Abstract
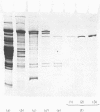
Ca2+-activated neutral proteinase was purified from rabbit skeletal muscle by a method involving DEAE-Sephacel chromatography, affinity chromatography on organomercurial–Sepharose and gel filtration on Sephacryl S-200 and Sephadex G-150. The SDS (sodium dodecyl sulphate)/polyacrylamide-gel-electrophoresis data show that the purified enzyme contains only one polypeptide chain of mol.wt. 73000. The purification procedure used allowed us to eliminate a contaminant containing two components of mol.wt. about 30000 each. Whole casein or α1-casein were hydrolysed with a maximum rate at 30°C, pH7.5, and with 5mm-CaCl2, but myofibrils were found to be a very susceptible substrate for this proteinase. This activity is associated with the destruction of the Z-discs, which is caused by the solubilization of the Z-line proteins. The activity of the proteinase in vitro is not limited to the removal of Z-line. SDS/polyacrylamide-gel electrophoresis on larger plates showed the ability of the proteinase to degrade myofibrils more extensively than previously supposed. This proteolysis resulted in the production of a 30000-dalton component as well as in various other higher- and lower-molecular-weight peptide fragments. Troponin T, troponin I, α-tropomyosin, some high-molecular-weight proteins (M protein, heavy chain of myosin) and three unidentified proteins are degraded. Thus the number of proteinase-sensitive regions in the myofibrils is greater than as previously reported by Dayton, Goll, Zeece, Robson & Reville [(1976) Biochemistry 15, 2150–2158]. The Ca2+-activated neutral proteinase is not a chymotrypsin- or trypsin-like enzyme, but it reacted with all the classic thiol-proteinase inhibitors for cathepsin B, papain, bromelain and ficin. Thus the proteinase was proved to have an essential thiol group. Antipain and leupeptin are also inhibitors of the Ca2+-activated neutral proteinase.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswanikumar S., Radhakrishnan A. N. Purification and properties of a peptidase acting on a synthetic collagenase substrate from experimental granuloma tissue in the rat. Biochim Biophys Acta. 1972 Jul 13;276(1):241–249. doi: 10.1016/0005-2744(72)90026-5. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch W. A., Stromer M. H., Goll D. E., Suzuki A. Ca 2+ -specific removal of Z lines from rabbit skeletal muscle. J Cell Biol. 1972 Feb;52(2):367–381. doi: 10.1083/jcb.52.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton W. R., Goll D. E., Zeece M. G., Robson R. M., Reville W. J. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry. 1976 May 18;15(10):2150–2158. doi: 10.1021/bi00655a019. [DOI] [PubMed] [Google Scholar]
- Ducastaing A., Etherington D. J. Purification of bovine spleen collagenolytic cathepsin, (cathepsin N). Biochem Soc Trans. 1978;6(5):938–940. doi: 10.1042/bst0060938. [DOI] [PubMed] [Google Scholar]
- Etherington D. J. The purification of bovine cathepsin B1 and its mode of action on bovine collagens. Biochem J. 1974 Mar;137(3):547–557. doi: 10.1042/bj1370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Zak R., Fischman D. A. Compositional studies of myofibrils from rabbit striated muscle. J Cell Biol. 1976 Jan;68(1):123–141. doi: 10.1083/jcb.68.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P., Etherington D. J. Characterisation of cathepsin B and collagenolytic cathepsin from human placenta. Eur J Biochem. 1978 Feb 1;83(1):87–97. doi: 10.1111/j.1432-1033.1978.tb12071.x. [DOI] [PubMed] [Google Scholar]
- Huston R. B., Krebs E. G. Activation of skeletal muscle phosphorylase kinase by Ca2+. II. Identification of the kinase activating factor as a proteolytic enzyme. Biochemistry. 1968 Jun;7(6):2116–2122. doi: 10.1021/bi00846a014. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Ishiura S., Murofushi H., Suzuki K., Imahori K. Studies of a calcium-activated neutral protease from chicken skeletal muscle. I. Purification and characterization. J Biochem. 1978 Jul;84(1):225–230. doi: 10.1093/oxfordjournals.jbchem.a132111. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. A calcium-activated neutral protease in normal and dystrophic human muscle. Clin Chim Acta. 1976 Dec 1;73(2):293–297. doi: 10.1016/0009-8981(76)90175-3. [DOI] [PubMed] [Google Scholar]
- Kopitar M., Brzin J., Zvonar T., Locnikar P., Kregar I., Turk V. Inhibition studies of an intracellular inhibitor on thiol proteinases. FEBS Lett. 1978 Jul 15;91(2):355–359. doi: 10.1016/0014-5793(78)81209-5. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Wong P. T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny I. F. The action of a muscle proteinase on the myofibrillar proteins of bovine muscle. J Sci Food Agric. 1974 Oct;25(10):1273–1284. doi: 10.1002/jsfa.2740251011. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jakábová M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+. J Biol Chem. 1977 Aug 25;252(16):5602–5605. [PubMed] [Google Scholar]
- Puca G. A., Nola E., Sica V., Bresciani F. Estrogen binding proteins of calf uterus. Molecular and functional characterization of the receptor transforming factor: A Ca2+-activated protease. J Biol Chem. 1977 Feb 25;252(4):1358–1366. [PubMed] [Google Scholar]
- Reddy M. K., Etlinger J. D., Rabinowitz M., Fischman D. A., Zak R. Removal of Z-lines and alpha-actinin from isolated myofibrils by a calcium-activated neutral protease. J Biol Chem. 1975 Jun 10;250(11):4278–4284. [PubMed] [Google Scholar]
- Reville W. J., Goll D. E., Stromer M. H., Robson R. M., Dayton W. R. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Subcellular localization of the protease in porcine skeletal muscle. J Cell Biol. 1976 Jul;70(1):1–8. doi: 10.1083/jcb.70.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer J. L., Welgus H. G., Jeffrey J. J., Eisen A. Z. The function of Ca+ in the action of mammalian collagenases. Arch Biochem Biophys. 1976 Mar;173(1):355–361. doi: 10.1016/0003-9861(76)90270-8. [DOI] [PubMed] [Google Scholar]
- Stracher A., McGowan E. B., Shafiq S. A. Muscular dystrophy: inhibition of degeneration in vivo with protease inhibitors. Science. 1978 Apr 7;200(4337):50–51. doi: 10.1126/science.635570. [DOI] [PubMed] [Google Scholar]
- Stromer M. H., Goll D. E., Roth L. E. Morphology of rigor--shortened bovine muscle and the effect of trypsin on pre- and postrigor myofibrils. J Cell Biol. 1967 Aug;34(2):431–445. doi: 10.1083/jcb.34.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo-Oka T., Shimizu T., Masaki T. Inhibition of proteolytic activity of calcium activated neutral protease by leupeptin and antipain. Biochem Biophys Res Commun. 1978 May 30;82(2):484–491. doi: 10.1016/0006-291x(78)90900-2. [DOI] [PubMed] [Google Scholar]