Abstract
Purpose
To further define anatomic criteria for resection and ablation using an expert panel–based three-dimensional liver model to objectively predict local treatment recommendations for colorectal liver metastases (CRLM).
Materials and Methods
This study analyzed data from participants with small CRLM (≤3 cm) considered suitable for resection, thermal ablation, or irreversible electroporation (IRE), according to a multidisciplinary expert panel, who were included in two prospective multicenter trials (COLLISION [NCT03088150] and COLDFIRE-2 [NCT02082782]) between August 2017 and June 2022. Ten randomly selected participants were used to standardize the model’s Couinaud segments. CRLM coordinates were measured and plotted in the model as color-coded lesions according to the treatment recommendations. Statistical validation was achieved through leave-one-out cross-validation.
Results
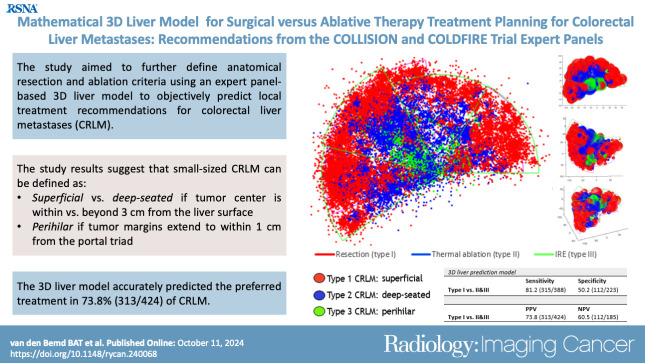
A total of 611 CRLM in 202 participants (mean age, 63 [range, 29–87] years; 138 male and 64 female) were included. Superficially located CRLM were considered suitable for resection, whereas more deep-seated CRLM were preferably ablated, with the transition zone at a subsurface depth of 3 cm. Ninety-three percent (25 of 27) of perihilar CRLM treated with IRE were at least partially located within 1 cm from the portal triad. Use of the model correctly predicted the preferred treatment in 313 of 424 CRLM (73.8%).
Conclusion
The results suggest that CRLM can be defined as superficial (preferably resected) and deep-seated (preferably ablated) if the tumor center is within versus beyond 3 cm from the liver surface, respectively, and as perihilar if the tumor margins extend to within 1 cm from the portal triad.
Keywords: Ablation Techniques, CT, MRI, Liver, Abdomen/GI, Metastases, Oncology
Supplemental material is available for this article.
© RSNA, 2024
Keywords: Ablation Techniques, CT, MRI, Liver, Abdomen/GI, Metastases, Oncology
Summary
A mathematical three-dimensional treatment prediction model, developed through the input of a multidisciplinary expert panel, performed well in categorizing treatment options for small (≤3 cm) colorectal liver metastases based on their anatomic location.
Key Points
■ In a study of 202 participants with 611 colorectal liver metastases (CRLM), the calculated transition zone between preferably resected superficial CRLM and preferably ablated deep-seated CRLM was 3 cm from the liver surface.
■ Ninety-three percent of CRLM for which irreversible electroporation was preferred were at least partially located within 1 cm from the portal triad.
■ Use of the three-dimensional liver model accurately predicted the preferred treatment in 73.8% (313 of 424) of small CRLM.
Introduction
The 2020 global cancer burden status report estimated more than 1.9 million new cases of colorectal cancer and 935 000 deaths, making colorectal cancer the second leading cause of cancer-related death worldwide (1–3). Roughly half of patients with colorectal cancer develop colorectal liver metastases (CRLM) (4–6), yet only a minority (10%–15%) can undergo curative-intent partial hepatectomy (7,8). Widely adopted alternative treatment options include heat-based thermal ablation techniques, such as radiofrequency ablation and microwave ablation, and non–heat-based techniques, such as irreversible electroporation (IRE), stereotactic ablative radiation therapy, histotripsy, high-intensity focused ultrasound, and percutaneous ethanol injection (9–13).
In previously published systematic reviews and meta-analyses, partial hepatectomy was considered superior to ablation in terms of overall survival, and, as a result, most national consensus guidelines state that thermal ablation should be reserved for unresectable disease (14–18). For patients with an uncompromised general health status, the decision to appoint CRLM as anatomically resectable depends predominantly on its distance from the surface, hepatic veins, and portal triad. This decision is likely influenced by the fact that thermal ablation offers a safe alternative for smaller and, especially, deep-seated CRLM that would otherwise require technically demanding surgery with unacceptable loss of healthy liver parenchyma.
The safety boundaries for resection are determined by both the volume and function of the future liver remnant; major hepatectomy without adequate functional liver remnant increases the risk of posthepatectomy liver failure (19,20). Although most superficial and deep-seated CRLM seem to be suitable for thermal ablation, close proximity to the hepatic hilum is an absolute contraindication because of the risk of biliary tract injury (21). For unresectable perihilar CRLM, nonthermal ablation techniques, such as stereotactic ablative radiation therapy and IRE, can be offered, with IRE gaining ground since the publication of the positive results from the COLDFIRE-2 (Colorectal Liver Metastatic Disease: Efficacy of Irreversible Electroporation–A Single-arm Phase II Clinical Trial) trial (22–25).
The multidisciplinary COLLISION (Colorectal Liver Metastases: Surgery vs Thermal Ablation) trial group has previously constructed per-patient and per-tumor tree-based consensus criteria (4,25). This group formulated recommendations on the basis of available evidence and a Delphi consensus protocol, after which they proposed a treatment algorithm with combined resection and ablation criteria for the treatment of CRLM (25,26). In the absence of widely accepted definitions, however, the treatment strategy chosen is variable and operator dependent, leading to widespread debate. Objective criteria to select the most suitable treatment option are relevant to structure tumor board decisions, to accurately define specific indications for future clinical research and to enhance intersociety discussions.
The purpose of this study was to further define and validate anatomic resection and ablation criteria for small (≤3 cm) CRLM using a mathematical three-dimensional (3D) liver model based on expert panel recommendations from two multicenter prospective trials, COLLISION (4) and COLDFIRE-2 (24,27), assessing eligibility for resection, thermal ablation, and nonthermal ablation for every individual tumor presented.
Materials and Methods
Study Design and Sample
Data from individuals with small liver-only CRLM (≤3 cm) were obtained from databases of two multicenter prospective clinical trials: COLLISION and COLDFIRE-2. The ongoing international phase III randomized controlled COLLISION trial (registered at clinicaltrials.gov as NCT03088150) (4) is comparing thermal ablation with surgical resection for small CRLM; the two-center phase IIb prospective clinical COLDFIRE-2 trial (registered at clinicaltrials.gov as NCT02082782) (24,27) investigated IRE for tumors anatomically not amenable for surgical resection or thermal ablation. Both study protocols have been approved by the medical ethical review boards of the Amsterdam University Medical Centers. The study designs and inclusion and exclusion criteria were previously published (4,27). By signing informed consent, participants granted permission to exchange and use their data.
Expert Panel and Participant Selection
Potential participants were initially reviewed by local multidisciplinary tumor boards before being assessed by a multidisciplinary expert panel, which consisted of representatives from 15 high-volume hepatobiliary cancer centers across three countries—the Netherlands, Belgium, and Italy—each performing at least 100 liver resections and/or ablations annually. The expert panel included 20 hepatobiliary surgeons, 20 interventional radiologists, one radiation oncologist, and one medical oncologist, each with a minimum of 5 years of professional experience in their respective fields. The panel received concise information, using the secured SIILO end-to-end encryption messaging application (28), regarding patients’ medical history, comorbidities, performance status, and treatment plan proposed by the local center. In addition, a short video of the diagnostic examinations, as shown in the picture archiving and communication system, was shared; this video included an audio recording by the treating physician focusing on the tumor’s location. This allowed the panel to review the patient’s information and proposed treatment plan concisely and provide immediate feedback through the secured platform (SIILO; Doctolib). In case of a challenging anatomic location of CRLM or complex liver anatomic features, the provision to transmit images via the picture archiving and communication system was offered.
Surgeons were asked whether individual CRLM were considered technically eligible for resection, and interventional radiologists were asked whether they considered the lesions technically eligible for thermal ablation or IRE. Consensus was reached when two independent interventional radiologists and two surgeons agreed on the technical eligibility and feasibility of the local treatment plan. Although the COLLISION trial is challenging this, resection was considered standard of care (25). Individuals with anatomically unresectable CRLM were preferably treated with thermal ablation. Furthermore, thermal ablation was routinely proposed to avoid major hepatectomy in deep-seated CRLM. IRE was considered for individuals with lesions that were unsuitable for both resection and thermal ablation. Both studies allowed the presence of concomitant CRLM greater than 3 cm as long as these larger lesions were considered resectable.
All individuals assessed by the panel were included in this study, regardless of the final local therapy received, including those who withdrew from study participation before randomization or before treatment because of extensive disease elsewhere. Individuals with aberrant anatomic features of the concerned liver segment, including variations or anomalies of hepatic veins, arteries, and bile ducts, and individuals from COLDFIRE-2 with a tumor size greater than 3 cm and a history of major hepatectomy or altered vascular anatomy were excluded. Twenty-seven of the 202 participants included in this study have been previously described in the COLDFIRE-2 report (24).
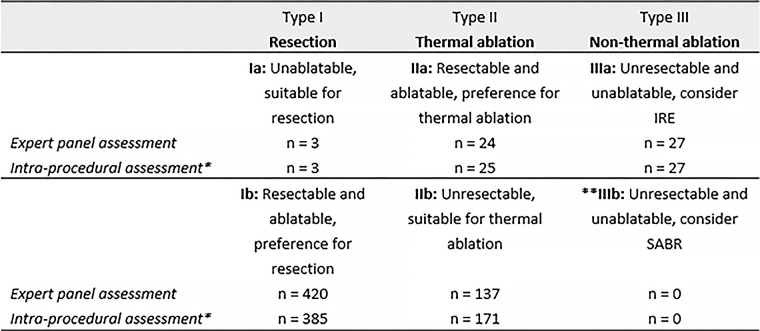
The panel’s assessments resulted in three anatomy-based categories: tumors that, according to current standard of care, should be resected (type I), tumors that should be thermally ablated (type II), and tumors that should be nonthermally ablated (type III). There were also five subcategories: resectable and not ablatable (type Ia: resect), resectable and ablatable (type Ib: resect), resectable and ablatable (type IIa: ablate), unresectable and ablatable (type IIb: ablate), and if resection and thermal ablation are not an option, prefer IRE (type IIIa: IRE).
Standardized 3D Liver Model
To create the 3D liver model, a randomization tool was used to select 10 livers from both trial imaging databases. A standardized liver model was subsequently constructed from composed pie chart slices representing the eight Couinaud segments (29). The adjacent inferior vena cava (IVC) wall represents the point-of-the-pie for segments I to VIII. The parameters used for model construction include the cross-sectional radius (r) of the segment, angle (α), and maximum height (h) perpendicular to the axial plane and width of the base. Measurements and construction of the liver model were performed in collaboration with an experienced interventional radiologist (M.R.M.) and a medical physics expert (H. Keijzers), each with more than 10 years of experience in their respective field. MATLAB, version 9.3.0.713579 (R2017b; MathWorks) was used for model construction. Data of the parameters used in the standardized 3D liver model are shown in Table S1, and MATLAB scripts along with detailed explanations are given in Appendix S1.
CRLM Coordinates
CRLM sizes were noted. Center coordinates were measured and color-coded according to the panel’s suggested treatment (red represented resectable and ablatable, blue represented unresectable and ablatable, and green represented unresectable and unsuitable for thermal ablation) and then plotted in the appropriate segments of the standardized 3D liver model using various MATLAB scripts. The following parameters were used to determine the relative virtual position of the tumor in the standardized liver: height (hcrlm = [htumor-bottom/htumor-top] × hsegment), radius (rcrlm = [r1/r2] × rsegment), and angle (αcrlm = [x1/x2] × αsegment). Here, r1 and r2 represent the distances from the tumor’s center to the IVC and to the liver surface, respectively. The perpendicular distances from the tumor’s center to the segment’s dorsal and ventral border are represented as ×1 and ×2, respectively. Figure S1 displays the three measurements.
Statistical Analysis
Participant baseline and tumor characteristics were obtained from the trial databases and are presented using descriptive statistics. Categorical variables are presented as frequencies and continuous variables as means (± SDs) or as medians with ranges. Statistical analyses were performed using SPSS software, version 28.0.1.0 (IBM).
To define the depth of the resection-to-ablation transition zone, the number of treated CRLM (y-axis) was plotted against the measured subsurface tumor depth (ie, coordinate r2 [x-axis]) for preferably resected versus preferably ablated (thermal and nonthermal) CRLM using Excel 2016 (KB4011684) 64-bit edition (Microsoft). Because the liver is anatomically structured into eight segments with several approaches to access the liver surface, affecting the choice of local therapy, the transition zone was also defined for the left and right hemiliver (30,31). To further define perihilar tumor localization for which nonthermal ablation (IRE) is eligible, the CRLM coordinates in segments IVa/b, V, and VIII were evaluated.
The area under the receiver operating characteristic (ROC) curve was used to assess the performance of subsurface tumor depth (ie, coordinate r2) in indicating the preferred treatment method (dichotomized as resection [type I] vs ablation [types II and III]).
The 3D liver prediction model was statistically validated by means of a leave-one-out cross-validation using various MATLAB scripts, which are provided in Appendix S1. Results were summed in a confusion matrix, which was used to calculate sensitivity, specificity, positive predictive value, and negative predictive value. Plotting new CRLM in the 3D liver model entails measuring tumor diameters and center coordinates and then entering these data into the designated software (NIfTI file). The software automatically plots the tumor in the model, with the color of the sphere indicating the treatment preference.
Results
Participant, Disease, and Treatment Characteristics
Figure 1 provides an overview of participant selection in this study. From August 2017 to June 2022, a total of 325 participants from COLLISION (n = 274) or COLDFIRE-2 (n = 51) were considered potentially eligible for inclusion in this study and were screened by the appointed expert panel. Of these 325 participants, 202 (62.2%) were included; 123 (37.8%) were excluded because of aberrant liver anatomic features, most commonly involving variations of the hepatic artery (n = 9), or because the shared cross-sectional source images were incompatible with the designated software program, rendering accurate tumor coordinate measurements infeasible (n = 90). Furthermore, participants from COLDFIRE-2 were excluded because of a history of major hepatectomy or altered vascular anatomy of the concerned liver segment (n = 18) or tumor size greater than 3 cm (n = 6). Altogether, 202 participants (mean age, 63 [range, 29–87] years; 138 male and 64 female) with 611 CRLM were included in this study.
Figure 1:
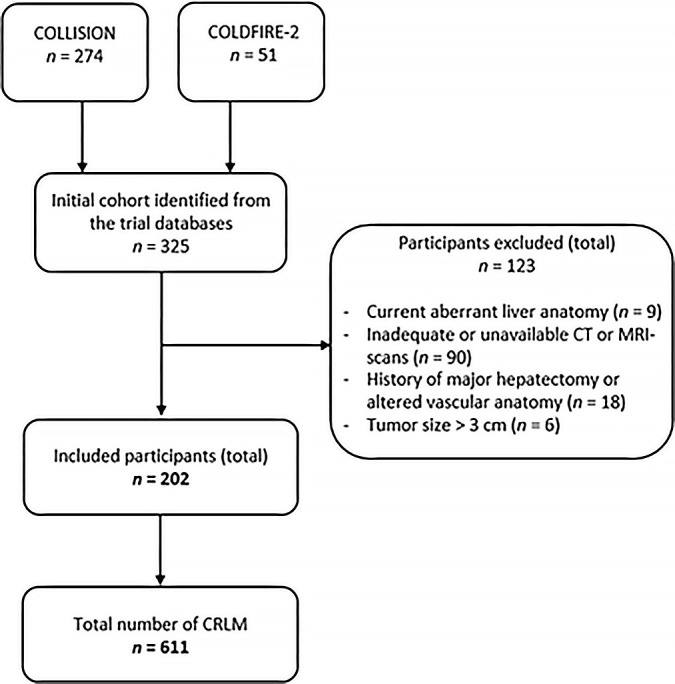
Flowchart of the study participant selection procedure. CRLM = colorectal liver metastases.
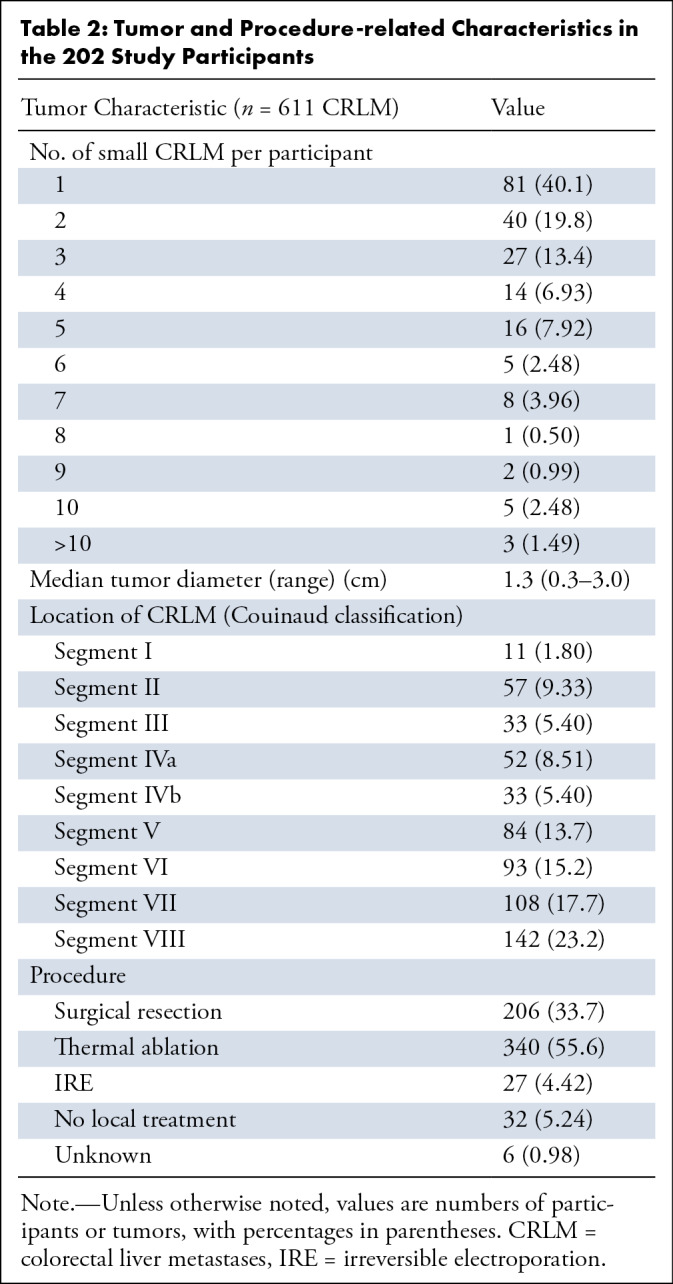
An overview of participant and disease characteristics is shown in Table 1. The median number of CRLM per participant was two (range, one to 13), with tumor sizes ranging from 0.3 to 3 cm in diameter (median, 1.3 cm). Of the 611 total CRLM, 206 (33.7%) were treated with resection, 340 (55.6%) with thermal ablation, and 27 (4.00%) with IRE. No local procedure was performed in 32 tumors (5.24%), and the treatment was unknown in six tumors (0.98%). Additional tumor- and procedure-related characteristics are summarized in Table 2.
Table 1:
Participant and Disease Characteristics
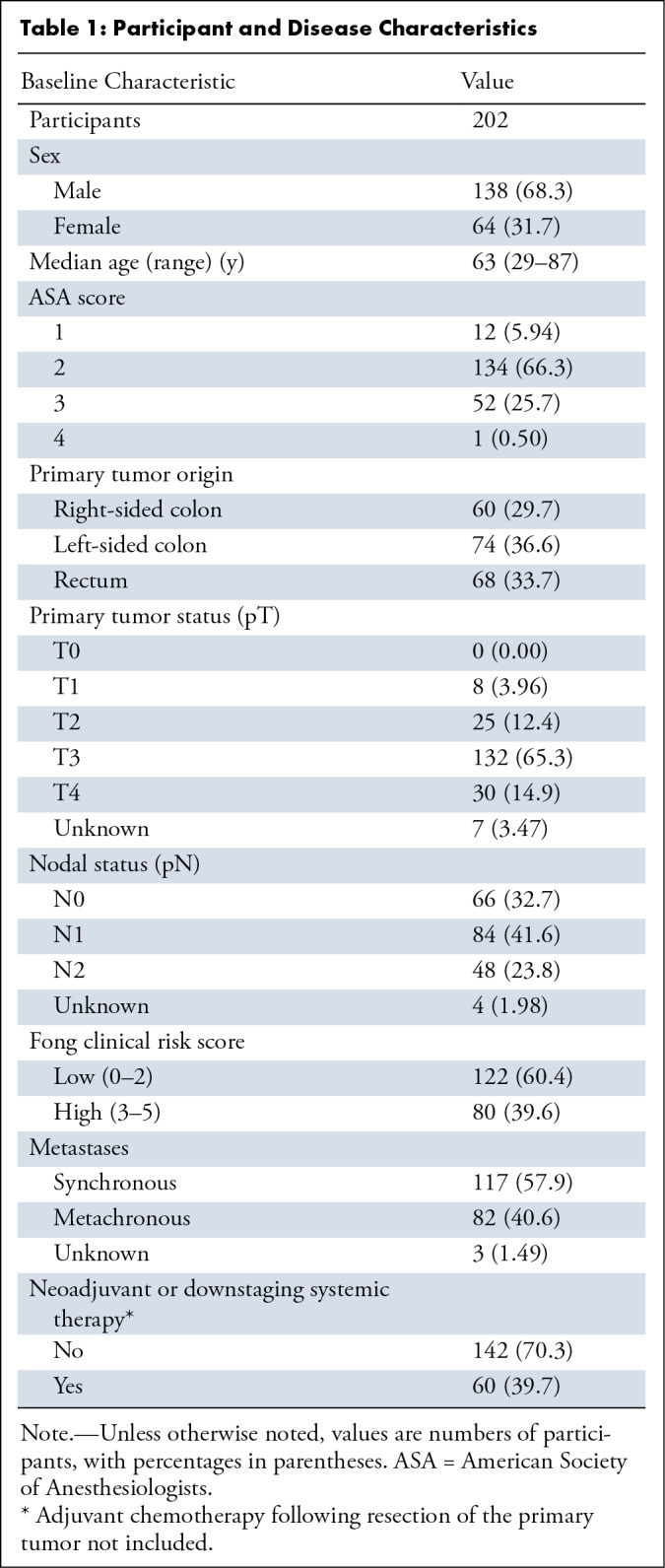
Table 2:
Tumor and Procedure-related Characteristics in the 202 Study Participants
Expert Panel and Intraprocedural Assessments
In 82.0% (n = 501) of the individual tumor assessments, the final advice of the expert panel concurred with the intraprocedural assessment and eventual treatment. The treatment plan changed in 18.0% (n = 110) of the 611 total tumors for the following reasons: unresectable because of a certain (unforeseen) difficult anatomic location (5.72%; n = 35); unresectable because of detection of multiple scattered lesions that would require major resection (0.98%; n = 6); and not ablatable because of tumor growth (>3 cm) (2.29%; n = 14) or the detection of additional CRLM in the same lobe or site, favoring all-in-one resection (2.78%; n = 17). In the other cases, participants were eventually considered not a candidate for local treatment because of disease progression (4.26%; n = 26) or because the lesion was not a metastasis or was no longer visible after chemotherapy (0.98%; n = 6). Data from the intraprocedural assessment were missing in 0.98% (n = 6). Figure 2 outlines the numbers per preferred local treatment category based on the panel’s assessment and based on the intraprocedural findings.
Figure 2:
Classification system for colorectal liver metastases (CRLM) based on preferred local treatment. Corresponding numbers of CRLM in this study are reported as “n“. *Additional and previously unknown CRLM detected during local treatment were not included in the analysis, although treatment plan modifications caused by these concomitant lesions were reported. **The absence of CRLM in the category where stereotactic ablative radiotherapy (SABR) was preferred is the result of including participants from two prospective trials that did not assess SABR. IRE = irreversible electroporation.
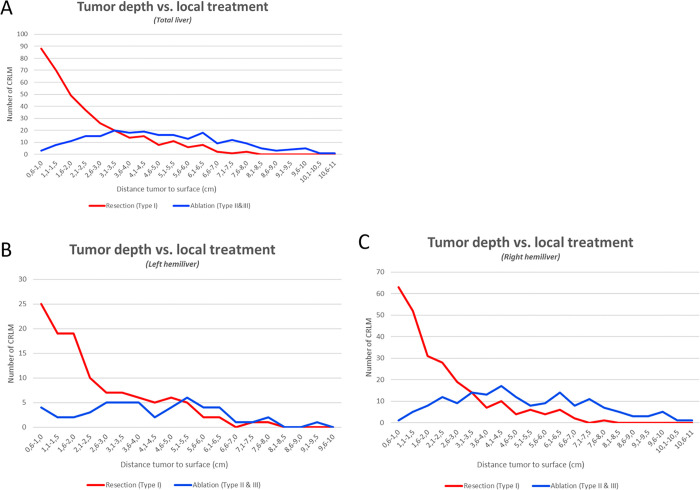
In Figure 3, the subsurface depth (range, 0.2–11.0 cm) of the tumor’s center was plotted against the number of tumors where the panel recommended resection or ablation (thermal and nonthermal). The calculated transition zone between preferably resected (type I) and preferably ablated (type II or III) tumors was 3.1–3.5 cm from the liver surface. In other words, small CRLM can be categorized as having a superficial location if their center is within 3 cm from the liver surface and as having a deep-seated location if the center is located more than 3 cm from the surface. Figure 3 also depicts the results for the left and right hemiliver. Figure 4 shows the ROC curve for performance of subsurface tumor depth in indicating the preferred treatment. The area under the ROC was 0.84 (95% CI: 0.81, 0.87), indicating a good overall predictive performance. According to coordinate analysis of perihilar tumors (type III), 25 of 27 (93%) CRLM treated with IRE were at least partially positioned within 1 cm of the portal triad.
Figure 3:
Distance from tumor to the free liver surface per treatment: (A) total liver, (B) left hemiliver, and (C) right hemiliver. CRLM = colorectal liver metastases.
Figure 4:
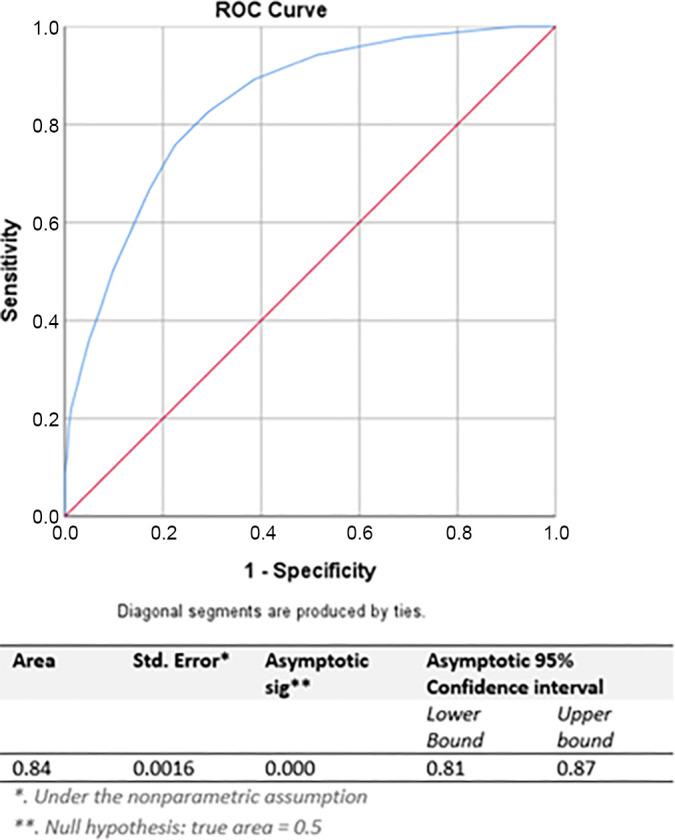
Receiver operating characteristic (ROC) curve for performance of subsurface tumor depth in indicating the preferred treatment (resection vs ablation).
3D Liver Treatment Prediction Model for Small CRLM
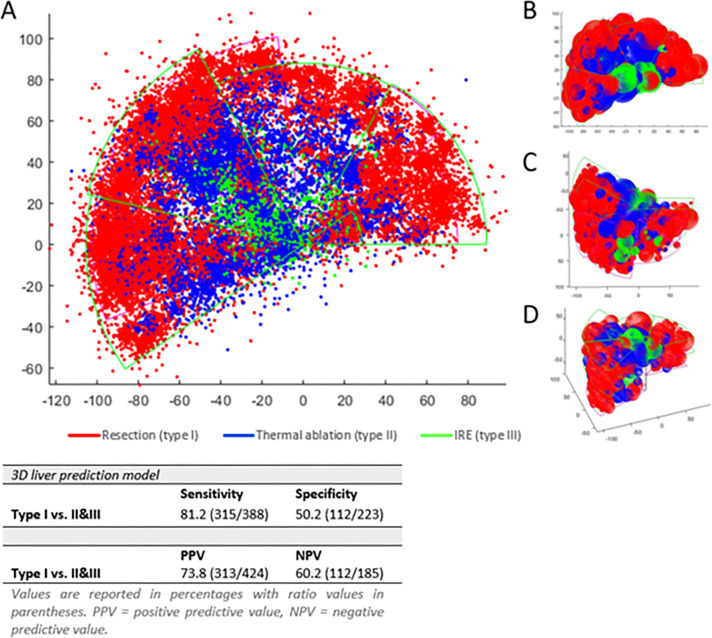
The 3D prediction model for the preferred local treatment of small CRLM with plotted color-coded CRLM is depicted in Figure 5. To generate a confusion matrix, type II and III were combined to reflect the separation of assessments between resection and ablation. The sensitivity and specificity were 81.2% (315 of 388) and 50.2% (112 of 223), respectively. According to the confusion matrix, the positive predictive value was 73.8% (313 of 424 CRLM). MATLAB depictions of the 3D model, including respective tumor sizes and perspectives from various angles, are also shown in Figure 5.
Figure 5:
(A) Three-dimensional (3D) liver treatment prediction model for small colorectal liver metastases (red: prefer resection; blue: prefer thermal ablation; green: prefer nonthermal ablation). (B) Top, (C) bottom, and (D) frontal views of the model. IRE = irreversible electroporation.
Discussion
This article details the creation and validation of a 3D liver model, developed through expert panel input within a multidisciplinary tumor board, to categorize treatment decisions for small (≤3 cm) CRLM based on anatomic location. The objective was to clarify the definitions of superficial, deep-seated, and perihilar CRLM and to semiautomatically determine the ideal treatment approach: resection, thermal ablation, or nonthermal ablation. The study demonstrates that superficial CRLM (type I), classically treated with resection, are characterized by a tumor center within 3 cm of the liver surface. Conversely, for deep-seated tumors (type II), which are more than 3 cm beneath the liver surface, ablation is usually favored. Perihilar tumors (type III), with margins less than 1 cm from the portal triad, are not eligible for thermal ablation. However, if they cannot be resected, they might qualify for IRE. Use of the 3D liver model accurately predicted the preferred treatment in 73.8% of small CRLM.
Because the resectable and nonablatable category (type Ia) comprises only three tumors, the model’s red zone largely signifies resectable and ablatable CRLM (type Ib). For this latter category (type Ib), which makes up 68.7% of all small CRLM, partial hepatectomy is now under review against thermal ablation in the ongoing COLLISION (NCT03088150), the NEW-COMET (Ablation vs Resection of Colorectal Cancer Liver Metastases; NCT05129787), and the HELARC (Comparison of Hepatectomy and Local Ablation for Resectable Synchronous and Metachronous Colorectal Liver Metastasis; NCT02886104) trials. This study’s lower specificity (50.2%) was predominantly the result of overlap regarding the preferred treatment option for deep-seated tumors. It illustrates that the choice between resection and ablation for these tumors is multifactorial and not solely based on subsurface depth and distance to blood vessels. However, the model showed high sensitivity in classifying CRLM as type I, where resection was predominantly advised. For future studies, iterative learning of convolutional neural networks could be used to improve specificity and, subsequently, achieve a higher positive predictive value.
Aside from the COLLISION group’s consensus-based treatment algorithm (25), no other previously reported study investigators proposed combined resection and ablation criteria for CRLM. In addition to this subjective algorithm, the current study provides a more objective and detailed classification of a tumor’s specific location within the liver, guiding discussions on the preferred local treatment. As represented by the ROC analysis, tumor depth performed well in indicating which method may be preferred by a panel of experts. Further investigation into such matters as treatment success and complications are warranted in future research to validate the model against more objective criteria.
Most deep-seated tumors in the left hemiliver were evaluated as resectable, indicating that the transition zone is deeply located within this hemiliver. This is most likely explained by the fact that the lateral section (segments II, III) is easily accessible because of the falciform ligament and umbilical fissure, allowing for a relatively simple resection with low risk of vascular or biliary injury (31,32). The transition zone for the right hemiliver was set as substantially more superficial. This is mostly owing to segments VII and VIII, which contained the highest number of CRLM, many of which were unresectable. Because of anatomic factors, such as the subdiaphragmatic location, the deeply situated Glissonean pedicle, and its intimate contact with the IVC and the right and middle hepatic veins, segment VIII resection is considered more difficult (33).
This study had several limitations. First and foremost, because most participants were recruited from COLLISION, its inclusion and exclusion criteria may have caused confounding by indication, as at least one CRLM eligible for both resection and thermal ablation (type Ib lesion) needed to be present in the study. However, because this lesion type is the most common in clinical practice (it covers the largest volume of the liver), the effect of this bias seems unlikely to be significant. Another shortcoming of the study’s inclusion criteria is that the model is primarily applicable to relatively healthy individuals who have not been heavily pretreated and have limited disease burden restricted to the liver. Furthermore, only metastases smaller than 3 cm were assessed in this study. The model is not intended for larger tumors because these lesions are preferably resected and can be superficial or deep-seated. In addition, the model is less suited for individuals with multiple tumors. For example, for tumors scattered throughout the liver, operators may favor ablation to spare healthy liver parenchyma, whereas for tumors aggregated in a single lobe, operators may prefer hemihepatectomy to eradicate disease.
A second limitation is that major aberrant anatomy precludes a certain subset of patients from the model, and the existence of minor variances is likely to have induced uncertainty in the predictive model. In several series, the occurrence of anatomic variations of the hepatic artery, biliary tract, and portal venous system ranged from 20% to 50% (32,34–37). Anatomic variations can occur in the course, size, or branching patterns of these structures, and when combined with variations in the size, shape, and configuration of the liver lobes, can result in aberrant patterns of segmentation and distribution of the liver lobes. Furthermore, the evidence presented was limited to the initial metastatic event. As a result, given the assumed higher complexity of surgical treatments in a previously accessed abdominal cavity and the reduced liver remnant volume, anatomic definitions are less applicable to patients with recurrences in the liver (38).
A third limitation of the study is the large number of patients excluded because of image incompatibility with the software program, which hindered accurate tumor coordinate measurements.
In conclusion, we propose a validated 3D liver treatment prediction model to objectively and semiautomatically classify small (≤3 cm) CRLM according to anatomic location. The main goals through use of this model are to (a) introduce a higher level of objectivity in multidisciplinary tumor board discussions by empirically predicting local treatment recommendations, (b) classify specific lesion types according to their anatomic location to help define inclusion and exclusion criteria for future registries and trials, and (c) improve intersociety communications (most resected CRLM are eligible for ablation and vice versa). We suggest defining superficial versus deep-seated CRLM as a depth of the lesion’s center within versus beyond 3 cm from the free liver surface and using the term perihilar if tumor margins reach within 1 cm from the portal triad. The model currently aids in objective preprocedural assessment during multidisciplinary discussions based on expert’s opinions. However, upcoming refinements to the model, incorporating feedback from more experts across different geographic and practice regions, may be necessary and could potentially influence global practice patterns. Future considerations, where the model provides risk assessments and oncologic outcomes per specific location, would further improve shared decision-making between the treating physicians and the patient. The ongoing randomized controlled trials will likely provide answers for whether thermal ablation, which is already recommended for small deep-seated CRLM, should also be favored over resection for more superficially located small CRLM. Eventually, these data could serve as a foundation for future artificial intelligence models using deep learning algorithms to adapt to evolving consensus on treatment options.
The complete list of authors and affiliations is at the end of this article.
Authors declared no funding for this work.
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Complete list of authors: Luca Aldrighetti, MD, PhD; Mark Arntz, MD, PhD; Laurens van Baardewijk, MD; Maarten W. Barentsz, MD, PhD; Peter van den Boezem, MD, PhD; Bart Bracke, MD; Rutger C. G. Bruijnen, MD, PhD; Mark C. Burgmans, MD, PhD; Thiery Chapelle, MD, PhD; Mariëlle M. E. Coolsen, MD, PhD; Veerle M. H. Coupé, MSc, PhD; Ronald M. van Dam, MD, PhD; Sanne de Boer, MD, PhD; Francesco de Cobelli, MD, PhD; Koert P. de Jong, MD, PhD; Jan J. J. de Vries, MD; Johannes H. W. de Wilt, MD, PhD; Otto M. van Delden, MD, PhD; Arjen Diederik, MD; Madelon Dijkstra, MD, PhD; Werner A. Draaisma, MD, PhD; Peter van Duijvendijk, MD, PhD; Hasan H. Eker, MD, PhD; Joris I. Erdmann, MD, PhD; Arian R. van Erkel, MD, PhD; Jurgen J. Futterer, MD, PhD; Bart Geboers, MD, PhD; Anne M. van Geel, MD; Nicole C. T. van Grieken, MD, PhD; Gerie Groot, MD; Jeroen Hagendoorn, MD, PhD; Tjarda van Heek, MD, PhD; Sjoerd F. M. Jenniskens, MD, PhD; Matthijs G. M. Kater, MD; Geert Kazemier, MD, PhD; Han Kruimer, MD; Wouter K. G. Leclercq, MD, PhD; Susan van der Lei, MD; Christiaan van der Leij, MD, PhD; Birgit I. Lissenberg-Witte, MSc, PhD; Eric R. Manusama, MD, PhD; Mark A. J. Meier, MD; Bram B. van der Meijs, MD; Quintus Molenaar, MD, PhD; Karin Nielsen, MD, PhD; Maarten W. Nijkamp, MD, PhD; Bart Op de Beeck, MD, PhD; Christiaan Overduin, MSc, PhD; Fons H. Potters, MD; Francesca Ratti, MD; Jeroen J. van der Reijden, MD; Floris J. Rietema, MD; Simeon J. S. Ruiter, MD, PhD; Hester J. Scheffer, MD, PhD; Evelien Schouten, MSc; Hermien W. H. Schreurs, MD, PhD; Hannah H. Schulz, MD, MD; Gian Piero Serafino, MD; Colin Sietses, MD, PhD; Gerrit Slooter, MD, PhD; Maarten L. J. Smits, MD, PhD; Gert-Jan Spaargaren, MD; Martijn W. J. Stommel, MD, PhD; Rutger-Jan Swijnenburg, MD, PhD; Floor E. F. Timmer, MD; Kathelijn S. Versteeg, MD, PhD; Ted Vink, MD; Danielle J. W. Vos, MD; Barbara A. Zonderhuis, MD
Author affiliations continued: Department of Radiology and Nuclear Medicine, Amsterdam UMC, Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, the Netherlands (J.J.J.d.V., O.M.v.D., M.D., B.G., S.v.d.L., B.B.v.d.M., H.J.S., H.H.S., F.E.F.T., D.J.W.V.); Cancer Center Amsterdam, Amsterdam, the Netherlands (J.J.J.d.V., O.M.v.D., M.D., J.I.E., B.G., N.C.T.v.G., G.K., S.v.d.L., B.B.v.d.M., H.J.S., H.H.S., R.J.S., F.E.F.T., K.S.V., D.J.W.V., B.A.Z.); Department of Radiology and Nuclear Medicine, OLVG Hospital, Oost, Amsterdam, the Netherlands (J.J.J.d.V.); Department of Surgical Oncology, San Raffaele, Milan, Italy (L.A., F.R.); Department of Radiology, Radboud UMC, Nijmegen, the Netherlands (M.A., J.J.F., S.F.M.J., C.O.); Department of Radiology, Máxima MC, Veldhoven, the Netherlands (L.v.B., M.W.B., J.J.v.d.R.); Department of Surgical Oncology, Radboud UMC, Nijmegen, the Netherlands (P.v.d.B., J.H.W.d.W., M.W.J.S.); Department of HPB, Transplantation and Endocrine Surgery, UZA, University of Antwerp, Belgium (B.B., T.C.); Department of Radiology and Nuclear Medicine, UMC Utrecht, Utrecht, the Netherlands (R.C.G.B., M.L.J.S.); Department of Radiology, Leiden UMC, Leiden, the Netherlands (M.C.B., A.R.v.E.); Department of Surgical Oncology, Maastricht UMC, Maastricht, the Netherlands (M.M.E.C., R.M.v.D.); Department of Epidemiology and Data Science, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands (V.M.H.C.); Department of Radiology, Maastricht UMC, Maastricht, the Netherlands (S.d.B., C.v.d.L.); Department of Radiology, San Raffaele, Milan, Italy (F.d.C.); Department of Surgical Oncology, UMC Groningen, Groningen, the Netherlands (K.P.d.J., M.W.N., S.J.S.R.); Department of Radiology and Nuclear Medicine, ZGV, Ede, the Netherlands (A.D., G.G., G.J.S.); Department of Surgical Oncology, Jeroen Bosch Ziekenhuis, ‘s-Hertogenbosch, the Netherlands (W.A.D.); Department of Surgical Oncology, Isala, Zwolle, the Netherlands (P.v.D.); Department of Surgery, UZG, Gent, Belgium (H.H.E.); Department of Surgical Oncology, Amsterdam UMC, Vrije Universiteit, Amsterdam, the Netherlands (J.I.E., G.K., R.J.S., B.A.Z.); Department of Radiology and Nuclear Medicine, NWZ Hospital Group, Alkmaar, the Netherlands (A.M.v.G., F.J.R., H.J.S.); Department of Pathology, Amsterdam UMC, Vrije Universiteit, Amsterdam, the Netherlands (N.C.T.v.G.); Department of Surgical Oncology, UMC Utrecht, Utrecht, the Netherlands (J.H., Q.M.); Department of Surgical Oncology, ZGV, Ede, the Netherlands (T.v.H., C.S.); Department of Radiology, UMC Groningen, Groningen, the Netherlands (M.G.M.K.); Department of Radiology, Flevoziekenhuis, Almere, the Netherlands (H. Kruimer); Department of Surgical Oncology, Máxima MC, Veldhoven, the Netherlands (W.K.G.L., G.S.); Department of Epidemiology and Biostatics, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands (B.I.L.W.); Department of Radiology, Isala, Zwolle, the Netherlands (M.A.J.M., F.H.P.); Department of Surgical Oncology, Erasmuc MC, Rotterdam, the Netherlands (K.N.); Department of Radiology, UZA, Antwerp, Belgium (B.O.d.B.); Department of Radiology and Nuclear Medicine, AvL, Amsterdam, the Netherlands (E.S.); Department of Surgical Oncology, NWZ Hospital Group, Alkmaar, the Netherlands (H.W.H.S.); Department of Radiology, Jeroen Bosch Ziekenhuis, ‘s-Hertogenbosch, the Netherlands (G.P.S.); Department of Medical Oncology, Amsterdam UMC, Vrije Universiteit, Amsterdam, the Netherlands (K.S.V.); and Department of Radiology, Medical Center Leeuwarden, Leeuwarden, the Netherlands (T.V.).
Disclosures of conflicts of interest: B.A.T.v.d.B. No relevant relationships. R.S.P. Consultancy for Medtronic-Covidien; payment or honoraria from Medtronic-Covidien; PhD thesis support from Medtronic-Covidien, AngioDynamics, Terumo, Sirtex, and MML Medica; support for attending meetings and/or travel from AngioDynamics; junior board member Dutch Society for Interventional Radiology; cofounder of Medpeers. H. Keijzers No relevant relationships. L.A. No relevant relationships. M.A. No relevant relationships. L.v.B. No relevant relationships. M.W.B. No relevant relationships. P.v.d.B. No relevant relationships. B.B. No relevant relationships. R.C.G.B. No relevant relationships. M.C.B. No relevant relationships. T.C. No relevant relationships. M.M.E.C. No relevant relationships. V.M.H.C. No relevant relationships. R.M.v.D. Unrestricted grants for other work from Dutch Cancer Society, ZonMw Netherlands, KCE Belgium, Guerbet, and Abbott; chairman of the board of Get It Cured Foundation. S.d.B. No relevant relationships. F.d.C. No relevant relationships. K.P.d.J. No relevant relationships. J.J.J.d.V. No relevant relationships. J.H.W.d.W. No relevant relationships. O.M.v.D. No relevant relationships. A.D. No relevant relationships. M.D. No relevant relationships. W.A.D. No relevant relationships. P.v.D. No relevant relationships. H.H.E. No relevant relationships. J.I.E. No relevant relationships. A.R.v.E. No relevant relationships. J.J.F. No relevant relationships. B.G. AngioDynamics research funding and educational grant. A.M.v.G. No relevant relationships. N.C.T.v.G. Advisory role for BMS, Diaceutics, and Astellas; lectures/education for BMS, Astellas, and MedTalks; scientific committees of Dutch Cancer Society and Sacha Swarttouw-Heijmans Foundation. G.G. No relevant relationships. J.H. No relevant relationships. T.v.H. No relevant relationships. S.F.M.J. No relevant relationships. M.G.M.K. No relevant relationships. G.K. No relevant relationships. H. Kruimer No relevant relationships. W.K.G.L. No relevant relationships. S.v.d.L. No relevant relationships. C.v.d.L. No relevant relationships. B.I.L.W. No relevant relationships. E.R.M. No relevant relationships. M.A.J.M. Sirtex Medical, collaboration for podcast (in Dutch) to educate medical oncologists and surgeons about the current indications and referral for radioembolization, fee paid for one time participation as compensation for time invested; Heymans (constructing company) personal stocks (nonmedical). B.B.v.d.M. No relevant relationships. Q.M. No relevant relationships. K.N. No relevant relationships. M.W.N. No relevant relationships. B.O.d.B. No relevant relationships. C.O. Stichting Hanarth Fonds. F.H.P. No relevant relationships. F.R. No relevant relationships. J.J.v.d.R. No relevant relationships. F.J.R. No relevant relationships. S.J.S.R. No relevant relationships. H.J.S. Paid consultant for AngioDynamics; patents planned, issued or pending by AngioDynamics and as faculty of medical conferences (CIRSE). E.S. No relevant relationships. H.W.H.S. No relevant relationships. H.H.S. Support during the manuscript writing. G.P.S. No relevant relationships. C.S. No relevant relationships. G.S. No relevant relationships. M.L.J.S. Payment or honoraria from Philips, Medtronic, and Terumo. G.J.S. No relevant relationships. M.W.J.S. No relevant relationships. R.J.S. Proctor for Robotic Liver Surgery for Intuitive Surgical, fees go into research fund. F.E.F.T. No relevant relationships. K.S.V. No relevant relationships. T.V. No relevant relationships. D.J.W.V. No relevant relationships. B.A.Z. No relevant relationships. P.M.v.d.T. No relevant relationships. M.R.M. Medtronic-Covidien (partially restricted research grant for the COLLISION trial from Medtronic Covidien); speaker and moderator honoraria from Medtronic-Covidien, AngioDynamics, Johnson & Johnson, and Philips; support to attend LIFE symposia from AngioDynamics; executive or past executive board for CIRSE, ECIO, and SIO.
Abbreviations:
- CRLM
- colorectal liver metastases
- IRE
- irreversible electroporation
- IVC
- inferior vena cava
- ROC
- receiver operating characteristic
- 3D
- three-dimensional
Contributor Information
Bente A. T. van den Bemd, Email: b.vandenbemd@amsterdamumc.nl.
Collaborators: Luca Aldrighetti, Mark Arntz, Laurens van Baardewijk, Maarten W. Barentsz, Peter van den Boezem, Bart Bracke, Rutger C. G. Bruijnen, Mark C. Burgmans, Thiery Chapelle, Mariëlle M. E. Coolsen, Veerle M. H. Coupé, Ronald M. van Dam, Sanne de Boer, Francesco de Cobelli, Koert P. de Jong, Jan J. J. de Vries, Johannes H. W. de Wilt, Otto M. van Delden, Arjen Diederik, Madelon Dijkstra, Werner A. Draaisma, Peter van Duijvendijk, Hasan H. Eker, Joris I. Erdmann, Arian R. van Erkel, Jurgen J. Futterer, Bart Geboers, Anne M. van Geel, Nicole C. T. van Grieken, Gerie Groot, Jeroen Hagendoorn, Tjarda van Heek, Sjoerd F. M. Jenniskens, Matthijs G. M. Kater, Geert Kazemier, Han Kruimer, Wouter K. G. Leclercq, Susan van der Lei, Christiaan van der Leij, Birgit I. Lissenberg-Witte, Eric R. Manusama, Mark A. J. Meier, Bram B. van der Meijs, Quintus Molenaar, Karin Nielsen, Maarten W. Nijkamp, Bart Op de Beeck, Christiaan Overduin, Fons H. Potters, Francesca Ratti, Jeroen J. van der Reijden, Floris J. Rietema, Simeon J. S. Ruiter, Hester J. Scheffer, Evelien Schouten, Hermien W. H. Schreurs, Hannah H. Schulz, Gian Piero Serafino, Colin Sietses, Gerrit Slooter, Maarten L. J. Smits, Gert-Jan Spaargaren, Martijn W. J. Stommel, Rutger-Jan Swijnenburg, Floor E. F. Timmer, Kathelijn S. Versteeg, Ted Vink, Danielle J. W. Vos, and Barbara A. Zonderhuis
References
- 1. Sung H , Ferlay J , Siegel RL , et al . Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries . CA Cancer J Clin 2021. ; 71 ( 3 ): 209 – 249 . [DOI] [PubMed] [Google Scholar]
- 2. Dyba T , Randi G , Bray F , et al . The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers . Eur J Cancer 2021. ; 157 : 308 – 347 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Estimated Age-Standardized Incidence Rates (World) in 2022, All Cancers, Both Sexes, All Ages . http://gco.iarc.fr/today/online-analysis-map. Published 2022. Accessed April 2023 .
- 4. Puijk RS , Ruarus AH , Vroomen LGPH , et al . Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial . BMC Cancer 2018. ; 18 ( 1 ): 821 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Engstrand J , Nilsson H , Strömberg C , Jonas E , Freedman J . Colorectal cancer liver metastases - a population-based study on incidence, management and survival . BMC Cancer 2018. ; 18 ( 1 ): 78 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manfredi S , Lepage C , Hatem C , Coatmeur O , Faivre J , Bouvier AM . Epidemiology and management of liver metastases from colorectal cancer . Ann Surg 2006. ; 244 ( 2 ): 254 – 259 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruers T , Van Coevorden F , Punt CJ , et al . Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial . J Natl Cancer Inst 2017. ; 109 ( 9 ): djx015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hackl C , Neumann P , Gerken M , Loss M , Klinkhammer-Schalke M , Schlitt HJ . Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma . BMC Cancer 2014. ; 14 ( 1 ): 810 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oki E , Ando K , Nakanishi R , et al . Recent advances in treatment for colorectal liver metastasis . Ann Gastroenterol Surg 2018. ; 2 ( 3 ): 167 – 175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu Z , Hall TL , Vlaisavljevich E , Lee FT Jr . Histotripsy: the first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound . Int J Hyperthermia 2021. ; 38 ( 1 ): 561 – 575 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ansari D , Andersson R . Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors . World J Gastroenterol 2012. ; 18 ( 10 ): 1003 – 1008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cervantes A , Martinelli E ; ESMO Guidelines Committee . Updated treatment recommendation for third-line treatment in advanced colorectal cancer from the ESMO Metastatic Colorectal Cancer Living Guideline . Ann Oncol 2024. ; 35 ( 2 ): 241 – 243 . [DOI] [PubMed] [Google Scholar]
- 13. Yang T , Chen Q , Kuang L , et al . Effectiveness and safety of ultrasound-guided high-intensity focused ultrasound ablation for the treatment of colorectal cancer liver metastases . Int J Hyperthermia 2022. ; 39 ( 1 ): 829 – 834 . [DOI] [PubMed] [Google Scholar]
- 14. Meijerink MR , Puijk RS , van Tilborg AAJM , et al . Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis . Cardiovasc Intervent Radiol 2018. ; 41 ( 8 ): 1189 – 1204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Amerongen MJ , Jenniskens SFM , van den Boezem PB , Fütterer JJ , de Wilt JHW . Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases - a meta-analysis . HPB (Oxford) 2017. ; 19 ( 9 ): 749 – 756 . [DOI] [PubMed] [Google Scholar]
- 16. Kron P , Linecker M , Jones RP , Toogood GJ , Clavien PA , Lodge JPA . Ablation or resection for colorectal liver metastases? A systematic review of the literature . Front Oncol 2019. ; 9 : 1052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akgül Ö , Çetinkaya E , Ersöz Ş , Tez M . Role of surgery in colorectal cancer liver metastases . World J Gastroenterol 2014. ; 20 ( 20 ): 6113 – 6122 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bai H , Huangz X , Jing L , Zeng Q , Han L . The effect of radiofrequency ablation vs. liver resection on survival outcome of colorectal liver metastases (CRLM): a meta-analysis . Hepatogastroenterology 2015. ; 62 ( 138 ): 373 – 377 . [PubMed] [Google Scholar]
- 19. Rassam F , Olthof PB , Richardson H , van Gulik TM , Bennink RJ . Practical guidelines for the use of technetium-99m mebrofenin hepatobiliary scintigraphy in the quantitative assessment of liver function . Nucl Med Commun 2019. ; 40 ( 4 ): 297 – 307 . [DOI] [PubMed] [Google Scholar]
- 20. Pulitano C , Crawford M , Joseph D , Aldrighetti L , Sandroussi C . Preoperative assessment of postoperative liver function: the importance of residual liver volume . J Surg Oncol 2014. ; 110 ( 4 ): 445 – 450 . [DOI] [PubMed] [Google Scholar]
- 21. van Tilborg AA , Scheffer HJ , de Jong MC , et al . MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient- and lesion-based analysis of two historical cohorts . Cardiovasc Intervent Radiol 2016. ; 39 ( 10 ): 1438 – 1446 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scheffer HJ , Melenhorst MC , Echenique AM , et al . Irreversible electroporation for colorectal liver metastases . Tech Vasc Interv Radiol 2015. ; 18 ( 3 ): 159 – 169 . [DOI] [PubMed] [Google Scholar]
- 23. Scheffer HJ , Nielsen K , van Tilborg AA , et al . Ablation of colorectal liver metastases by irreversible electroporation: results of the COLDFIRE-I ablate-and-resect study . Eur Radiol 2014. ; 24 ( 10 ): 2467 – 2475 . [DOI] [PubMed] [Google Scholar]
- 24. Meijerink MR , Ruarus AH , Vroomen LGPH , et al . Irreversible electroporation to treat unresectable colorectal liver metastases (COLDFIRE-2): a phase II, two-center, single-arm clinical trial . Radiology 2021. ; 299 ( 2 ): 470 – 480 . [DOI] [PubMed] [Google Scholar]
- 25. Nieuwenhuizen S , Puijk RS , van den Bemd B , et al . Resectability and ablatability criteria for the treatment of liver only colorectal metastases: multidisciplinary consensus document from the COLLISION trial group . Cancers (Basel) 2020. ; 12 ( 7 ): 1779 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsu CC , Sandford BA . The Delphi technique: making sense of consensus . Pract Assess Res Eval 2007. ; 12 : 10 . [Google Scholar]
- 27. Scheffer HJ , Vroomen LG , Nielsen K , et al . Colorectal liver metastatic disease: efficacy of irreversible electroporation--a single-arm phase II clinical trial (COLDFIRE-2 trial) . BMC Cancer 2015. ; 15 ( 1 ): 772 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siilo: The free secure messaging app for healthcare professionals . https://www.siilo.com. Accessed September 2023 .
- 29. Couinaud C . The Liver: Anatomical and Surgical Studies . Paris, France: : Masson, 1957. . [Google Scholar]
- 30. Inoue Y , Suzuki Y , Fujii K , et al . Laparoscopic liver resection using the lateral approach from intercostal ports in segments VI, VII, and VIII . J Gastrointest Surg 2017. ; 21 ( 12 ): 2135 – 2143 . [DOI] [PubMed] [Google Scholar]
- 31. Ishizawa T , Gumbs AA , Kokudo N , Gayet B . Laparoscopic segmentectomy of the liver: from segment I to VIII . Ann Surg 2012. ; 256 ( 6 ): 959 – 964 . [DOI] [PubMed] [Google Scholar]
- 32. Lowe MC , D’Angelica MI . Anatomy of hepatic resectional surgery . Surg Clin North Am 2016. ; 96 ( 2 ): 183 – 195 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anselmo A , Sensi B , Bacchiocchi G , Siragusa L , Tisone G . All the routes for laparoscopic liver segment VIII resection: a comprehensive review of surgical techniques . Front Oncol 2022. ; 12 : 864867 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fonseca-Neto OCLD , Lima HCS , Rabelo P , Melo PSV , Amorim AG , Lacerda CM . Anatomic variations of hepatic artery: a study in 479 liver transplantations . Arq Bras Cir Dig 2017. ; 30 ( 1 ): 35 – 37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Noussios G , Dimitriou I , Chatzis I , Katsourakis A . The Main anatomic variations of the hepatic artery and their importance in surgical practice: review of the literature . J Clin Med Res 2017. ; 9 ( 4 ): 248 – 252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prado Neto EV , Petroianu A . Anatomical variations of portal venous system: importance in surgical clinic . Arq Bras Cir Dig 2022. ; 35 : e1666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keplinger KM , Bloomston M . Anatomy and embryology of the biliary tract . Surg Clin North Am 2014. ; 94 ( 2 ): 203 – 217 . [DOI] [PubMed] [Google Scholar]
- 38. Valdimarsson VT , Hellberg K , Brismar TB , Sparrelid E , Sturesson C . Repeat procedures for recurrent colorectal liver metastases: analysis of long-term liver regeneration and outcome . Cancer Manag Res 2019. ; 11 : 2617 – 2622 . [DOI] [PMC free article] [PubMed] [Google Scholar]