ABSTRACT
Introduction
Guillain‐Barré Syndrome (GBS) is a spectrum of peripheral neuropathies characterized by rapid symmetrical limb weakness and sensory symptoms. GBS can be life‐threatening and requires intensive care, particularly for patients with imminent respiratory failure. In Africa, limited research and high therapy costs pose challenges. This literature review aims to comprehensively address GBS in Africa to improve understanding and outcomes.
Aim
This literature review aims to provide an extensive overview of GBS in Africa, encompassing its clinical presentation, impact, management approaches, and challenges faced. It also highlights the need for increased research and awareness to enhance patient care and outcomes.
Methods
A comprehensive review of existing literature on GBS in Africa was conducted, focusing on clinical presentation, diagnosis, management, and its impact on patients and communities. Data sources included medical databases, research articles, and reports. Data was scoured from databases such as PubMed, Medline and Embase. A total of four hundred and fifty‐five articles and case studies were screened, with broader topic margins into GBS and different triggers, demographics, statistics, and variations in treatments across the world. These articles were further screened to match our inclusion criteria which focused on articles published after 2000 and which gave clearer insights into the presentations and situation of GBS in the African continent.
Results
GBS in Africa is characterized by a range of clinical presentations, with limited diagnostic resources and healthcare infrastructure. Patients often face long intervals between symptom onset and hospitalization, impacting outcomes. The syndrome's impact extends beyond physical symptoms, affecting patients' quality of life, employment, and community roles. Management involves immunotherapy, physiotherapy, and psychosocial support, but high therapy costs and incomplete recovery pose challenges. Research in Africa has grown in recent years but remains limited compared to other regions. Efforts are needed to expand research capacity, introduce early screening programs, and improve healthcare infrastructure.
Conclusion
GBS presents a significant healthcare challenge in Africa, with the potential for severe clinical outcomes. This literature review underscores the importance of enhancing research, awareness, and healthcare infrastructure. African‐led research initiatives offer hope for improved patient outcomes and healthcare system strengthening. By advocating for increased government support and resources, Africa can address the pressing needs of GBS patients and foster a brighter and healthier future for affected individuals on the continent.
Keywords: Africa, Guillain‐Barré Syndrome, healthcare challenges, peripheral neuropathies, research and management
1. Introduction
Guillain‐Barré Syndrome (GBS) represents a spectrum of peripheral neuropathies characterized by the sudden onset of symmetrical weakness in the extremities. This article aims to shed light on the landscape of GBS within the African continent, emphasizing the need for a comprehensive understanding of the condition.
GBS manifests with initial sensory symptoms, such as distally distributed paresthesia or numbness, indicating a symmetrical pattern of weakness [1]. The severity of the syndrome can escalate rapidly, potentially becoming life‐threatening and necessitating intensive care unit (ICU) admission along with mechanical ventilation [1]. ICU admission is particularly recommended for patients facing imminent respiratory insufficiency, severe autonomic dysfunction, pronounced swallowing impairment, diminished cough reflex, or rapidly progressing weakness [2].
The triggering factors of GBS, particularly in the context of the African continent, 30% of cases of GBS are attributed to Campylobacter jejuni. Growing evidence also suggests the likely involvement of Helicobacter pylori infection in the development of GBS [3]. Diseases that are endemic in the African continent like Malaria and Dengue fever have also been incriminated in initiating the post‐infectious sequelae that is GBS [4, 5]. Understanding these triggers becomes crucial in the comprehensive assessment of the syndrome.
The annual incidence of GBS varies from 0.81 to 1.89 cases per 100,000 population, displaying a linear increase over time [2]. While clinical evaluation is typically sufficient for diagnosis, additional procedures such as lumbar puncture and electrophysiological studies can further substantiate the diagnosis and differentiate between demyelinating and axonal subtypes of GBS [1]. The management of GBS requires a multidisciplinary approach, encompassing supportive medical care and immunotherapy. Both intravenous immunoglobulin (IVIg) and plasma exchange have proven efficacy as treatments for GBS [2].
In the African context, the limited body of research on GBS, coupled with the prohibitive costs associated with therapy, poses significant challenges to patient prognosis. This article seeks to bridge this gap by offering a comprehensive exploration of GBS in Africa, addressing both its clinical aspects and the contextual challenges that impact its management (Figure 1).
Figure 1.
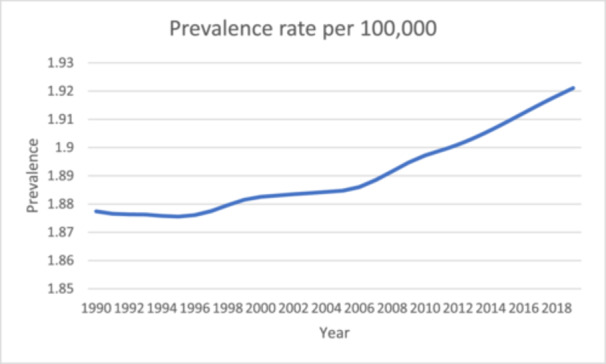
Prevalence rate of Guillain Barré Syndrome in Africa from 1990 to 2019 [4].
2. Methods
A literature review on GBS was conducted on African countries. The primary aim was to focus on clinical presentation of GBS, diagnosis, management, and its impact on patients and communities. Databases such as PubMed, Medline and Embase were scoured with certain keywords such as “Guillian Barre Syndrome.” “Africa,” “Diagnosis,” “Management,” and “Presentation.” The inclusion criteria were pre‐determined and all articles from 1999 to 2024 were considered. The criteria included published articles, government audits, documents from the ministry of health, and official statistics of the WHO. Exclusion criteria included preprints and articles before 1999, for better relevance and significance of data. A total of four hundred and fifty‐five articles and case studies were screened, with broader topic margins into GBS and different triggers, demographics, statistics, and variations in treatments across the world. These articles were further screened to match our inclusion criteria which focused on articles published after 1999 and which gave clearer insights into the presentations and situation of GBS in the African continent and finally twenty‐six references were selected.
3. Understanding GBS
GBS stands as a prevalent cause of acute flaccid paralysis, characterized by symmetrical limb weakness, along with hyporeflexia or areflexia [1]. Predominant GBS subtypes include acute inflammatory demyelinating polyneuropathy and acute motor axonal neuropathy [1]. Despite the syndrome's variable clinical course, a significant number of patients report a preceding infection as a trigger for symptom onset with Campylobacter Jejuni being the most commonly associated pathogen. Development of GBS has also been noted following Malaria and Dengue fever [4, 5], conditions endemic in African countries. GBS has also been reported following infection by COVID‐19 and Hepatitis E viruses [6, 7].
This post‐infectious phenomenon is attributed to antibodies, originally intended to combat the infection, mistakenly attacking the peripheral nervous system, a concept known as molecular mimicry. The specific nature of the antecedent infection and patient‐related host factors appear to influence the disease's form and severity. Among available treatment modalities, both IVIg and plasma exchange have proven effective in managing GBS, with IVIg often preferred for practical reasons [3]. Nonetheless, GBS frequently retains its severity; approximately 3%–10% of patients succumb to the condition, while 20% remain unable to ambulate even after 6 months. Furthermore, many patients endure persistent pain and fatigue for extended durations [3].
It is important to emphasize the importance of a thorough clinical evaluation in the diagnosis of GBS and early recognition of initial symptoms. Furthermore, supplementary diagnostic modalities such as lumbar puncture and nerve conduction studies are important in diagnosing as well as classifying GBS. The management of GBS encompasses a wide variety of specialties. This multidisciplinary approach to treatment and recovery includes immunotherapy, rehabilitative physiotherapy, and supportive care. A study conducted on patients admitted to a neurology department at a tertiary hospital in Burkina Faso revealed that weakness in the extremities was the most frequently reported initial symptom, occurring in 32 patients (91.4%), while 15 patients (42.9%) presented with paresthesia. Motor deficits involved all four limbs in 21 patients (65.6%) and both lower limbs in 14 patients (11.4%). The onset of symptoms was consistently progressive in all patients [2]. (Figure 2).
Figure 2.
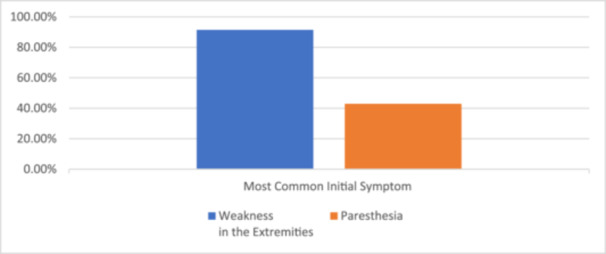
The most common initial symptom in patients with Guillain‐Barré Syndrome GBS admitted to the neurology department of a tertiary hospital in Burkina Faso [2].
4. Diagnosis and Clinical Presentation
GBS is characterized by a swift and symmetrical weakening of the limbs, coupled with hyporeflexia or areflexia. Nevertheless, GBS exhibits considerable heterogeneity concerning the occurrence, scope, and severity of cranial nerve deficits, sensory manifestations, muscular weakness, ataxia, pain, autonomic dysfunction, and the disease's overall progression [1]. Timely diagnosis and intervention hold critical significance in GBS management. However, the primary hurdles in diagnosing GBS within the African context are the limited availability of diagnostic facilities, healthcare infrastructure, and a shortage of adequately trained healthcare professionals (Table 1).
Table 1.
Diagnostic criteria of GBS; acute inflammatory demyelinating polyneuropathy and acute motor axonal neuropathy [1].
| Features required for diagnosis of GBS | |
| Progressive weakness in legs and arms (sometimes initially only in legs) | |
| Areflexia (or decreased tendon reflexes) in weak limbs |
| Acute inflammatory demyelinating polyneuropathy (AIDP) | |
|---|---|
| Additional symptoms | Nerve conduction study findings |
| Progressive phase lasts days to 4 weeks | Features of demyelination (only assessable if distal CMAP amplitude is > 10% LLN) |
| Relative symmetry of symptoms | Prolonged distal motor latency |
| Mild sensory symptoms or signs | Decreased motor nerve conduction velocity |
| Cranial nerve involvement, especially bilateral weakness of facial muscles | Increased F‐wave latency, conduction blocks and temporal dispersion |
| Autonomic dysfunction | |
| Pain (often) | |
| Acute motor axonal neuropathy (AMAN) | |
|---|---|
| Additional symptoms | Nerve conduction study findings |
| Progressive phase lasts days to 4 weeks | No features of demyelination (or, one demyelinating feature in one nerve if distal CMAP amplitude is < 10% LLN) |
| Relative symmetry of symptoms | Distal CMAP amplitude is < 80% LLN in at least two nerves |
| No sensory symptoms or signs | Transient motor nerve conduction block may be present (possibly caused by antiganglioside antibodies) |
| Cranial nerve involvement (rarely) | No features of demyelination (or, one demyelinating feature in one nerve if distal CMAP amplitude is < 10% LLN) |
| Autonomic dysfunction | — |
| Pain (sometimes) | — |
Certain qualitative tools have been developed to diagnose GBS) known as the Brighton Criteria. The criteria include: physical exam, clinical history, laboratory, and imaging findings. The Brighton criteria also classifies patients based on the completeness of the data. Some of the key diagnostic characteristics for GBS include symmetrical limb weakness at admission, decreased reflexes in weak limbs at admission, increased protein level in cerebrospinal fluid (CSF) in 77% of patients.
5. Impact of GBS
The most profound impact of GBS is evident in the enduring consequences it imposes on individuals' daily lives, significantly affecting their overall quality of life [5]. An estimated 20% of GBS survivors find themselves reliant on external assistance for mobility [5, 8]. Additionally, many individuals grapple with lingering pain and fatigue, leading to occupational shifts, including reduced work capacity or transition to lower‐paying positions. These changes are primarily attributed to the loss of physical strength, diminished concentration, heightened anxiety, apathy, depression, and emotional instability, often culminating in unemployment and a subsequent reduction in income [5, 6, 7, 9]. GBS can also result in the loss of sexual function, prompting adjustments in leisure activities to align with the individual's altered health status [7, 9]. Partners of GBS patients often need to adapt their lifestyles to provide emotional support and assume additional responsibilities in household management and income generation [6]. On a broader scale, communities experience the loss of a productive workforce and the social contributions of these individuals, who also grapple with anxiety regarding the potential recurrence of the disease [6] (Table 2).
Table 2.
A summary of the effects of GBS to the different sectors.
| Effects on | |
|---|---|
| The individual | Inability to walk |
| Residual pain | |
| Fatigue | |
| Change or loss of job | |
| Impaired concentration | |
| Anxiety | |
| Apathy | |
| Depression | |
| Emotional instability | |
| Loss of sexual function | |
| Change of leisure | |
| To the partners | Change in lifestyle to support patients emotionally |
| Adapting to fill in the house keeping and income generation | |
| To the communities | Loss of workforce |
| Loss of social contribution of the individuals |
6. Treatment and Management Approaches Used in Africa
The approach to managing GBS revolves around comprehensive medical and supportive care [1]. During the acute phase, immunotherapy plays a central role, typically employing IVIg and/or plasma exchange administered over sessions, often up to five sessions within a 2‐week timeframe based on body weight considerations [5, 8, 10, 11]. Immunotherapy is typically initiated for individuals unable to walk unaided for a distance of 10 m [8, 12]. Close monitoring of respiratory, hemodynamic, and cardiac functions serves as critical indicators of autonomic function during this phase, guiding decisions regarding potential transfer to the ICU [5, 13]. The progressive phase, extending beyond the initial 2 weeks, necessitates management of secondary complications stemming from immobility, such as deep venous thrombosis, which is typically addressed with low molecular weight heparin [5, 13]. Proper management of potential bladder and bowel dysfunction is also essential during this phase [5, 13].
Amidst these medical interventions, the early initiation of physiotherapy, rehabilitation, and psychosocial support, including physical therapy sessions and exercise, should not be overlooked. It's worth noting that in Africa, the cost of therapy remains a significant challenge, along with treatment failures, incomplete recovery, and treatment‐related fluctuations that extend the duration of care. There is ongoing promise in the use of recombinant antibodies, sialylated IgG, anti‐C1q, anti‐C5, Eculizumab, and Erythropoietin in GBS management [11, 13]. However, it's important to highlight that corticosteroids have not shown significant efficacy in the treatment of GBS [11]. (Table 3).
Table 3.
A summary of various treatment modalities used.
| Acute phase | Major modalities | Intravenous Immunoglobulin (IVIg) |
| Plasma exchange | ||
| Monitoring for | Respiratory function | |
| Hemodynamic function | ||
| Cardiac function | ||
| Progressive phase | Management of complications | Deep vein thrombosis with LMWH |
| Bladder and bowel dysfunction | ||
| Other modalities | Physical therapy | Physiotherapy |
| Rehabilitation | ||
| Psychosocial support | ||
| Hopeful modalities | Recombinant antibodies | |
| Sialylated IgG | ||
| Anti‐C1q | ||
| Anti‐C5 | ||
| Eculizumab | ||
| Erythropoietin |
The cost of immunotherapy for GBS can vary based on factors such as the specific treatment regimen, the country's healthcare infrastructure, and whether the treatment is provided in public or private healthcare settings. Additionally, prices for medications and medical services can change over time. Currently, there is no official information about the price of immunotherapy in Africa. But, there is a research paper where it's calculated the cost of IVIg and TPE per kg. The cheapest one is R 59728.50 (2917eur) for 30 kg, while for 75 kg is R 100134.00 (4890eur). The currency used is Rands (used in South Africa). The percentage of how many people can actually afford to be treated for GBS is not available, but because of the expensive treatments, lots of people are getting treated with cheaper options–like painkillers and physical rehabilitation which is evident based on comparison studies [14].
A table elucidating a vivid comparison between Epidemiology, Clinical Features and Treatment of GBS in Africa versus HIC versus LMIC is formulated to better understand the discrepancies. (Table 4).
Table 4.
Comparison between HIC, LMIC and Africa in terms of GBS epidemiology, clinical features and treatment [15].
| Epidemiology | ||
| High‐Income Countries (HIC): GBS is relatively well‐documented in HICs, where comprehensive healthcare systems often contribute to better surveillance and reporting. In HICs, incidence rates of GBS vary but are generally estimated to be around 1‐2 cases per 100,000 individuals per year. Low‐ and Middle‐Income Countries | Lower‐Middle Income Countries (LMIC): Epidemiological data in LMICs may be less reliable due to challenges in healthcare infrastructure, underreporting, and varying access to medical care. Incidence rates may be lower or less accurately documented compared to HICs. | Africa: GBS epidemiology in Africa is not as extensively studied as in some HICs, and data may be limited in certain regions. Challenges such as underreporting, varying healthcare access, and differences in infectious disease prevalence may impact the incidence and understanding of GBS. |
| Clinical Features | ||
| High‐Income Countries (HIC): GBS typically presents with acute onset of symmetric weakness and can progress rapidly. Common preceding infections include Campylobacter jejuni, cytomegalovirus, and Epstein‐Barr virus. | Low‐ and Middle‐Income Countries (LMIC): Clinical features in LMICs may be influenced by the prevalence of infectious diseases specific to those regions, potentially impacting the spectrum of preceding infections. | Africa: Similar clinical features are expected in Africa, with variations influenced by regional infectious disease patterns. Limited healthcare access may contribute to delayed presentation and diagnosis. |
| Treatment | ||
| High‐Income Countries (HIC): Treatment often involves either Intravenous Immunoglobulin (IVIG) or Plasmapheresis (PE). HICs generally have more resources for timely diagnosis and access to these treatments. | Low‐ and Middle‐Income Countries (LMIC): Affordability and accessibility are challenges in LMICs, and some patients may face difficulties in receiving IVIG or PE due to high costs. | Africa: Similar challenges to LMICs may exist in Africa, with a subset of patients potentially struggling with the cost and availability of immunotherapy. Treatment accessibility may vary within the continent based on healthcare infrastructure and economic disparities. |
7. Adressing the Challenges in Africa
Africa faces numerous challenges in the healthcare sector, but there are specific areas that hold the potential for positive transformation. One crucial aspect is the improvement of awareness and education surrounding GBS. GBS often remains undiagnosed or misdiagnosed and untreated due to limited awareness among healthcare professionals and the general population [2]. The authors believe that this lack of awareness extends not only to the syndrome itself but also to the infectious diseases that can induce GBS. Access to healthcare services is a crucial aspect, with many regions facing shortages of trained medical personnel and essential medical equipment. Improving the capacity of healthcare facilities to diagnose and treat GBS is essential. Telemedicine and mobile health initiatives can play a vital role in bridging the gap, enabling remote consultation and support.
Furthermore, establishing regional and national GBS registries can enhance surveillance and data collection, aiding in understanding the epidemiology of GBS in Africa. This data‐driven approach can inform public health policies and resource allocation. Investing in healthcare infrastructure can address these challenges by attracting and employing healthcare professionals, particularly specialists in neurology. This investment can significantly enhance Africa's capacity to diagnose and effectively treat GBS [15]. (Figure 3) Launching awareness campaigns, providing training for healthcare workers, and disseminating information can significantly enhance understanding of GBS. This, in turn, can facilitate early detection and more effective management of the condition [15].
Figure 3.
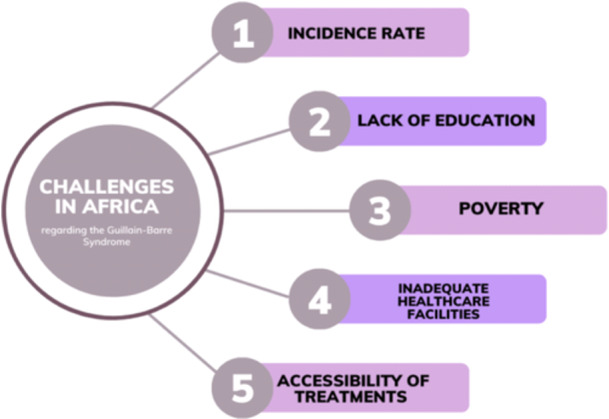
Challenges in Africa pertaining to Guillain Bare syndrome.
In Africa, one of the initial signs of GBS that is often unnoticed is altered mental status, along with paralysis of the extremities, facial palsy, and weakness and tingling in the hands and feet [16]. While GBS primarily affects motor nerves, some African patients have noted experiences of sensory deficits. GBS frequently presents with facial or pharyngeal weakness, and a substantial portion of hospitalized GBS patients, approximately one‐third, may require mechanical ventilation due to respiratory or oropharyngeal muscle weakness [17]. Oropharyngeal muscle weakness, on the other hand, affects swallowing and airway protection, increasing the risk of aspiration pneumonia. The onset of these complications can be rapid and life‐threatening, emphasizing the need for vigilant monitoring and intervention, especially pertaining to the African cases [18]. Patients with GBS in Africa have reported their most significant weakness phase within 2 weeks after symptom onset [19, 20].
Strengthening healthcare infrastructure and expertize is another vital facet. Many African countries, including low‐income nations, grapple with inadequate healthcare facilities, poor hygiene, insufficient beds, a shortage of skilled professionals, and limited resources. Enhancing the accessibility of treatments and therapies is essential for improving health outcomes. The authors have noted that high costs, limited availability, and geographical barriers often prevent Africans from accessing necessary treatments and therapies. Governments and healthcare organizations should work together to negotiate affordable prices for medications and collaborate with pharmaceutical companies to ensure a consistent supply of essential drugs. (Figure 4) (Figure 5).
Figure 4.

Symptoms for early detection of GBS.
Figure 5.
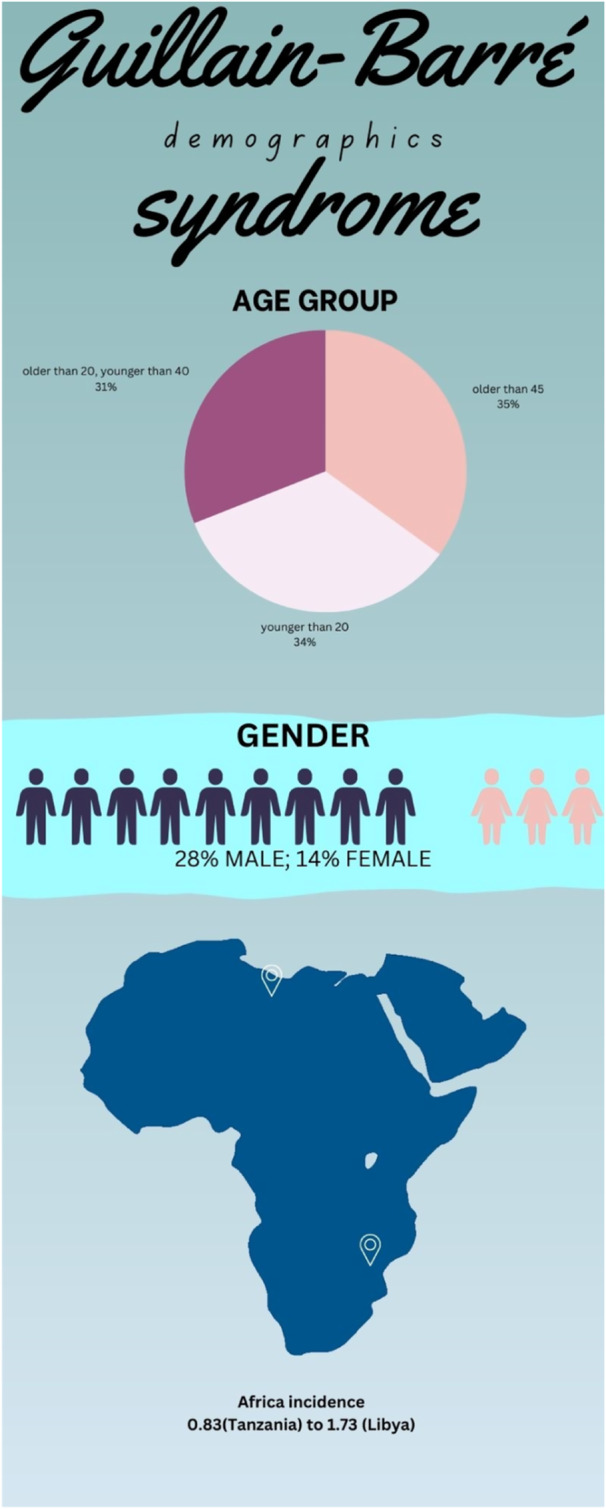
– Demographics of Guillain Barre Syndrome.
Empowering patient support networks and advocacy groups also have a significant role. These organizations provide a platform for individuals affected by GBS to share their experiences, access information, help each other and advocate for their rights. Strengthening these networks by involving them in policy‐making processes will address patient needs effectively [15].
8. Research and Innovation in GBS
As of 2021, the data on GBS in lower‐middle‐income countries including. Africa is limited due to fewer studies conducted. African and Latin American countries are underrepresented in global research and Africa currently contributes only 2% of global research output [15, 21]. However, the past decade has been promising for the continent in increasing scientific research due to capacity‐building investment, regional and local financing from the government and the global north, and national and international collaborations [21]. Although a firm scientific research foundation has been entrenched in Africa, expansion and improvement of GBS research capacity is required. More studies such as case‐control, cohort, observational cohort studies, and systemic population‐based surveillance must be conducted to understand GBS's incidence and overall burden. Clinical intervention studies to devise affordable treatment must be designed considering the specific health challenges faced in Africa. Papri et al. outlines the significance of programs for early screening which must be introduced and the need for financial investments in health care infrastructure to upgrade diagnostic facilities which can help avoid selection bias at the hospital level due to long intervals between onset of weakness and hospitalizations, often observed in GBS patients [15]. The key ongoing challenge faced by Low‐to‐Middle Income countries (LMICs) like Africa in treating GBS is the lack of early recognition and escalation of treatment [22, 23, 24, 25, 26]. Existing and newer prognostic models should be validated and used in LMIC as they will help clinicians accurately identify patients who need ICU care at the earliest thereby improving the management of individual patients and increasing the efficiency of ICU services in low‐resource settings. A sustainable clinical trial infrastructure must be established to support research. High‐quality diagnostic laboratories and training programs for healthcare professionals must be curated for better management of patients of GBS and for clinical research [15] (Figure 6).
Figure 6.
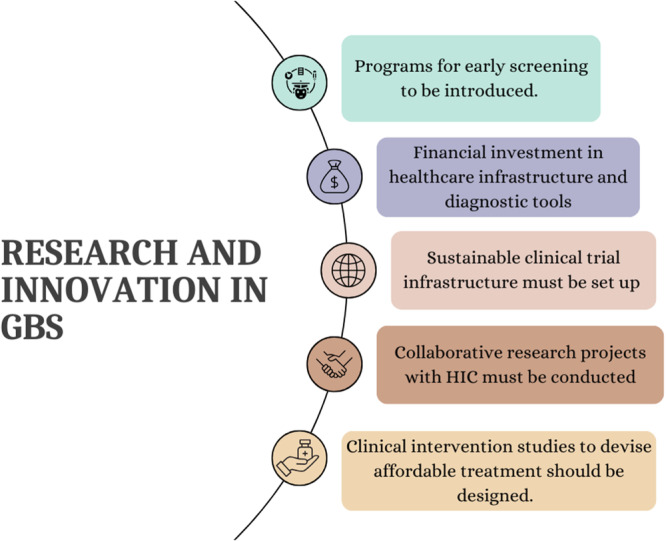
Research and Innovation in Guillain Barré Syndrome.
9. Conclusion
In conclusion, GBS, despite being underreported in Africa, poses a significant health challenge with the potential for severe clinical outcomes if not addressed effectively. To mitigate the impact of GBS on the continent, it is imperative to enhance the scope and quality of research efforts. This necessitates a concerted effort to advocate for increased government support and allocation of resources towards GBS research in Africa.
The significance of African‐led research cannot be overstated. By fostering and promoting research initiatives originating within the continent, there is a unique opportunity to provide hope and tangible improvements in outcomes and quality of life for GBS patients in Africa. This approach not only addresses the pressing healthcare needs of the region but also empowers local expertize and strengthens healthcare systems to better manage and mitigate the effects of GBS. Ultimately, advancing research and increasing government support for GBS research in Africa will contribute to a brighter and healthier future for those affected by this condition on the continent.
Author Contributions
All authors have approved the final manuscript for submission. Olivier Uwishema: conceptualization, writing–reviewing, editing, supervising and designing, project administration. Burhan Kantawala: supervising the draft, reviewing and editing, project administration. Anamarija Minova: writing the first draft and revising. Jonathan Babuya: writing the first draft and revising. Essey Ketema Wodajo: writing the first draft and revising. Sucharu Asri: writing the first draft and revising. Magda Wojtara: reviewed and edited the second draft. Olivier Uwishema: reviewed and edited the final draft.
Disclosure
The corresponding author (Olivier UWISHEMA) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Ethics Statement
Tha authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank Oli Health Magazine Organization (OHMO)'s members for their contributions and support for this manuscript.
Data Availability Statement
Tha authors have nothing to report.
References
- 1. van den Berg B., Walgaard C., Drenthen J., Fokke C., Jacobs B. C., and van Doorn P. A., “Guillain–Barré Syndrome: Pathogenesis, Diagnosis, Treatment and Prognosis,” Nature Reviews Neurology 10 (2014): 469–482, 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 2. Dabilgou A. A., Kaboré R., Dravé A., et al., “Guillain‐Barré Syndrome (GBS) in Sub‐Saharan Africa: Experience From a Tertiary Level Hospital in Burkina Faso,” PAMJ Clinical Medicine 8 (2022): 1–8, 10.11604/pamj-cm.2022.8.15.31957. [DOI] [Google Scholar]
- 3. Dardiotis E., Sokratous M., Tsouris Z., et al., “Association Between Helicobacter pylori Infection and Guillain‐Barré Syndrome: A Meta‐Analysis,” European Journal of Clinical Investigation 50, no. 5 (2020): 1–9, https://pubmed.ncbi.nlm.nih.gov/32124432/. [DOI] [PubMed] [Google Scholar]
- 4. Ahmed A., El‐Amin R., Musa A. M., et al., “Guillain‐Barre Syndrome Associated With COVID‐19 Infection: A Case Series,” Clinical Case Reports 11, no. 2 (2023): 1–6, https://pubmed.ncbi.nlm.nih.gov/36852114/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmed A., EL‐Sadig S. M., and Siddig E. E., “Guillain–Barre Syndrome Associated With Hepatitis E Virus Infection: A Case Report,” Clinical Case Reports 11, no. 9 (2023): 1–4, https://pubmed.ncbi.nlm.nih.gov/37655129/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uwishema O., Frederiksen K. S., Correia I. F. S., Mahmoud A., Onyeaka H., and Dost B., “The Impact of COVID‐19 on Patients With Neurological Disorders and Their Access to Healthcare in Africa: A Review of the Literature,” Brain and Behavior 12, no. 9 (2022): e2742, 10.1002/brb3.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willison H. J., Jacobs B. C., and van Doorn P. A., “Guillain‐Barré Syndrome,” The Lancet 388 (2016): 717–727, 10.1016/s0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 8. GBD Results 2023. Institute for Health Metrics and Evaluation, https://vizhub.healthdata.org/gbd-results/.
- 9. Hughes R. A. C., Swan A. V., Raphael J.‐C., Annane D., van Koningsveld R., and van Doorn P. A., “Immunotherapy for Guillain‐Barre Syndrome: A Systematic Review,” Brain 130 (2007): 2245–2257, 10.1093/brain/awm004. [DOI] [PubMed] [Google Scholar]
- 10. Bernsen R. A. J. A. M., de Jager A. E. J., Schmitz P. I. M., and van der Meché F. G. A., “Long‐Term Impact on Work and Private Life After Guillain–Barré Syndrome,” Journal of the Neurological Sciences 201 (2002): 13–17, 10.1016/s0022-510x(02)00158-2. [DOI] [PubMed] [Google Scholar]
- 11. Bersano A., Carpo M., Allaria S., Franciotta D., Citterio A., and Nobile‐Orazio E., “Long Term Disability and Social Status Change After Guillain–Barré Syndrome,” Journal of Neurology 253 (2006): 214–218, 10.1007/s00415-005-0958-x. [DOI] [PubMed] [Google Scholar]
- 12. Bernsen R. A. J. A. M., de Jager A. E. J., Schmitz P. I. M., and van der Meché F. G. A., “Residual Physical Outcome and Daily Living 3 to 6 Years After Guillain‐Barre Syndrome,” Neurology 53 (1999): 409, 10.1212/wnl.53.2.409. [DOI] [PubMed] [Google Scholar]
- 13. Hughes R. A. C., Swan A. V., and van Doorn P. A., “Intravenous Immunoglobulin for Guillain‐Barré Syndrome,” Cochrane Library 2019 (2014): 1–49, 10.1002/14651858.cd002063.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poole C., Model of Direct Cost Comparison of Polyvalent Human Normal Immunoglobulin and Therapeutic Plasma Exchange as First Line Treatment for Guillain Barre Syndrome in the Public Sector in South Africa. Conference Paper (2014), https://www.researchgate.net/publication/261991112_Model_of_direct_cost_comparison_of_polyvalent_human_normal_immunoglobulin_and_therapeutic_plasma_exchange_as_first_line_treatment_for_Guillain_Barre_Syndrome_in_the_Public_Sector_in_South_Africa.
- 15. Papri N., Islam Z., Leonhard S. E., Mohammad Q. D., Endtz H. P., and Jacobs B. C., “Guillain–Barré Syndrome in Low‐Income and Middle‐Income Countries: Challenges and Prospects,” Nature Reviews Neurology 17 (2021): 285–296, 10.1038/s41582-021-00467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulroy E. and Anderson N. E., “Altered Mental Status in “Guillain‐Barré Syndrome” –A Noteworthy Clinical Clue,” Annals of Clinical and Translational Neurology 7 (2020): 2489–2507, 10.1002/acn3.51226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meena A., Khadilkar S., and Murthy J. M. K., “Treatment Guidelines for Guillain‐Barré Syndrome,” Annals of Indian Academy of Neurology 14 (2011): 73, 10.4103/0972-2327.83087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shang P., Zhu M., Baker M., Feng J., Zhou C., and Zhang H.‐L., “Mechanical Ventilation in Guillain–Barré Syndrome,” Expert Review of Clinical Immunology 16, no. 11 (2020): 1053–1064, 10.1080/1744666x.2021.1840355. [DOI] [PubMed] [Google Scholar]
- 19. Guillain‐Barre syndrome . Mayo Clinic 2022, https://www.mayoclinic.org/diseases-conditions/guillain-barre-syndrome/symptoms-causes/syc-20362793.
- 20. Gordon P. H. and Wilbourn A. J., “Early Electrodiagnostic Findings in Guillain‐Barré Syndrome,” Archives of Neurology 58 (2001): 913, 10.1001/archneur.58.6.913. [DOI] [PubMed] [Google Scholar]
- 21. Kasprowicz V. O., Chopera D., Waddilove K. D., et al., “African‐Led Health Research and Capacity Building ‐ Is It Working?,” BMC Public Health 20 (2020): 1104, 10.1186/s12889-020-08875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onofrey L., Naus C., Thakur K. T., Kadyaudzu C., and Prin M., “Two Success Stories in the Management of Guillain–Barré Syndrome Illustrate the Challenges of Intensive Care Unit Care in Malawi,” Tropical Doctor 51 (2021): 19–24, 10.1177/0049475520962757. [DOI] [PubMed] [Google Scholar]
- 23. Uwishema O., Rai A., Nicholas A., et al., “Childhood Tuberculosis Outbreak in Africa: Is It a Matter of Concern?,” International Journal of Surgery 109 (2023): 1539–1542, 10.1097/js9.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin J., Gao Q., Xiao K., Tian D., Hu W., and Han Z., “Efficacy of Therapies in the Treatment of Guillain‐Barre Syndrome: A Network Meta‐Analysis,” Medicine 100 (2021): e27351, 10.1097/md.0000000000027351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuki N. and Hartung H.‐P., “Guillain–Barré Syndrome,” New England Journal of Medicine 366 (2012): 2294–2304, 10.1056/nejmra1114525. [DOI] [PubMed] [Google Scholar]
- 26. Shahrizaila N., Lehmann H. C., and Kuwabara S., “Guillain‐Barré Syndrome,” The Lancet 397 (2021): 1214–1228, 10.1016/s0140-6736(21)00517-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Tha authors have nothing to report.


