Abstract
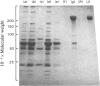
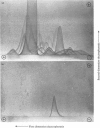
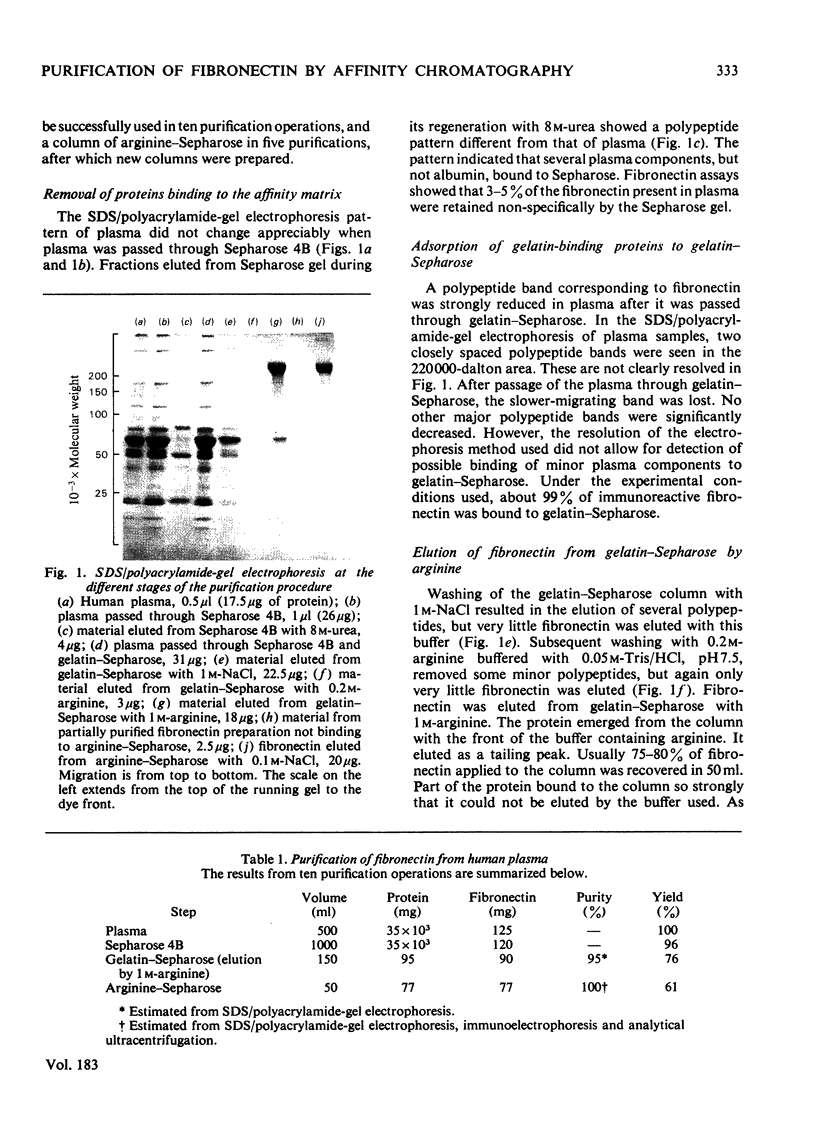
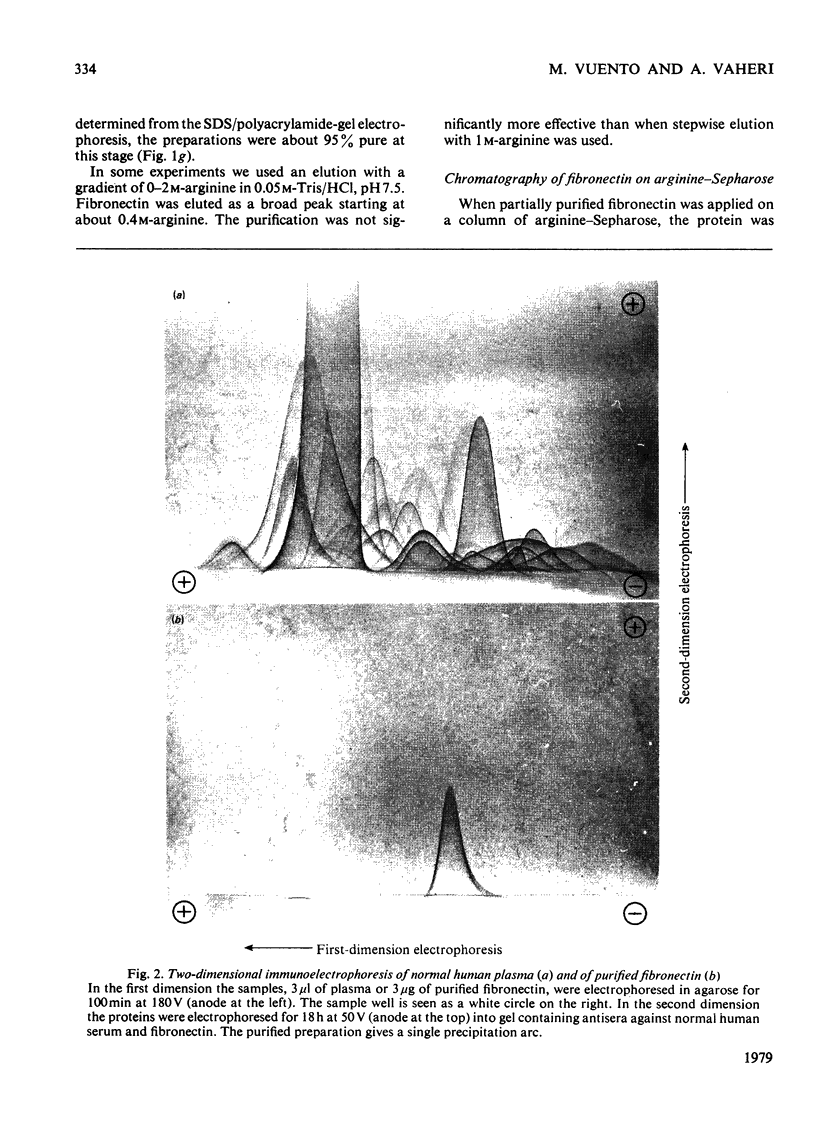
Fibronectin was purified from human plasma by affinity chromatography under nondenaturing conditions. The method was based on the previously known binding of fibronectin to gelatin. The novel features of our method are the use of arginine in the elution of fibronectin from immobilized gelatin [Vuento & Vaheri (1978) Biochem. J. 175, 333-336] and the use of arginine-agarose as second affinity step. The purified protein was homogeneous as judged by polyacrylamide-gel electrophoresis, analytical ultracentrifugation and two-dimensional immunoelectrophoresis. The yield was 60%. We propose that the method would be useful in preparation of fibronectin for studies on its biological activities, where it is important that the protein is obtained in a native state.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenstock F. A., Saba T. M., Weber P., Laffin R. Biochemical and immunological characterization of human opsonic alpha2SB glycoprotein: its identity with cold-insoluble globulin. J Biol Chem. 1978 Jun 25;253(12):4287–4291. [PubMed] [Google Scholar]
- Chen A. B., Mosesson M. W. An improved method for purification of the cold-insoluble globulin of human plasma (CLg). Anal Biochem. 1977 May 1;79(1-2):144–151. doi: 10.1016/0003-2697(77)90388-8. [DOI] [PubMed] [Google Scholar]
- Clarke H. G., Freeman T. Quantitative immunoelectrophoresis of human serum proteins. Clin Sci. 1968 Oct;35(2):403–413. [PubMed] [Google Scholar]
- Dessau W., Jilek F., Adelmann B. C., Hörmann H. Similarity of antigelatin factor and cold insoluble globulin. Biochim Biophys Acta. 1978 Mar 28;533(1):227–237. doi: 10.1016/0005-2795(78)90566-4. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hedman K., Vaheri A., Wartiovaara J. External fibronectin of cultured human fibroblasts is predominantly a matrix protein. J Cell Biol. 1978 Mar;76(3):748–760. doi: 10.1083/jcb.76.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga S., Suzuki K., Hashimoto S. Bovine plasma cold-insoluble globulin: gross structure and function. Ann N Y Acad Sci. 1978 Jun 20;312:56–73. doi: 10.1111/j.1749-6632.1978.tb16793.x. [DOI] [PubMed] [Google Scholar]
- Jilek F., Hörmann H. Cold-insoluble globulin, II. Plasminolysis of cold-insoluble globulin. Hoppe Seylers Z Physiol Chem. 1977 Jan;358(1):133–136. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linder E., Stenman S., Lehto V. P., Vaheri A. Distribution of fibronectin in human tissues and relationship to other connective tissue components. Ann N Y Acad Sci. 1978 Jun 20;312:151–159. doi: 10.1111/j.1749-6632.1978.tb16800.x. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Mosher D. F. Action of fibrin-stabilizing factor on cold-insoluble globulin and alpha2-macroglobulin in clotting plasma. J Biol Chem. 1976 Mar 25;251(6):1639–1645. [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Dissociation of fibronectin from gelatin-agarose by amino compounds. Biochem J. 1978 Oct 1;175(1):333–336. doi: 10.1042/bj1750333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Wrann M., Ruoslahti E. Similarity of fibronectins isolated from human plasma and spent fibroblast culture medium. FEBS Lett. 1977 Oct 15;82(2):227–231. doi: 10.1016/0014-5793(77)80590-5. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]