Abstract
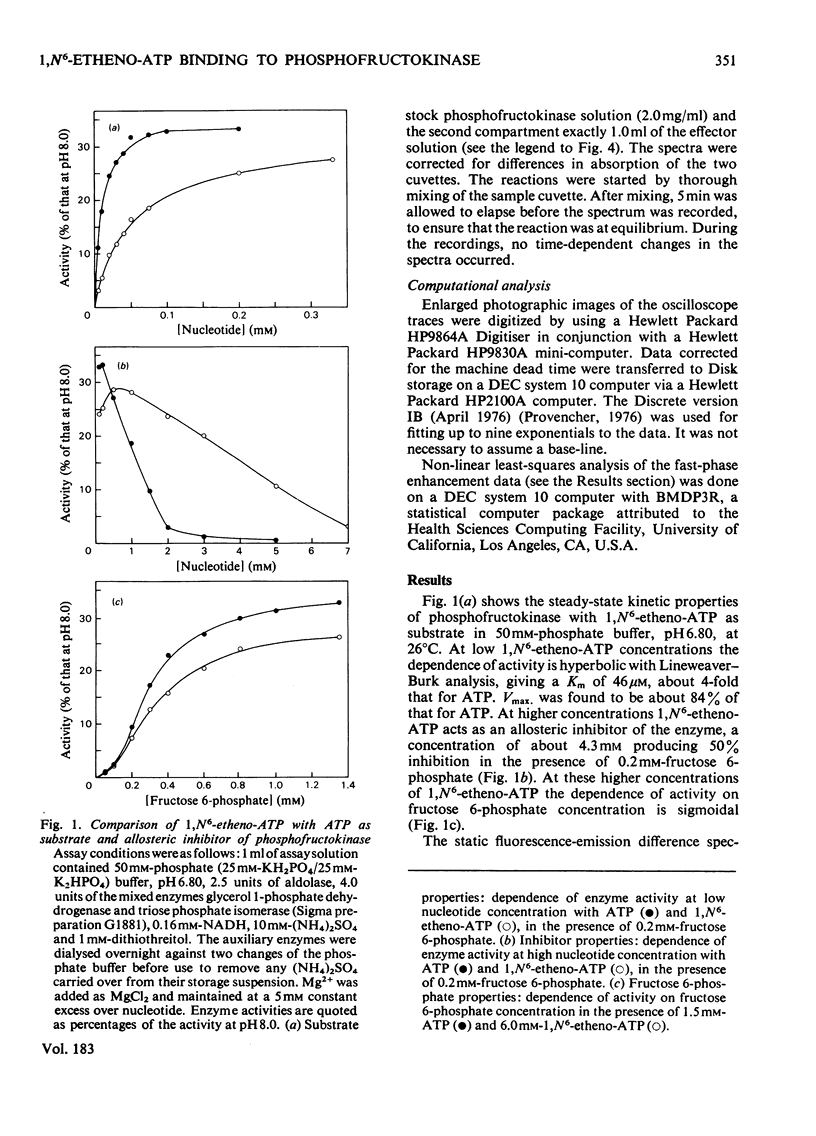
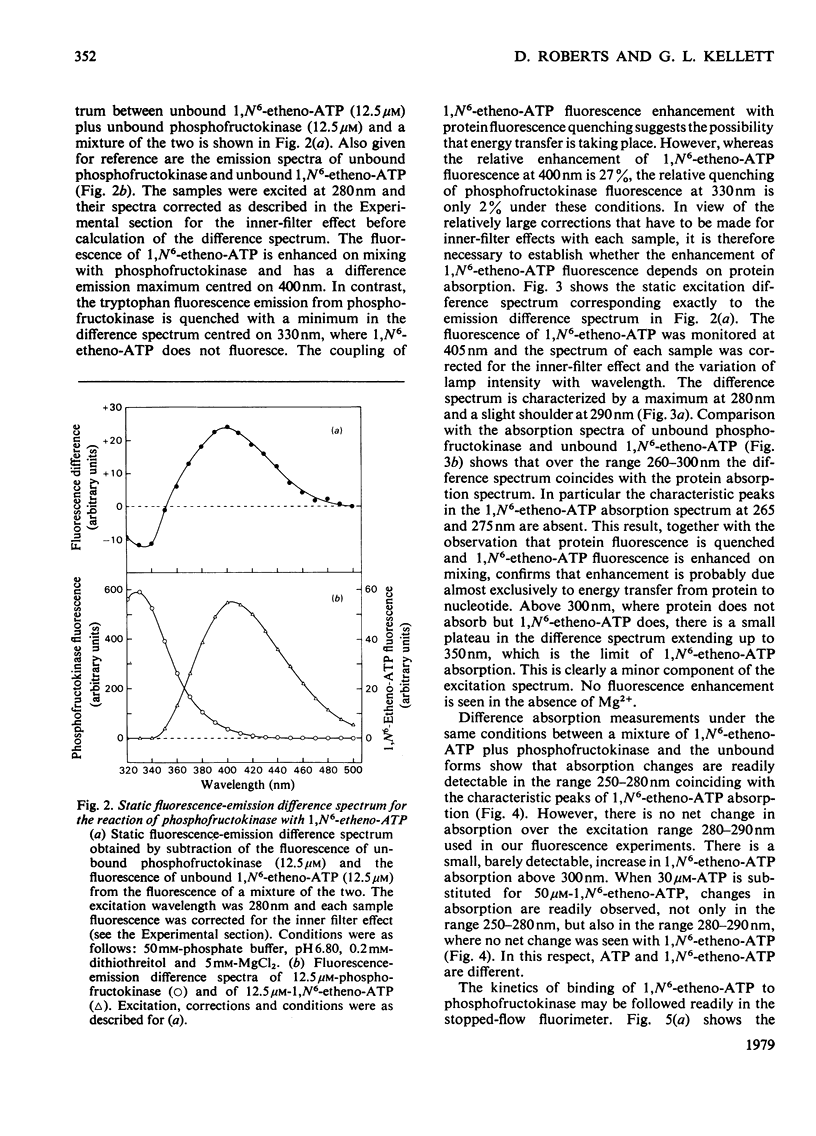
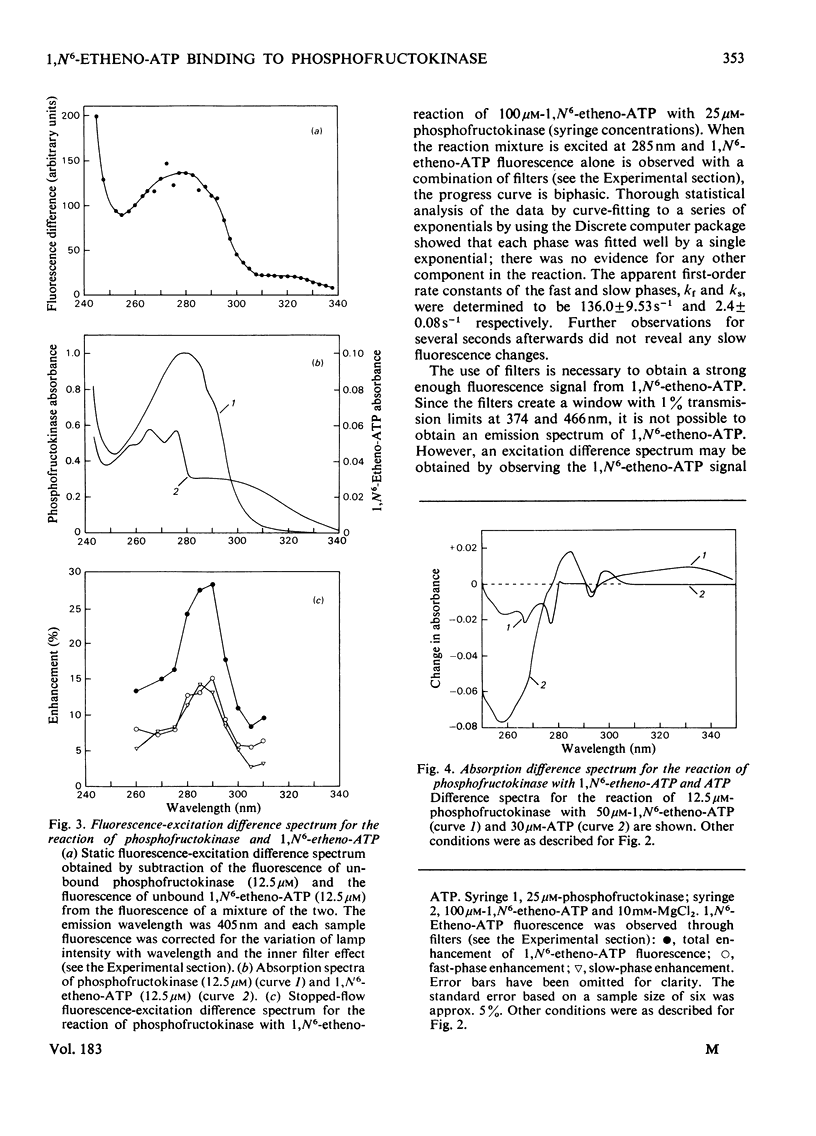
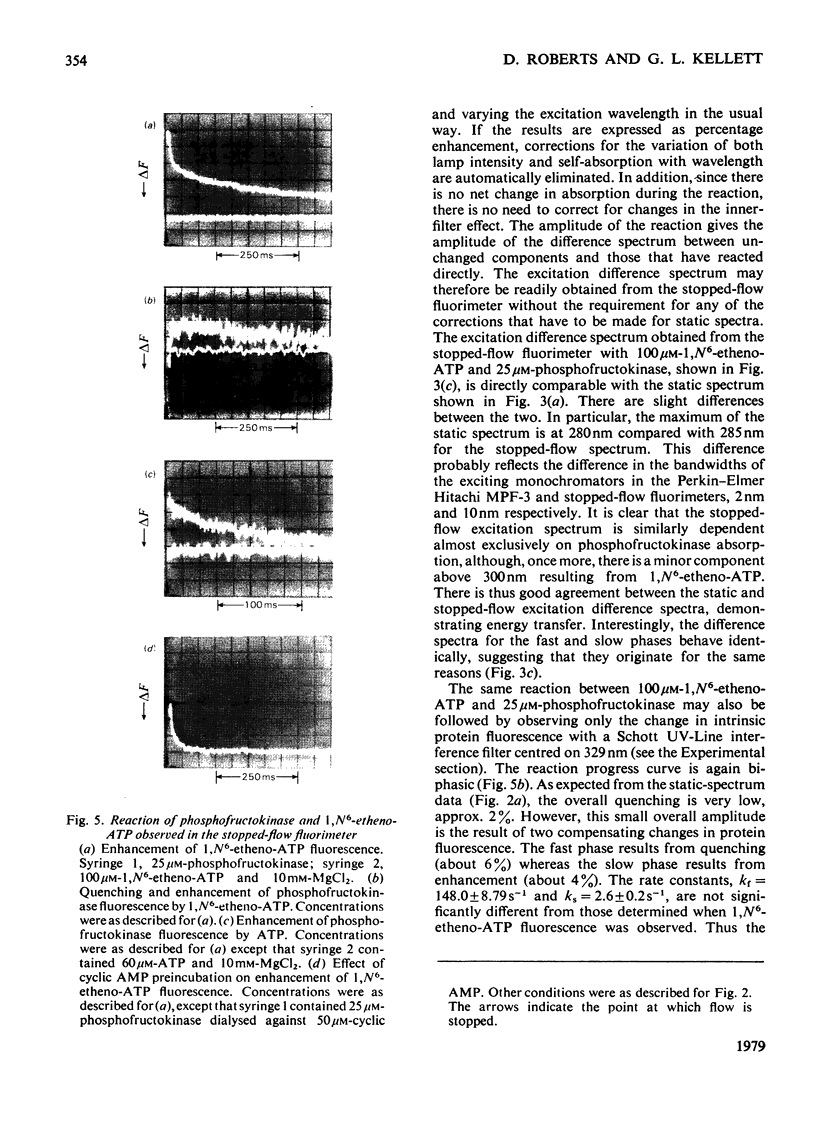
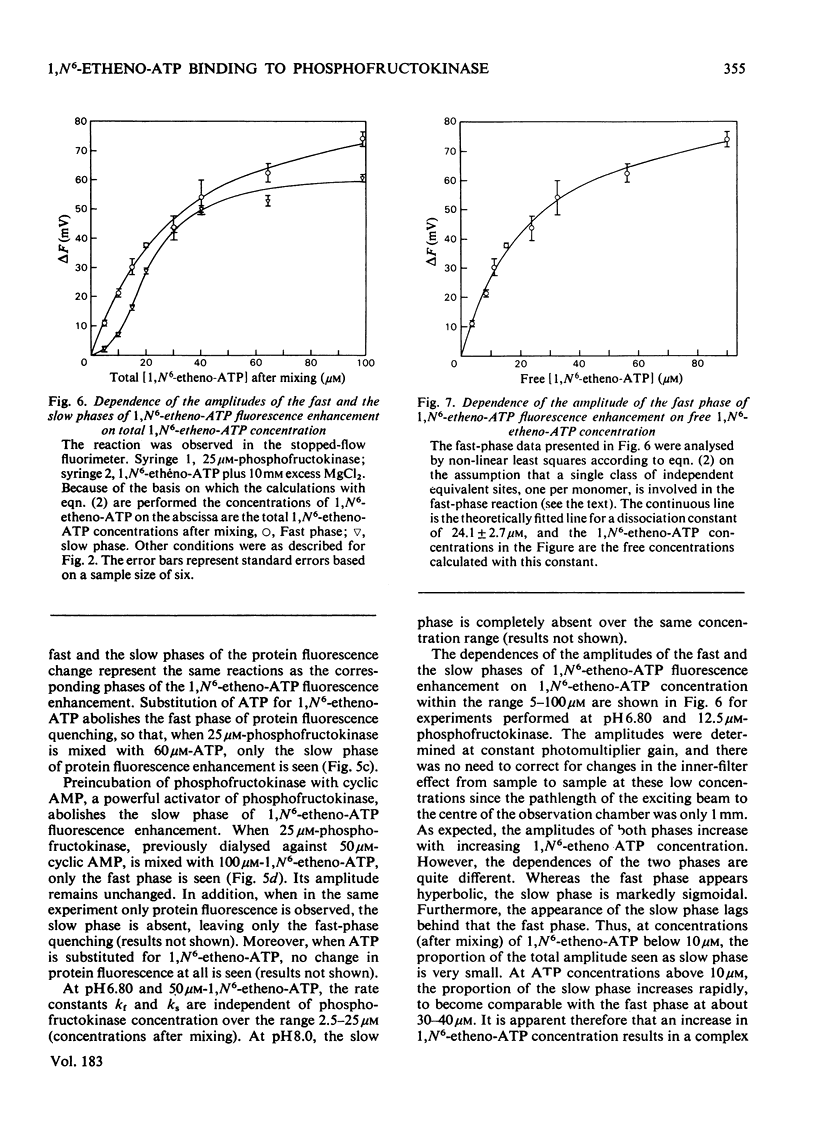
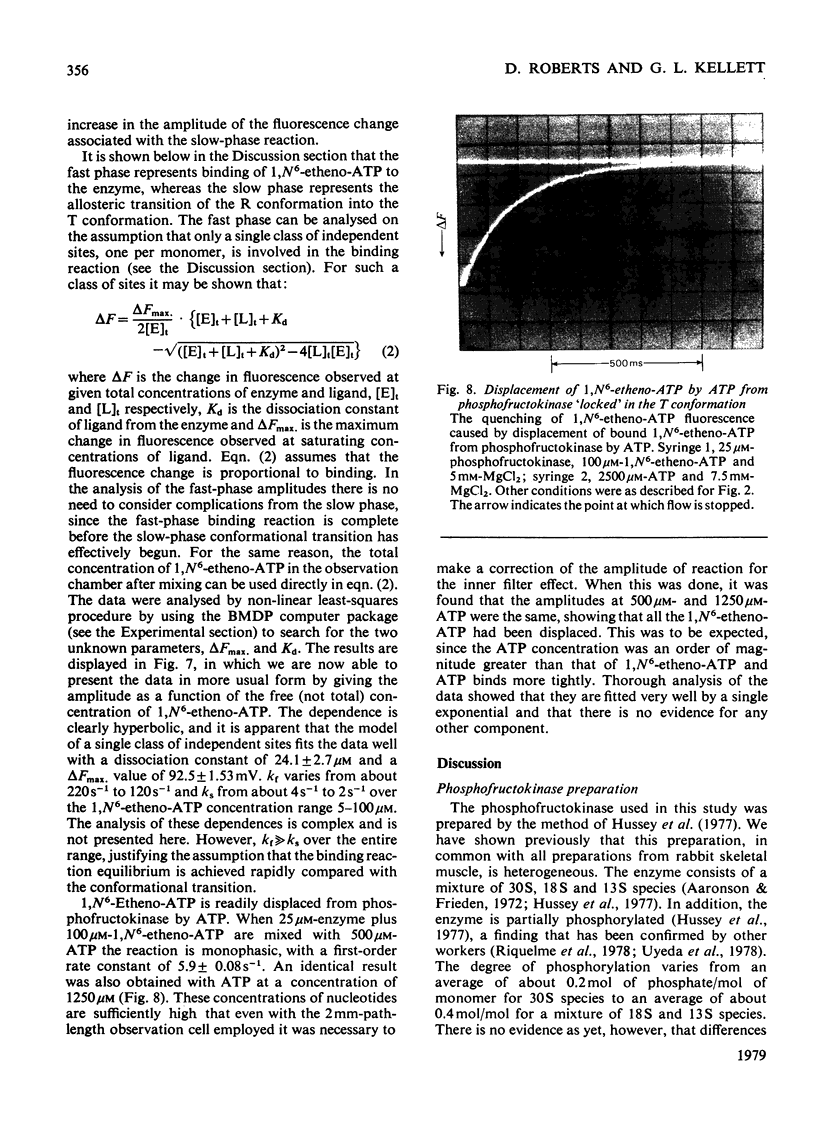
1. The fluorescent ATP analogue 1,N6-etheno-ATP is a good substrate and an efficient allosteric inhibitor of rabbit skeletal-muscle phosphofructokinase. 2. Fluorescence energy transfer occurs between bound 1,N6-etheno-ATP and phosphofructokinase. 1,N6-Etheno-ATP fluorescence is enhanced, intrinsic protein fluorescence is quenched, and the excitation spectrum of 1,N6-etheno-ATP fluorescence is characteristic of protein absorption. 3. The binding reaction of 1,N6-etheno-ATP observed by stopped-flow fluorimetry is biphasic. The fast phase results from binding to the catalytic site alone. The slow phase results from the allosteric transition of the R conformation into the T conformation induced by the binding of 1,N6-etheno-ATP to the regulatory site. 4. The fluorescence signal that allows the transition of the R conformation into the T conformation to be observed does not arise from 1,N6-etheno-ATP bound to the regulatory site. It arises instead from 1,N6-etheno-ATP bound to the catalytic site as a consequence of changes at the catalytic site caused by the transition of the R conformation into the T conformation. 5. In the presence of excess of Mg2+, the affinity of 1,N6-etheno-ATP for the regulatory site is very much greater in the T state than in the R state.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Frieden C. Rabbit muscle phosphofructokinase: studies on the polymerization. The behavior of the enzyme at pH 8, pH 6, and intermediate pH values. J Biol Chem. 1972 Dec 10;247(23):7502–7509. [PubMed] [Google Scholar]
- Colombo G., Tate P. W., Girotti A. W., Kemp R. G. Interaction of inhibitors with muscle phosphofructokinase. J Biol Chem. 1975 Dec 25;250(24):9404–9412. [PubMed] [Google Scholar]
- Goldhammer A. R., Hammes G. G. Steady-state kinetic study of rabbit muscle phosphofructokinase. Biochemistry. 1978 May 16;17(10):1818–1812. doi: 10.1021/bi00603a002. [DOI] [PubMed] [Google Scholar]
- Hill D. E., Hammes G. G. An equilibrium binding study of the interaction of fructose 6-phosphate and fructose 1,6-bisphosphate with rabbit muscle phosphofructokinase. Biochemistry. 1975 Jan 28;14(2):203–213. doi: 10.1021/bi00673a003. [DOI] [PubMed] [Google Scholar]
- Hussey C. R., Liddle P. F., Ardron D., Kellett G. L. The isolation and characterization of differentially phosphorylated fractions of phosphofructokinase from rabbit skeletal muscle. Eur J Biochem. 1977 Nov 1;80(2):497–506. doi: 10.1111/j.1432-1033.1977.tb11905.x. [DOI] [PubMed] [Google Scholar]
- Kemp R. G. Allosteric properties of muscle phosphofructokinase. I. Binding of magnesium adenosine triphosphate to the inhibitory site. Biochemistry. 1969 Aug;8(8):3162–3168. doi: 10.1021/bi00836a005. [DOI] [PubMed] [Google Scholar]
- Kemp R. G., Krebs E. G. Binding of metabolites by phosphofructokinase. Biochemistry. 1967 Feb;6(2):423–434. doi: 10.1021/bi00854a009. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Leonard K. R., Walker I. O. The self-association of rabbit-muscle phosphofructokinase. Eur J Biochem. 1972 Apr 11;26(3):442–448. doi: 10.1111/j.1432-1033.1972.tb01785.x. [DOI] [PubMed] [Google Scholar]
- Liddle P. F., Ardron D., Jacobs D. J., Kellett G. L. The kinetics of effector-induced association-dissociation reactions of phosphofructokinase. Biochem Soc Trans. 1976;4(1):87–88. doi: 10.1042/bst0040087. [DOI] [PubMed] [Google Scholar]
- Liddle P. F., Jacobs D. J., Kellett G. L. A stopped-flow laser light-scattering photometer for the study of the kinetics of macromolecular association-dissociation reactions. Anal Biochem. 1977 May 1;79(1-2):276–290. doi: 10.1016/0003-2697(77)90403-1. [DOI] [PubMed] [Google Scholar]
- Liou R. S., Anderson S. R. Binding of ATP and of 1,N6-ethenoadensone triphosphate to rabbit muscle phosphofructokinase. Biochemistry. 1978 Mar 21;17(6):999–1004. doi: 10.1021/bi00599a009. [DOI] [PubMed] [Google Scholar]
- Lorenson M. Y., Mansour T. E. Studies on heart phosphofructokinase. Binding properties of native enzyme and of enzyme desensitized to allosteric control. J Biol Chem. 1969 Dec 10;244(23):6420–6431. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mathias M. M., Kemp R. G. Allosteric properties of muscle phosphofructokinase. 3. Thiol reactivity as an indicator of conformational state. Biochemistry. 1972 Feb 15;11(4):578–584. doi: 10.1021/bi00754a016. [DOI] [PubMed] [Google Scholar]
- Onishi H., Otsuka E., Ikehara M., Tonomura Y. Energy transfer from tryptophan residues to a fluorescent ATP analog, 1,N6-ethenoadenosine triphosphate, bound to H-meromyosin. J Biochem. 1973 Sep;74(3):435–450. doi: 10.1093/oxfordjournals.jbchem.a130263. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A., Luft J. H., Love D. S., Krebs E. G. Crystallization and properties of rabbit skeletal muscle phosphofructokinase. J Biol Chem. 1966 Oct 25;241(20):4625–4637. [PubMed] [Google Scholar]
- Pavelich M. J., Hammes G. G. Aggregation of rabbit muscle phosphofructokinase. Biochemistry. 1973 Mar 27;12(7):1408–1414. doi: 10.1021/bi00731a022. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Riquelme P. T., Hosey M. M., Marcus F., Kemp R. G. Phosphorylation of muscle phosphofructokinase by the catalytic subunit of cyclic AMP-dependent protein kinase. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1480–1487. doi: 10.1016/0006-291x(78)91170-1. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J., Weber G. Fluorescent modification of adenosine-containing coenzymes. Biological activities and spectroscopic properties. Biochemistry. 1972 Sep 12;11(19):3499–3506. doi: 10.1021/bi00769a001. [DOI] [PubMed] [Google Scholar]
- Setlow B., Mansour T. E. Studies on heart phosphofructokinase. Binding of cyclic adenosine 3',5'-monophosphate, adenosine monophosphate, and of hexose phosphates to the enzyme. Biochemistry. 1972 Apr 11;11(8):1478–1486. doi: 10.1021/bi00758a024. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Bardsley R. G., Eccleston J. F., Weeds A. G. Elementary processes of the magnesium ion-dependent adenosine triphosphatase activity of heavy meromyosin. A transient kinetic approach to the study of kinases and adenosine triphosphatases and a colorimetric inorganic phosphate assay in situ. Biochem J. 1972 Feb;126(3):635–644. doi: 10.1042/bj1260635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K., Miyatake A., Luby L. J., Richards E. G. Isolation and characterization of muscle phosphofructokinases with varying degrees of phosphorylation. J Biol Chem. 1978 Nov 25;253(22):8319–8327. [PubMed] [Google Scholar]
- Uyeda K. Studies on the reaction mechanism of skeletal muscle phosphofructokinase. J Biol Chem. 1970 May 10;245(9):2268–2275. [PubMed] [Google Scholar]
- Walker I. D., Harris J. I., Runswick M. J., Hudson P. The subunits of rabbit-muscle phosphofructokinase. A search for sequence repetition. Eur J Biochem. 1976 Sep;68(1):255–269. doi: 10.1111/j.1432-1033.1976.tb10785.x. [DOI] [PubMed] [Google Scholar]
- Wolfman N. M., Thompson W. R., Hammes G. G. Study of the interaction of adenylyl imidodiphosphate with rabbit muscle phosphofructokinase. Biochemistry. 1978 May 16;17(10):1813–1817. doi: 10.1021/bi00603a001. [DOI] [PubMed] [Google Scholar]