbed and then undergo conjugation to glucuronides, sulfates and di‐conjugated forms which are mainly excreted in urine. The Panel noted that hesperetin dihydrochalcone has the same fate in the organism as that of neohesperidine dihydrochalcone and considered that hesperetin dihydrochalcone can be anticipated to be metabolised to innocuous products only and therefore it can be evaluated along the A‐side of the Procedure. However, the applicant, provided more toxicity data (prenatal developmental toxicity study) than required by the A‐side and these data were also evaluated.
In the prenatal developmental toxicity study, no developmental toxicity was observed with dose levels of hesperetin dihydrochalcone up to nominal 1000 mg/kg bw per day (actual dose 821 mg/kg bw per day).
In the 90‐day toxicity study, indications were obtained that hesperetin dihydrochalcone affects thyroid hormone levels dose‐relatedly at all doses tested (100, 300 and 1000 mg/kg bw per day). However, these changes were not accompanied by apical findings indicative of hypothyroidism, e.g. changes in thyroid histopathology, body weight, motor activity and neurobehavioral parameters. The Panel, therefore, considered these hormonal effects as not adverse.
Using 1000 mg/kg bw per day as a reference point from the 90‐day toxicity study, at step A4 of the Procedure, MoEs of 71 × 103 and 28 × 103 were calculated for adults and children, respectively, when considering the chronic APET dietary exposure estimates.
Therefore, the use of hesperetin dihydrochalcone as food flavouring at the proposed uses and use levels, as specified in Appendix B, is of no safety concern.
The Panel noted that no data on acute toxicity were available for hesperetin dihydrochalcone. However, considering the results from an acute toxicity study for the structurally related substance neohesperidine dihydrochalcone [FL‐no: 16.061] (EFSA CEF Panel, 2010a, EFSA FAF Panel, 2022a) and from repeated dose toxicity studies on hesperetin dihydrochalcone, there is no concern for acute toxicity.
Cumulative chronic exposure estimates to hesperetin dihydrochalcone [FL‐no: 16.137] and the structurally related substances [FL‐no: 16.061, 16.109, 16.110, 16.112] of 2.5 and 6.0 mg/kg bw per day for adults and children, respectively, are above the TTC for structural class (III). However, considering 1000 mg/kg bw per day, as a reference point, the MoEs are 400 and 167, respectively. In addition, the Panel noted that the contribution of hesperetin dihydrochalcone [FL‐no: 16.137] to the cumulative exposure is minor (approximately 0.6%) and that approximately 55% of the cumulative exposure estimate comes from the chronic APET estimate for trilobatin [FL‐no: 16.112]. Overall, the Panel considered that the cumulative exposure to these five substances does not raise a safety concern.
5. CONCLUSIONS
The Panel concluded that the use of hesperetin dihydrochalcone [FL‐no: 16.137] as a flavouring substance under the proposed conditions of use (to be dissolved in 1,2‐propanediol up to 20% w/w prior to its addition to food) does not raise a safety concern at the dietary exposure estimates calculated using the APET approach. The Panel also concluded that the cumulative exposure to [FL‐no: 16.137] and four structurally related substances [FL‐no: 16.061, 16.109, 16.110, 16.112] does not raise a safety concern.
6. DOCUMENTATION AS PROVIDED TO EFSA
Application for authorisation of a new food flavouring substance, hesperetin dihydrochalcone, in accordance with Regulation (EC) No 1331/2008. 7 November 2022. Submitted by Symrise AG. 19
Additional information received on 08 February 2024, submitted by Symrise AG in response to a request from EFSA (26 June 2023).
Additional information received on 14 June 2024, submitted by Symrise AG in response to a request from EFSA (19 March 2024).
Covance, 2020a. Hesperetin Dihydrochalcone 95%: A 2‐Week Preliminary Toxicity Study via Dietary Administration in Rats. Covance CRS, LLC, study number BW49VW. April 2020. Unpublished study report submitted by Symrise AG.
Covance, 2020b. Hesperetin Dihydrochalcone 95%: A 13‐Week Toxicity Study via Dietary Administration in Rats. Covance CRS, LLC, study number BF45FF. September 2020. Unpublished study report submitted by Symrise AG.
Covance, 2020c. Hesperetin Dihydrochalcone: Preliminary Embryo‐Fetal Development in Sprague Dawley Rats. Covance study number 8451008. December 2020. Unpublished study report submitted by Symrise AG.
Gentronix, 2018. Hesperetin Dihydrochalcone (CAS # 35400–60‐3): in vitro Mammalian Cell Micronucleus Test in Human Lymphocyte Cells. Gentronix study no. MNT00457. December 2018. Unpublished study report submitted by Symrise AG.
Labcorp, 2022. Hesperetin Dihydrochalcone: Study for Effects on Embryo‐Fetal Development in Sprague Dawley Rat. Labcorp study number 8451011. August 2022. Unpublished study report submitted by Symrise AG.
LPT (Laboratory of Pharmacology and Toxicology), 2016. Mutagenicity study of 3‐(3‐hydroxy‐4‐methoxy‐phenyl)‐1‐(2,4,6,‐trihydroxyphenyl) propane‐1‐one in the salmonella typhimurium and Escherichia coli reverse mutation assay (in vitro). LPT Report no. 33678. August 2016. Unpublished study report submitted by Symrise AG.
Noack Laboratorien, 2022. Hesperetin Dihydrochalcone, estimation of the water solubility via preliminary testing. Study ID SO21723/ CWF19888N. February 2018. Unpublished study report submitted by Symrise AG.
Symrise AG, 2021. Hesperetin Dihydrochalcone, Determination of the Partition Coefficient (n‐octanol/water) using the HPLC Method. Report no. 2021127ARC. December 2021. Unpublished study report submitted by Symrise AG.
ABBREVIATIONS
- ADME
absorption, distribution, metabolism and excretion
- ALT
alanine aminotransferase
- APET
added portions exposure technique
- AST
aspartate transaminase
- BW
body weight
- CAS
Chemical Abstract Service
- CBPI
Cytokinesis Block Proliferation Index
- CEF
Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CoE
Council of Europe
- DMSO
dimethylsulfoxide
- EDX
energy‐dispersive X‐ray spectroscopy
- EINECS
European Inventory of Existing Commercial chemical Substances
- FAF
Food Additives and Flavourings
- FAO
Food and Agriculture Organization of the United Nations
- FEEDAP
Additives and Products or Substances used in Animal Feed
- FEMA
Flavour and Extract Manufactures Association
- FGE
Flavouring Group Evaluation
- FL‐no
FLAVIS number
- FLAVIS
Flavour Information System database
- GLP
Good Laboratory Practice
- GRAS
generally regarded as safe
- HPLC‐DAD
high‐performance liquid chromatography–diode array detector
- HPLC‐UV
high‐performance liquid chromatography–ultraviolet
- IR
infrared
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LC/MS
liquid chromatography/mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantification
- MN
micronucleus
- MS
mass spectrometry
- MW
molecular weight
- NHDC
Neohesperidine dihydrochalcone
- NMR
nuclear magnetic resonance
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- QSAR
quantitative structure–activity relationship
- SEM
scanning electron microscopy
- SPET
single portion exposure technique
- TG
Test Guideline
- TSH
thyroid stimulating hormone
- TTC
threshold of toxicological concern
- T3
triiodothyronine
- T4
thyroxine
- VCF
volatile compounds in food
- WHO
World Health Organization
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2022‐00825
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Monica Andreassen, Gabriele Aquilina, Maria Bastos, Polly Boon, Laurence Castle, Biagio Fallico, Reginald FitzGerald, Maria Jose Frutos Fernandez, Bettina Grasl‐Kraupp, Ursula Gundert‐Remy, Rainer Gürtler, Eric Houdeau, Marcin Kurek, Henriqueta Louro, Patricia Morales, and Sabina Passamonti.
LEGAL NOTICE
The scientific output published implements EFSA's decision on the confidentiality requests submitted on specific items. As certain items have been awarded confidential status by EFSA they are consequently withheld from public disclosure by redaction.
ACKNOWLEDGEMENTS
The Panel wishes to thank the following for the support provided to this scientific output: Ana Maria Rincon, Camilla Smeraldi and Alexandra Tard.
APPENDIX A. Procedure for the safety evaluation of ‘stand‐alone’ chemically defined flavouring substances
A.1.
FIGURE A.1.
Procedure applied for the safety evaluation of hesperetin dihydrochalcone according to EFSA Guidance on the data required for the risk assessment of flavourings to be used in or on foods (EFSA CEF Panel, 2010b).
APPENDIX B. Food categories and use levels provided for hesperetin dihydrochalcone and five structurally and metabolically related substances
B.1.
TABLE B.1.
Food categories and use levels for hesperetin dihydrochalcone [FL‐no: 16.137] and five related substances as provided by the applicant (only these food categories are included for which use levels were provided). Portion sizes are according to the EFSA Guidance on the data required for the risk assessment of flavourings to be used in or on foods (EFSA CEF Panel, 2010b) (Documentation provided to EFSA no. 1, 2, 3).
| CODEX code | Food categories a | Standard portions b , c (g) | Occurrence level as added flavouring substance (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Maximum | Normal | Normal | Normal | Normal | Normal | |||
| Flavouring substances FL‐no: | 16.137 d | 16.061 e | 16.097 e | 16.110 e | 16.112 e | 16.109 e | |||
| 01.1 | Milk and dairy‐based drinks | 200 | 2 | 10 | 2 | 200 | 50 | 250 | 40 |
| 01.2 | Fermented and renneted milk products (plain), excluding food category 01.1.2 (dairy‐based drinks) | 200 | – | – | 2 | 200 | 50 | 250 | 40 |
| 01.3 | Condensed milk and analogues (plain) | 70 | – | – | 2 | 200 | 50 | 250 | 40 |
| 01.4 | Cream (plain) and the like | 15 | – | – | 2 | 200 | 50 | 250 | 40 |
| 01.5 | Milk powder and cream powder and powder analogues (plain) | 30 | – | – | 2 | 200 | 50 | 250 | 40 |
| 01.6 | Cheese and analogues | 40 | – | – | 2 | 200 | 50 | 250 | 40 |
| 01.7 | Dairy‐based desserts (e.g. pudding, fruit or flavoured yoghurt) | 125 | 3 | 10 | – | – | – | – | – |
| 01.8 | Whey and whey products, excluding whey cheeses | 200 | – | – | 2 | 200 | 50 | 250 | 40 |
| 02.1 | Fats and oils essentially free from water | 15 | – | – | 4 | 100 | – | – | 40 |
| 02.2 | Fat emulsions mainly of type water‐in‐oil | 15 | – | – | 4 | 100 | – | – | 40 |
| 02.3 | Fat emulsions mainly of type water‐in‐oil, including mixed and/or flavoured products based on fat emulsions | 15 | – | – | 4 | 100 | – | – | 40 |
| 02.4 | Fat‐based desserts excluding dairy‐based dessert products of category 1.7 | 50 | – | – | 4 | 100 | – | – | 40 |
| 03.0 | Edible ices, including sherbet and sorbet | 50 | 5 | 10 | 1 | 100 | 50 | – | 30 |
| 4.1.2 | Processed Fruit | 125 | 3 | 10 | 2 | – | 50 | – | – |
| 4.1.2.5 | Jams, jellies, marmalade | 30 | – | – | 2 | – | 50 | – | – |
| 04.2.2 | Processed vegetables (including mushrooms and fungi, roots and tubers, pulses and legumes and aloe vera), seaweeds and nuts and seeds | 200 | 2 | 10 | – | – | 50 | – | – |
| 05.1 | Cocoa products and chocolate products, including imitations and chocolate substitutes | 40 | – | – | 2 | 100 | 50 | – | 20 |
| 05.2 | Confectionery, including hard and soft candy, nougats, etc., other than 05.1, 05.3 and 05.4 | 30 | 5 | 10 | 2 | 100 | 50 | – | 20 |
| 05.3 | Chewing gum | 3 | 5 | 10 | 2 | 100 | 50 | – | 20 |
| 05.4 | Decorations (e.g. for fine bakery wares), toppings (non‐fruit) and sweet sauces | 35 | – | – | 2 | 100 | 50 | – | 20 |
| 06.1 | Whole, broken or flaked grain, including rice | 200 | – | – | 3 | 150 | 150 | 100 | 3 |
| 06.2 | Flours and starches (including soyabean powder) | 30 | – | – | 3 | 150 | 150 | 100 | 3 |
| 06.3 | Breakfast cereals, including rolled oats | 30 | 5 | 10 | 3 | 150 | 150 | 100 | 3 |
| 06.4 | Pastas and noodles and like products (e.g. rice paper, rice vermicelli, soya bean pastas and noodles) | 200 | – | – | 3 | 150 | 150 | 100 | 3 |
| 06.5 | Cereal and starch‐based desserts (e.g. rice pudding, tapioca pudding) | 200 | – | – | 3 | 150 | 150 | 100 | 3 |
| 06.6 | Batters (e.g. for breading or batters for fish or poultry) | 30 | – | – | 3 | 150 | 150 | 100 | 3 |
| 06.7 | Pre‐cooked or processed rice products, including rice cakes (Oriental type only) | 200 | – | – | 3 | 150 | 150 | 100 | 3 |
| 06.8 | Soya bean products (excluding soya bean products of food category 12.9 and fermented soya bean products of food category 12.10) | 100 | – | – | 3 | 150 | 150 | 100 | 3 |
| 07.1 | Bread and ordinary bakery wares | 50 | 5 | 10 | 4 | 200 | – | – | 30 |
| 07.2 | Fine bakery wares (sweet, salty, savoury) and mixes | 80 | – | – | 4 | 200 | – | – | 30 |
| 08.1 | Fresh meat, poultry and game | 200 | – | – | 2 | 100 | – | – | 20 |
| 08.2 | Processed meat, poultry and game products in whole pieces or cuts | 100 | – | – | 2 | 100 | – | – | 20 |
| 08.3 | Processed comminuted meat, poultry and game products | 100 | – | – | 2 | 100 | – | – | 20 |
| 08.4 | Edible casings (e.g. sausage casings) | 1 | – | – | 2 | 100 | – | – | 20 |
| 09.1.1 | Fresh fish | 200 | – | – | 2 | 100 | – | – | 30 |
| 09.1.2 | Fresh molluscs, crustaceans and echinoderms | 200 | – | – | 2 | 100 | – | – | 30 |
| 09.2 | Processed fish and fish products, including molluscs, crustaceans and echinoderms | 100 | – | – | 2 | 100 | – | – | 30 |
| 09.3 | Semi‐preserved fish and fish products, including molluscs, crustaceans and echinoderms | 100 | – | – | 2 | 100 | – | – | 30 |
| 09.4 | Fully preserved, including canned or fermented, fish and fish products, including molluscs, crustaceans and echinoderms | 100 | – | – | 2 | 100 | – | – | 30 |
| 10.2 | Egg products | 100 | – | – | 2 | – | – | – | – |
| 10.3 | Preserved eggs, including alkaline. salted and canned eggs | 100 | – | – | 2 | – | – | – | – |
| 10.4 | Egg‐based desserts (e.g. custard) | 125 | – | – | 2 | – | – | – | – |
| 12.1 | Salt and salt substitutes | 1 | – | – | 2 | 200 | 50 | – | 30 |
| 12.2 | Herbs, spices, seasonings and condiments (e.g. seasoning for instant noodles) | 1 | 4 | 10 | 2 | 200 | 50 | – | 30 |
| 12.3 | Vinegars | 15 | – | – | 2 | 200 | 50 | – | 30 |
| 12.5 | Soups and broths | 200 | 1 | 10 | 2 | 200 | 50 | – | 30 |
| 12.6 | Sauces and like products | 30 | 4 | 10 | 2 | 200 | 50 | – | 30 |
| 12.7.1 | Salads 120 g (e.g. macaroni salad, potato salad) excluding cocoa‐ and nut‐based spreads of food categories | 120 | – | – | 2 | 200 | 50 | – | 30 |
| 12.7.2 | Sandwich spreads (20 g), excluding cocoa‐ and nut‐based spreads of food categories | 20 | – | – | 2 | 200 | 50 | – | 30 |
| 12.8 | Yeast and like products | 1 | – | – | 2 | 200 | 50 | – | 30 |
| 12.9 | Protein products | 15 | – | – | 2 | 200 | 50 | – | 30 |
| 12.10 | Fermented soya bean products | 40 | – | – | 2 | 200 | 50 | – | 30 |
| 14.1 | Non‐alcoholic (‘soft’) beverages | 300 | 1.5 | 10 | 2 | 100 | 50 | 100 | 20 |
| 14.2.1 | Beer and malt beverages | 300 | 1.5 | 10 | 2 | 200 | 50 | – | 40 |
| 14.2.2 | Grape wines | 150 | – | – | 2 | 200 | 50 | – | 40 |
| 14.2.3 | Mead | 150 | – | – | 2 | 200 | 50 | – | 40 |
| 14.2.4 | Spirituous beverages | 30 | – | – | 2 | 200 | 50 | – | 40 |
| 15.1 | Snacks, potato‐, cereal‐, flour‐ or starch‐based (from roots and tubers, pulses and legumes) | 30 | 6 | 10 | 2 | 200 | 50 | – | 30 |
| 15.2 | Processed nuts, including coated nuts and nut mixtures (with e.g. dried fruit) | 30 | – | – | 2 | 200 | 50 | – | 30 |
| 15.3 | Snacks – fish based | 30 | – | – | 2 | 200 | 50 | – | 30 |
Most of the categories reported are the subcategories of Codex GSFA (General Standard for Food Additives, available at https://www.fao.org/gsfaonline/foods/index.html) used by the JECFA in the SPET technique (FAO/WHO, 2008).
For Adults. In case of foods marketed as powder or as concentrates, occurrence levels must be reported for the reconstituted product, considering the instructions reported on the product label or one of the standard dilution factors established by the JECFA (FAO/WHO 2008): ‐ 1/25 for powder used to prepare water‐based drinks such as coffee, containing no additional ingredients, − 1/10 for powder used to prepare water‐based drinks containing additional ingredients such as sugars (ice tea, squashes, etc.), − 1/7 for powder used to prepare milk, soups and puddings, − 1/3 for condensed milk.
Portion sizes are according to the EFSA Guidance on the data required for the risk assessment of flavourings to be used in or on foods (EFSA CEF Panel, 2010b). According to the guidance, standard portion sizes for children are obtained by multiplying the adult standard portion sizes by a factor of 0.63.
The candidate substance in FGE.420.
Supporting substances for which only normal use levels have been provided.
APPENDIX C. Genotoxicity studies
C.1.
TABLE C.1.
Summary of new in vitro genotoxicity studies on hesperetin dihydrochalcone [FL‐no: 16.137] (Documentation provided to EFSA no. 1).
| Chemical name [FL‐no] | Test system in vitro | Test object | Concentrations a and test conditions | Result | Reliability/comments | Relevance of test system/relevance of the result | Reference |
|---|---|---|---|---|---|---|---|
|
Hesperetin dihydrochalcone [16.137] |
Reverse Mutation test |
S. typhimurium TA98, TA100, TA1535, TA1537 E. Coli WP2 uvrA [pKM101] |
Experiment 1 (plate incorporation, +S9, –S9): 3.16–1000 μg/plate Experiment 2 (pre‐incubation, +S9, –S9): 3.16–1000 μg/plate |
Negative |
Reliable without restrictions. Study performed according to OECD TG 471 and in compliance with GLP. |
High/High | LPT (2016) |
| Micronucleus assay | Human Peripheral Blood Lymphocytes |
260, 273, 287 μg/mL (3 + 21 h, +S9) 214, 225, 236 μg/mL (3 + 21h, –S9) 17, 26, 39 μg/mL (24h, –S9) |
Negative | Reliable without restrictions. Study performed according to OECD TG 487 and in compliance with GLP. | High/High | Gentronix (2018) |
Abbreviations: GLP, Good Laboratory Practice; OECD, Organisation for Economic Co‐operation and Development.
For the in vitro MN study, the given concentrations are those for the cultures that were scored for micronuclei.
APPENDIX D. Toxicity data
D.1.
TABLE D.1.
Summary of toxicity studies for hesperetin dihydrochalcone [FL‐no: 16.137] (Documentation provided to EFSA no. 1, 2).
| Species; sex No./group | Route of administration | Dose levels (mg/kg bw per day) | Duration (days) | Results | Reference | Comments |
|---|---|---|---|---|---|---|
| Repeated dose toxicity studies | ||||||
|
Sprague–Dawley Rats; M, F 5/sex/group |
Oral (feed) | 0, 300, 1000 | 14 | No toxicity observed | Covance (2020a) | Dose range‐finding study. Doses up to 1000 mg/kg bw per day were considered appropriate for testing in the 90‐day toxicity study. |
|
Sprague–Dawley Rats; M, F 10/sex/group |
Oral (feed) | 0, 100, 300, 1000 | 90 | Treatment and dose‐related changes in T3 and T4 were observed, but not considered adverse. | Covance (2020b) |
Study performed according to OECD TG 408 and in compliance with GLP. The Panel considered the highest dose tested (1000 mg/kg bw per day) as a reference point. |
| Prenatal developmental toxicity studies | ||||||
|
Sprague–Dawley Rats; F 6/group |
Oral (feed) | 0, 100, 300, 1000 | During GD 5–20 | No maternal or fetal toxicity observed | Covance (2020c) | Preliminary embryo–foetal development study. Study performed according to OECD TG 414, but not in compliance with GLP. |
|
Sprague–Dawley Rats; F 22/group |
Oral (feed) | 0, 100, 300, 1000 | During GD 5–20 | No maternal or fetal toxicity observed | Labcorp (2022) | Study performed according to OECD TG 414 and in compliance with GLP. The highest dose level (actual dose of 821 mg/kg bw per day) can be set as a NOAEL in this study. |
Abbreviations: F, female; GD, gestation day; GLP, Good Laboratory Practice; M, male; OECD, Organisation for Economic Co‐operation and Development.
EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Castle, L. , Andreassen, M. , Aquilina, G. , Bastos, M. , Boon, P. , Fallico, B. , FitzGerald, R. , Frutos Fernandez, M. J. , Grasl‐Kraupp, B. , Gundert‐Remy, U. , Gürtler, R. , Houdeau, E. , Kurek, M. , Louro, H. , Morales, P. , Passamonti, S. , Degen, G. , Engel, K.‐H. , … Martino, C. (2024). Flavouring group evaluation 420 (FGE.420): Hesperetin dihydrochalcone. EFSA Journal, 22(12), e9091. 10.2903/j.efsa.2024.9091
Adopted: 23 October 2024
The declarations of interest of all scientific experts active in EFSA's work are available at https://open.efsa.europa.eu/experts
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–‐50.
Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings. OJ L 354, 31.12.2008, p. 1–6.
Online Edition: ‘Specifications for Flavourings’ https://www.fao.org/food/food‐safety‐quality/scientific‐advice/jecfa/jecfa‐flav/details/en/c/2280/.
JECFA reported as NOAEL 760 mg/kg bw per day, indicating ‘Previously rounded to 750 mg/kg bw per day’ (JECFA, 2012). However, for the evaluation of hesperetin dihydrochalcone, JECFA used the same study (Lina et al., 1990) on neohespredine dihydrochalcone and reported the NOAEL of 750 mg/kg bw per day (JECFA, 2021, 2022).
NHDC is the abbreviation used for neohesperidine dihydrochalcone in the scientific opinions by the FEEDAP Panel (EFSA FEEDAP Panel, 2011; EFSA FEEDAP Panel, 2014).
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1.
Decision available online https://www.efsa.europa.eu/en/corporate‐pubs/transparency‐regulation‐practical‐arrangements.
The non‐confidential version of the dossier, following EFSA's assessment of the applicant's confidentiality requests, is published on Open.EFSA and is available at the following link: https://open.efsa.europa.eu/dossier/FFL‐2022‐9311.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Determined with OECD Toolbox (version 4.7 available at https://qsartoolbox.org/).
Available online: https://www.efsa.europa.eu/en/data‐report/compendium‐botanicals.
Available online: https://chem.echa.europa.eu/.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
Based on the EFSA European Food Consumption Database (Comprehensive Database), toddlers are defined as the age class ranging from 12 months up to and including 35 months of age (EFSA, 2011).
Determined with OECD QSAR Toolbox (version 4.7 available at https://qsartoolbox.org/).
The non‐confidential version of the dossier, following EFSA's assessment of the applicant's confidentiality requests, is published on Open.EFSA and is available at the following link: https://open.efsa.europa.eu/dossier/FFL‐2022‐9311.
REFERENCES
- Borrego, F. , & Montijano, H. (2001). Neohesperidin dihydrochalcone. In Nabors L. O. (Ed.), Alternative sweeteners (pp. 85–99). Marcel Dekker, Inc. [Google Scholar]
- Braune, A. , Engst, W. , & Blaut, M. (2005). Degradation of neohesperidin dihydrochalcone by human intestinal bacteria. Journal of Agricultural and Food Chemistry, 53, 1782–1790. [DOI] [PubMed] [Google Scholar]
- Brown, J. P. , & Dietrich, P. S. (1979). Mutagenicity of plant flavonols in the Salmonella/mammalian microsome test: Activation of flavonol glycosides by mixed glycosidases from rat cecal bacteria and other sources. Mutation Research, Genetic Toxicology, 66, 223–240. [DOI] [PubMed] [Google Scholar]
- Cramer, G. M. , Ford, R. A. , & Hall, R. L. (1978). Estimation of toxic hazard: A decision tree approach. Food and Cosmetics Toxicology, 16(3), 255–276. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2011). Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal, 9(3), 2097. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2012). Proposed template to be used in drafting scientific opinion on flavouring substances (explanatory notes for guidance included). EFSA Supporting Publications, 9(1), EN‐218. 10.2903/sp.efsa.2012.en-218 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2020). Outcome of the public consultation on a draft protocol for assessing exposure to sweeteners as part of their safety assessment under the food additives re‐evaluation programme. EFSA Supporting Publications, 17(8), EN‐1913. 10.2903/sp.efsa.2020.EN-1913 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) . (2010a). Scientific Opinion on Flavouring group evaluation 32 (FGE.32): Flavonoids (flavanones and dihydrochalcones) from chemical groups 25 and 30. EFSA Journal, 8(9), 1065. 10.2903/j.efsa.2010.1065 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) . (2010b). Guidance on the data required for the risk assessment of flavourings to be used in or on foods. EFSA Journal, 8(6), 1623. 10.2093/j.efsa.2010.1623 [DOI] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes, M. , Aquilina, G. , Castle, L. , Degen, G. , Engel, K.‐H. , Fowler, P. J. , Frutos Fernandez, M. J. , Fürst, P. , Gundert‐Remy, U. , Gürtler, R. , Husøy, T. , Manco, M. , Mennes, W. , Moldeus, P. , Passamonti, S. , Shah, R. , Waalkens‐Berendsen, I. , Wright, M. , … Vianello, G. (2022a). Scientific Opinion on the re‐evaluation of neohesperidine dihydrochalcone (E 959) as a food additive. EFSA Journal, 20(11), 7595. 10.2903/j.efsa.2022.7595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) . (2022b). Scientific guidance on the data required for the risk assessment of flavourings to be used in or on foods. EFSA Journal, 20(12), 7673. 10.2903/j.efsa.2022.7673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) . (2011). Scientific Opinion on the safety and efficacy of neohesperidine dihydrochalcone when used as a sensory additive for piglets, pigs for fattening, calves for rearing and fattening, lambs for rearing and fattening, dairy sheep, ewes for reproduction, salmonids and dogs. EFSA Journal, 9(12), 2444. 10.2903/j.efsa.2011.2444 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) . (2014). Scientific Opinion on the safety of neohesperidine dihydrochalcone as a sensory additive for fish. EFSA Journal, 12(5), 3669. 10.2903/j.efsa.2014.3669 [DOI] [Google Scholar]
- EFSA Scientific Committee . (2009). Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: General principles. EFSA Journal, 7(7), 1051. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee . (2011). Scientific opinion on Genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal, 9(9), 2379. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy, A. , Benford, D. , Halldorsson, T. , Jeger, M. J. , Knutsen, H. K. , More, S. , Naegeli, H. , Noteborn, H. , Ockleford, C. , Ricci, A. , Rychen, G. , Schlatter, J. R. , Silano, V. , Solecki, R. , Turck, D. , Younes, M. , Bresson, J.‐L. , Griffin, J. , … Alexander, J. (2017). Guidance on the assessment of the biological relevance of data in scientific assessments. EFSA Journal, 15(8), 4970. 10.2903/j.efsa.2017.4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , More, S. , Bampidis, V. , Benford, D. , Bragard, C. , Halldorsson, T. , Hernández‐Jerez, A. , Bennekou, S. H. , Koutsoumanis, K. , Lambré, C. , Machera, K. , Naegeli, H. , Nielsen, S. , Schlatter, J. , Schrenk, D. , Silano, V. , Turck, D. , Younes, M. , Castenmiller, J. , … Schoonjans, R. (2021). Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA Journal, 19(8), 6769. 10.2903/j.efsa.2021.6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO . (2008). Joint FAO/WHO expert committee on food additives (pp. 17–26). Sixty‐ninth meeting. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) . (2012). Safety evaluation of certain food additives prepared by the seventy‐sixth meeting of the Joint FAO/WHO expert committee on food additives, WHO Food additives Series 67, Geneva. https://www.who.int/publications/i/item/9789241660679
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) . (2021). Evaluation of certain food additives: Eighty‐ninth report of the joint FAO/WHO expert committee on food additives. World Health Organization and food and agriculture Organization of the United Nations, Geneva (WHO Technical Report Series, No. 1027).
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) . (2022). Safety evaluation of certain food additives: prepared by the eighty‐ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). World Health Organization and Food and Agriculture Organization of the United Nations, Geneva (WHO Food Additives Series, No. 80).
- Lina, B. A. R. , Dreef‐van der Meulen, H. C. , & Leegwater, D. C. (1990). Subchronic (13‐week) oral toxicity of neohesperidindihydrochalcone in rats. Food Chemistry and Toxicology, 26, 507–513. 10.1016/0278-6915(90)90121-3 [DOI] [PubMed] [Google Scholar]
- MacGregor, J. T. , & Jurd, L. (1978). Mutagenicity of plant flavonoids: Structural requirements for mutagenic activity in salmonella typhimurium . Mutation Research, Environmental Mutagenesis and Related Subjects, 54, 297–309. [DOI] [PubMed] [Google Scholar]
- MacGregor, J. T. , Wehr, C. M. , Manners, G. D. , Jurd, L. , Minkler, J. L. , & Carrano, A. V. (1983). In vivo exposure to plant flavonols: Influence on frequencies of micronuclei in mouse erythrocytes and sister‐chromatid exchange in rabbit lymphocytes. Mutation Research, Genetic Toxicology, 124, 255–270. [DOI] [PubMed] [Google Scholar]
- Montijano, H. , Borrego, F. , Canales, I. , & Tomás‐Barberán, F. A. (1997). Validated high‐performance liquid chromatographic method for quantitation of neohesperidine dihydrochalcone in foodstuffs. Journal of Chromatography A, 758, 163–166. 10.1016/S0021-9673(96)00697-8 [DOI] [PubMed] [Google Scholar]
- Munro, I. C. , Ford, R. A. , Kennepohl, E. , & Sprenger, J. G. (1996). Correlation of structural class with no‐observed‐effect levels: A proposal for establishing a threshold of concern. Food and Chemical Toxicology, 32(9), 829–867. [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) . (2016). Test No. 487: In vitro mammalian cell micronucleus test. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co‐operation and Development) . (1995). Test No. 105: Water Solubility. OECD Guidelines for the Testing of Chemicals, Section 1. 10.1787/9789264069589-en [DOI]
- OECD (Organisation for Economic Co‐operation and Development) . (1997). Test No. 471: Bacterial Reverse Mutation Test. OECD Guidelines for the Testing of Chemicals, Section 4. 10.1787/9789264071247-en [DOI]
- OECD (Organisation for Economic Co‐operation and Development) . (2004). Test No. 117: Partition Coefficient (n‐octanol/water), HPLC Method. OECD Guidelines for the Testing of Chemicals, Section 1. 10.1787/9789264069824-en [DOI]
- OECD (Organisation for Economic Co‐operation and Development) . (2009). Guidance manual for the testing of manufactured nanomaterials: OECD's sponsorship programme; First revision. Series on the safety of manufactured nanomaterials, ENV/JM/MONO(2009)20/REV.
- OECD (Organisation for Economic Co‐operation and Development) . (2018a). Test No. 408: Repeated Dose 90‐Day Oral Toxicity Study in Rodents. OECD Guidelines for the Testing of Chemicals, Section 4 10.1787/9789264070707-en [DOI]
- OECD (Organisation for Economic Co‐operation and Development) . (2018b). Test No. 414: Prenatal Developmental Toxicity Study. OECD Guidelines for the Testing of Chemicals, Section 4. 10.1787/9789264070820-en [DOI]
- VCF (Volatile Compounds in Food) . (2024). BeWiDo BV. Version 16.10, 1991–2024. https://www.vcf‐online.nl/VcfHome.cfm
- Zhang, F. X. , Yuan, Y. L. , Cui, S. S. , Li, M. , Tan, X. , Qiu, Z. C. , & Li, R. M. (2021). Dissection of the potential pharmacological function of neohesperidin dihydrochalcone—a food additive—by in vivo substances profiling and network pharmacology. Food & Function, 12, 4325–4336. [DOI] [PubMed] [Google Scholar]


