Abstract
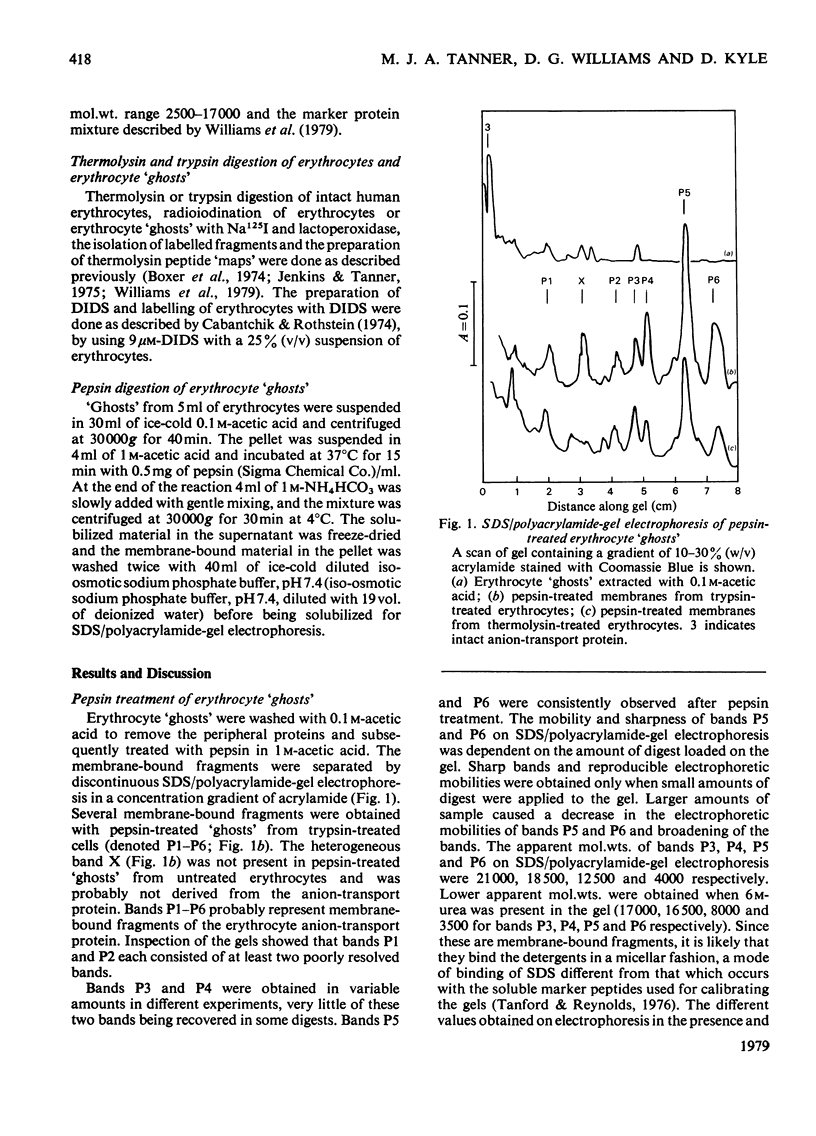
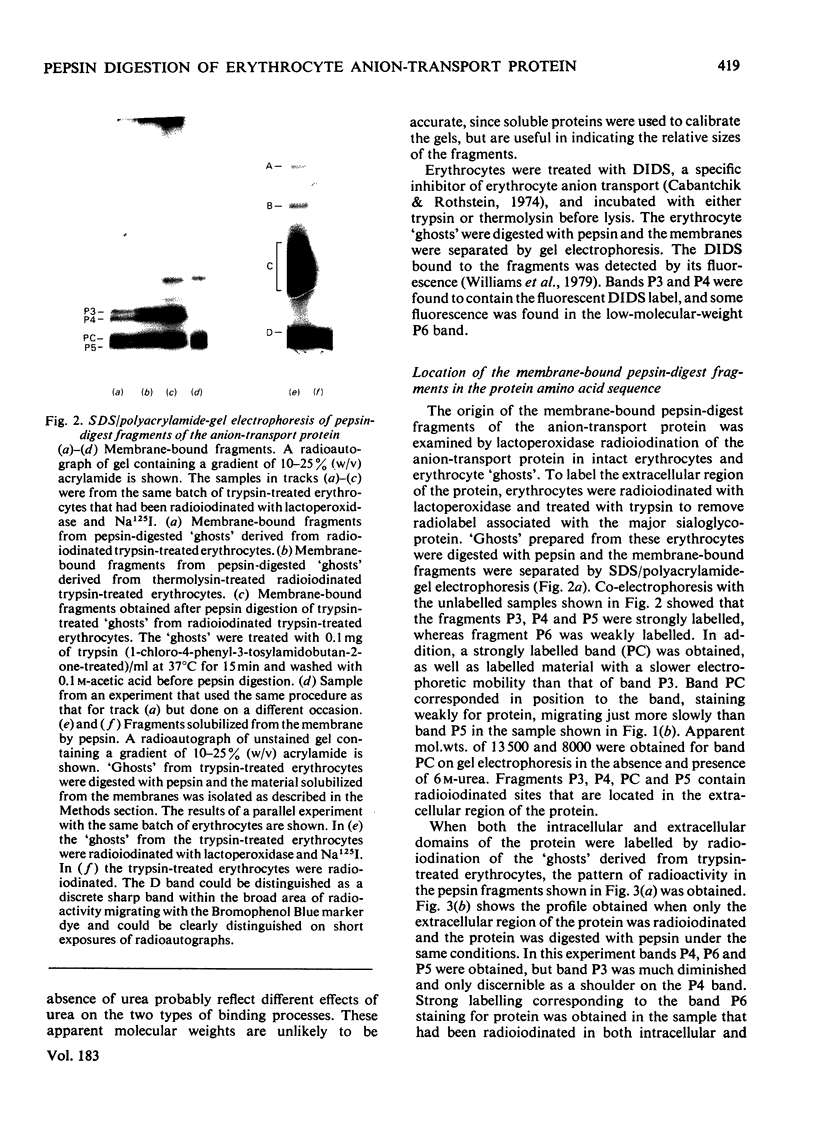
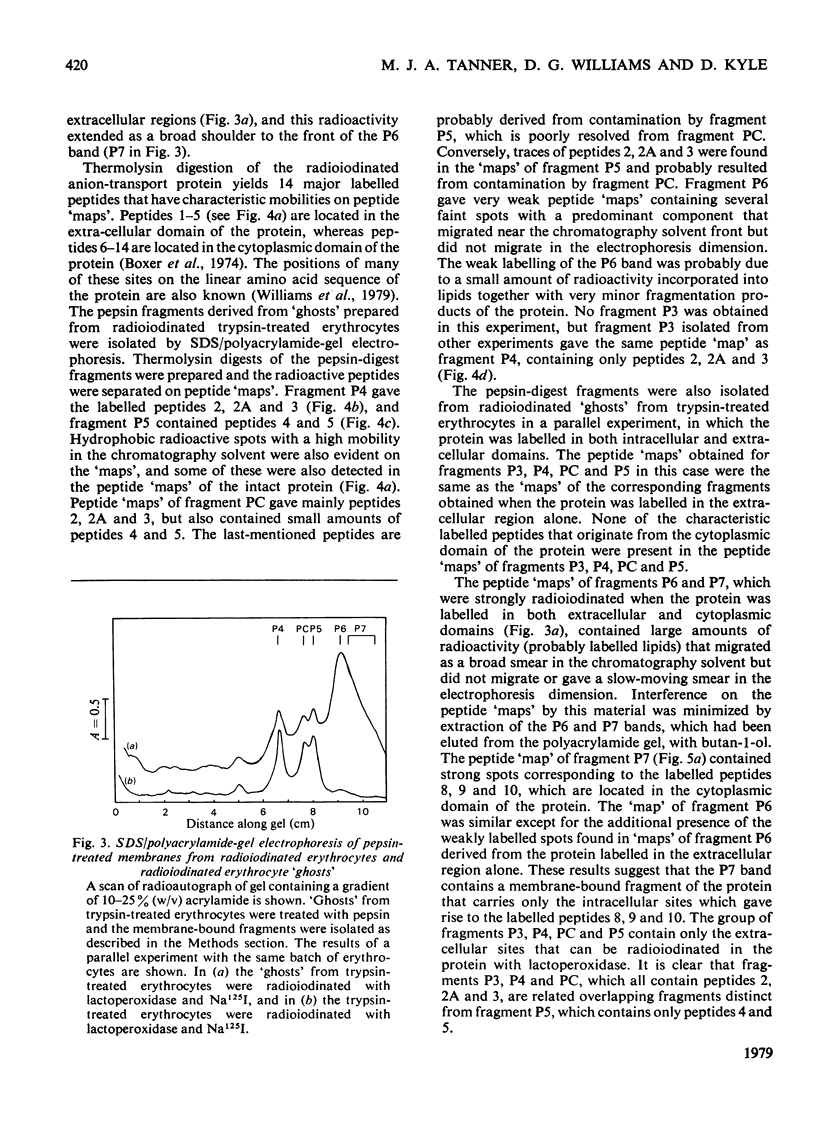
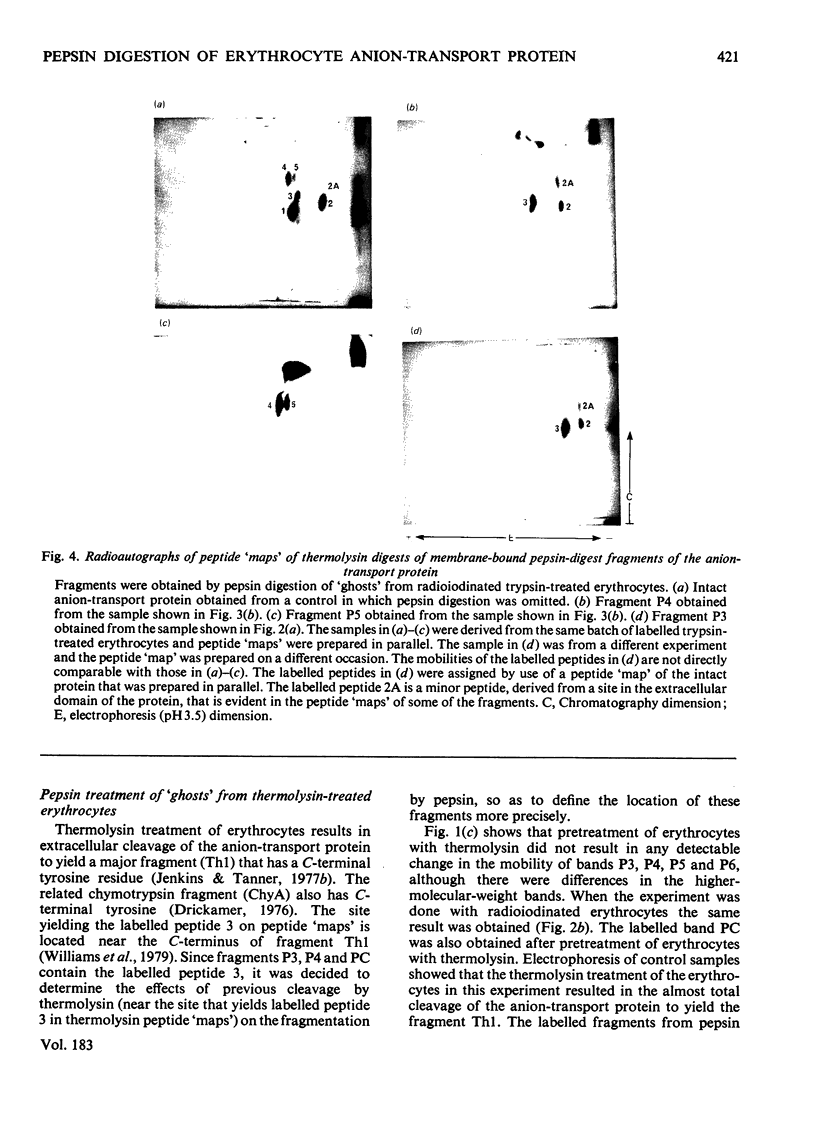
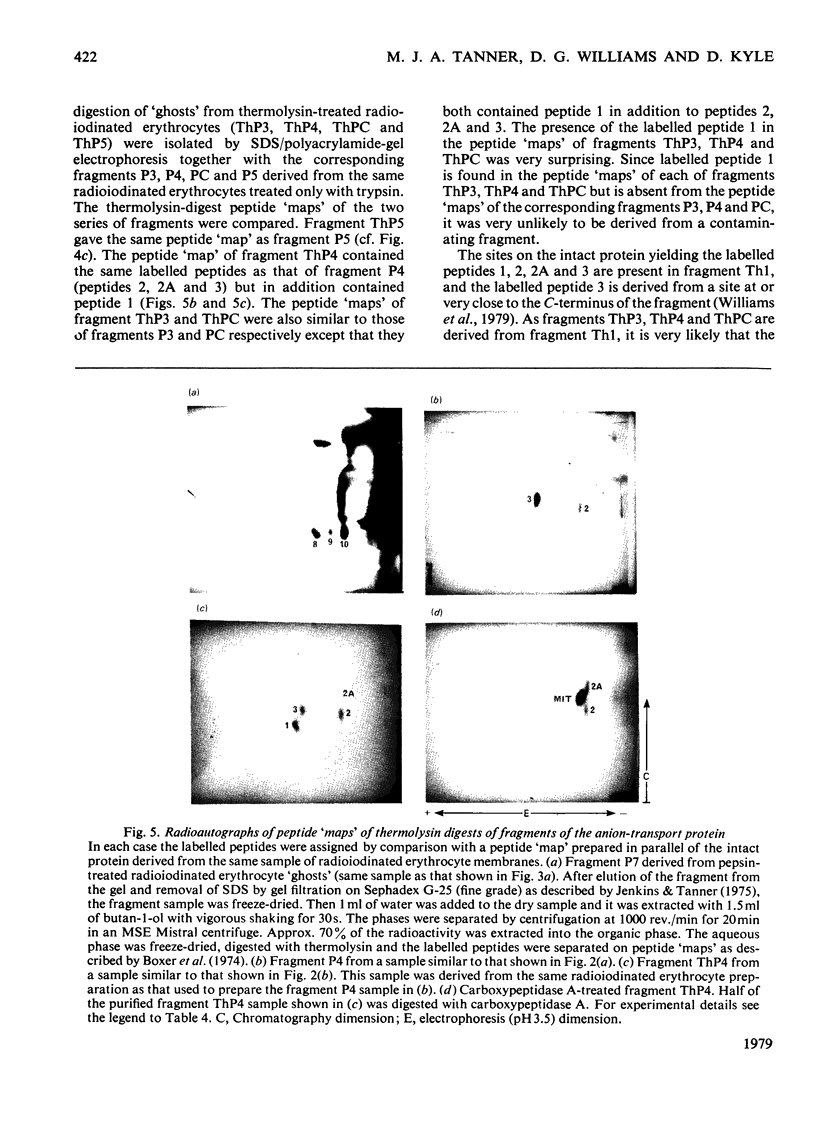
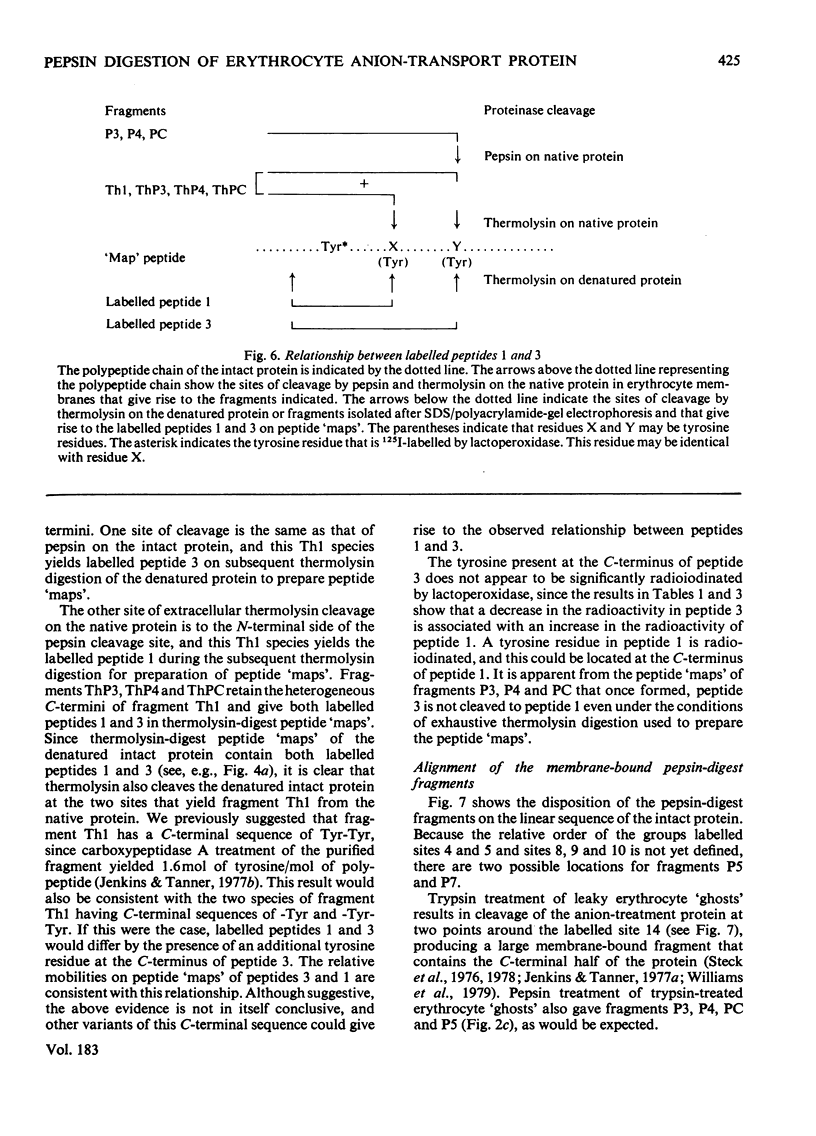
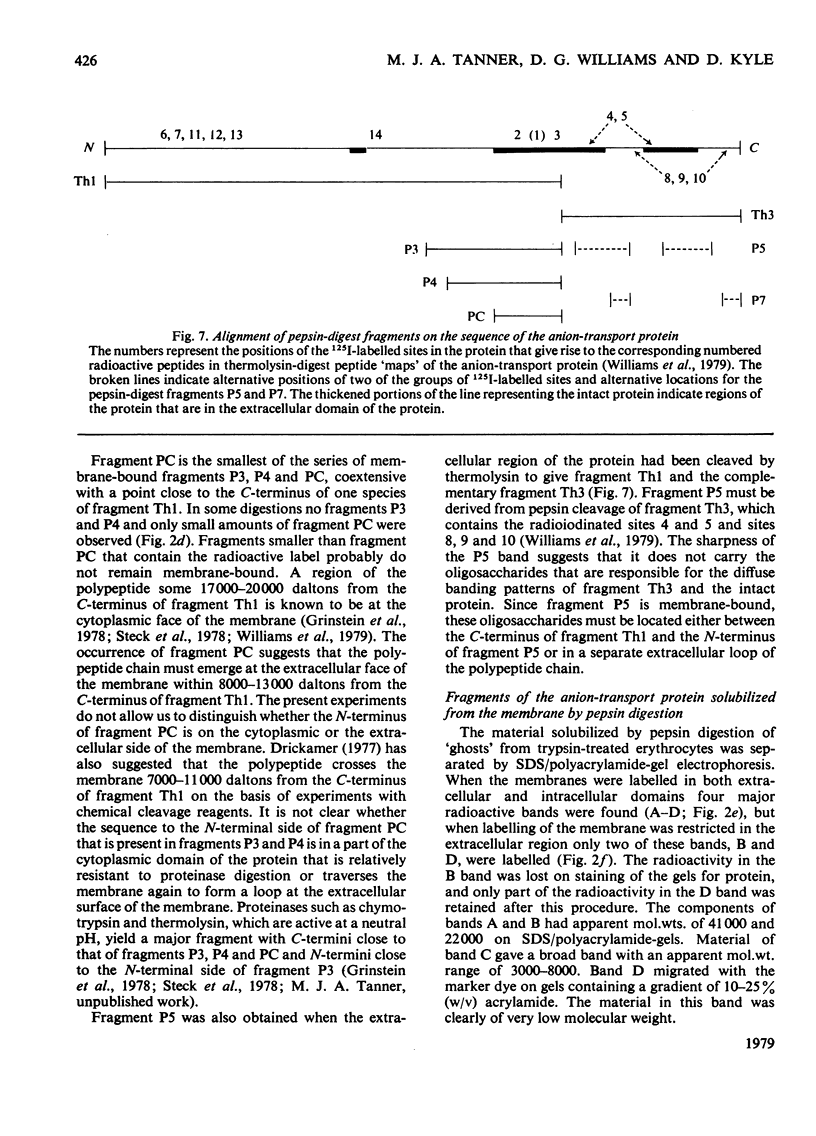
We have studied the fragmentation by pepsin in 1 M-acetic acid of the erythrocyte anion-transport protein in erythrocyte membranes. The location of the fragments obtained was determined by radioiodinating the protein with the use of lactoperoxidase, and identifying the labelled peptides obtained in peptide "maps" of thermolysin digests of the fragments. Three of the fragments were found to be related overlapping products, and shared a common C-terminus. The major site of pepsin cleavage leading to the C-termini of these fragments was shown to be close to the major site of extracellular cleavage of the protein by proteinases active at a neutral pH. Another two fragments were isolated and shown to be derived from the C-terminal portion of the protein. No well-defined large radioactive fragments of the protein were solubilized from the membrane by pepsin in 1 M-acetic acid, the bulk of the radioactivity attributable to the anion transport protein being recovered in very small fragments that could not be resolved by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Our results suggest that the polypeptide chain of the anion-transport protein emerges at the extracellular face of the membrane 8000-13000 daltons on the N-terminal side of the major site of extracellular cleavage of the protein by proteinases that are active at a neutral pH.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstee D. J., Mawby W. J., Tanner M. J. Abnormal blood-group-Ss-active sialoglycoproteins in the membrane of Miltenberger class III, IV and V human erythrocytes. Biochem J. 1979 Nov 1;183(2):193–203. doi: 10.1042/bj1830193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer D. H., Jenkins R. E., Tanner M. J. The organization of the major protein of the human erythrocyte membrane. Biochem J. 1974 Mar;137(3):531–534. doi: 10.1042/bj1370531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Drickamer L. K. Fragmentation of the 95,000-dalton transmembrane polypeptide in human erythrocyte membranes. J Biol Chem. 1976 Sep 10;251(17):5115–5123. [PubMed] [Google Scholar]
- Drickamer L. K. Fragmentation of the band 3 polypeptide from human erythrocyte membranes. Identification of regions likely to interact with the lipid bilayer. J Biol Chem. 1977 Oct 10;252(19):6909–6917. [PubMed] [Google Scholar]
- Grinstein S., Ship S., Rothstein A. Anion transport in relation to proteolytic dissection of band 3 protein. Biochim Biophys Acta. 1978 Feb 21;507(2):294–304. doi: 10.1016/0005-2736(78)90424-8. [DOI] [PubMed] [Google Scholar]
- Jenkins R. E., Tanner J. A. The major human erythrocyte membrane protein. Evidence for an S-shaped structure which traverses the membrane twice and contains a duplicated set of sites. Biochem J. 1975 Jun;147(3):393–399. doi: 10.1042/bj1470393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. E., Tanner J. A. The structure of the major protein of the human erythrocyte membrane. Characterization of the intact protein and major fragments. Biochem J. 1977 Jan 1;161(1):139–147. doi: 10.1042/bj1610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. E., Tanner M. J. Ionic-strength-dependent changes in the structure of the major protein of the human erythrocyte membrane. Biochem J. 1977 Jan 1;161(1):131–138. doi: 10.1042/bj1610131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Koziarz J. J., Singh M. K., Reddy G., Köhler H. Preparation and analysis of seven major, topographically defined fragments of band 3, the predominant transmembrane polypeptide of human erythrocyte membranes. Biochemistry. 1978 Apr 4;17(7):1216–1222. doi: 10.1021/bi00600a013. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The band 3 protein of the human red cell membrane: a review. J Supramol Struct. 1978;8(3):311–324. doi: 10.1002/jss.400080309. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Williams D. G., Jenkins R. E., Tanner M. J. Structure of the anion-transport protein of the human erythrocyte membrane. Further studies on the fragments produced by proteolytic digestion. Biochem J. 1979 Aug 1;181(2):477–493. doi: 10.1042/bj1810477. [DOI] [PMC free article] [PubMed] [Google Scholar]