Abstract
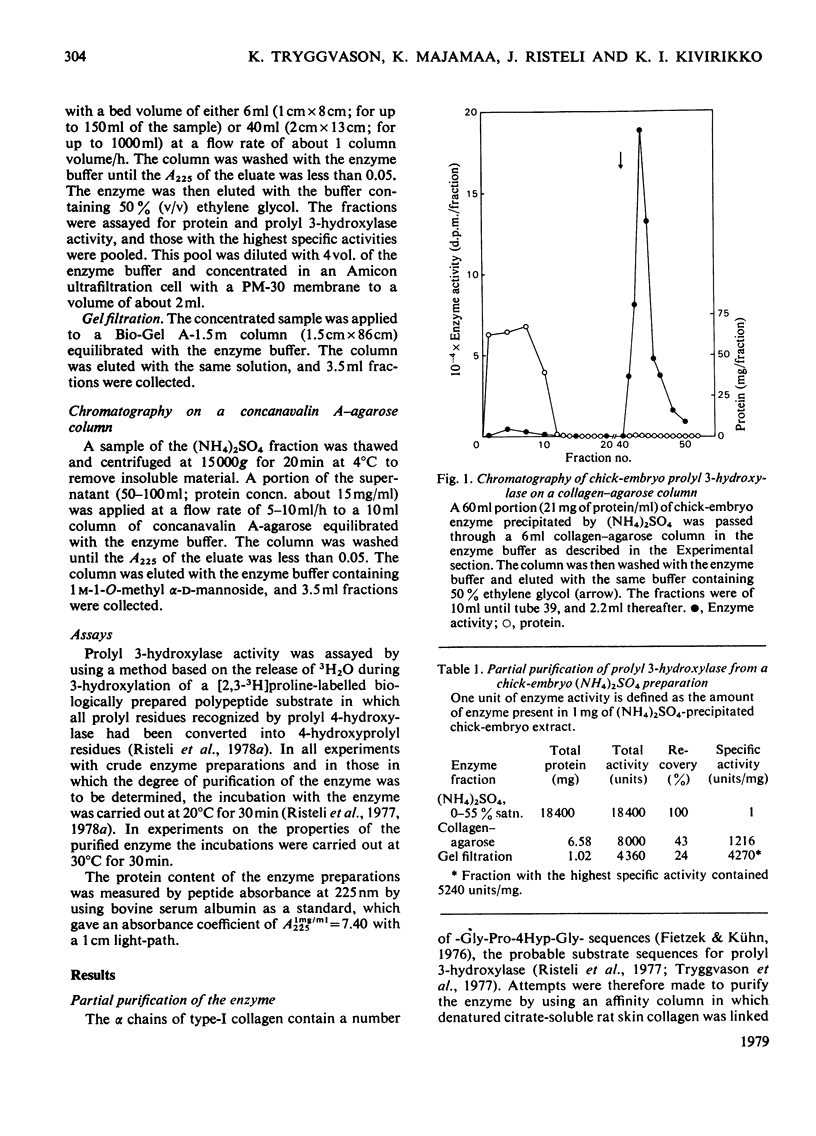
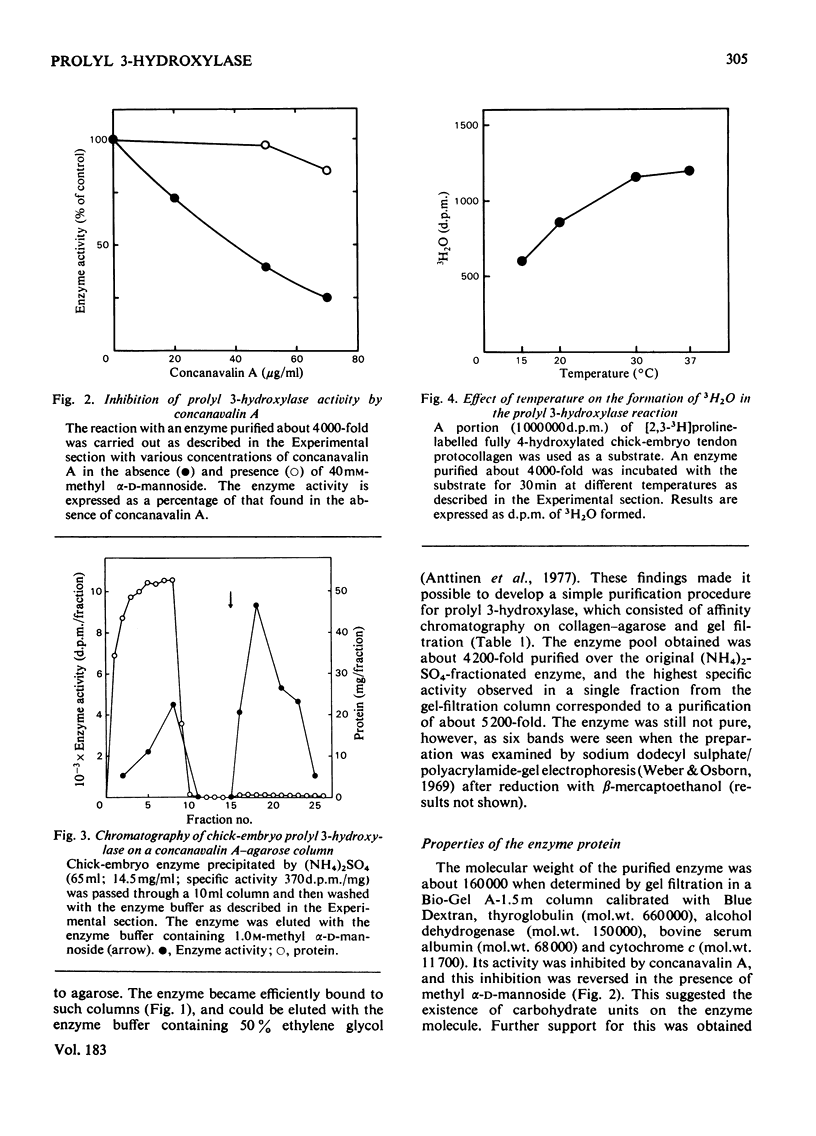
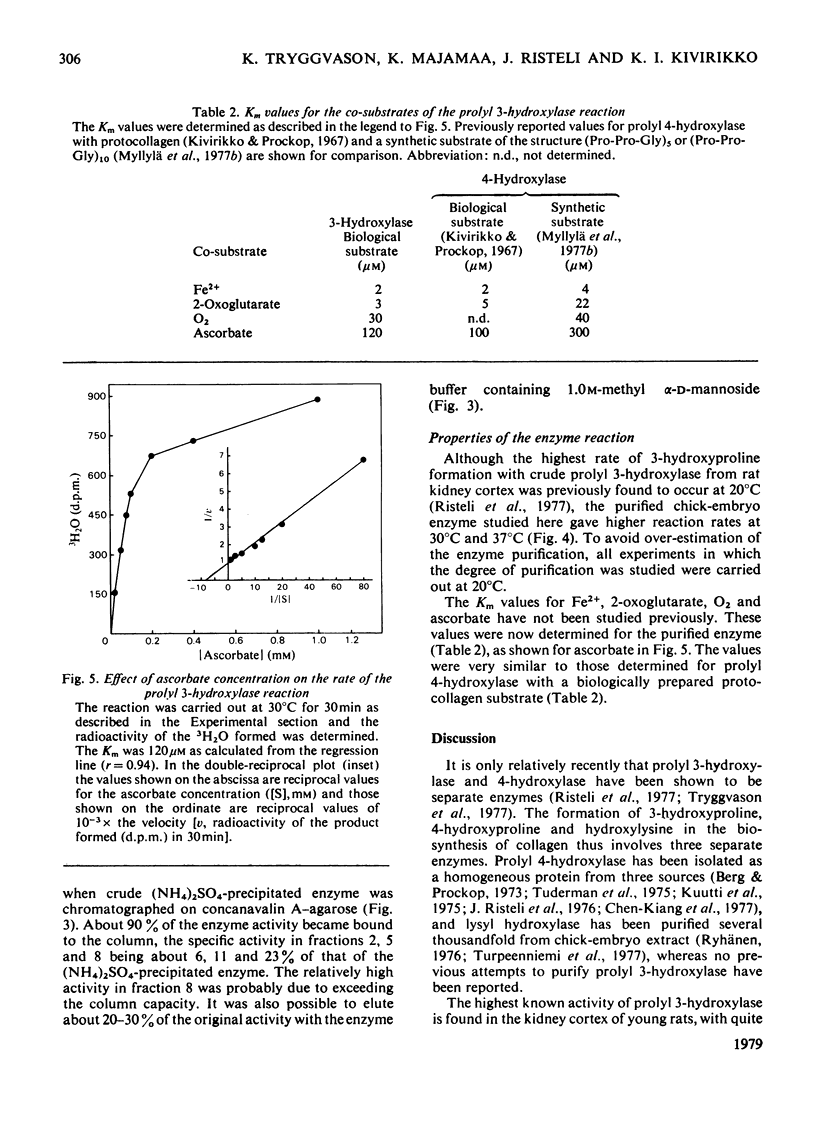
Prolyl 3-hydroxylase was purified up to about 5000-fold from an (NH4)2SO4 fraction of chick-embryo extract by a procedure consisting of affinity chromatography on denatured collagen linked to agarose, elution with ethylene glycol and gel filtration. The molecular weight of the purified enzyme is about 160000 by gel filtration The enzyme is probably a glycoprotein, since (a) its activity is inhibited by concanavalin A, and (b) the enzyme is bound to columns of this lectin coupled to agarose and can be eluted with a buffer containing methyl alpha-D-mannoside. The Km values for Fe2+, 2-oxoglutarate, O2 and ascorbate in the prolyl 3-hydroxylase reaction were found to be very similar to those previously reported for these co-substrates in the prolyl 4-hydroxylase and lysyl hydroxylase reactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttinen H., Myllylä R., Kivirikko K. I. Hydrophobic and carbohydrate-recognition chromatographies of collagen glucosyltransferase. Eur J Biochem. 1977 Aug 15;78(1):11–17. doi: 10.1111/j.1432-1033.1977.tb11708.x. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Chen-Kiang S., Cardinale G. J., Udenfriend S. Homology between a prolyl hydroxylase subunit and a tissue protein that crossreacts immunologically with the enzyme. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4420–4424. doi: 10.1073/pnas.74.10.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietzek P. P., Kühn K. The primary structure of collagen. Int Rev Connect Tissue Res. 1976;7:1–60. doi: 10.1016/b978-0-12-363707-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Guzman N. A., Berg R. A., Prockop D. J. Concanavalin A binds of purified prolyl hydroxylase and partially inhibits its enzymic activity. Biochem Biophys Res Commun. 1976 Nov 22;73(2):279–285. doi: 10.1016/0006-291x(76)90704-x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Enzymatic hydroxylation of proline and lysine in protocollagen. Proc Natl Acad Sci U S A. 1967 Mar;57(3):782–789. doi: 10.1073/pnas.57.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Partial purification and characterization of protocollagen lysine hydroxylase from chick embryos. Biochim Biophys Acta. 1972 Feb 28;258(2):366–379. doi: 10.1016/0005-2744(72)90228-8. [DOI] [PubMed] [Google Scholar]
- Kuutti E. R., Tuderman L., Kivirikko K. I. Human prolyl hydroxylase. Purification, partial characterization and preparation of antiserum to the enzyme. Eur J Biochem. 1975 Sep 1;57(1):181–188. doi: 10.1111/j.1432-1033.1975.tb02289.x. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Anttinen H., Risteli L., Kivirikko K. I. Isolation of collagen glucosyltransferase as a homogeneous protein from chick embryos. Biochim Biophys Acta. 1977 Jan 11;480(1):113–121. doi: 10.1016/0005-2744(77)90326-6. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Tuderman L., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 2. Kinetic analysis of the reaction sequence. Eur J Biochem. 1977 Nov 1;80(2):349–357. doi: 10.1111/j.1432-1033.1977.tb11889.x. [DOI] [PubMed] [Google Scholar]
- Pänkäläinen M., Aro H., Simons K., Kivirikko K. I. Protocollagen proline hydroxylase: molecular weight, subunits and isoelectric point. Biochim Biophys Acta. 1970 Dec 22;221(3):559–565. doi: 10.1016/0005-2795(70)90227-8. [DOI] [PubMed] [Google Scholar]
- Risteli J., Tryggvason K., Kivirikko K. I. A rapid assay for prolyl 3-hydroxylase activity. Anal Biochem. 1978 Feb;84(2):423–431. doi: 10.1016/0003-2697(78)90060-x. [DOI] [PubMed] [Google Scholar]
- Risteli J., Tryggvason K., Kivirikko K. I. Prolyl 3-hydroxylase: partial characterization of the enzyme from rat kidney cortex. Eur J Biochem. 1977 Mar 1;73(2):485–492. doi: 10.1111/j.1432-1033.1977.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Risteli J., Tuderman L., Kivirikko K. I. Intracellular enzymes of collagen biosynthesis in rat liver as a function of age and in hepatic injury induced by dimethylnitrosamine. Purification of rat prolyl hydroxylase and comparison of changes in prolyl hydroxylase activity with changes in immunoreactive prolyl hydroxylase. Biochem J. 1976 Aug 15;158(2):369–376. doi: 10.1042/bj1580369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli J., Tuderman L., Tryggvason K., Kivirikko K. I. Effect of hepatic injury on prolyl 3-hydroxylase and 4-hydroxylase activities in rat liver and on immunoreactive prolyl 4-hydroxylase concentrations in the liver and serum. Biochem J. 1978 Jan 15;170(1):129–135. doi: 10.1042/bj1700129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli L. Further characterization of collagen galactosyltransferase from chick embryos. Biochem J. 1978 Jan 1;169(1):189–196. doi: 10.1042/bj1690189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli L., Myllylä R., Kivirikko K. I. Affinity chromatography of collagen glycosyltransferases on collagen linked to agarose. Eur J Biochem. 1976 Aug 1;67(1):197–202. doi: 10.1111/j.1432-1033.1976.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Ryhänen L. Lysyl hydroxylase. Further purification and characterization of the enzyme from chick embryos and chick embryo cartilage. Biochim Biophys Acta. 1976 Jun 7;438(1):71–89. doi: 10.1016/0005-2744(76)90224-2. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Majamaa K., Kivirikko K. I. Prolyl 3-hydroxylase and 4-hydroxylase activities in certain rat and chick-embryo tissues and age-related changes in their activities in the rat. Biochem J. 1979 Jan 15;178(1):127–131. doi: 10.1042/bj1780127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryggvason K., Risteli J., Kivirikko K. I. Glomerular basement membrane collagen and activities of the intracellular enzymes of collagen biosynthesis in congenital nephrotic syndrome of the Finnish type. Clin Chim Acta. 1978 Jan 16;82(3):233–240. doi: 10.1016/0009-8981(78)90005-0. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Risteli J., Kivirikko K. I. Separation of prolyl 3-hydroxylase and 4-hydroxylase activities and the 4-hydroxyproline requirement for synthesis of 3-hydroxyproline. Biochem Biophys Res Commun. 1976 May 23;76(2):275–281. doi: 10.1016/0006-291x(77)90722-7. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Turpeenniemi T. M., Puistola U., Anttinen H., Kivirikko K. I. Affinity chromatography of lysyl hydroxylase on concanavalin A-agarose. Biochim Biophys Acta. 1977 Jul 8;483(1):215–219. doi: 10.1016/0005-2744(77)90024-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



