Abstract
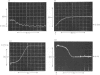
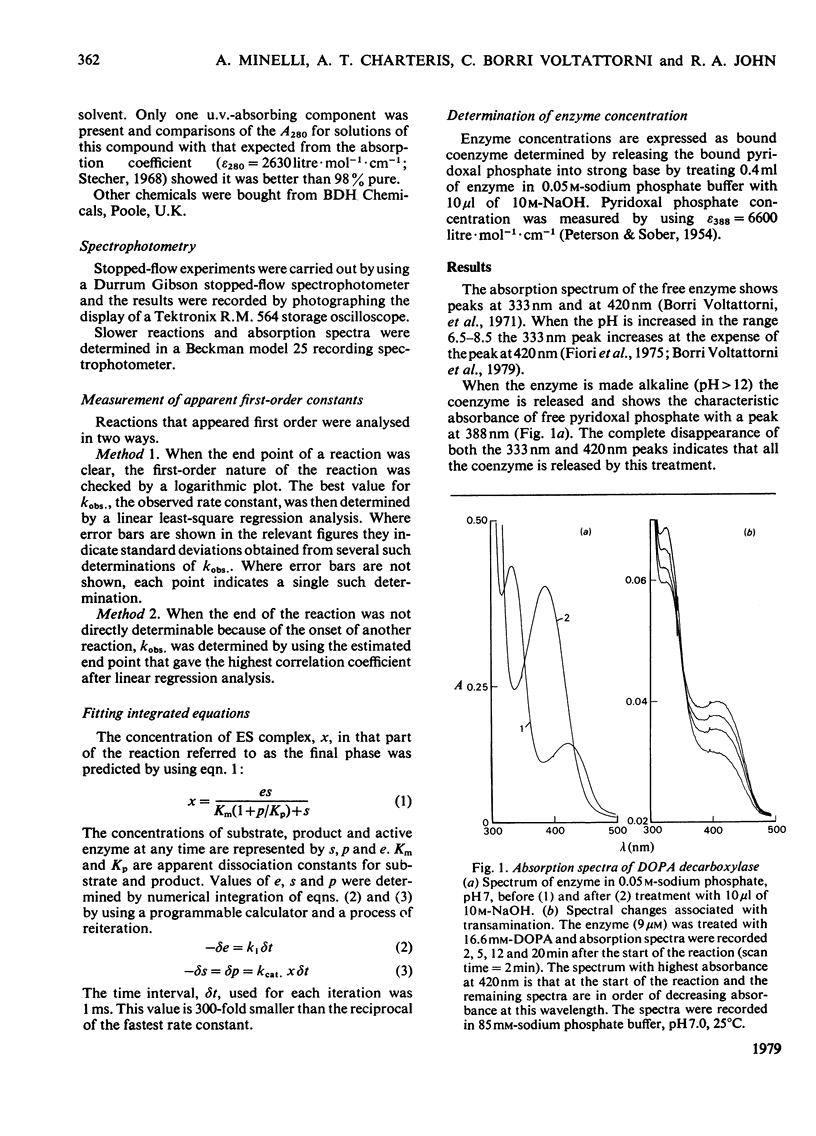
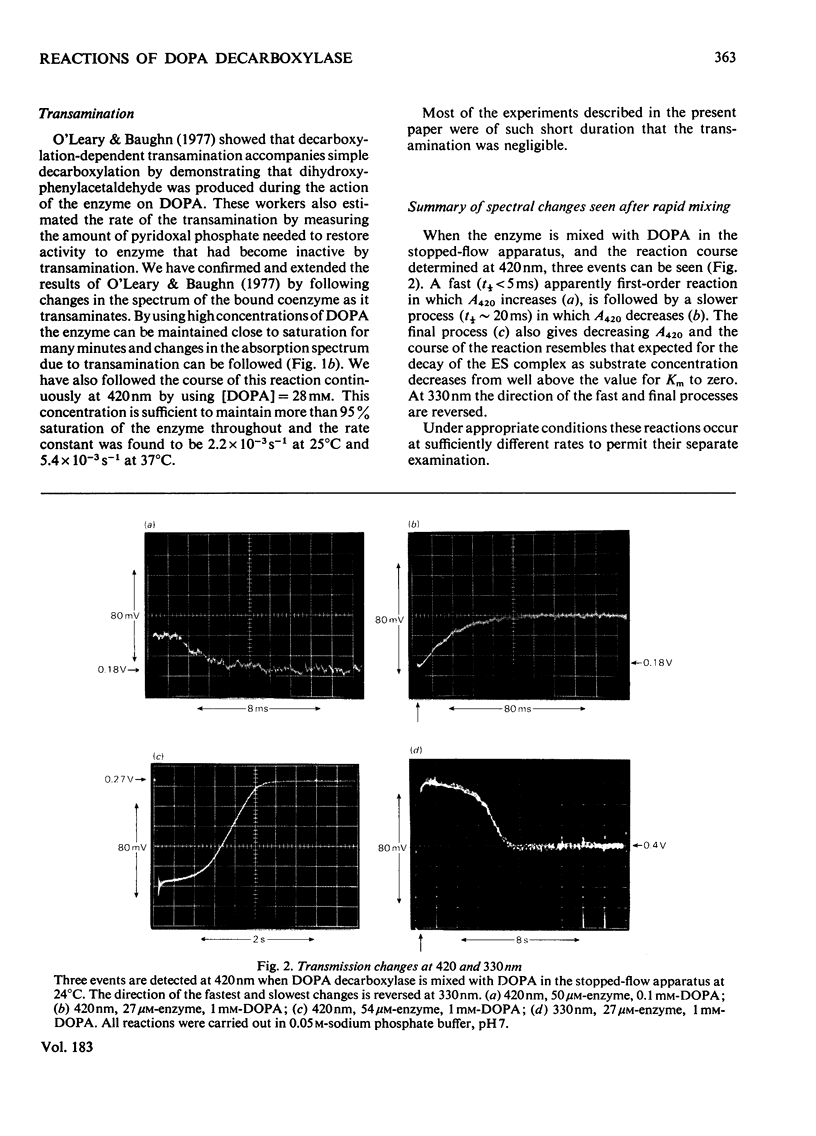
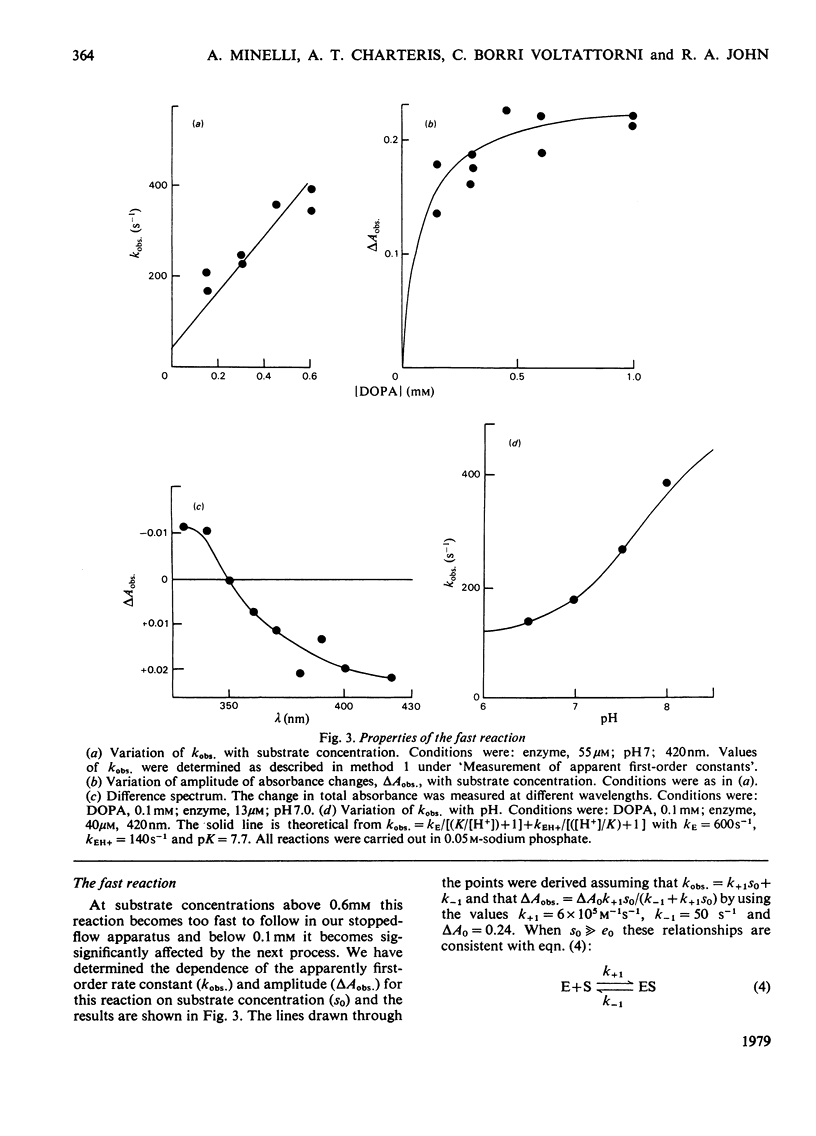
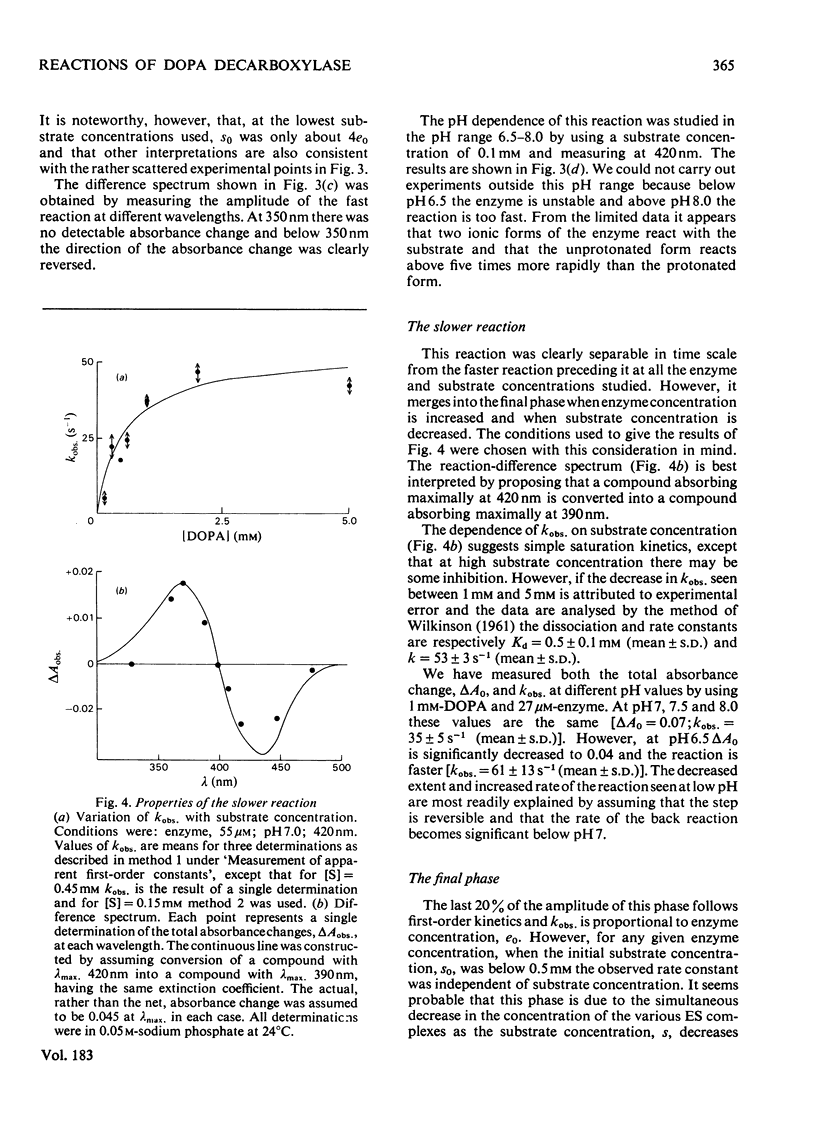
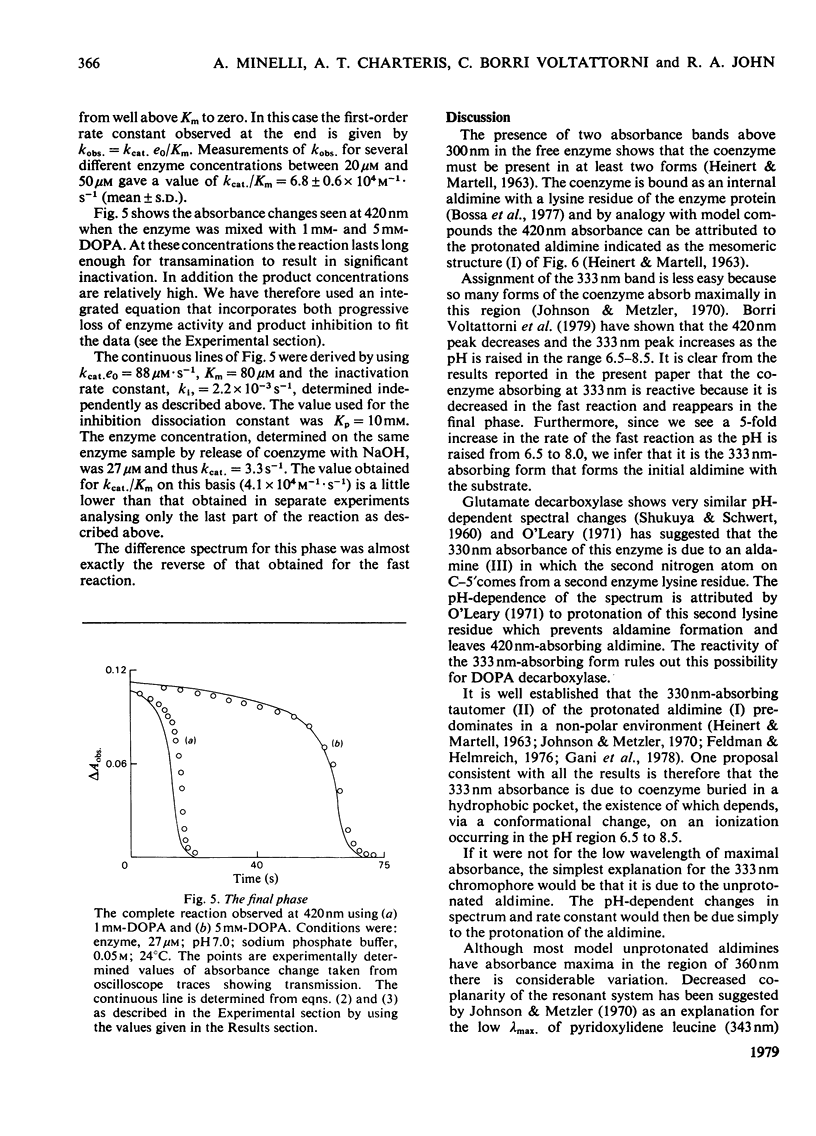
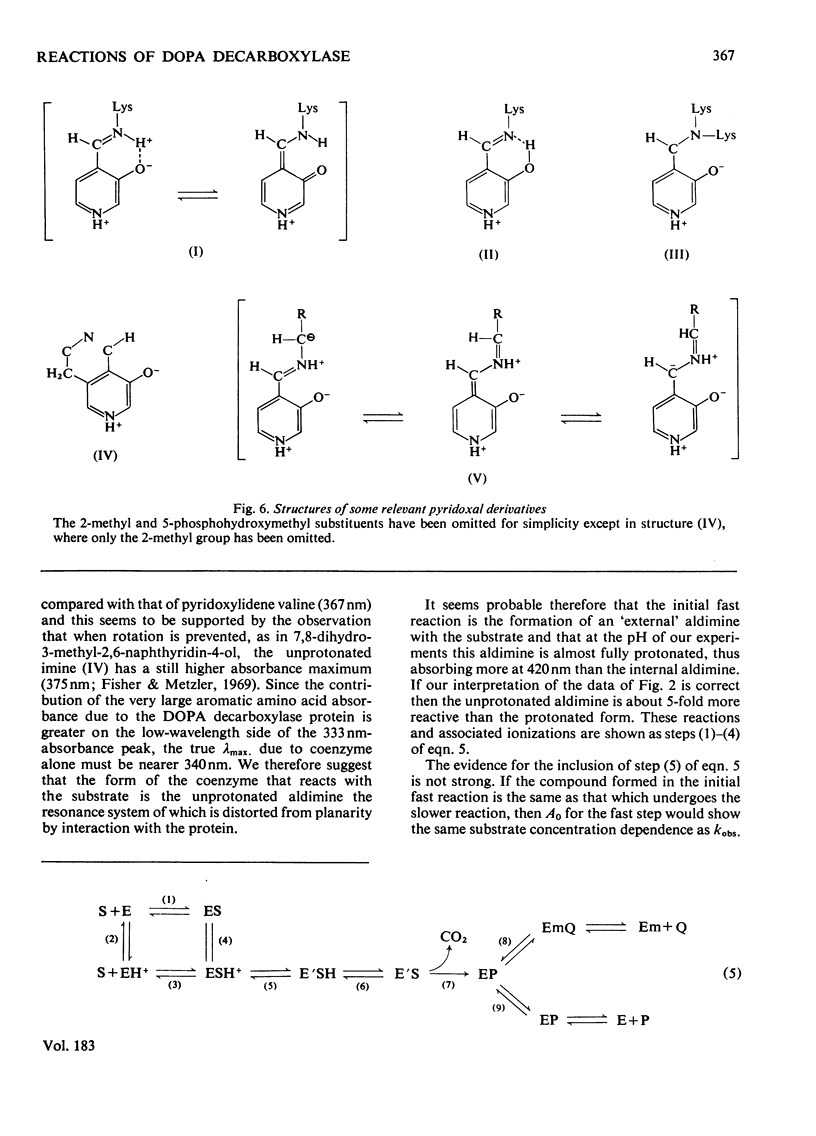
The study of DOPA (3,4-dihydroxyphenylalanine) decarboxylase by steady-state methods is difficult because multiple reactions occur. The reaction with DOPA was studied at enzyme concentrations between 20 and 50 micrometer by direct observation of the bound coenzyme by using stopped-flow and conventional spectrophotometry. Four processes were observed on different time scales and three of these were attributed to stages in the decarboxylation. The fourth was attributed to an accompanying transamination that renders the enzyme inactive. It was clear that much, if not all, of the 330 nm-absorbing coenzyme present in the free enzyme plays an active part in the decarboxylation, since it is converted into 420 nm-absorbing material in the first observable step. An intermediate absorbing maximally at 390 nm is formed in a slower step. Rate and equilibrium constants have been determined and the ratio of decarboxylation to transamination was estimated to be 1200:1.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bossa F., Martini F., Barra D., Voltattorni C. B., Minelli A., Turano C. The chymotryptic phosphopyridoxyl peptide of DOPA decarboxylase from pig kidney. Biochem Biophys Res Commun. 1977 Sep 9;78(1):177–184. doi: 10.1016/0006-291x(77)91237-2. [DOI] [PubMed] [Google Scholar]
- Christenson J. G., Dairman W., Udenfriend S. On the identity of DOPA decarboxylase and 5-hydroxytryptophan decarboxylase (immunological titration-aromatic L-amino acid decarboxylase-serotonin-dopamine-norepinephrine). Proc Natl Acad Sci U S A. 1972 Feb;69(2):343–347. doi: 10.1073/pnas.69.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann K., Helmreich E. J. The pyridoxal 5' -phosphate site in rabbit skeletal muscle glycogen phosphorylase b: an ultraviolet and 1H and 31P nuclear magnetic resonance spectroscopic study. Biochemistry. 1976 Jun 1;15(11):2394–2401. doi: 10.1021/bi00656a023. [DOI] [PubMed] [Google Scholar]
- Fiori A., Turano C., Borri-Voltattorni C., Minelli A., Codini M. Interaction of L-DOPA decarboxylase with substrates: a spectrophotometric study. FEBS Lett. 1975 Jun 15;54(2):122–125. doi: 10.1016/0014-5793(75)80057-3. [DOI] [PubMed] [Google Scholar]
- Gani V., Kupfer A., Shaltiel S. A micellar model for the pyridoxal 5'-phosphate site of glycogen phosphorylase. Biochemistry. 1978 Apr 4;17(7):1294–1300. doi: 10.1021/bi00600a025. [DOI] [PubMed] [Google Scholar]
- John R. A., Charteris A. The reaction of amino-oxyacetate with pyridoxal phosphate-dependent enzymes. Biochem J. 1978 Jun 1;171(3):771–779. doi: 10.1042/bj1710771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster G. A., Sourkes T. L. Purification and properties of hog-kidney 3,4-dihydroxyphenylalanine decarboxylase. Can J Biochem. 1972 Jul;50(7):791–797. doi: 10.1139/o72-110. [DOI] [PubMed] [Google Scholar]
- Matsushima Y., Martell A. E. Pyridoxal analogs. IX. Electron absorption spectra and molecular species in methanol solution. J Am Chem Soc. 1967 Mar 15;89(6):1322–1330. doi: 10.1021/ja00982a008. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H. A proposed structure for the 330 -nm chromophore of glutamate decarboxylase and other pyridoxal 5'-phosphate dependent enzymes. Biochim Biophys Acta. 1971 Aug 20;242(2):484–492. doi: 10.1016/0005-2744(71)90241-5. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H., Baughn R. L. Decarboxylation-dependent transamination catalyzed by mammalian 3,4-dihydroxyphenylalanine decarboxylase. J Biol Chem. 1977 Oct 25;252(20):7168–7173. [PubMed] [Google Scholar]
- O'Leary M. H., Baughn R. L. New pathway for metabolism of dopa. Nature. 1975 Jan 3;253(5486):52–53. doi: 10.1038/253052a0. [DOI] [PubMed] [Google Scholar]
- SCHIRCH L., JENKINS W. T. SERINE TRANSHYDROXYMETHYLASE. PROPERTIES OF THE ENZYME-SUBSTRATE COMPLEXES OF D-ALANINE AND GLYCINE. J Biol Chem. 1964 Nov;239:3801–3807. [PubMed] [Google Scholar]
- SCHOTT H. F., CLARK W. G. DOPA decarboxylase inhibition through the interaction of coenzyme and substrate. J Biol Chem. 1952 May;196(1):449–462. [PubMed] [Google Scholar]
- SHUKUYA R., SCHWERT G. W. Glutamic acid decarboxylase. II. The spectrum of the enzyme. J Biol Chem. 1960 Jun;235:1653–1657. [PubMed] [Google Scholar]
- Voltattorni C. B., Minelli A., Turano C. Spectral properties of the coenzyme bound to DOPA decarboxylase from pig kidney. FEBS Lett. 1971 Oct 1;17(2):231–235. doi: 10.1016/0014-5793(71)80153-9. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]