Abstract
CARBON MONOXIDE POISONING IS AN ENIGMATIC ILLNESS. The symptoms are often nonspecific or masked by an exacerbation of an underlying illness, such as congestive heart failure, that has been triggered by carbon monoxide inhalation. The effects can range from mild, annoying symptoms relieved by removal of the source to severe morbidity with profound central nervous system dysfunction, acute complications and delayed sequelae. Estimates suggest that about one-third of nonfatal cases of carbon monoxide poisoning go undetected and undiagnosed. We present a case of residential carbon monoxide poisoning to illustrate these points and to demonstrate the usefulness of a simple tool based on the CH2OPD2 mnemonic (Community, Home, Hobbies, Occupation, Personal habits, Diet and Drugs) that physicians can use to obtain an environmental exposure history. We outline the clinical management of carbon monoxide poisoning and provide strategies and resources to prevent exposure.
Case
A woman brings her 15-year-old son to their family physician in late November because he has had frequent headaches for 6 weeks. They are dull, frontal headeaches that occur 2 or 3 times a week, usually in the morning and evening, and often resolve by the time the patient arrives at school in the morning. Associated symptoms include occasional dizziness, nausea, difficulty concentrating and fatigue. The boy has no previous history of headaches and no other remarkable medical history. The only medication he takes is acetaminophen on occasion to relieve the headaches. The patient has no known drug allergies and does not smoke or use recreational drugs or alcohol. The only significant family history is that the patient's mother occasionally has migraines; she reports no recent increase in frequency. The patient appears healthy. The findings on physical examination are normal, as are the findings on examination of his ears, nose and throat, and cardiovascular, neurological and musculoskeletal systems. Because of the nonspecific signs and symptoms, the physician takes a brief environmental exposure history using the CH2OPD2 mnemonic (Community, Home, Hobbies, Personal habits, Diet and Drugs) to identify possible new sources of environmental contaminants (Table 1).1 The exposure history reveals that the patient's bedroom was moved to the basement 4 months earlier, after he and his father had insulated and dry-walled the basement. The house is heated by a gas-burning furnace, and the family uses the wood-burning fireplace in the living room several times a week. The physician considers the possibility of exposure to carbon monoxide. The patient's venous blood carboxyhemoglobin level is 5%. He advises the family to call the gas company and have the basement checked for carbon monoxide before they spend another night there. The gas company finds that the level in the home is normal.
Table 1

How should the physician interpret this information and proceed?
Carbon monoxide is the product of incomplete combustion of hydrocarbons. It is colourless, odourless and nonirritating yet toxic. Readily absorbed through the lungs, carbon monoxide binds to hemoglobin as carboxyhemoglobin (COHb). Hemoglobin's affinity for carbon monoxide is 200 to 250 times stronger than its affinity for oxygen. The absorption of carbon monoxide causes a leftward shift in the oxygen–hemoglobin dissociation curve, which results in decreased oxygen-carrying capacity and impaired release of oxygen to the tissues. This effect, together with impaired perfusion from hypoxic cardiac function, leads to cellular hypoxia.2 Fetuses are especially vulnerable to carbon monoxide exposure. Fetal hemoglobin has a higher affinity than adult hemoglobin for carbon monoxide, and therefore fetal COHb levels may significantly exceed maternal levels.
A low baseline level of 1%–3% carboxyhemoglobin in venous blood is detectable in all people from endogenous production.2 Tobacco smoke elevates this baseline level,2,3 as does exposure to carbon monoxide in the environment (Table 2). Motor vehicle exhaust is the most common outdoor source of carbon monoxide. Indoor sources other than tobacco smoke include exhaust from poorly ventilated gas- and oil-burning appliances and the infiltration of polluted outdoor air.7 The amount of carbon monoxide absorbed depends on the relative concentration in the environment, the duration of exposure and the patient's minute volume. In relatively unpolluted ambient air, the concentration of carbon monoxide is 0.02–1.0 ppm. However, in urban areas it can be 10–20 times higher than that during periods of atmospheric stagnation8,9 (i.e., when temperature inversions occur in winter or when sedentary air masses occur in summer). In general, indoor levels rise and fall with outdoor levels, except in buildings with poorly ventilated combustion appliances. Carbon monoxide levels of about 115 mg/m3 (100 ppm) have been detected in the kitchens of some houses immediately after gas stoves were used for cooking.7 After reviewing the evidence, the Federal–Provincial Advisory Committee on Environmental and Occupational Health recommended that the acceptable short-term exposure ranges for carbon monoxide in residential indoor air not exceed an average concentration of 11 ppm over 8 hours or 25 ppm over 1 hour.7
Table 2
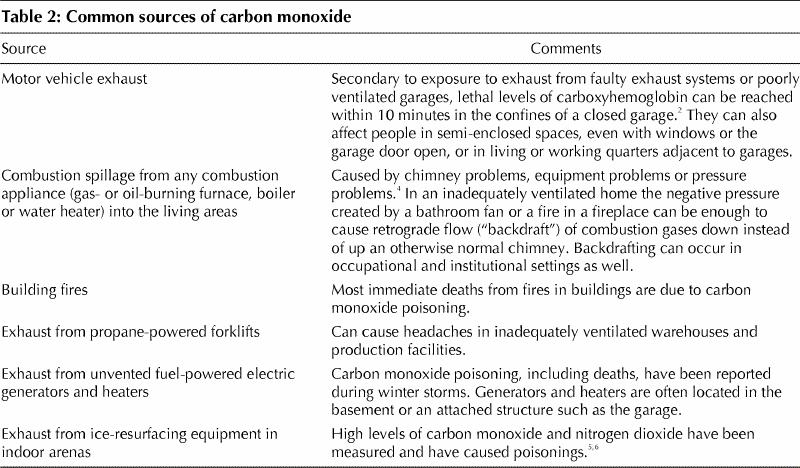
Multiple epidemiological studies have shown that elevated concentrations of carbon monoxide in urban ambient air are associated with increases in daily mortality rates10,11,12,13,14,15,16,17 and in rates of hospital admission because of diseases such as congestive heart failure.18 The true incidence of carbon monoxide poisoning is unknown, since many mildly symptomatic exposures go undetected19 or are masked by underlying disease. One estimate suggests that as many as one-third of all cases of carbon monoxide poisoning go undiagnosed.3
Clinical management
History-taking tools such as the CH2OPD2 mnemonic1 can help physicians direct general and more specific questions when screening patients for carbon monoxide poisoning (see box). Physicians may wish to use the more comprehensive environmental exposure history forms developed by the Environmental Health Clinical of Sunnybrook & Women's College Health Sciences Centre (information on where to find these forms appears at the end of the article). Clues that point to an environmental exposure include more than one person being affected, pets being affected, the temporal relation of the symptoms to the use of the furnace, fireplace or venting fans, or a history of smoke inhalation.
Box 1.

The main indoor air pollutant that can cause headaches is carbon monoxide. Environmental tobacco smoke and volatile organic compounds can also cause headaches, but irritant symptoms of the eyes and throat are likely to be more prominent with those sources. The most common symptoms of carbon monoxide inhalation include headache, dizziness, weakness, nausea, difficulty in concentrating, shortness of breath and visual changes. Less frequent symptoms include chest pain, loss of consciousness, abdominal pain and muscle cramping.2 The circulatory and nervous systems are often affected because of their fixed oxygen needs: patients who have inhaled carbon monoxide may present with signs of myocardial ischemia, hypotension, congestive heart failure, arrhythmias, mental confusion, clumsiness, emotional lability, impaired judgement, diminished visual acuity, stupor or coma.20 Carbon monoxide poisoning is considered a “disease with a thousand faces.” 20 Its classic mask — cherry-red lips, cyanosis and retinal hemorrhages — occurs only rarely.2 The signs and symptoms of nonlethal carbon monoxide poisoning, especially from intermittent, low-level exposures, are easily mistaken for other illnesses commonly seen in primary care, such as viral illness, depression, chronic fatigue syndrome, migraine or other headaches.21,22 Because viral illness and carbon monoxide poisoning both peak in incidence during the winter, the chance that the latter will be misdiagnosed increases in this season.23
There appears to be no marker or constellation of signs or symptoms at presentation that predicts long-term outcome following carbon monoxide poisoning.24 Physicians must be alert to the possibility of delayed effects. An array of delayed neuropsychiatric signs and symptoms — the “neuropsychiatric syndrome” — has been observed in patients from 3 to 240 days after acute exposure, even in patients without initial signs of cerebral impairment immediately after exposure. The syndrome is estimated to occur in 10%–30% of carbon monoxide poisonings, but the reported incidence varies widely.2 Up to 10% of survivors show gross neurological or psychiatric impairment (e.g., parkinsonism, incontinence, dementia or psychosis), which is obvious to the physician.25 More frequent is the development of subtle, persistent neuropsychiatric deficits, including personality changes and cognitive deficits.
When carbon monoxide poisoning is suspected, COHb levels in venous blood should be measured.26 Normal levels are 1%–3% in nonsmokers and 4%–5% in smokers (levels as high as 9%–15% in smokers have been reported2). Increased levels are diagnostic. However, the mean half-life of COHb is 320 minutes (range 128–409 minutes) in young healthy volunteers in room air, 80.3 minutes in people breathing 100% oxygen at 1 atmosphere and 23.3 minutes in those breathing 100% oxygen at 3 atmospheres.27 COHb levels may fall substantially by the time of presentation, especially in cases of recurrent, low-level exposures. Therefore, a normal COHb level does not rule out carbon monoxide poisoning.2 Arterial blood gas measurements may reveal a metabolic acidosis, a normal partial pressure of oxygen, a variable partial pressure of carbon dioxide and decreased oxygen saturation.20 However, in cases of mild carbon monoxide poisoning without respiratory distress, the evidence from retrospective chart reviews has shown only a weak correlation between degree of acidosis, COHb levels and extent of neurological disability.28,29 Arterial blood gas analysis may not be as useful as the clinical impression of neurological disability in guiding treatment of mild carbon monoxide poisoning.28,29
A detailed neurological examination and neuropsychological testing should be performed to document any subtle abnormalities.2 CT and MRI scans may show widespread changes in the brain. The globus pallidus is the commonest site of abnormality.30 Changes in white matter are also commonly seen.30 The extent to which imaging changes correlate with clinical outcome, and the clinical value of neuroimaging, is disputed.30,31
People exposed to carbon monoxide must be treated immediately with high-flow oxygen, preferably 100%, at normal atmospheric pressure. There are no guidelines as to the length of the observation period. Most patients, including the case subject we have described earlier, can be treated in the primary care or emergency setting.2 Patients with severe carbon monoxide poisoning or those with associated underlying medical problems, especially cardiovascular disease, should be admitted to hospital, given oxygen as described above and monitored for cardiac arrhythmias. Classifying and treating patients primarily on the basis of COHb levels is inappropriate and potentially misleading.
The use of hyperbaric oxygen to treat severe cases is controversial.32,33,34 There is no evidence that unselected use of hyperbaric oxygen to treat such cases reduces the frequency of neurological symptoms at 1 month, and evidence from available randomized controlled trials is insufficient to provide clear guidelines for practice.35 The Undersea and Hyperbaric Medical Society recommends treatment with hyperbaric oxygen for patients with signs of serious intoxication regardless of their COHb levels.36 This includes patients with a history of unconsciousness and those with neurological signs, cardiovascular dysfunction or severe acidosis.
Whenever carbon monoxide poisoning is suspected, firefighters or the local gas company should be notified to investigate and correct the source of the problem; the investigators should be equipped with a breathing apparatus to protect against poisoning.2 Improperly designed or maintained household venting systems can cause a reversal of the continuous flow of flue gases and outdoor air, pulling them down the chimney instead of up — a process known as “backdrafting.” 4 Elevations in ambient carbon monoxide levels at the source can be transient, especially if the problem is backdrafting with spillage of combustion product into the house.4 This can result in a normal reading that fails to identify a true problem. Consequently, it is sometimes necessary to take repeated measures at different times.
Prevention
The problems with diagnosis, treatment and delayed sequelae make the prevention of carbon monoxide poisoning most important. Prevention should include public education and regulations in the following areas:
· Frequent inspection and routine maintenance of vented combustion appliances, fireplaces, flues and chimneys.
· Avoidance of conditions that lead to retrograde flow (“backdrafting”), and testing for backdrafting with the use of a simple chimney flow test.4,37
· No idling of automobiles in closed or even open garages, especially those attached to the home.
· No use of unvented combustion appliances such as space heaters and cooking devices (e.g., gas barbeques and Hibachis) indoors.
· Installation of carbon monoxide detectors.38,39,40
· Media campaigns to warn the public about dangers of exposure during periods of increased risk, such as anticipated cold spells and snow or ice storms.2
· Reduced exposure to and use of fuel-powered ice-resurfacing equipment in indoor skating and curling facilities.5,6,8
The Canada Mortgage and Housing Corporation has published a number of educational materials.4,37,41
Carbon monoxide detectors save lives.38 They are widely available, and their installation in new structures containing potential carbon monoxide sources is now legislated in building codes in many jurisdictions. Detectors are certified to a UL2034 or CSA 6.19 standard4,39,40,41 and should be installed close to combustion sources and close to bedrooms.4,41 Standards for carbon monoxide detectors were revised in 1997 to ensure that alarms will sound before most people experience the adverse effects (loss of ability to react) of carbon monoxide poisoning, with the alarm sounding within 3 hours 9 minutes if 70 ppm of carbon monoxide is present, within 50 minutes if 150 ppm is present and within 15 minutes if 400 ppm is present.42 The level of 70 ppm is equivalent to a COHb concentration in blood of 10%.43 However, these concentrations of carbon monoxide are significantly higher than those specified in the Canadian Health Guidelines.7,41 The Canada Mortgage and Housing Corporation advises that people at risk of reacting to lower levels of carbon monoxide (fetuses, infants, elderly people and people with respiratory or heart disease) may need to install a detector that displays both high and low levels.41
The case revisited
How should the physician interpret the information and proceed?
There are 2 important findings: the case subject's COHb level is 5%, and the carbon monoxide level in the basement, where the subject's bedroom is located, is normal.
A COHb level of 5% in a person who does not smoke is definitely considered elevated, although headaches do not usually start until the level is over 16%. The half-life of COHb is short, about 3 hours, so it is likely that the patient's level fell off substantially after he left the basement. A normal level would not have definitively ruled out carbon monoxide poisoning as a cause of his headaches.
With regard to the normal carbon monoxide level in the basement, the important clue in this case is the timing of the headaches. They started in the fall, at the beginning of the heating season. On further questioning, a relationship is established between the headaches and the evenings when the fireplace was used. A heating, ventilation and air-conditioning contractor conducts a chimney flow test4 with the fireplace in use and finds a backdraft and concurrent carbon monoxide level of 75 ppm in the basement. When the lit fire in the livingroom consumed oxygen it caused a negative pressure in the inadequately ventilated house, including the furnace room in the basement, which resulted in the reversed flow of combustion gases, including carbon monoxide, down the chimney into the living area in the basement. The renovation that increased the insulation in the basement may also have contributed to increasing the backdrafting by reducing air flow and ventilation to the furnace. Reduced ventilation may also have allowed the concentration of carbon monoxide to increase.
It is imperative to treat the building as well as the patient. The family is advised to remedy the backdrafting by providing a fresh-air intake to the furnace and to install a carbon monoxide detector in the basement.
At a follow-up appointment the patient reports that he has had no more headaches and is feeling generally much better.
Additional resources .
American Academy of Asthma and Allergy: www.aaaai.org
Canada Mortgage and Housing Corporation: www.cmhc-schl.gc.ca
International Joint Commission Health Professionals Task Force: www.ijc.org/boards/health.html
Lung Association: www.lung.ca/asthma
National Institute for Environmental Health Sciences (NIEHS): www.niehs.nih.gov/airborne/home.htm
Ontario College of Family Physicians Environmental Health Committee: www.cfpc.ca/ocfp/commit.html (click on “Environment” in left margin)
Physicians for a Smoke-free Canada: www.smokefree.ca
Pollution Probe: www.pollutionprobe.org
US Environmental Protection Agency: www.epa.gov/iaq/co.html
Articles to date in this series .
Weir E. Identifying and managing adverse environmental health effects: a new series. CMAJ 2002;166(8):1041-3.
Marshall L, Weir E, Abelsohn A, Sanborn MD. Identifying and managing adverse environmental health effects: 1. Taking an exposure history. CMAJ 2002;166(8):1049-55.
Abelsohn A, Stieb D, Sanborn MD, Weir E. Identifying and managing adverse environmental health effects: 2. Outdoor air pollution. CMAJ 2002;166(9):1161-7.
Sanborn MD, Abelsohn A, Campbell M, Weir E. Identifying and managing adverse environmental health effects: 3. Lead exposure. CMAJ 2002;166(10):1287-92.
Sanborn MD, Cole D, Abelsohn A, Weir E. Identifying and managing adverse environmental health effects: 4. Pesticides. CMAJ 2002;166(11):1431-6.
Abelsohn A, Gibson BL, Sanborn MD, Weir E. Identifying and managing adverse environmental health effects: 5. Persistent organic pollutants. CMAJ 2002;166 (12): 1549-54.
Footnotes
[A detailed exposure history questionnaire is available on the Ontario College of Family Physicians Web site (www.cfpc.ca/ocfp/index.html — click on “Exposure History Sheets in MS Word” in the scrolling menu located in the middle of the page). The different components (Community, Home and Hobbies, Occupation, Personal habits, Diet and Drugs) can be printed on coloured paper for easy identification in patient charts. The questionnaire may be given to a patient to complete at home and bring to the next appointment for review and interpretation.]
This article has been peer reviewed.
Contributors: Dr. Abelsohn conceived of and drafted the article. Drs. Gibson, Sanborn and Weir contributed to the conception of the review. All of the authors contributed to the revising of the manuscript and approved the final version.
Competing interests: None declared.
Correspondence to: Dr. Alan Abelsohn, 1-1735 Bathurst St., Toronto ON M5P 3K4; fax 416 483-8182; alan.abelsohn@utoronto.ca
References
- 1.Marshall L, Weir E, Abelsohn A, Sanborn MD. Identifying and managing adverse environmental health effects: 1. Taking an exposure history. CMAJ 2002;166(8):1049-55. Available: www.cmaj.ca/cgi/content/full/166/8/1049 [PMC free article] [PubMed]
- 2.Ernst A, Zibrak J. Carbon monoxide poisoning. N Engl J Med 1998;339(22):1603-8. [DOI] [PubMed]
- 3.Varon J, Marik P, Fromm R, Gueler A. Carbon monoxide poisoning: a review for clinicians. J Emerg Med 1999:17(1):87-93. [DOI] [PubMed]
- 4.Combustion gases in your home [About your house fact sheet]. Ottawa: Canada Mortgage and Housing Corporation; 1996. Available: www.cmhc-schl.gc.ca/en/burema/gesein/abhose/abhose_ce02.cfm (accessed 2002 May 22).
- 5.Lee K, Yanagisawa Y, Spengler JD. Carbon monoxide and nitrogen dioxide levels in an indoor ice skating rink with mitigation methods. J Air Waste Manag Assoc 1993;43:769-71.
- 6.Brauer M. Recreational buildings. In: Spengler JD, Samet JM, McCarthy JF, editors. Indoor air quality handbook. New York: McGraw-Hill; 2001. p. 67.1-67.18.
- 7.Exposure guidelines for residential indoor air quality: a report of the Federal–Provincial Advisory Committee on Environmental and Occupational Health. Ottawa: Health Canada; 1995. Cat no H46-2/90-156E. Available: www.hc-sc.gc.ca/ehp/ehd/catalogue/bch_pubs/90ehd156.htm (accessed 2002 May 28).
- 8.National ambient air quality objectives for carbon monoxide: desirable, acceptable and tolerable levels. Ottawa: Environment Canada and Health Canada; 1994.
- 9.Campbell ME, Benson BA, Muir MA. Urban air quality and human health: a Toronto perspective. Can J Public Health 1995;86(5):351-7. [PubMed]
- 10.Burnett RT, Brook JR, Cakmak S, Philips O, Raizenne ME, Stieb DM, et al. The association between ambient carbon monoxide levels and daily mortality in Toronto, Canada. J Air Waste Manag Assoc 1998;48:689-700. [DOI] [PubMed]
- 11.Gwynn RC, Burnett RT, Thurston GD. A time-series analysis of acidic particulate matter and daily mortality and morbidity in the Buffalo, New York, region. Environ Health Perspect 2000;108(2):125-33. [DOI] [PMC free article] [PubMed]
- 12.Hong YC, Leem JH, Ha EH, Christiani DC. PM(10) exposure, gaseous pollutants, and daily mortality in Inchon, South Korea. Environ Health Perspect 1999;107(11):873-8. [DOI] [PMC free article] [PubMed]
- 13.Mar, TF, Norris, GA, Koenig JQ, Larson TV. Association between air pollution and mortality in Phoenix, 1995–1997. Environ Health Perspect 2000;108(4):347-53. [DOI] [PMC free article] [PubMed]
- 14.Moolgavkar SH. Air pollution and daily mortality in three US counties. Environ Health Perspect 2000;108(8):777-84. [DOI] [PMC free article] [PubMed]
- 15.Pereira LA, Loomis D, Conceicao GM, Braga AL F, Arcas RM, Kishi HS, et al. Association between air pollution and intrauterine mortality in Sao Paulo, Brazil. Environ Health Perspect 1998;106(6):325-9. [DOI] [PMC free article] [PubMed]
- 16.Peters A, Skorkovsky J, Kotesovec F, Brynda J, Spix C, Wichmann HE. Associations between mortality and air pollution in central Europe. Environ Health Perspect 2000;108(4):283-7. [DOI] [PMC free article] [PubMed]
- 17.Samet JM, Zeger SL, Kelsall JE, Xu J, Kalkstein LS. Particulate air pollution and daily mortality: analyses of the effects of weather and multiple air pollutants. The phase I.B report of the Particle Epidemiology Evaluation Project. Boston: Health Effects Institute; 1997. Summary statement available: www.healtheffects.org/Pubs/peepib.htm (accessed 2002 May 22).
- 18.Morris RD, Naumova EN. Carbon monoxide and hospital admissions for congestive heart failure: evidence of an increased effect at low temperatures. Environ Health Perspect 1998;106(10):649-53. [DOI] [PMC free article] [PubMed]
- 19.Thom SR, Keim LW. Carbon monoxide poisoning: a review. Epidemiology, pathophysiology, clinical findings, and treatment options including hyperbaric oxygen therapy. J Toxicol Clin Toxicol 1989;27(3):141-56. [DOI] [PubMed]
- 20.Fisher J. Carbon monoxide poisoning: a disease of a thousand faces. Chest 1999;115(2):322-32. [DOI] [PubMed]
- 21.Knobeloch L, Jackson R. Recognition of chronic carbon monoxide poisoning. WMJ 1999;98(6):26-9. [PubMed]
- 22.Heckerling P, Leakin J, Maturen A, Terzian C, Segarra D. Screening hospital admissions from the emergency department for occult carbon monoxide poisoning. Am J Emerg Med 1990;8:301-4. [DOI] [PubMed]
- 23.Kales SN. Carbon monoxide intoxication. Am Fam Physician 1993;48:1100-4. [PubMed]
- 24.Weaver LK. Carbon monoxide poisoning. Crit Care Clin 1999;15(2):297-317. [DOI] [PubMed]
- 25.Choic IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol 1983;40:433-5. [DOI] [PubMed]
- 26.Turner M. Carbon monoxide poisoning. Carboxyhemoglobin can be measured with standard blood tests [letter]. BMJ 2000;320:804. [PMC free article] [PubMed]
- 27.Petersen J, Stewart R. Absorption and elimination of carbon monoxide by active young men. Arch Environ Health 1970;21:165-71. [DOI] [PubMed]
- 28.Lebby TI, Zalenski R, Hryhorczuk DO, Leikin JB. The usefulness of the arterial blood gas in pure carbon monoxide poisoning. Vet Hum Toxicol 1989;31 (2):138-40. [PubMed]
- 29.Myers RA, Britten JS. Are arterial blood gases of value in treatment decisions for carbon monoxide poisoning? Crit Care Med 1989;17(2):139-42. [DOI] [PubMed]
- 30.O'Donnell P, Buxton PJ, Pitkin A, Jarvis LJ. The magnetic resonance imaging appearances of the brain in acute carbon monoxide poisoning. Clin Radiol 2000;55(4):273-80. [DOI] [PubMed]
- 31.Prockop LD. Naidu KA. Brain CT and MRI findings after carbon monoxide toxicity. J Neuroimaging 1999;9(3):175-81. [DOI] [PubMed]
- 32.Weaver LK. Hyperbaric oxygen in carbon monoxide poisoning [editorial]. BMJ 1999;319:1083-4. [DOI] [PMC free article] [PubMed]
- 33.Scheinkestel CD, Bailey M, Myles PS, Jones K, Cooper DJ, Millar IL, et al. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: a randomised controlled clinical trial. Med J Aust 1999;170(5):203-10. [DOI] [PubMed]
- 34.Hawkins M, Harrison J, Charters P. Severe carbon monoxide poisoning: outcome after hyperbaric oxygen therapy. Br J Anaesth 2000;84(5):584-6. [DOI] [PubMed]
- 35.Juurlink DN, Stanbrook MB, McGuigan MA. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev 2000;(2):CD002041. [DOI] [PubMed]
- 36.Camporesi EM. Hyperbaric oxygen therapy: a committee report. Kensington (MD): Undersea and Hyperbaric Medical Society; 1996.
- 37.The clean air guide. How to identify and correct indoor air problems in your home. Ottawa: Canada Mortgage and Housing Corporation; 1998.
- 38.Yoon SS, Macdonald SC, Parrish RG. Deaths from unintentional carbon monoxide poisoning and potential for prevention with carbon monoxide detectors. JAMA 1998;279:685-7. [DOI] [PubMed]
- 39.Carbon monoxide. What every householder should know. Toronto: Enbridge Consumers Gas; 2000. Available (pdf format): www.cgc.enbridge.com/pdf/carbon-monoxide.pdf (accessed 2002 May 15).
- 40.Leikin JB, Clifton J, Hanashiro P. Carbon monoxide poisoning. N Engl J Med 1999; 40 (16):1290-1. [PubMed]
- 41.Carbon monoxide [About your house fact sheet]. Ottawa: Canada Mortgage and Housing Corporation; 2001. Available: www.cmhc-schl.gc.ca/en/burema/gesein/abhose/abhose_ce25.cfm (accessed 2002 May 22).
- 42.UL announces changes to carbon monoxide alarm standard [press release]. Northbrook (IL): Underwriters Laboratories Inc.; 1997. Available: www.ul.com/about/newsrel/chngs.htm (accessed 2002 May 27).
- 43.Raub JA, Mathieu-Nolf M, Hampson NB, Thom SR. Carbon monoxide poisoning — a public health perspective [review]. Toxicology 2000;145:1-14. [DOI] [PubMed]


