Abstract
Chinese cabbage is a vital while perishable leafy vegetable. Blue-green light combined treatment was found to be better to retain high nutritional value in Chinese cabbage than blue or green monochromatic light treatments by increasing the contents of soluble protein, indol-3-ylmethyl (I3M), flavonoids, total phenols and carotene. Gene expression study further revealed that blue-green light treatment increased the expression of biosynthetic genes in the metabolic pathways of glucosinolates (BrCYP79B2, BrIGMT1), flavonoids (BrCHS.1, BrF3H, BrCHI) and carotenoids (BrPSY1, BrLCYB, BrLCYE, BrVDE), as well as the light signal master regulator gene BrHY5.2. In addition, the blue-green light treatment up-regulated the activities of antioxidant enzymes, as well as 2,2-diphenyl-1-picrylhydrazyl and ferric-reducing antioxidant power activities, while reducing the levels of superoxide anion, hydrogen peroxide (H2O2), polyphenol oxidase (PPO) activity and malondialdehyde (MDA). Overall, our findings confirmed that blue-green light treatment can effectively enrich the nutritional value and prolong the shelf life of Chinese cabbage.
Keywords: Chinese cabbage, Blue-green light treatment, Glucosinolates, Flavonoids, Antioxidant activity
Graphical abstract

Highlights
-
•
Blue-green light treatment (BGLT) prolonged the shelf life of Chinese cabbage.
-
•
BGLT increased the antioxidant capacity of postharvest Chinese cabbage (PCC).
-
•
The beneficial effects of BGLT on PCC was greater than those of monochromatic light.
-
•
BGLT induced the accumulation of I3M, NMOI3M and 4MT at the early stage of storage.
-
•
BGLT induced the expression of genes in GLS, flavonoids and carotenoids pathways.
List of compounds
Glucosinolates (Compound CID: 6537198)
Progoitrin (Compound CID: 5281139)
Flavonoids (Compound CID: 5281616)
Indol-3-ylmethyl glucosinolate (Compound CID: 6602378)
Methanol (Compound CID: 887)
4-Methoxyindol-3-ylmethyl glucosinolate (Compound CID: 9576738)
4-Hydroxyindol-3-ylmethyl glucosinolate (Compound CID: 9548896)
4-Pentenyl glucosinolate (Compound CID: 656527)
Trichloroacetic acid (Compound CID: 6421)
5-Methylthiopentyl glucosinolate (Compound CID: 656525)
1. Introduction
Chinese cabbage (Brassica rapa L. ssp. pekinensis) is a highly nutritious cruciferous vegetable (Zhang et al., 2016). During storage, Chinese cabbage leaves are susceptible to yellowing, wilting, and rotting, leading to a rapid deterioration in both appearance and quality. This issue can be addressed using light emitting diode (LED) lighting as a green, economical and efficient technology for extending the shelf life of vegetables and enhancing their post-harvest quality (Xie, Tang, Xiao, Geng, & Guo, 2022).
Blue light treated strawberry fruit may have stronger free radical scavenging properties because they contain more total phenols, ascorbic acid, and anthocyanins (Xu et al., 2014). Moreover, the application of blue LEDs during post-harvest re-culturing of barley leaves induced total phenolic compounds and to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals (Lee et al., 2010). Consequently, LED lighting has emerged as a practical and effective tool for improving the nutritional quality of harvested vegetables.
The wavelength and intensity of LED lights during postharvest treatment are critical factors for their beneficial effects, and the optimal setting of LED lights needs to be experimentally determined for each specific vegetable (Thilini Deepashika Perera, Navaratne, & Wickramasinghe, 2022). Research has demonstrated that implementing low-intensity LED lighting at a rate of 20 μmol m−2 s−1 is an ecologically sound approach formaintaining the phytonutrient levels and upholding the overall quality of broccoli throughout the postharvest storage process, while high intensity LED lighting has been shown to induce abiotic stress and induced reactive oxygen species (ROS) in crops. The study of Jin, Ding, and Xie (2021) has illustrated the efficacy of white, green, and blue LED lights with a range of 10 to 35 μmol m−2 s−1 in preserving the chlorophyll content of postharvest vegetables. According to Wang, Xu, and Cui (2015), the utilization of blue LEDs can effectively prolong the duration of active photosynthesis and enhance photosynthetic capacity, making them an efficient tools to prolong shelf life by delaying aging. After harvest, the blue light treatment of broccoli has been found to delay the chlorophyll degradation, increase total phenolic and GLS content, prevent spoilage and extend shelf life (Jin, Yao, Xu, Wang, & Zheng, 2015). The application of blue light to strawberry fruit increased its antioxidant enzyme activities (catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX)) (Xu, Shi, et al., 2014). Similarly, mangoes treated with blue LED (75 μmol s−1 m−2), exhibited significantly higher activities of CAT, and guaiacol peroxidase (POD) during storage for 15 d (Chávez-Zaragoza et al., 2022). Furthermore, the combined treatment of blue light and SA significantly improve the activities of enzymatic antioxidants (SOD and APX) in strawberries stored for 9 d (Zhang et al., 2022). Thus, LED light with experimentally determined wavelength and light intensity could effectively maintain the shelf life and quality of postharvest vegetables.
The molecular mechanisms of LED irradiation effects in postharvest crops have received increasing attention in recent years. Initially, our prior investigations have demonstrated that monochromatic blue light can induce the synthesis and accumulation of GLS, flavonoids, carotenoids, and phenolic compounds (Zhang et al., 2023). This not only enhances the antioxidant enzyme activity but also contributes to the elevation of bioactive substances in vegetables. However, due to the nature of blue light as high-energy, short-wavelength radiation, intense exposure to monochromatic blue light can adversely affect plant growth (Izzo, Hay Mele, Vitale, Vitale, & Arena, 2020). For instance, studies have shown that blue LEDs (440–450 nm) were reported to delay the formation of tomato pigments and enhance the amounts of lycopene after 21 d of treatment on green tomatoes (Dhakal and Baek, 2014a, Dhakal and Baek, 2014b). However, reports on the impact of green light on the accumulation of secondary metabolites in plants are scarce. In cabbage samples treated with green LEDs, the chlorophyll content was higher than that of white, red, blue, and non-irradiated groups (Lee, Ha, Oh, & Cho, 2014), and green light significantly aids in photosynthetic carbon assimilation, potentially boosting crop yields. It has been demonstrated that green light can promptly reverse the stomatal opening induced by blue light (Talbott, Nikolova, Ortiz, Shmayevich, & Zeiger, 2002), and it also actively drives the photosynthesis. Recent research suggests beneficial interactions between the perception of ultraviolet (UV) and green light, indicating that green light responses may be mediated by photoreceptors or cryptochromes, meanwhile, green light has been demonstrated to promptly reverse the stomatal opening induced by blue light (Palma et al., 2020). Additionally, the application of green light represents a novel technique that can penetrate the outermost leaves of cabbage and reach the inner leaves, thereby promoting the photochemical reaction of overlapping leaves. Light treatment has also been identified as a crucial factor in preserving the pigment content and fresh weight of Chinese cabbage during storage (Wang, Liu, Tian, Ma, & Wang, 2022). However, the physiological and biochemical effects of blue-green light combined treatment on postharvest Chinese cabbage have not been reported yet. We hypothesize that green and blue light may play complementary roles in inducing stomatal opening, regulating photosynthesis, and sustaining carbon sources.
Here, we have investigated that the biophysical and biochemical responses to the Chinese cabbage under different quality and wavelength of LED treatments during postharvest stage. Our main objectives are: (i) to investigate the influence of green light and blue light combined treatment on antioxidant enzymes activities and the accumulation of GLS, flavonoids and carotenoids, (ii) to study the molecular mechanisms and interactions between multiple secondary metabolic pathways, and (iii) to explore the roles of different antioxidant substances in prolonging the shelf life and improve the commerciality and nutrition of the postharvest Chinese cabbage.
2. Material and methods
2.1. Plant materials and light treatments
In fall 2024, the Chinese cabbage was planted at Taigu experimental farm in Taigu (N: 37°25′, E: 112°32′). The seeds were sowed on Sep 25th, transplanted on Aug 27th, and selected for maturity and absence of pests and diseases. Oval-shaped Chinese cabbage was used for the post-harvest light treatment test at the commodity's maturity stage (Fig. S1). The light treatment test was divided into two parts, as shown in Fig. 1. Firstly, the “Shanye1” with uniform growth was selected and treated with a maintained light intensity (120 μmol m−2 s−1), and blue (λ = 460 nm) and green (λ = 520 nm) light, compound blue light, green light (1,1) (Fig. 1A), the beads of each light type were uniformly distributed, and the ratio of light quality was the ratio of the number of light sources providing the light quality, meanwhile, the dark conditions as the control group. The light intensity was controlled by adjusting the distance between the plant and the light source, and the position of the plant was randomly adjusted every day, so that the light is illuminated evenly. The spectrum of the light source was monitored daily by a spectrum analyzer (Li-cor, PS300, U.S), and the light intensity was measured with a photo-quantum meter (MQ-100, Apogee Instruments Inc., Logan, UT, USA). Other culture conditions were kept constant (temperature: 20 ± 3 °C, relative humidity: 30 %, photoperiod 16/8 h). Samples of the Chinese cabbage were taken with 0, 7, 14, 21, and 28 d after treatment for analysis of the inner leaf color using a colorimeter and for sample collection. Secondly, the “Shanye1” lines were treated with three distinct light intensities (60, 120, 300 umol m−2 s−1) between 0 and 10 d (Fig. 1B), and the inner leaf color was analyzed using a colorimeter, and samples were collected. The sampling was conducted with a random sampling of plants, and three biological replicates were selected for all assays.
Fig. 1.
Schematic diagram of the experimental setup for postharvest light treatment on Chinese cabbage. A. Blue light, green light, and blue-green light combined treatments were used to treat the postharvest Chinese cabbage. B. Treat postharvest Chinese cabbage with 60, 120, and 300 umol m−2 s−1 light intensities, and take samples at 10 d. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2. Surface color measurement
During storage, the L*, a*, and b* values of Chinese cabbage leaves were measured using a colorimeter (CR-410, Konica Minolta, NIC., Japan). The color values were reported as L*, a*, b*, and chroma (C*), with chroma (C*) being calculated using the formula (a*2 + b*2)1/2, as suggested by (Jing et al., 2015), which indicates color intensity. Additionally, the yellow index (YI), which reflects the degree of yellowness, was calculated using the formula 142.86 b*/L*.
2.3. Weight loss
During the storage period of Chinese cabbage, measurements were taken every 7 d starting from the time of harvest. Each Chinese cabbage was weighed three times to determine the weight reduction rate. The results were shown as a percentage of the starting weight.
2.4. Total flavonoids, total phenolics, and carotenoids
Total phenols (TP) and flavonoids (TF) contents were determined according to Pirie and Mullins (1976). In brief, 0.5 g of the sample material were subjected to mechanical maceration in a solution comprising 1 % hydrochloric acid (v/v) and methanol, under conditions devoid of ambient light, to facilitate the extraction process. Following complete homogenization, the mixture was permitted to undergo solvent extraction for a period of 20 min. Subsequently, the resultant supernatant from each sample was transferred via filtration into a 15 mL volumetric test tube, in preparation for subsequent analytical procedures. The results were given as OD280 kg−1 and OD325 kg−1, respectively. Total carotenoids were determined using the method of He, Yang, Wu, and Hu (2015). Fresh leaf tissue (0.2 g) was macerated in 95 % (v/v) ethanol supplemented with CaCO3 under dark conditions and chilled at 4 °C for 12 h. The homogenate was then centrifuged at 6000g for 20 min, and the supernatant was analyzed using a UV-1800 ultraviolet spectrophotometer (Shimadzu, Kyoto, Japan). The units of total carotenoids content were expressed in g kg−1.
2.5. Measurement of physiological characters and histochemical staining
The soluble protein of the Chinese cabbage was determined following the method of Bradford (1976). Add 0.5 g of fresh Chinese cabbage leaves into the mortar, then add 2 mL of 0.05, pH = 7.8 phosphoric acid buffer, and cool down with liquid nitrogen. Then centrifuge at 4 °C, 12000 g for 20 min. The supernatant was added with 3 mL coomassie brilliant blue, placed for 2 min, and determined at 595 nm. The result was presented as g kg−1. The content of total soluble solids (TSS) was determined with a digital refractometer (ATAGO, PAL-1, Model 3810, Japan). The determination of soluble sugar was conducted according to Yan et al. (2013). Weigh 0.5 g of Chinese cabbage leaves, add quartz sand and 5 mL of trichloroacetic acid (TCA), and grind thoroughly. Centrifuge the mixture at 5000 g for 10 min at room temperature. Transfer 2 mL of the supernatant to a test tube, add 2 mL of 0.6 % dithiobarbituric acid (TBA) solution, and heat in a boiling water bath for 15 min. Rapidly cool the reaction mixture and centrifuge at 5000 g for 10 min. Measure the absorbance of the supernatant at 532 nm and 450 nm. The hydrogen peroxide (H2O2) contents were measured as follows the 0.5 g of plant leaf tissue was rapidly homogenized in liquid nitrogen and combined with 2 mL acetone. After centrifugation at 12,000 rpm for 10 min at 4 °C, 1 mL of the supernatant was treated with 0.2 mL ammonia and 0.1 mL concentrated hydrochloric acid. The precipitate was washed with pre-cooled acetone until colorless and then resuspended in 3 mL of 2 M sulfuric acid. The H2O2 content was shown in mmol kg−1. The determination of MDA content refers to Feng's method (Feng et al., 2019). Briefly, 0.2 g of plant leaves were extracted with 5 % trichloroacetic acid, centrifugated at 10000 g for 15 min, 0.5 % thiobarbituric acid was added in the reaction solution of the same volume, and then boiled in water for 10 min. After cooling, the absorbance was determined. The MDA content of Chinese cabbage is shown in mol kg−1. A 0.5 g fresh-leaf sample was pulverized to a fine powder using liquid nitrogen in a precooled mortar and pestle, then homogenized in 3 mL of 50 mM phosphate buffer (pH 7.8) on ice. Subsequent centrifugation was performed at 12,000 g for 20 min at 4 °C. The supernatant was used for enzyme activity determination, and all operations were carried out at 4 °C. The procedure of Aebi (1984) was used to measure the CAT activity. The POD and SOD activities were measured in accordance with Feng et al. (2019) method. The method of Xu, Shi, et al., 2014 was used for the determination APX in Chinese cabbage. Polyphenol oxidase (PPO) was determined according to the method of Ma et al. (2021). 2.8 mL of 0.04 M catechol solution was added to 0.2 mL of enzyme extract, and the absorbance was measured at 398 nm. The results of the CAT, POD, SOD, APX, and PPO are shown in g kg−1. The Nitro blue tetrazolium (NBT) staining for O2− (superoxide) was conducted as described previously (Feng et al., 2019). Intact fresh leaves were submerged in NBT solution and stained at 28 °C for 3 h. Following staining, then the leaves were boiled in a water bath for 15 min to decolorize. Post-decolorization, the leaf color was examined and photographed. The quantification of the ROS (NBT stained area) was obtained by following the method of Feng et al. (2019).
2.6. Antioxidant activity
With reference to He, Zhang, and Zhang (2016) determined the ion of ferric-reducing antioxidant power (FRAP) by thoroughly pulverizing 0.5 g of fresh frozen tissue using absolute ethanol, fixing the resulting homogenate to a volume of 10 mL, subsequently dissolved and centrifuged the solution at 4500 ×g for 10 min. The supernatant was collected and stored in the dark for future use. To measure FRAP, 200 uL of sample extract was mixed uniformly with 2.8 mL of FRAP reaction solution, and the resulting mixture was reacted at 37 °C for 1 h. The determination of DPPH refers to the method of He et al. (2016). In a 2 mL centrifuge tube, and 100 uL of the sample extract to 1.9 mL of 0.1 mM DPPH solution in methanol. After 1 h of light protection at room temperature, the absorbance at 517 nm was measured with a UV-1800 spectrophotometer. The calculation method refers to He et al. (2016), and the regression equation of the standard curves was y = 0.1187 x + 2.2324 (R2 = 0.99). The FRAP and DPPH were shown as Trolox equivalent antioxidant capacity (mM kg−1).
2.7. GLS measurements
The GLS were extracted and analyzed from Chinese cabbage leaf tissue according to the work (Kliebenstein et al., 2001). In brief, 0.1 g of the sample was pulverized in a tissue grinder with 1 mL of a 90 % methanol solution. Then, 210 μL of Sulfatase solution was added in tissue grinder. The supernatant was centrifuged at 8000 ×g for1 a minute to prepare it for HPLC analysis. In order to conduct the analysis, the HPLC system (LC-2030 Plus Shimadzu Japan) was employed in accordance with a previously established procedure (Kliebenstein et al., 2001). Based on retention time, the composition of GLS was established (Table S1). The GLS content was shown as mmol kg−1.
2.8. Real-time quantitative PCR (qRT-PCR) analysis
The total RNA was extracted from the collected Chinese cabbage samples using a total RNA kit (Tiangen Biotech, Xi'an, China), and cDNA was synthesized and detected by PrimeScript™ kit (Takara, Dalian, China). QRT-PCR was conducted as described in our previous study (Zhang et al., 2023). The GAPDH gene (GO0048316) of the Chinese cabbage was used as the control gene. The primer sequences used were shown in Table S2. The expression levels of carotenoid, flavonoid, GLS metabolism, and resistance-related genes, including the BrHY5.2, BrPSY1, BrLCYB, BrLCYE, BrVDE, BrPDS, BrZEP, BrCCD4, BrCHS.1, BrF3H, BrCHI, BrCYP79B2, BrIGMT1, BrMYB4, BrPIF4, BrMYB12.1 were assessed using three independent biological replicates.
2.9. Statistical analysis
Each value point is expressed as the mean ± standard error (SE) of three biological replicates. Statistical software SPSS version 23 (IBM Corp., Armonk, NY, USA) was used, and the statistical comparisons were performed using one-way analysis of variance (ANOVA) followed by Duncan's multiple range test. For all tests, a significance level of P < 0.05 was employed.
3. Results
3.1. Exploring the optimal condition of LED light on treating Chinese cabbage in the postharvest stage
The leaf head of Chinese cabbage during postharvest stage showed variable responses under light treatments. There was no significant difference in antioxidant enzyme activity between the 60 umol m−2 s−1 light treatment and the control group, while the 300 umol m−2 s−1 light treatment resulted in a substantial accumulation of H2O2, which was significantly higher than that of the control group (Fig. S2). Moderate-intensity blue and green light (120 umol m−2 s−1) were found to significantly reduce the H2O2 content and increase the activities of antioxidant enzymes SOD, APX and total soluble solids content (Fig. S2). Thus, we used medium-intensity light of 120 umol m−2 s−1 for all the subsequent postharvest light treatment studies. The blue-green light treatment significantly induced the color intensity during the first 14 d of postharvest Chinese cabbage storage (Fig. 2A). Moreover, the blue light treatment led to an initial increase and subsequent decline in the yellow index (YI) value, which the yellowness was higher than that of the dark group (Fig. 2B). Blue light and blue-green light treatments on postharvest Chinese cabbage up-regulated the content of soluble protein, soluble sugar, and soluble solids, while green light had no significant effect (Fig. 3A-C). Furthermore, during the storage, blue light and blue-green light treatments reduced the water loss, compared to the dark control Chinese cabbage (Fig. 3D). The TP and TF content was increased by blue-green light combined treatment (Fig. 3E-F). In conclusion, the blue-green light treatment increased the intrinsic quality of postharvest Chinese cabbage in the early storage period (0–14 d) and delayed the quality decline of postharvest Chinese cabbage in the late storage period (14–28 d).
Fig. 2.
The effects of blue, green, and blue-green light treatment on the color index of Chinese cabbage during storage after harvest. A. Chroma (C*). B. Yellow index (YI). Statistical significant differences between treatment groups at the same period of storage are shown by values with distinct letters (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
The effects of blue, green and blue-green light treatments of postharvest physiological parameters in Chinese cabbage during storage. A. Soluble protein. B. Soluble sugar. C. Total soluble solids. D. Water loss. E. Total flavonoids. F. Total phenolic compounds. Statistical significant differences between treatment groups at the same period of storage are shown by values with distinct letters (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Antioxidant enzymes activity and carotenoid content
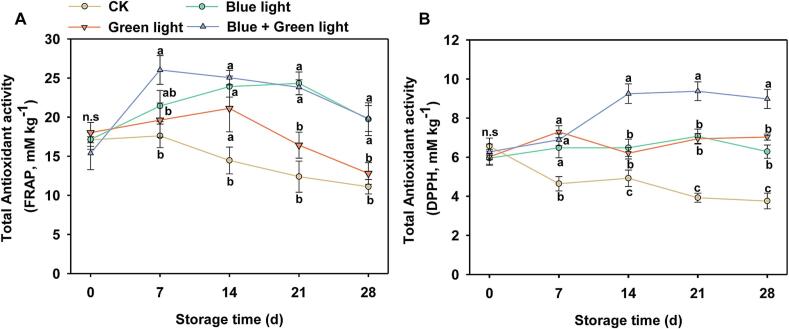
Additionally, blue-green light treatment inhibited the PPO enzyme activity but increased the POD, SOD, APX and CAT enzymes activities (Fig. 4A-E). During the storage, blue light and blue-green light treatments reduced the MDA content, while induced H2O2 content, compared to the dark control Chinese cabbage (Fig. 4F-G). Blue light treatment also increased the FRAP and DPPH activities, while the dark control showed the opposite trend in postharvest Chinese cabbage (Fig. 5A-B). After 28 d of blue-green light treatment, the development of leaf bulbs was almost complete, and the leaf bulbs were fine and healthy, while the control Chinese cabbage during dark storage showed wilting and yellowing (Fig. S3). Additionally, postharvest blue-green light treatment resulted in significant accumulation of carotenoid content, while the ROS (NBT stained area) was significantly lower than that of dark-treated Chinese cabbage for 21 d (Fig. S4). These results suggested that the blue and green combined light could delay pigment breakdown, increase antioxidant activity, and enhance the appearance quality of postharvest Chinese cabbage.
Fig. 4.
The effects of blue, green and blue-green light treatments of postharvest antioxidant enzyme activity in Chinese cabbage during storage. A. PPO activity. B. POD activity. C. SOD activity. D. APX activity. E. CAT activity. F. H2O2 content. G. MDA content. Statistical significant differences between treatment groups at the same period of storage are shown by values with distinct letters (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
The effects of blue, green, and blue-green light treatments of Chinese cabbage's postharvest total antioxidant capacity. A. FRAP activity. B. DPPH activity. Statistical significant differences between treatment groups at the same period of storage are shown by values with distinct letters (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. The GLS content
As shown in Fig. 6, the GLS content was found to be affected by postharvest light treatments. Overall, the GLS exhibited a decreasing trend during the postharvest stage. The total GLS and indolic GLS were induced rapidly within 7 d of blue light and green light irradiation and then showed a downward trend, while there was no significantly change in the GLS content of Chinese cabbage in 28 d (Fig. 6A-B). However, aliphatic GLS showed a fluctuating state during storage (Fig. 6C). Specifically, under blue-green light treatment, the content of 4MT reached its peak at 7 d, but no significant change was observed in both blue light treatment and dark-treated groups after 28 d (Fig. S5B). In a short period of 7 d, the blue light induced the accumulation of 2OH-4-Pentenyl, and 4-Pentyl (Fig. S5C—D). The pattern of accumulation of 5MT induced by blue light was similar to that of 4MT, reaching its peak at 7 d and then declined (Fig. S5E). Indole GLS, namely I3M, NMOI3M, and 4OHI3M of postharvest Chinese cabbage induced by blue light first increased and then decreased, while 4MOI3M exhibited dynamic fluctuations (Fig. S5F—I). These findings suggested that blue light and blue-green treatments initially increased the indolic GLS content of the postharvest Chinese cabbage, with extended storage time, while the GLS gradually decreased to the control level, and long-term treatment has minimal effect on the GLS content in postharvest Chinese cabbage.
Fig. 6.
The effects of blue, green and blue-green light treatments of postharvest GLS content during storage in Chinese cabbage. A. total GLS content. B. indolic GLS. C. aliphatic GLS. The indolic GLS have inducing 1-methoxyindol-3-ylmethyl (NMOI3M), Indol-3-ylmethyl (I3M), 4-methoxyindol-3-ylmethyl (4MOI3M), and 4-hydroxyindol-3-ylmethyl (4OHI3M). The aliphatic GLS includes (2R)-2-hydroxy-3-butenyl (progoitrin), 4-methylthiopentyl (4MT), 2-hydroxy-4-pentenyl (2-OH-4-pentenyl), 4-pentenyl (4-pentyl), and 5-methylthiopentyl (5MT). Statistical significant differences between treatment groups at the same period of storage are shown by values with distinct letters (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Gene expression patterns of GLSs, carotenoids and MEP pathways
Our study revealed that light signals, carotenoids and GLS-related genes were significantly induced by light treatments. Specifically, the BrHY5.2 was significantly induced by blue-green light at 7 and 21 d (Fig. 7A), while carotenoid metabolism-related genes BrPSY1, BrLCYB, BrLCYE, BrVDE, BrPDS, and BrZEP were induced and carotenoid degradation gene BrCCD4 was significantly repressed (Fig. 7B-H). Additionally, flavonoid synthesis genes BrCHS.1, BrF3H and BrCHI were significantly induced by green light and blue-green light, compared with the control stored in the dark (Fig. 7I-K). On the other hand, blue light induced BrCYP79B2 and BrIGMT1 of GLS pathway, while stress-related transcription factors were significantly induced by blue-green light treatment at postharvest 21 d in Chinese cabbage (Fig. 7L-P). These findings demonstrated that the synergistic application of blue-green light could significantly induce metabolic genes during the postharvest storage of Chinese cabbage, which corroborates our observations on metabolic and nutritional outcomes.
Fig. 7.
The effects of blue, green and blue-green light treatments of postharvest carotenoid, flavonoid, GLS metabolism-related gene and resistance-related gene expression pattern in Chinese cabbage during storage. A-H. The transcriptional level of carotenoid metabolism-related genes (BrHY5.2, BrPSY1, BrLCYB, BrLCYE, BrVDE, BrPDS, BrZEP, BrCCD4). I—K. The transcriptional level of flavonoid metabolism-related genes (BrCHS.1, BrF3H, BrCHI). L-M. The transcriptional level of GLS metabolism-related genes (BrCYP79B2, BrIGMT1). N—P. The transcriptional level of resistance-related genes (BrMYB4, BrPIF4, BrMYB12.1). Statistical significant differences between treatment groups at the same period of storage are shown by values with distinct letters (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Correlation analysis
To further explore the complex crosstalk among the content of GLS and pigments, antioxidant enzyme, and gene expression data in blue-green treated Chinese cabbage in postharvest stage, a comprehensive correlation analysis was presented in Fig. 8. Employing a principal component analysis (PCA) model, the first principal component (PC1) accounted for 35.29 % of the variance. The four different light-treatment groups can be roughly separated in the PC1 direction (Fig. 8A). During storage, we have observed a positive correlation between soluble solids and the antioxidant enzymes SOD (r = 0.89) and POD (r = 0.87). Additionally, I3M was significantly correlated positively between NMOI3M (r = 0.89), 4OHI3M (r = 0.96) and 5MT (r = 0.82). Furthermore, there was a significant positive correlation with 5MT and POD (r = 0.66), as well as between 4-PentyI and SOD (r = 0.59). The soluble sugar concentration was significantly negatively correlated with H2O2 (r = −0.92) and MDA (r = −0.72). Total flavonoids and carotenoids also showed negative correlations with H2O2 and MDA (Fig. 8B). These findings suggested that blue-green light combined treatment could induce a synergistic relationship among the accumulation of GLS substances, antioxidant enzymes, soluble sugars and pigments. The coordinated expression of pertinent structural genes and specific metabolic pathways mitigated ROS activity, thereby impeding the processes of aging, and yellowing in Chinese cabbage during postharvest storage.
Fig. 8.
Analysis of correlation between physiological indicators, reactive oxygen species metabolism, antioxidant activity, GLS metabolism, carotenoid, flavonoid, GLS metabolism-related gene and resistance-related gene in Chinese cabbage during storage for 21 d. A. Principal component analysis of 12 sample scores diagram from different light-treatment groups B. The correlation network diagram. When comparing the treatment group (blue, green, and blue-green light) to the control group (dark) during storage, asterisks indicate significant changes (*P < 0.05; **P < 0.01; *** P < 0.001). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
4.1. Blue-green light treatment significantly increased the quality of Chinese cabbage during postharvest storage
Post-harvest storage of Chinese cabbage often leads to a descend in nutrition, rotting and yellowing of outer leaves, as well as weight loss and loss of leaf bulbs. Blue light treatment is an effective tool to increase the soluble solids and total sugar content in postharvest vegetables and fruit, such as tomatoes (Dhakal and Baek, 2014a, Dhakal and Baek, 2014b) and strawberry fruit (Xu, Shi, et al., 2014). Conversely, darkness promotes senescence in leafy vegetables. Furthermore, the YI value of appearance quality initially increased, but then decreased (Fig. 2B), indicating that blue-green light treatment synergistically delays the deterioration of appearance quality during postharvest storage. Concurrently, the burst of ROS enhances the synthesis of carotenoids, leading to an increase in the yellow index of Chinese cabbage leaves. Throughout the 14–28 days storage period, the Chinese cabbage produces a plethora of antioxidant enzymes, such as POD, SOD, and APX (Fig. 4B-D). Our findings indicated that the NBT value after 21 days of blue light treatment was lower than that of the control, confirming the inhibitory effect on intracellular ROS (Fig. S4C). We hypothesize that under monochromatic blue light treatment, the photooxidative decomposition of carotenoids is accelerated during the later stages of Chinese cabbage during storage. These carotenoids are also consumed in large quantities as quenching agents for the burst of ROS, which is a significant contributor to the decline in the yellow index. Additionally, we observed that the increase in L* value during the later storage period of Chinese cabbage coincided with a decrease in the yellow index. The elevated L* value may be attributed to the maintenance of high levels of osmotic regulatory substances, such as soluble sugars and proteins, within the leaf cells. In this study, blue-green light treatment increased soluble protein, soluble sugar content (Fig. 3A-B), and improved its quality and nutritional value in postharvest Chinese cabbage. Moreover, compared to monochromatic blue or green light treatments, the combined blue-green light treatment is more stable, reducing the water loss rate (Fig. 3D), preserving a relatively stable internal leaf environment, and thereby extending the shelf life of postharvest Chinese cabbage. Additionally, we also observed that the induction of MDA and H2O2 content was mitigated by reducing water loss (Fig. 4F-G). The application of blue light treatment reduced the content of MDA, thereby extending the postharvest shelf life, as reported in fresh cut flowers in which blue light treatment significantly delayed senescence and improved vase life (Aalifar et al., 2020).
4.2. Blue-green light treatment reduces ROS levels in Chinese cabbage during postharvest storage
Plants have developed a sophisticated system of both enzymatic and non-enzymatic antioxidants to mitigate ROS produced during growth and development (Xu, Shi, et al., 2014). Here, postharvest Chinese cabbage was exposed to blue and blue-green light treatments, resulting in the accumulation of antioxidant enzymes such as POD, SOD, APX and CAT (Fig. 4), which could help to remove excess ROS produced during storage. In particular, the blue-green light treatment led to peak values of POD and CAT activity at 21 d (Fig. 4B, E), compared to postharvest Chinese cabbage treated with monochromatic blue light. At the same time, the rate of increase in SOD and APX activity under blue-green light treatment was higher than that of postharvest Chinese cabbage treated with monochromatic blue or green light in the early stage, and it maintained a high activity level throughout (Fig. 4C-D). APX plays an important role in catalyzing the decomposition of H2O2 into water. During the 21d storage period of Chinese cabbage, the application of blue-green light treatment resulted in a significant 23.85 % enhancement in catalase (CAT) activity in comparison to the monochromatic blue light treatment, as depicted in Fig. 4E. The elevated CAT levels are instrumental in the efficient decomposition of H2O2, thereby mitigating the oxidative damage to membrane lipids. Collectively, the blue-green light treatment was found to specifically induce a rapid increase in the activity of antioxidant enzymes in the initial stages of storage, followed by the maintenance of heightened antioxidant enzyme activity throughout the subsequent middle and late stages of storage. This effect was more pronounced when compared to the treatments involving monochromatic blue or green light, as well as the control group. The observed enhancement in antioxidant response may be attributed to the synergistic effects of blue and green light treatments. Blue light has been documented to swiftly activate the leaves of Chinese cabbage, thereby escalating the activity of antioxidant enzymes, a phenomenon that has been corroborated in various species as referenced in the literature (Chávez-Zaragoza et al., 2022; Jin et al., 2021). Furthermore, green light penetrates more deeply into the leaf cells, engaging in the photosynthetic reaction process and augmenting the absorption of green quanta, as elucidated by Palma et al. (2020). The immediate reversal of blue light-induced stomatal opening by green light, along with the reduction of water dissipation, is imperative for preserving the stability of intracellular enzyme activity. These findings underscore the potential of blue-green light treatment in enhancing the antioxidant defense mechanisms in Chinese cabbage during storage.
Previous studies have also demonstrated that blue light treatment could activate the POD, SOD and APX in fresh-cut amaranth (Amaranthus dubius L.) under 12 d, thereby enhances the quality and lengthen the shelf life of the postharvest food (Jin et al., 2021). LED lighting irradiation has also been demonstrated to reduce PPO activity and leaf browning levels in Chrysanthemum carinatum after 4 d of the storage (Zhou et al., 2019). In summary, the application of a blue and green light combination to postharvest Chinese cabbage is essential for preserving antioxidant enzyme activity. Future research will explore the underlying induction mechanisms through an integrative approach utilizing molecular biological techniques. Furthermore, the blue-green light irradiation after harvest significantly up-regulated the accumulation of TP and TF during the postharvest Chinese cabbage, which further delays the senescence of the leaves and extend the shelf life (Fig. 3E-F). Additionally, blue LED treatment has also been shown was significantly increased the concentration for total phenolic and antioxidant enzymes in strawberry fruit (Xu, Shi, et al., 2014). These findings were closely consistent with previous studies reporting higher levels of nutritional and bioactive compounds (e.g., ascorbic acid, carotenoids) and longer shelf life in spinach (Spinacia oleracea L.) after exposing to light treatment compared with those stored in darkness (Lester, Makus, & Hodges, 2010).
4.3. GLS metabolites in Chinese cabbage induced by blue-green light
The light treatment can improve vegetable quality and nutrition value by retaining the GLS content while also improving the appearance of leafy green vegetables in postharvest storage under light/dark cycles (Lee et al., 2016). Blue light has been reported to stimulate the GLS accumulation (Lee et al., 2016), and treatment of broccoli with blue light can enhance aliphatic GLS (Kopsell & Sams, 2013). Additionally, LED green light is more effective than other light sources in retaining high levels of bioactive compounds, such as GLS, in postharvest broccoli (Jin et al., 2015). After 7 days of treatment with blue and green light, the total indolic GLS content in Chinese cabbage increased by 32.45 %, followed by a decline (Fig. 6B). The results of this study also demonstrated that most indolic GLS (I3M, NMOI3M, 4MOI3M, 4OHI3M) and aliphatic GLS (4MT, 4-Pentyl) exhibited an increasing trend during the early storage period (Fig.S5). The combined treatment of blue and green light for 7 days resulted a significant increase of 32.45 % in the total GLS content in Chinese cabbage, followed by a subsequent decrease (Fig. 6B). Notably, the accumulation of specific indolic GLS, such as I3M, NMOI3M, and 4MOI3M, was enhanced by 16.02 %, 64.85 %, and 34.53 %, respectively (Fig. S5F—H). However, the inducing effect of the blue and green light combination on aliphatic GLS was not pronounced. The content of Progoitrin, 2-OH-4-Pentenyl, and 5MT decreased after 14 days of storage in Chinese cabbage (Fig. S5). The combined treatment of blue and green light significantly induced the accumulation of indolic GLS in Chinese cabbage, with increases of 16.02 %, 64.85 %, and 34.53 % for I3M, NMOI3M, and 4MOI3M, respectively (Fig. S5F—H). However, the effect on aliphatic GLS content was less pronounced. Studies have established an antagonistic relationship between the indolic and aliphatic branches of GLS biosynthesis (Mitreiter & Gigolashvili, 2021). Additionally, the metabolic allocation costs associated with the production of GLS may limit the induction effect on aliphatic GLS. Postharvest Chinese cabbage may synthesize a large amount of indolic GLS in response to light induction, and the accumulation of indolic GLS can effectively inhibit the occurrence of fungal and postharvest vegetable diseases. This may be applicable to the optimal defense theory in plants (Malhotra, Kumar, & Bisht, 2023), which helps to balance the adaptive costs of GLS synthesis. Furthermore, after 21 d of storage, there were no significant changes of GLS metabolites in both the treatment and the control groups (Fig. 6C), likely due to the degradation effects of GLS during the later stages of storage. Meanwhile, the biosynthesis of GLS consumingly depends on the supply of nitrogen and sulfur (Yan & Chen, 2007), and the sudden cessation of these nutrients during harvest may contribute to the reduction of the GLS content. Furthermore, during long-term storage of Chinese cabbage, the accumulation of MDA content leads to cell integrity loss and damage, which may expose myrosinase enzymes to the GLS by rupturing vacuoles, potentially explaining the rapid decline in GLS concentrations in the control group. In addition, blue-green light combination treatment has a certain effect on reducing the content of progoitrin, because progoitrin has been found to be the cause of bitter taste of Brassica vegetables (Cartea & Velasco, 2008), which also helps to reduce the bitterness of Chinese cabbage, thereby enhancing its taste appeal and potentially improving consumer acceptance. The study also found that GLS could be induced by blue-green light, and maintained high accumulation for 7 d during postharvest stage, suggesting that light-induced health-promoting GLS substances may further enhance the quality of postharvest Chinese cabbage.
For example, indole-3-carbinol (I3C), which produced from the breakdown of I3M, has the potential to prevent and treat various cancers, such as leukemia, breast and prostate cancer (Lin et al., 2022). Our findings highlighted the effectiveness of blue-green light treatment in promoting the accumulation of beneficial GLS in postharvest Chinese cabbage. Additionally, the accumulation of aliphatic GLS (4MT, 5MT and 4-PentyI) may also act as a deterrent for herbivorous insects and certain bacterial formation, making it a valuable bio-fumigant for agricultural production (Sotelo, Lema, Soengas, Cartea, & Velasco, 2015).
4.4. Molecular mechanisms of blue-green light treatment during the postharvest stage
Blue light greatly induced the transcription level of the BrHY5.2 gene throughout the 7-day postharvest storage period (Fig. 7A). Meanwhile, the BrHY5.2 gene may also activate downstream flavonoid metabolic pathways (Zhang et al., 2023). Furthermore, blue light greatly induced the transcription levels of mainly structural genes of the carotenoid metabolic pathway, such as BrPSY1 and BrLCYB (Fig. 7B-C), while decreasing the transcription levels of the carotenoid degradation gene BrCCD4. When compared with the dark-treated Chinese cabbage line, this resulted in a considerable buildup of carotenoids caused by blue-green light at 7 and 21 d (Fig. S4A). Recent studies have revealed that blue LEDs were also discovered to enhance the expression of genes likely PSY, LCYB and ZEP in the flavedo of satsuma mandarin. This upregulation of carotenogenic genes leads to an increase in the content of β-cryptoxanthin (Ma et al., 2012), demonstrating that blue light-mediated enhancement in carotenoid production may be attributed to the upregulation of key enzyme genes within the carotenoid metabolic pathway. The investigation also discovered induction of flavonoid biosynthesis-related genes, including the BrCHS.1, BrF3H, and BrCHI (Fig. 7I-K), which is consistent with the results of total flavonoid accumulation. It has also been reported that exposing postharvest strawberry fruit to blue light for 12 d increases flavonoid concentration, which were pivotal for the fruit's antioxidant properties (Xu et al., 2014). Furthermore, blue-green light treatment of postharvest Chinese cabbage induced GLS biosynthetic genes at 7 d, such as BrCYP79B2 and BrIGMT1 (Fig. 7L-M), the cytochrome P450 enzyme CYP79B2 catalyzing conversion of tryptophan to form indole-3-acetaldoxime, and leading to accumulation of GLS metabolites. Extant literature similarly indicates that the process of photoinduction positively influences the biosynthesis of GLS in broccoli (Kopsell & Sams, 2013). This effect is manifested through the activation of key enzymes involved in the GLS metabolic pathway, thereby enhancing the production of these phytochemicals known for their health-promoting attributes. To encapsulate the findings, the synergistic application of blue and green light markedly enhanced the expression levels of enzymes associated with postharvest metabolism of carotenoids, flavonoids, and glucosides, thereby facilitating the synthesis and accumulation of these metabolites during the early storage phase of Chinese cabbage, by enhancing the quality and nutritional value of postharvest Chinese cabbage while mitigating damage caused by ROS during storage.
The maintenance of total antioxidant capacity in plants relies on the synergistic action of various antioxidant enzymes and non-enzymatic antioxidant compounds (Xu, Shi, et al., 2014). This study identified significant positive correlations between soluble sugars, soluble proteins, carotenoids and GLS composition (I3M, 5MT, 4OHI3M), as well as negative correlations with H2O2 and MDA induced by blue-green light (Fig. 8B). Monochromatic blue light treatment alone enhances the activities of soluble sugars, soluble proteins, and antioxidant enzymes in postharvest vegetable crops. However, it also leads to stomatal opening in leaves, increasing the water loss rate and diminishing the marketable quality of postharvest vegetables. In contrast, the combined treatment of blue and green light exhibits a synergistic effect, with green light penetrating more deeply into the mesophyll, enhancing the photosynthetic carbon assimilation of inner mesophyll cells (Palma et al., 2020). Ultimately, the blue-green light combined treatment effectively activated the antioxidant enzyme system and increased the levels of non-enzymatic antioxidants, including GLS, total phenols, total flavonoids, and carotenoids. This treatment, compared to monochromatic blue or green light treatments, also effectively inhibited the surge of reactive oxygen species, corroborating the negative correlations with MDA and H2O2. The reduction in MDA and H2O2 content is instrumental in mitigating membrane lipid peroxidation and cellular damage, thereby significantly enhancing the storage value of Chinese cabbage. There are a few possible reasons given below: polyphenols and flavonoids can increase the stability of carotenoids; antioxidant properties of carotenoids include scavenging 1O2 and generating heat as a by-product; flavonoids in scavenging 1O2 and alleviate the damages caused to the outer envelope of the chloroplast membranes; indole GLS are also directly involved in the process of scavenging ROS (Cabello-Hurtado, Gicquel, & Esnault, 2012), possibly by regulating glutathione sulfotransferase and thioredoxin reductase to clear free radicals and block the peroxide chain reaction. These findings suggested that multiple metabolites worked together to effectively manage the H2O2 burst that occurs during postharvest storage of Chinese cabbage, providing strong defense mechanisms against oxidative damage. Considering the multifaceted and nuanced nature of photoinduced secondary metabolic pathways, subsequent investigative efforts will be concentrated on dissecting the molecular underpinnings of the interactions between photoresponsive regulatory factors and the array of secondary metabolites.
5. Conclusion
Here, the combined treatment of blue and green light was found to effectively delay the aging of postharvest Chinese cabbage, while also greatly delaying the loss in quality and prolonging the shelf life of the vegetable. This was achieved by preventing weight loss, leaf yellowing, and protein degradation during the storage period of the Chinese cabbage. Additionally, the blue-green light treatment was observed to enhance the levels of DPPH and FRAP, as well as to stimulate the accumulation of several antioxidant enzymes, including CAT, POD, SOD, and APX. The induction of antioxidant capacity has the potential to improve nutritional quality and promote human health. Comparatively, green light treatment was found to be less effective than blue-green light treatment, which could induce light signal related genes, secondary metabolic biosynthesis related gene expression, enzyme activity, and biosynthesis of antioxidant compounds, including I3M, NMOI3M, 4MOI3M, carotenoids, and flavonoids during storage. Our work added to the current understanding of how combined blue-green light treatment could be improved the post-harvest Chinese cabbage quality.
CRediT authorship contribution statement
Ruixing Zhang: Writing – review & editing, Writing – original draft, Validation, Software, Resources, Formal analysis, Data curation, Conceptualization. Qianqian He: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Data curation, Conceptualization. Qiming Pan: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Investigation. Yizhe Feng: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Yu Shi: Writing – original draft, Supervision, Software, Methodology, Formal analysis. Gaizhen Li: Writing – original draft, Methodology, Investigation, Data curation. Yi Zhang: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation. Yulin Liu: Writing – review & editing, Writing – original draft, Visualization, Supervision, Software, Methodology, Investigation, Formal analysis, Conceptualization. Abid Khan: Writing – original draft, Supervision, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was supported by the Doctoral Research Starting Project at Shanxi Agricultural University (2024BQ10), the Basic Research Program of Shanxi Province (202403021212306), the Taiyuan Comprehensive Experimental Station of the National Bulk Vegetable Industry Technology System (CARS-23-G09), and the Earmarked Fund for Shanxi Modern Agro-industry Technology Research System (2024CYJSTX08).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.102004.
Contributor Information
Ruixing Zhang, Email: zrxlyl@sxau.edu.cn.
Qianqian He, Email: 20231033@stu.sxau.edu.cn.
Qiming Pan, Email: 2020055340@nwsuaf.edu.cn.
Yizhe Feng, Email: 20232214@stu.sxau.edu.cn.
Yu Shi, Email: shiyu@sxau.edu.cn.
Yi Zhang, Email: yyzy@sxau.edu.cn.
Yulin Liu, Email: zrxnihao@163.com.
Abid Khan, Email: khanabid@uoh.edu.pk.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
Data availability
Data will be made available on request.
References
- Aalifar M., Aliniaeifard S., Arab M., Zare Mehrjerdi M., Dianati Daylami S., Serek M., et al. Blue light improves vase life of carnation cut flowers through its effect on the antioxidant defense system. Frontiers in Plant Science. 2020;11:511. doi: 10.3389/fpls.2020.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105(C):121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72(1–2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cabello-Hurtado F., Gicquel M., Esnault M.-A. Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chemistry. 2012;132(2):1003–1009. doi: 10.1016/j.foodchem.2011.11.086. [DOI] [Google Scholar]
- Cartea M.E., Velasco P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochemistry Reviews. 2008;7(2):213–229. doi: 10.1007/s11101-007-9072-2. [DOI] [Google Scholar]
- Chávez-Zaragoza K., Morales-Guerrero A., Colín-Chávez C., Tovar-Díaz L., Ornelas-Paz J.D.J., Osuna-Castro J.A., et al. Improving the nutraceutical value of mango during ripening by postharvest irradiation with blue LEDs via enhancing of antioxidant enzyme activities. International Journal of Food Science & Technology. 2022;57(4):2498–2509. doi: 10.1111/ijfs.15623. [DOI] [Google Scholar]
- Dhakal R., Baek K.H. Short period irradiation of single blue wavelength light extends the storage period of mature green tomatoes. Postharvest Biology and Technology. 2014;90:73–77. doi: 10.1016/j.postharvbio.2013.12.007. [DOI] [Google Scholar]
- Dhakal R., Baek K.-H. Metabolic alternation in the accumulation of free amino acids and γ-aminobutyric acid in postharvest mature green tomatoes following irradiation with blue light. Horticulture, Environment, and Biotechnology. 2014;55(1):36–41. doi: 10.1007/s13580-014-0125-3. [DOI] [Google Scholar]
- Feng X.H., Zhang H.X., Ali M., Gai W.X., Cheng G.X., Yu Q.H., et al. A small heat shock protein CaHsp25.9 positively regulates heat, salt, and drought stress tolerance in pepper (Capsicum annuum L.) Plant Physiology and Biochemistry. 2019;142:151–162. doi: 10.1016/j.plaphy.2019.07.001. [DOI] [PubMed] [Google Scholar]
- He Q., Yang H., Wu L., Hu C. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresource Technology. 2015;191:219–228. doi: 10.1016/j.biortech.2015.05.021. [DOI] [PubMed] [Google Scholar]
- He Q., Zhang Z., Zhang L. Anthocyanin accumulation, antioxidant ability and stability, and a transcriptional analysis of anthocyanin biosynthesis in purple heading Chinese cabbage (Brassica rapa L. ssp. pekinensis) Journal of Agricultural and Food Chemistry. 2016;64(1):132–145. doi: 10.1021/acs.jafc.5b04674. [DOI] [PubMed] [Google Scholar]
- Izzo L.G., Hay Mele B., Vitale L., Vitale E., Arena C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environmental and Experimental Botany. 2020;179 doi: 10.1016/j.envexpbot.2020.104195. [DOI] [Google Scholar]
- Jin P., Yao D., Xu F., Wang H., Zheng Y. Effect of light on quality and bioactive compounds in postharvest broccoli florets. Food Chemistry. 2015;172:705–709. doi: 10.1016/j.foodchem.2014.09.134. [DOI] [PubMed] [Google Scholar]
- Jin S., Ding Z., Xie J. Study of postharvest quality and antioxidant capacity of freshly cut amaranth after blue LED light treatment. Plants (Basel) 2021;10(8):1614. doi: 10.3390/plants10081614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing G.X., Li T.T., Qu H.X., Yun Z., Jia Y.X., Zheng X.L., Jiang Y.M. Carotenoids and volatile profiles of yellow- and red-fleshed papaya fruit in relation to the expression of carotenoid cleavage dioxygenase genes. Postharvest Biology and Technology. 2015;109:114–119. doi: 10.1016/j.postharvbio.2015.06.006. [DOI] [Google Scholar]
- Kliebenstein D.J., Kroymann J., Brown P., Figuth A., Pedersen D., Gershenzon J., Mitchell-Olds T. Genetic control of natural variation in arabidopsis glucosinolate accumulation. Plant Physiology. 2001;126(2):811–825. doi: 10.1104/pp.126.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopsell D.A., Sams C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. Journal of the American Society for Horticultural Science. 2013;138(1):31–37. doi: 10.21273/Jashs.138.1.31. [DOI] [Google Scholar]
- Lee M.K., Arasu M.V., Park S., Byeon D.H., Chung S.O., Park S.U., et al. LED lights enhance metabolites and antioxidants in Chinese cabbage and kale. Brazilian Archives of Biology and Technology. 2016;59 ARTN e1615054610doi:1590/1678-4324-2016150546. [Google Scholar]
- Lee N.Y., Lee M.J., Kim Y.K., Park J.C., Park H.K., Choi J.S., et al. Effect of light emitting diode radiation on antioxidant activity of barley leaf. Journal of Korean Society for Applied Biological Chemistry. 2010;53(6):685–690. doi: 10.3839/jksabc.2010.104. [DOI] [Google Scholar]
- Lee Y.J., Ha J.Y., Oh J.E., Cho M.S. The effect of LED irradiation on the quality of cabbage stored at a low temperature. Food Science and Biotechnology. 2014;23(4):1087–1093. doi: 10.1007/s10068-014-0149-6. [DOI] [Google Scholar]
- Lester G.E., Makus D.J., Hodges D.M. Relationship between fresh-packaged spinach leaves exposed to continuous light or dark and bioactive contents: Effects of cultivar, leaf size, and storage duration. Journal of Agricultural and Food Chemistry. 2010;58(5):2980–2987. doi: 10.1021/jf903596v. [DOI] [PubMed] [Google Scholar]
- Lin L.P., Liu D., Qian J.C., Wu L., Zhao Q., Tan R.X. Post-ingestion conversion of dietary indoles into anticancer agents. National Science Review. 2022;9(4) doi: 10.1093/nsr/nwab144. nwab144ARTN nwab144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G., Zhang L., Kato M., Yamawaki K., Kiriiwa Y., Yahata M., Ikoma Y., Matsumoto H. Effect of blue and red LED light irradiation on β-cryptoxanthin accumulation in the flavedo of citrus fruits. Journal of Agricultural and Food Chemistry. 2012;60:197–201. doi: 10.1021/jf203364m. [DOI] [PubMed] [Google Scholar]
- Ma Y.R., Wang H.Y., Yan H., Malik A.U., Dong T.T., Wang Q.G. Pre-cut NaCl solution treatment effectively inhibited the browning of fresh-cut potato by influencing polyphenol oxidase activity and several free amino acids contents. Postharvest Biology and Technology. 2021;178 doi: 10.1016/j.postharvbio.2021.111543. ARTN 111543. [DOI] [Google Scholar]
- Malhotra B., Kumar P., Bisht N.C. Defense versus growth trade-offs: Insights from glucosinolates and their catabolites. Plant, Cell & Environment. 2023;46(10):2964–2984. doi: 10.1111/pce.14462. [DOI] [PubMed] [Google Scholar]
- Mitreiter S., Gigolashvili T. Regulation of glucosinolate biosynthesis. Journal of Experimental Botany. 2021;72(1):70–91. doi: 10.1093/jxb/eraa479. [DOI] [PubMed] [Google Scholar]
- Palma C.F.F., Castro-Alves V., Morales L.O., Rosenqvist E., Ottosen C.O., Strid A. Spectral composition of light affects sensitivity to UV-B and photoinhibition in cucumber. Frontiers in Plant Science. 2020;11 doi: 10.3389/fpls.2020.610011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirie A., Mullins M.G. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiology. 1976;58(4):468–472. doi: 10.1104/pp.58.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo T., Lema M., Soengas P., Cartea M.E., Velasco P. In vitro activity of glucosinolates and their degradation products against Brassica-pathogenic bacteria and fungi. Applied and Environmental Microbiology. 2015;81(1):432–440. doi: 10.1128/Aem.03142-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott L.D., Nikolova G., Ortiz A., Shmayevich I., Zeiger E. Green light reversal of blue-light-stimulated stomatal opening is found in a diversity of plant species. American Journal of Botany. 2002;89(2):366–368. doi: 10.3732/ajb.89.2.366. [DOI] [PubMed] [Google Scholar]
- Thilini Deepashika Perera W.P., Navaratne S., Wickramasinghe I. Impact of spectral composition of light from light-emitting diodes (LEDs) on postharvest quality of vegetables: A review. Postharvest Biology and Technology. 2022;191 doi: 10.1016/j.postharvbio.2022.111955. [DOI] [Google Scholar]
- Wang T., Liu S., Tian S., Ma T., Wang W. Light regulates chlorophyll biosynthesis via ELIP1 during the storage of Chinese cabbage. Scientific Reports. 2022;12(1):11098. doi: 10.1038/s41598-022-15451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.Y., Xu X.M., Cui J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica. 2015;53(2):213–222. doi: 10.1007/s11099-015-0083-8. [DOI] [Google Scholar]
- Xie C., Tang J., Xiao J., Geng X., Guo L. Purple light-emitting diode (LED) lights controls chlorophyll degradation and enhances nutraceutical quality of postharvest broccoli florets. Scientia Horticulturae. 2022;294 doi: 10.1016/j.scienta.2021.110768. [DOI] [Google Scholar]
- Xu F., Cao S., Shi L., Chen W., Su X., Yang Z. Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. Journal of Agricultural and Food Chemistry. 2014;62(20):4778–4783. doi: 10.1021/jf501120u. [DOI] [PubMed] [Google Scholar]
- Xu F., Shi L.Y., Chen W., Cao S.F., Su X.G., Yang Z.F. Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Scientia Horticulturae. 2014;175:181–186. doi: 10.1016/j.scienta.2014.06.012. [DOI] [Google Scholar]
- Yan W., Sheng H., Cheng F.W., Nan Z.Q., De W.F., Wei Q.N. A new method for fast determination of total soluble sugar content in plant tissue: TBA-method. Journal of Jinggangshan University(Natural Science) 2013;34(3):37–40. doi: 10.3969/j.issn.1674-8085.2013.03.009. [DOI] [Google Scholar]
- Yan X., Chen S. Regulation of plant glucosinolate metabolism. Planta. 2007;226(6):1343–1352. doi: 10.1007/s00425-007-0627-7. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang Y., Gao Y., Jiang X.L., Zhang M.D., Wu H., et al. Effects of histone deacetylase inhibitors on microspore embryogenesis and plant regeneration in Pakchoi (Brassica rapa ssp chinensis L.) Scientia Horticulturae. 2016;209:61–66. doi: 10.1016/j.scienta.2016.05.001. [DOI] [Google Scholar]
- Zhang R., Liu Y., Pan Q., Khan A., Bai X., Ali M., et al. The effects of short term blue light treatment on promoting nutrition value in Chinese cabbage. Food Chemistry. 2023;412 doi: 10.1016/j.foodchem.2023.135542. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li S., Deng M., Gui R., Liu Y., Chen X., et al. Blue light combined with salicylic acid treatment maintained the postharvest quality of strawberry fruit during refrigerated storage. Food Chemistry: X. 2022;15 doi: 10.1016/j.fochx.2022.100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.H., Gu S.T., Zuo J.H., Gao L.P., Wang Q., Jiang A.L. LED irradiation delays the postharvest senescence of garland chrysanthemum (chrysanthemum carinatum Schousb.) Journal of Food Measurement and Characterization. 2019;13(4):3005–3014. doi: 10.1007/s11694-019-00221-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Data Availability Statement
Data will be made available on request.