Abstract
Several drugs of abuse may exert their action by modulating the immune system. Despite this, individuals using substances of abuse are often excluded from immunopsychiatry studies. We conducted a retrospective, single-center study to examine differences in circulating immune/inflammatory parameters (i.e., total and differential white blood cell (WBC) counts, neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte (MLR) ratio, platelet-to-lymphocyte ratio, and C-reactive protein) between psychiatric inpatients with a positive urine test to cannabinoids, opioids, or cocaine, and those with negative toxicology. A total of 927 inpatients were included. Patients with positive toxicology (n = 208) had significantly higher WBC counts (P < 0.001, η2p = 0.02), as well as increased neutrophils (P = 0.002, η2p = 0.01), monocytes (P < 0.001, η2p = 0.02), lymphocytes (P < 0.001, η2p = 0.02), and eosinophils (P = 0.01, η2p = 0.01) compared to those with negative toxicology (n = 719). The increase in neutrophil counts was particularly evident in patients who tested positive for cannabinoids (n = 168; P < 0.001, η2p = 0.02). In contrast, eosinophil counts were particularly increased in the cocaine-positive subgroup (n = 27; P = 0.004, η2p = 0.01). Patients with a positive urine test to opioids (n = 13) were characterized by a significantly lower MLR (P = 0.03, η2p = 0.005). The type of psychiatric diagnosis moderated the differences in neutrophil counts between patients with a positive and negative toxicology to cannabinoids. Notably, significantly higher neutrophil counts were found only in patients diagnosed with a psychotic disorder (P < 0.001, η2p = 0.03). Taken together, our findings suggest that drugs of abuse may differently impact the immune/inflammatory response system in individuals diagnosed with psychiatric conditions. Specifically, recent cannabinoids use may be associated with an acute activation of the inflammatory response system, particularly in individuals with a psychotic disorder, while cocaine and opioid use may be associated with eosinophilia and a decrease in the MLR, respectively, regardless of the primary psychiatric diagnosis.
Keywords: Schizophrenia, Inflammation, Cannabinoids, Cocaine, Opioids, Drugs of abuse, Neutrophils
Highlights
-
•
Cannabinoids, opioids, and cocaine use may differently impact the immune system in psychiatric inpatients.
-
•
Patients under cocaine use showed higher eosinophil counts.
-
•
Patients under opioids use showed a decreased MLR.
-
•
Higher neutrophil counts were particularly observed in patients with psychotic disorders using cannabinoids.
1. Introduction
Accumulating evidence suggests that immunological and/or inflammatory changes may underly several psychiatric conditions, such as schizophrenia, major depressive disorder, bipolar disorder, autism spectrum disorder and/or personality disorders. An increased expression of different pro-inflammatory genes, abnormalities in the number and/or function of several immune cells (e.g., monocytes, lymphocytes), and increased blood and/or cerebrospinal fluid levels of pro-inflammatory compounds (e.g., cytokines, chemokine and/or C-reactive protein (CRP)) have been repeatedly reported in individuals diagnosed with these conditions (Goldsmith et al., 2016; Vogels et al., 2017; Becking et al., 2018; Garcia-Rizo et al., 2019; Jackson and Miller, 2020; Simon et al., 2023; Bioque et al., 2022; Arteaga-Henríquez et al., 2022; López-Villatoro et al., 2023; Sørensen et al., 2023). The relevance of these findings lies in their potential use as biological diagnostic and therapeutic markers in individuals with psychiatric symptoms (personalized psychiatry) (Drexhage et al., 2010; Benedetti et al., 2017; Becking et al., 2018; Arteaga-Henríquez et al., 2019; Ioannou et al., 2024; Llorca-Bofí et al., 2024). For a better understanding of the whole picture, it is important to investigate how several factors, such as age (Grosse et al., 2015), previous viral infections (Simon et al., 2023), childhood trauma (Schiweck et al., 2020) and/or obesity (Milaneschi et al., 2020; Arteaga-Henriquez et al., 2021) may influence the immune/inflammatory changes found in individuals diagnosed with psychiatric conditions (Pillinger et al., 2019).
Substance abuse is highly comorbid in individuals diagnosed with psychiatric disorders (Ross and Peselow, 2012; Toftdahl et al., 2016), i.e., about 10–50% of the subjects diagnosed with a psychiatric disorder will also experience a comorbid substance use disorder (SUD) at some point in their lives (Ross and Peselow, 2012; Toftdahl et al., 2016). Accumulating research suggests that individuals who use substances of abuse may be at a higher risk of treatment resistance and suicidal behavior. On the contrary, other reports also suggest the potential beneficial effect of substances like cannabinoids in the management of anxiety (Schott, 2019). The mechanisms by which these agents impact mood and behavior are not fully understood but may be related to their capability to impact the immune/inflammatory response system exerting, depending on the agent and probably, on the target population, and anti-inflammatory and/or pro-inflammatory action (Tanasescu and Constantinescu, 2010; Bidwell et al., 2020; Henshaw et al., 2021). Despite this, patients meeting criteria for a SUD or under sporadic substance of abuse use are frequently excluded from Immunopsychiatry studies representing an important gap in the literature.
The aim of this retrospective study was to investigate the differences in relation to the levels of a set of immune/inflammatory parameters (i.e., total and differential white blood cell (WBC) counts, platelet counts, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), and CRP), between patients with a psychiatric diagnosis and a positive toxicology to cannabinoids, opioids or cocaine and those with a negative toxicology.
2. Material and methods
2.1. Study participants
For this retrospective study, electronic medical records of all patients admitted to the Inpatient Psychiatric Unit (Santa María University Hospital, Lleida, Spain) between January 1, 2010, and December 31, 2020, were extensively reviewed by two experienced psychiatrists. In order to avoid duplications (and also, to indirectly control for other factors, such as chronicity and/or polypharmacy), we only included data referred to the first time of admission.
Included were acutely ill psychiatric inpatients aged 18 or over, from whom a blood and urine sample was collected at admission (i.e., within the first 24 h). Excluded were subjects diagnosed with a psychiatric disorder secondary to a known medical condition or with a non-specified psychiatric disorder. Patients admitted after a suicide attempt, pregnant or breastfeeding women, as well as individuals diagnosed with a comorbid autoimmune and/or acute/chronic inflammatory, metabolic, cardiovascular, or neurological (including neurocognitive) condition were excluded, too. Subjects testing positive for other agents of abuse than benzodiazepines (BZD), cannabinoids, opioids and/or cocaine (i.e., those testing positive for amphetamines/amphetamine derivatives) were also not included. Given the described association between alcohol consumption and immune system/inflammatory dysfunction (Calleja-Conde et al., 2021), subjects under an alcohol substance use disorder and/or under alcohol use were excluded.
The study protocol was approved by the Local Ethics Committee belonging to the Santa María's University Hospital (Lleida, Spain).
2.2. Study procedures
Blood and urine were collected between 8.00 and 10.00 a.m. by an experienced nurse belonging to the Department of Psychiatry at Santa María's University Hospital (Lleida, Spain) after an overnight fasting. Selected blood markers included: total and differential WBC counts (i.e., basophils, eosinophils, neutrophils, monocytes, lymphocytes), platelet counts and CRP levels. In addition, the following indexes were calculated: NLR, MLR, and PLR. Total and differential WBC counts were assessed by flow cytometry using a Sysmex XN analyzer; the detection range, as determined by the assay manufacturer was set at 0-440 × 109/L. Platelet counts were assessed by impedance also by a Sysmex XN analyzer; the detection range was in this case set at 0-5000 × 109/L. CRP levels were assessed by an immunoturbidimetric assay on a Beckman Coulter automated analyzer; the lower limit of detection, as determined by the assay manufacturer was set on 2 mg/L. Positivity in urine (no/yes) was determined by immunochromatography (i.e., Multi-line Drug Screen Test Device (MONLAB)), and established according to both Substance Abuse Mental Health Administration (SAMHSA) (Verstraete and Pierce, 2001), and United Nations International Drug Control Program criteria (SAMHSA, 2004). The cut-off limits for considering a patient as “positive” were 50 ng/mL for cannabinoids, 300 ng/mL for opioids and 300 ng/mL for cocaine (Verstraete and Pierce, 2001).
2.3. Statistical analyses
Statistical analyses were performed using IBM-SPSS v.23 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp., USA). Continuous data were expressed as mean ± standard deviation (SD), while categorical data were expressed as absolute values and percentages (%). Data were tested for normal distribution by the Kolmogorov-Smirnov test (n ≥ 30). Group comparisons of sample characteristics were analyzed by using Mann-Whitney U tests (i.e., continuous data), or Pearson's chi-square (χ2) tests (i.e., categorical data). A univariate analysis of covariance (ANCOVA) model was used to compare mean immune/inflammatory parameter levels between patients with a negative vs. those with a positive toxicology. Age (years), consumption of BZD (no/yes) and the type of primary psychiatric diagnosis were introduced as covariables. Sensitivity subgroup analyses were additionally performed, in order to explore the effect of the type of agent of use, as well as the type of primary psychiatric diagnosis on immune/inflammatory parameters. Effect sizes are reported as partial eta-squared (η2p). All tests were two-tailed, with P-values equal or less than 0.05 being considered as of statistical significance.
3. Results
3.1. Baseline characteristics of study participants
Baseline characteristics of the 927 patients included are reported in Table 1. For a complete description of the diagnoses included, see Table S1.
Table 1.
Sample characteristics of study participants.
| Negative n = 719 | Positive n = 208 | Negative vs. Positive: | Cannabinoids n = 168 | Negative vs. Positive: | Opioids n = 13 | Negative vs. Positive: | Cocaine n = 27 | Negative vs. Positive: | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years); mean (SD) | 44.93 (16.16) | 35.11 (10.75) | <0.001 | 33.89 (10.25) | <0.001 | 42.95 (15.12) | 0.66 | 38.93 (9.22) | 0.06 |
| Females; n (%) | 366 (51%) | 111 (53%) | 0.53 | 91 (54%) | 0.45 | 9 (69%) | 0.19 | 11 (41%) | 0.30 |
| Agent of use, n (%) | |||||||||
|
396 (55%) | 136 (66%) | 0.01 | 105 (63%) | 0.08 | 9 (69%) | 0.32 | 22 (81%) | 0.005 |
|
0 (0%) | 168 (81%) | |||||||
|
0 (0%) | 13 (6%) | |||||||
|
0 (0%) | 27 (13%) | |||||||
| Primary Psychiatric; n (%) | |||||||||
|
0 (0%) | 46 (22%) | <0.001 | 29 (17%) | <0.001 | 5 (38%) | <0.001 | 12 (44%) | <0.001 |
|
312 (43%) | 81 (39%) | 0.25 | 73 (43%) | 0.99 | 1 (8%) | 0.01 | 7 (26%) | 0.07 |
|
108 (15%) | 7 (3%) | <0.001 | 4 (2%) | <0.001 | 3 (23%) | 0.31 | 0 (0%) | 0.01 |
|
130 (18%) | 26 (12%) | 0.06 | 23 (14%) | 0.17 | 2 (15%) | 0.57 | 1 (4%) | 0.03 |
|
85 (12%) | 14 (7%) | 0.02 | 10 (6%) | 0.03 | 2 (15%) | 0.47 | 2 (7%) | 0.48 |
|
46 (6%) | 23 (11%) | 0.02 | 18 (11%) | 0.05 | 0 (0%) | 0.43 | 5 (18%) | 0.03 |
|
7 (1%) | 1 (1%) | 0.43 | 1 (1%) | 0.64 | 0 (0%) | 0.88 | 0 (0%) | 0.77 |
|
7 (1%) | 0 (0%) | 0.17 | 0 (0%) | 0.23 | 0 (0%) | 0.88 | 0 (0%) | 0.77 |
|
17 (2%) | 1 (1%) | 0.06 | 1 (1%) | 0.12 | 0 (0%) | 0.73 | 0 (0%) | 0.53 |
|
7 (1%) | 9 (4%) | 0.001 | 9 (5%) | 0.001 | 0 (0%) | 0.88 | 0 (0%) | 0.77 |
Abbreviations: BZD: benzodiazepines; NDV: Neurodevelopmental Disorder; OCD: Obsessive-Compulsive Disorder; PD: Personality Disorder; SD: standard deviation; SUD: Substance Use Disorder. P-values were based on Mann Whitney U tests (continuous variables), and on χ2−Squared test (categorical variables). Significant values are highlighted in bold.
In total, 719 (78%) of patients had a negative toxicology; 208 (22%) had a positive toxicology (i.e., cannabinoids (n = 168, 18%), opioids (n = 13, 1%), cocaine (n = 27, 3%)). Among the total of patients with a positive toxicology, 46 (22%) were diagnosed with a SUD.
Patients with a positive toxicology were statistically significantly younger (P<0.001) and showed a statistically significantly higher consumption of BZD (P = 0.01) compared to those with a negative urine test. In addition, subjects with a positive toxicology were diagnosed with a conduct (i.e., 9% vs 4%; P = 0.001) or a personality disorder (i.e., 11% vs. 6%; P = 0.02) at a statistically significantly higher proportion; and with a depressive (i.e., 3% vs. 15%; P < 0.001) or an adjustment disorder (7% vs. 12%; P = 0.02) at a statistically significantly lower proportion, (Table 1).
Significant differences were not found between patients testing negative and those testing positive in relation to sex or to the proportion of psychotic, bipolar, neurodevelopmental, obsessive-compulsive or eating disorder diagnoses (Table 1).
3.2. Blood levels of immune/inflammatory parameters in individuals with a negative urine test vs. individuals with a positive urine test
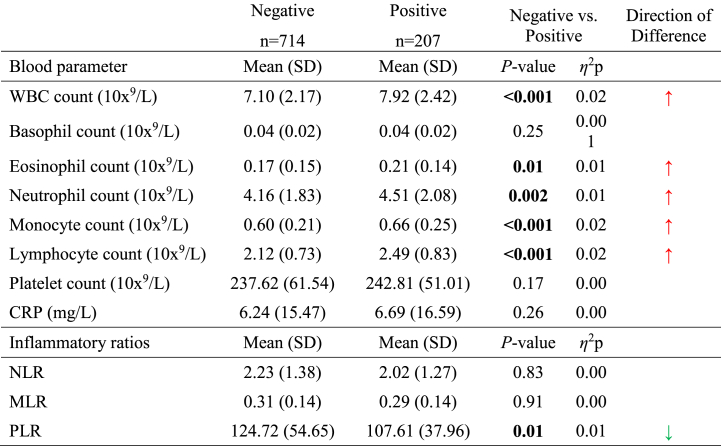
Overall, patients with a positive toxicology were characterized by a statistically significantly higher WBC count (P < 0.001, η2p = 0.02) and by statistically significantly higher blood levels of neutrophils (P = 0.002, η2p = 0.01), monocytes (P < 0.001, η2p = 0.02), lymphocytes (P < 0.001, η2p = 0.02) and eosinophils (P = 0.01, η2p = 0.01) compared to those with a negative toxicology (Table 2). Patients with a positive toxicology were on the contrary characterized by a statistically significantly lower PLR (P = 0.01, η2p = 0.01) compared to those testing negative (Table 2). Significant differences were not found between patients with a positive toxicology and those with a negative toxicology in relation to any of the other immune/inflammatory parameters assessed (Table 2).
Table 2.
Blood levels of immune/inflammatory parameters in individuals with a negative urine test vs. individuals with a positive urine test.
Abbreviations: CRP: C-reactive protein; MLR: monocyte-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; WBC: white blood cell count. Analyses were based on an analysis of covariance (ANCOVA) model with the corresponding blood parameter as the dependent variable, group (negative/positive) as fixed effect variable, and age, consumption of BZD (yes/no), and the type of primary psychiatric diagnosis, as covariates. Significant P-values are highlighted in bold and marked with an asterisk (i.e., ∗∗∗P≤ 0.001, ∗∗P≤ 0.01, ∗P≤ 0.05).
3.3. Blood levels of immune/inflammatory parameters in individuals testing negative vs. those testing positive, stratified by the agent of use
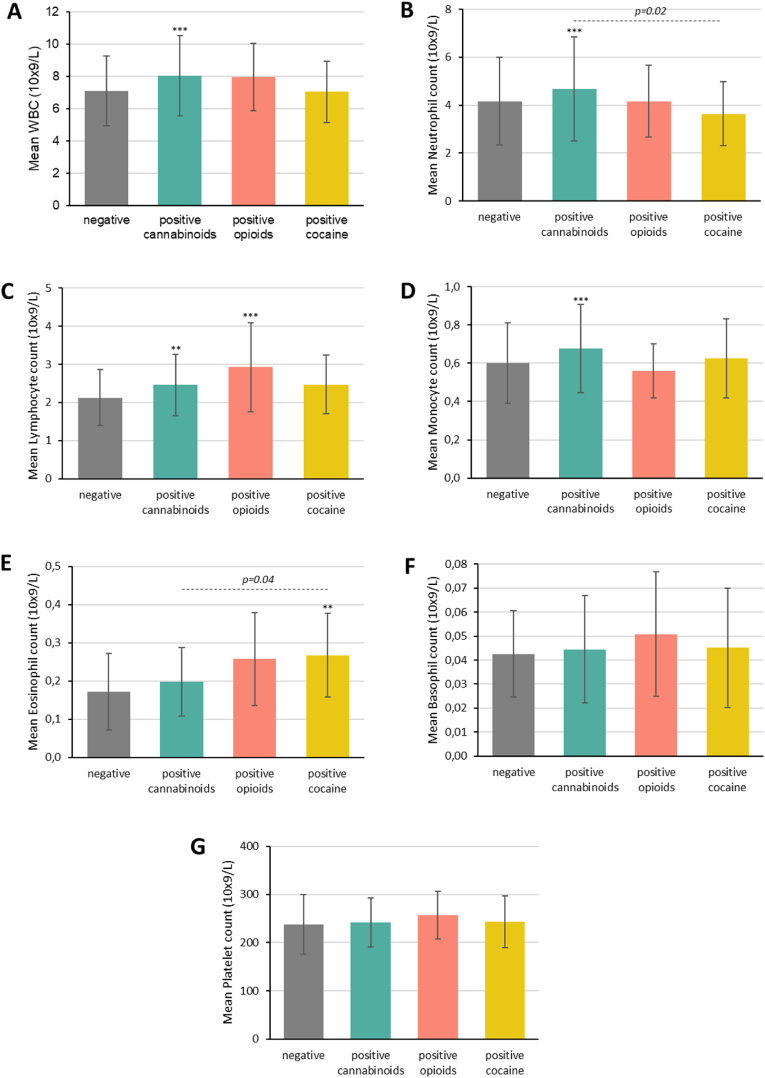
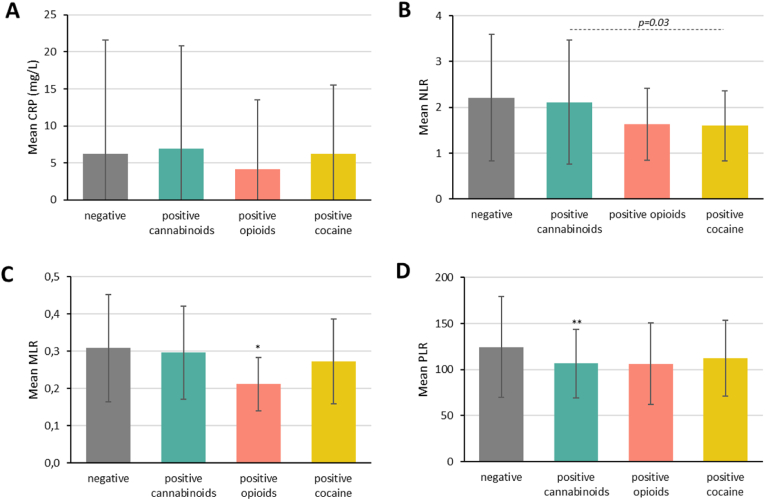
As a sensitivity analysis, we stratified patients with a positive urine test according to their agent of use and then compared them to those with a negative urine test (Fig. 1, Fig. 2, Table S2).
Fig. 1.
Blood levels of immune parameters in individuals with a negative urine test vs. individuals with positive urine test (stratified by agent of consumption; cannabinoids, opioids, or cocaine).
Abbreviations: WBC: white blood cell count. Analyses were based on an analysis of covariance (ANCOVA) model with the corresponding blood parameter as the dependent variable, group as fixed effect variable, and age, consumption of BZD, and type of primary psychiatric diagnosis, as covariates. Significant values are marked with an asterisk (i.e., ∗∗∗P ≤ 0.001, ∗∗P ≤ 0.01, ∗P ≤ 0.05) when comparing negative vs. specific positive urine tests, and with the corresponding p-value (---) when comparing the positive urine tests among themselves.
Fig. 2.
Blood levels of inflammatory parameters in individuals with a negative urine test vs. individuals with positive urine test (stratified by agent of consumption; cannabinoids, opioids, or cocaine).
Abbreviations: CRP: C-reactive protein; MLR: monocyte-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio. Analyses were based on an analysis of covariance (ANCOVA) model with the corresponding blood parameter as the dependent variable, group as fixed effect variable, and age, consumption of BZD, and type of primary psychiatric diagnosis, as covariates. Significant values are marked with an asterisk (i.e., ∗∗∗P ≤ 0.001, ∗∗P ≤ 0.01, ∗P ≤ 0.05) when comparing negative vs. specific positive urine tests, and with the corresponding p-value (---) when comparing the positive urine tests among themselves.
By doing so, we found that patients with a positive toxicology to cannabinoids were characterized by a statistically significantly higher WBC count (P < 0.001, η2p = 0.03) and by statistically significantly higher neutrophil (P < 0.001, η2p = 0.02), monocyte (P < 0.001, η2p = 0.02) and lymphocyte (P = 0.002, η2p = 0.01) counts compared to those with a negative toxicology (Fig. 1A, B, C, D, Table S2). Patients testing positive in urine to cannabinoids were also characterized by a statistically significantly lower PLR (P = 0.02, η2p = 0.01) compared to those with a negative toxicology (Fig. 2D–Table S2). A moderator effect for age and for the type of primary psychiatric diagnosis was suggested for the differences in neutrophil counts (P = 0.02, η2p = 0.01 and P = 0.02, η2p = 0.01, respectively). Age also seemed to moderate the findings for the differences in the PLR (P < 0.001, η2p = 0.02) and in lymphocyte counts (P < 0.001, η2p = 0.004); a moderator effect of BZD consumption was additionally suggested for the differences in lymphocyte counts (P = 0.05, η2p = 0.004).
In relation to patients under opioids use, a statistically significantly lower MLR and a statistically significantly higher lymphocyte counts were found when compared to patients with a negative toxicology (P = 0.03, η2p = 0.005 and P < 0.001, η2p = 0.01, respectively) (Fig. 1, Fig. 2C, Table S2). Age seemed to moderate the findings for the differences in the MLR and in lymphocyte counts (P < 0.01, η2p = 0.02 and P < 0.01, η2p = 0.03, respectively); a moderator effect of BZD consumption was also suggested for the differences in lymphocyte counts (P = 0.04, η2p = 0.004).
In addition, patients with a positive toxicology to cocaine were characterized by statistically significantly higher eosinophil counts compared to those testing negative (P = 0.004, η2p = 0.01) (Fig. 1E–Table S2). Again, age seemed to moderate the findings for the differences in eosinophil counts (P = 0.01, η2p = 0.01).
Statistically significant differences were only found for the NLR and for neutrophil and eosinophil counts when comparing patients with a positive toxicology to cannabinoids, opioids or cocaine among themselves. Specifically, patients testing positive to cannabinoids showed the highest NLR and neutrophil counts, and those testing positive to cocaine showed the highest eosinophil counts (Fig. 1, Fig. 2, Table S2).
3.4. Blood levels of immune/inflammatory parameters in individuals testing negative vs. those testing positive to cannabinoids, stratified by primary psychiatric diagnosis
A moderator effect of the type of primary psychiatric diagnosis was suggested for the differences in neutrophil counts between patients with a positive toxicology to cannabinoids and those with a negative urine test (see 3.3). This may indicate that neutrophil counts may vary depending on the primary psychiatric diagnosis. Accordingly, we investigated the existence of differences in the levels of neutrophil counts between patients with a negative toxicology to cannabinoids and those with a positive toxicology, after stratifying them according to their primary psychiatric diagnosis (Table 3). Since a statistically significant correlation was found between neutrophil counts and the WBC (rs = 0.88, P < 0.001), monocyte counts (rs = 0.55, P < 0.001), basophil counts (rs = 0.22, P < 0.001) and platelet counts (rs = 0.24, P < 0.001), as well as between neutrophil counts and CRP levels (rs = 0.29, P < 0.001), the NLR (rs = 0.74, P < 0.001), MLR (rs = 0.44, P < 0.001) and PLR (rs = 0.13, P < 0.001), the existence of differences in these parameters were also investigated. Due to a low sample size (see Table 1), patients with a depressive disorder and an adjustment disorder were grouped into one diagnostic category, and patients with a personality disorder and a those with a conduct disorder, into another one. Analyses on patients with a neurodevelopmental disorder, an eating disorder, and an obsessive-compulsive disorder could unfortunately not be performed.
Table 3.
Blood levels of immune/inflammatory parameters in individuals with a negative urine test vs. those with positive urine test (stratified by primary psychiatric diagnosis).
| Psychotic Disorder (1) |
Bipolar Disorder (2) |
Depressive + Adjustment Disorder (3) |
Personality + Conduct Disorder (4) |
SUD (5) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative n = 312 | Positive n = 73 | Negative n = 130 | Positive n = 23 | Negative n = 193 | Positive n = 14 | Negative n = 53 | Positive n = 27 | Negative n = 17 | Positive n = 29 | |
| Blood parameter | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| WBC count (10x9/L) | 7.16 (2.14) | 8.38 (2.83)∗∗∗ | 7.23 (2.51) | 8.35 (2.11) | 6.95 (1.94) | 7.61 (2.17) | 7.47 (2.40) | 7.88 (2.41) | 7.68 (1.39) | 7.45 (2.07) |
| Basophil count (10x9/L) | 0.04 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.04 (0.03) | 0.04 (0.02) | 0.05 (0.02) | 0.05 (0.03) | 0.04 (0.02) |
| Neutrophil count (10x9/L) | 4.22 (1.83) | 5.08 (2.59)∗∗∗ | 4.29 (2.16) | 4.82 (1.75) | 4.04 (1.61) | 4.43 (1.59) | 4.34 (1.77) | 4.23 (1.93) | 3.94 (1.29) | 4.17 (1.69) |
| Monocyte count (10x9/L) | 0.61 (0.22) | 0.71 (0.28)∗∗ | 0.59 (0.22) | 0.66 (0.21) | 0.58 (0.17) | 0.59 (0.19) | 0.64 (0.25) | 0.72 (0.31) | 0.63 (0.18) | 0.61 (0.15) |
| Platelet count (10x9/L) | 236.48 (63.26) | 241.18 (47.39) | 240.30 (59.48) | 247.08 (41.25) | 241.82 (59.72) | 238.14 (49.33) | 235.67 (61.45) | 239.18 (59.24) | 149.23 (55.62) | 236.38 (55.62) |
| CRP (mg/L) | 5.40 (11.26) | 7.45 (19.84) | 5.67 (9.53) | 8.30 (22.73) | 6.74 (15.42) | 2.31 (1.55) | 12.10 (37.08) | 5.40 (7.81) | 9.26 (12.62) | 7.04 (16.59) |
| Inflammatory ratios | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| NLR | 2.30 (1.47) | 2.28 (1.55) | 2.27 (1.38) | 2.06 (1.38) | 2.14 (1.35) | 1.94 (0.59) | 2.19 (1.08) | 1.72 (0.95) | 1.64 (0.94) | 1.99 (1.11) |
| MLR | 0.32 (0.16) | 0.31 (0.15) | 0.30 (0.27) | 0.27 (0.15) | 0.29 (0.13) | 0.28 (0.13) | 0.32 (0.14) | 0.28 (0.11) | 0.26 (0.12) | 0.28 (0.14) |
| PLR | 126.51 (56.72) | 107.90 (34.46) | 124.49 (44.45) | 97.36 (27.98)∗ | 124.66 (57.39) | 112.85 (56.46) | 122.44 (62.50) | 101.55 (42.35) | 100.24 (27.18) | 109.42 (43.16) |
Abbreviations: CRP: C-reactive protein; MLR: monocyte-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; WBC: white blood cell count. Analyses were based on an analysis of covariance (ANCOVA) model with the corresponding blood parameter as the dependent variable, group (negative/positive), as fixed effect variable, and age, consumption of BZD (yes/no), and agent of use (cannabinoids/opioids/cocaine), as covariates. Significant P-values are highlighted in bold and marked with an asterisk (i.e., ∗∗∗P ≤ 0.001, ∗∗P ≤ 0.01, ∗P ≤ 0.05).
In the subgroup of patients diagnosed with a psychotic disorder, patients testing positive in urine to cannabinoids showed a statistically significantly higher WBC (P < 0.001, η2p = 0.04) and statistically significantly higher neutrophil (P < 0.001, η2p = 0.03) and monocyte (P = 0.002, η2p = 0.02) counts compared to those testing negative (Table 3).
Patients with a positive toxicology to cannabinoids and a diagnosis of either bipolar disorder, depressive/adjustment disorder, personality/conduct disorder or SUD did not statistically significantly differ in relation to any of the immune/inflammatory parameters assessed when compared with those with a negative toxicology except for the PLR, with patients with a bipolar disorder diagnosis and a positive urine test showing a statistically significantly PLR compared to those testing negative (Table 3).
Statistically significant differences in relation to the WBC, neutrophil, monocyte and platelet counts were not found when comparing patients with a psychotic disorder, bipolar disorder, depressive/adjustment disorder, personality/conduct disorder or SUD among themselves (data not shown).
4. Discussion
To the best of our knowledge, this is the first study to date aimed at investigating differences in a set of immune/inflammatory parameters between psychiatric inpatients with a positive urine test to cannabinoids, opioids or cocaine, and those with a negative urine test.
Overall, patients with a positive toxicology were characterized by a significantly higher WBC counts, as well as significantly higher blood levels of neutrophils, monocytes, lymphocytes and eosinophils compared to those with a negative one. The increase in eosinophil counts was particularly evident in the subgroup of patients who tested positive for cocaine. In contrast, patients who tested positive for cannabinoids were characterized by significantly higher WBC, neutrophil and monocyte counts compared to those with a negative toxicology. In addition, a statistically significantly lower MLR was found in the subgroup of patients testing positive in urine to opioids when compared to those with a negative toxicology. These findings are supported by previous existing human studies where an association between cocaine consumption and an increase in eosinophil counts (Berman et al., 2016; Reyes et al., 2018; Gill et al., 2023), opioids consumption and a lower MLR (Banerjee and Sarkar, 1994; Orum et al., 2018), and cannabis use and higher neutrophil counts (Lorenz et al., 2017; Alshaarawy, 2019; Romeo et al., 2023) has been reported, suggesting that cannabinoids, opioids and cocaine may differently impact the immune/inflammatory response system in individuals with and without psychiatric conditions.
Something which makes our study novel is the fact that analyses were not restricted to patients diagnosed with a specific psychiatric disorder, i.e., patients with different psychiatric conditions were included. Thereby, we were able to investigate the influence of the type of primary psychiatric diagnosis on the differences found between patients with a positive toxicology to any of the agents of abuse assessed, and those with a negative toxicology. Interestingly, a moderator effect for the type of psychiatric diagnosis was found for the differences in neutrophil counts in individuals under cannabinoids use, suggesting a differential effect of cannabinoids on neutrophil counts, depending on the primary psychiatric diagnosis. More specific, we found that only among patients with a psychotic disorder diagnosis did cannabinoid users have statistically significantly higher neutrophil counts compared to non-users. Psychotic patients under cannabinoids use were also the only ones characterized by a statistically significantly higher WBC and monocyte counts when compared to non-users.
While the association between cannabis use and non-psychotic psychiatric conditions is still under debate, exogenous cannabis is considered as one of the major environmental risk factors for psychosis. Consumption of cannabis has been repeatedly associated with not only a worsening in psychotic symptoms and/or with an increased risk of psychotic relapse (Kuepper et al., 2011; Haney and Evins, 2016; Bioque et al., 2022) but also, with an increased risk of developing a psychotic disorder in the future (Moore et al., 2007; Di Forti et al., 2019).
The mechanisms for such associations are not fully understood. However, accumulating recent evidence suggests that cannabinoids may increase the risk of psychosis and/or worsen psychotic symptoms by impacting the immune/inflammatory response system of predisposed individuals (Gibson et al., 2020).
Individuals with a psychotic disorder and/or predisposed to are characterized by a dysregulated endocannabinoid system (ECS) (Bioque et al., 2013). The ECS constitutes a complex regulatory network of neurotransmitters, receptors and enzymes that plays a crucial role in maintain inflammatory balance in the human body. While the ECS is widely expressed throughout the body and can be found in almost all organs, the human nervous system and the immune system have interestingly been found to represent the highest expression levels of cannabinoid receptors (Lu and Mackie, 2021). In individuals with psychosis, a decreased expression of the cannabinoid receptor 2 (CB2) and both endocannabinoids synthesizing enzymes (N-acyl phosphatidylethanolamine phospholipase and diacylglycerol lipase) have been found when compared to healthy controls (Bioque et al., 2013).
Activation of CB2 receptors has been associated with the inhibition of microglial activation (Olabiyi et al., 2023) and with the blockade of neutrophil and/or monocyte migration (Gómez et al., 2021), that is, with a systemic deactivation of the inflammatory response system. Therefore, abnormalities in the expression and/or of function of CB2 receptors (which may occur in psychosis) may be associated with both microglial activation and with an activation of the inflammatory response system, something which has been repeatedly described in subjects with psychosis (Bioque et al., 2024; Chen et al., 2024).
In psychotic patients under cannabis use, dysregulation of the ECS may be particularly accentuated (Bioque et al., 2013). Therefore, these patients may exhibit heightened activation of their immune/inflammatory response system, as supported by our findings.
The WBC is nowadays considered as a reliable measure of the overall immune system activity (i.e., a high WBC count, or leukocytosis, suggests the existence of an activated immune response system) (Soehnlein et al., 2017). While acute inflammation is characterized by an increase in neutrophil levels, chronic inflammation is also associated with elevated levels of mononuclear cells, such as monocytes (Soehnlein et al., 2017).
Taken all this together, we hypothesize that (recent) cannabinoids use may be associated with a significantly higher (acute) activation of the inflammatory response system in, particularly, individuals diagnosed with a psychotic disorder.
This may have important potential therapeutic implications. An association between an activated inflammatory response system and treatment-resistance has been reported in patients diagnosed with psychotic disorders (Llorca-Bofí et al., 2021; Bioque et al., 2022; Howes and Onwordi, 2023). Interestingly clozapine (i.e., an antipsychotic agent indicated for the management of treatment-resistant schizophrenia) may exert tits action by, among other mechanisms, modulating neutrophil levels (Jones et al., 2023; Llorca-Bofí et al., 2024). Indeed, one of the most serious adverse effects of clozapine is neutropenia, a potentially serious and mortal side effect characterized by a significant reduction in neutrophil counts, which are crucial for, for example, combating infections (Silva et al., 2020). Therefore, we hypothesize modulation of neutrophil counts as a novel treatment strategy in individuals with psychosis under cannabinoids use. Interestingly, accumulating research has suggested clozapine as the first-line treatment for the management of patients with schizophrenia and a history of cannabis use (Brzozowska et al., 2017; Tang et al., 2017).
Although encouraging, our findings must be considered in light of several important limitations. First, immune and/or inflammatory parameters were assessed in the blood, and findings may thus differ from those in the brain. Data about a healthy control group and/or about potential confounding factors, such as body mass index (Pangrazzi et al., 2020), childhood maltreatment and/or the use of psychotropic medication were also not available and therefore, not included in our analyses. However, we controlled for other potential confounding factors, such as type of primary psychiatric diagnosis, illness chronicity, BZD use and/or the presence of comorbid somatic conditions and have made an effort to review the existing literature on the topic, finding a concordance between previous findings, and ours.
Second, information about consumption periodicity was not collected. Thereby, and since measures in urine inform about recent use, findings may vary in patients with a history of chronic use. Unfortunately, immune/inflammatory parameters were assessed at a single timepoint, preventing us from assessing the effects of drug abstinence on these markers over time. In line with this, in a recent study performed by Romeo et al. (2023), tetrahydrocannabinol (THC) cessation was interestingly associated with an increase in inflammatory markers, including WBC count, as well as lymphocyte and monocyte levels, all this correlating with symptomatology of patients with psychosis. However, and as a strength of our study, information about drug consumption relied on quantitative (objective) information, rather than solely on self-reported substance patterns, and a standardized blood sampling procedure in all included patients was implemented, reducing the potential for technical and biological biases (Raouf et al., 2018). Third, data about cannabinoids composition were not collected. Cannabis sativa contains not only delta-9-tetrahydrocannabinol (Δ9-THC) and/or cannabidiol (CBD), but more than 70 different cannabinoids that are also found in the cannabis products available on the market (Elsohly and Slade, 2005). This may be of relevance, since different animal (Malfait et al., 2000) and human studies have repeatedly suggested that Δ9-THC and CBD may exert opposing immunomodulatory effects (Bidwell et al., 2020). Exogenous cannabinoids containing a high percentage of CBD may exert an immunosuppressor and/or anti-inflammatory action, decreasing the numbers of different leukocyte subpopulations, such as monocytes (McDougle et al., 2017; Henshaw et al., 2021).
Both the type and/or concentrations of cannabinoids may differ greatly by, among other factors, the place of origin (Potter et al., 2008; Mehmedic et al., 2010). Specifically in Spain, THC content in the cannabis usually consumed on the streets is between 15 and 28% on average. On the contrary, the mean CBD content has been set at 5% on average (Santos-Álvarez et al., 2021). Accordingly, we assumed that our patients were under consumption of exogenous cannabinoids containing a high percentage of Δ9-THC, but unfortunately, we cannot ensure this, something which could have biased our findings and may be responsible of the inconsistencies found in relation to previous existing literature.
Also, the possibility of a type II error (i.e., false negative) should be taken into account when interpreting our findings. Despite including a relatively large total sample size (n = 927), sensitivity subgroup analyses based on the agent of use or on the primary psychiatric diagnosis led to small sample sizes. This could contribute to failing to detect statistically significant differences when actually present. Finally, the retrospective nature of our study, coupled with the relatively small sample size, limited the inferences that could be drawn about causation.
5. Conclusions
Overall, our findings suggest that recent cannabinoids use may be associated with a significantly higher activation of the immune/inflammatory response system, particularly in individuals diagnosed with a psychotic disorder. On the contrary, recent cocaine and opioid use may be respectively associated with eosinophilia and with a significantly lower MLR, regardless of the primary psychiatric diagnosis.
We believe our findings may provide important novel valuable insights into how cannabinoids, opioids and cocaine may impact the inflammatory response system of patients diagnosed with different psychiatric conditions and may thus have important clinical and therapeutic implications. For example, modulation of neutrophil counts may represent a novel treatment strategy in individuals with a psychotic disorder and a comorbid cannabis use. However, larger prospective studies, including data about symptoms severity, consumption periodicity and/or about cannabinoids composition are warranted to confirm our findings.
CRediT authorship contribution statement
Vicent Llorca-Bofí: Writing – original draft, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Maria Mur: Writing – review & editing, Supervision, Funding acquisition. Maria Font: Writing – review & editing, Project administration, Methodology, Investigation. Roberto Palacios-Garrán: Writing – review & editing. Maite Sellart: Writing – review & editing. Enrique del Agua-Martínez: Writing – review & editing. Miquel Bioque: Writing – review & editing, Supervision. Gara Arteaga-Henríquez: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization.
Submission declaration
The present work has not been published previously, has not been submitted to another journal while under consideration for Brain, Behavior, and Immunity-Health, and will not be published elsewhere upon acceptance. The manuscript in its current form has been approved by all co-authors.
Funding
GA-H was supported by the European Commission, and funded by the European's Union Horizon 2020 research and innovation program under grant agreement numbers 728018 and 754740.
The funding source had no role in the study design, the data collection, the analysis and interpretation of data, the manuscript writing, and in the decision to submit the article for publication. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
VL-B reported receiving financial support for continuing medical education from Adamed, Advanz Pharma, Angelini, Casen Recordati, Exeltis, Janssen, Lundbeck, Neurocrine Biosciences and Rovi outside the submitted work. GA-H reported receiving personal fees from Janssen outside the submitted work.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the staff of the Department of Psychiatry of Hospital Universitari Santa Maria, Lleida, Spain.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2024.100898.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
Data availability
Data will be made available on request.
References
- Alshaarawy O. Total and differential white blood cell count in cannabis users: Results from the cross-sectional National Health and Nutrition Examination Survey, 2005-2016. J. Cannabis Res. 2019;1:1–7. doi: 10.1186/S42238-019-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga-Henriquez G., Burger B., Weidinger E., Grosse L., Moll N., Schuetze G., et al. Activation and deactivation steps in the tryptophan breakdown pathway in major depressive disorder: a link to the monocyte inflammatory state of patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;107 doi: 10.1016/J.PNPBP.2020.110226. [DOI] [PubMed] [Google Scholar]
- Arteaga-Henríquez G., Lugo-Marín J., Gisbert L., Setién-Ramos I., Martínez-Gallo M., Pujol-Borrell R., et al. Activation of the monocyte/Macrophage system and abnormal blood levels of lymphocyte subpopulations in individuals with autism spectrum disorder: a systematic review and meta-analysis. Int. J. Mol. Sci. 2022;23:14329. doi: 10.3390/IJMS232214329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga-Henríquez G., Simon M.S., Burger B., Weidinger E., Wijkhuijs A., Arolt V., et al. Low-grade inflammation as a Predictor of Antidepressant and anti-inflammatory Therapy response in MDD patients: a systematic review of the literature in Combination with an analysis of Experimental data collected in the EU-MOODINFLAME Consortium. Front. Psychiatry. 2019;10:458. doi: 10.3389/FPSYT.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D., Sarkar N.K. A study of some blood parameters in chronic heroin smokers. Med. Sci. Res. 1994;22:379–380. [Google Scholar]
- Becking K., Haarman B.C.M., Grosse L., Nolen W.A., Claes S., Arolt V., et al. The circulating levels of CD4+ t helper cells are higher in bipolar disorder as compared to major depressive disorder. J. Neuroimmunol. 2018;319:28–36. doi: 10.1016/J.JNEUROIM.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Poletti S., Hoogenboezem T.A., Locatelli C., De Wit H., Wijkhuijs A.J.M., et al. Higher Baseline Proinflammatory cytokines Mark poor Antidepressant response in bipolar disorder. J. Clin. Psychiatry. 2017;78:e986–e993. doi: 10.4088/JCP.16M11310. [DOI] [PubMed] [Google Scholar]
- Berman M., Paran D., Elkayam O. Cocaine-Induced Vasculitis. Rambam Maimonides Med. J. 2016;7 doi: 10.5041/RMMJ.10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell L.C., Ellingson J.M., Karoly H.C., Yorkwilliams S.L., Hitchcock L.N., Tracy B.L., et al. Association of Naturalistic administration of cannabis flower and Concentrates with Intoxication and Impairment. JAMA Psychiatry. 2020;77:787–796. doi: 10.1001/JAMAPSYCHIATRY.2020.0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioque M., García-Bueno B., MacDowell K.S., Meseguer A., Saiz P.A., Parellada M., et al. Peripheral endocannabinoid system dysregulation in first-episode psychosis. Neuropsychopharmacology. 2013;38:2568–2577. doi: 10.1038/NPP.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioque M., Llorca-Bofí V., Salmerón S., García-Bueno B., MacDowell K.S., Moreno C., et al. Association between neutrophil to lymphocyte ratio and inflammatory biomarkers in patients with a first episode of psychosis. J. Psychiatr. Res. 2024;172:334–339. doi: 10.1016/J.JPSYCHIRES.2024.02.044. [DOI] [PubMed] [Google Scholar]
- Bioque M., Matias-Martins A.C., Llorca-Bofí V., Mezquida G., Cuesta M.J., Vieta E., et al. Neutrophil to lymphocyte ratio in patients with a first episode of psychosis: a two-year Longitudinal follow-up study. Schizophr. Bull. 2022;48:1327–1335. doi: 10.1093/SCHBUL/SBAC089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioque M., Mezquida G., Amoretti S., García-Rizo C., López-Ilundain J.M., Diaz-Caneja C., et al. Clinical and treatment predictors of relapse during a three-year follow-up of a cohort of first episodes of schizophrenia. Schizophr. Res. 2022;243:32–42. doi: 10.1016/j.schres.2022.02.026. [DOI] [PubMed] [Google Scholar]
- Brzozowska N.I., De Tonnerre E.J., Li K.M., Wang X.S., Boucher A.A., Callaghan P.D., et al. The differential Binding of antipsychotic drugs to the ABC Transporter P-Glycoprotein Predicts cannabinoid-antipsychotic drug Interactions. Neuropsychopharmacology. 2017;42:2222–2231. doi: 10.1038/NPP.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja-Conde J., Echeverry-Alzate V., Bühler K.M., Durán-González P., Morales-García J.Á., Segovia-Rodríguez L., et al. The immune system through the Lens of alcohol Intake and Gut Microbiota. Int. J. Mol. Sci. 2021;22:7485. doi: 10.3390/IJMS22147485. 2021;22:7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Tan Y., Tian L. Immunophenotypes in psychosis: is it a premature inflamm-aging disorder? Mol Psychiatry. 2024;29:2834–2848. doi: 10.1038/S41380-024-02539-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexhage R.C., Knijff E.M., Padmos R.C., Van Der Heul-Nieuwenhuijzen L., Beumer W., Versnel M.A., et al. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev. Neurother. 2010;10:59–76. doi: 10.1586/ERN.09.144. [DOI] [PubMed] [Google Scholar]
- ElSohly M.A., Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/J.LFS.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Di Forti M., Quattrone D., Freeman T.P., Tripoli G., Gayer-Anderson C., Quigley H., et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatr. 2019;6:427–436. doi: 10.1016/S2215-0366(19)30048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rizo C., Casanovas M., Fernandez-Egea E., Oliveira C., Meseguer A., Cabrera B., et al. Blood cell count in antipsychotic-naive patients with non-affective psychosis. Early Interv. Psychiatry. 2019;13:95–100. doi: 10.1111/EIP.12456. [DOI] [PubMed] [Google Scholar]
- Gibson C.L., Bassir Nia A., Spriggs S.A., DeFrancisco D., Swift A., Perkel C., et al. Cannabinoid use in psychotic patients impacts inflammatory levels and their association with psychosis severity. Psychiatry Res. 2020;293 doi: 10.1016/j.psychres.2020.113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill C., Sturman J., Ozbek L., Henderson S.R., Burns A., Hamour S., et al. Cocaine-induced granulomatosis with polyangiitis-an under-recognized condition. Rheumatol. Adv. Pract. 2023;7 doi: 10.1093/RAP/RKAD027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez C.T., Lairion F., Repetto M., Ettcheto M., Merelli A., Lazarowski A., et al. Cannabidiol (CBD) Alters the Functionality of neutrophils (PMN). Implications in the Refractory Epilepsy treatment. Pharmaceuticals. 2021;14:220. doi: 10.3390/PH14030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse L., Carvalho L.A., Wijkhuijs A.J.M., Bellingrath S., Ruland T., Ambrée O., et al. Clinical characteristics of inflammation-associated depression: monocyte gene expression is age-related in major depressive disorder. Brain Behav. Immun. 2015;44:48–56. doi: 10.1016/J.BBI.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Haney M., Evins A.E. Does cannabis Cause, Exacerbate or Ameliorate psychiatric disorders? An Oversimplified debate Discussed. Neuropsychopharmacology. 2016;41:393–401. doi: 10.1038/NPP.2015.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw F.R., Dewsbury L.S., Lim C.K., Steiner G.Z. The effects of cannabinoids on pro- and anti-inflammatory cytokines: a systematic review of in Vivo studies. Cannabis Cannabinoid Res. 2021;6:177–195. doi: 10.1089/CAN.2020.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O.D., Onwordi E.C. The synaptic hypothesis of schizophrenia version III: a master mechanism. Mol. Psychiatry. 2023;28:1843–1856. doi: 10.1038/s41380-023-02043-w. 2023;28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou M., Simon M.S., Borkent J., Wijkhuijs A., Berghmans R., Haarman B.C.M., et al. Higher T central and lower effector memory cells in bipolar disorder: a differentiation abnormality? Brain Behav. Immun. Health. 2024;38:100764. doi: 10.1016/J.BBIH.2024.100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A.J., Miller B.J. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr. Scand. 2020;142:18–26. doi: 10.1111/ACPS.13140. [DOI] [PubMed] [Google Scholar]
- Jones R., Morales-Munoz I., Shields A., Blackman G., Legge S.E., Pritchard M., et al. Early neutrophil trajectory following clozapine may predict clozapine response – Results from an observational study using electronic health records. Brain Behav. Immun. 2023;113:267–274. doi: 10.1016/J.BBI.2023.07.012. [DOI] [PubMed] [Google Scholar]
- Kuepper R., Van Os J., Lieb R., Wittchen H.U., Höfler M., Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:537. doi: 10.1136/BMJ.D738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca-Bofí V., Bioque M., Madero S., Mallorquí A., Oliveira C., Garriga M., et al. Blood cell count ratios at Baseline are associated with initial clinical response to clozapine in treatment-resistant, clozapine-Naïve, schizophrenia-spectrum disorder. Pharmacopsychiatry. 2024;57:173–179. doi: 10.1055/A-2290-6386. [DOI] [PubMed] [Google Scholar]
- Llorca-Bofí V., Palacios-Garrán R., Rey Routo D., Buil-Reiné E., Adrados-Pérez M., Gich I., et al. High neutrophil-lymphocyte ratio upon admission is associated with better response in psychotic depression. J. Psychiatr. Res. 2021;143:38–42. doi: 10.1016/j.jpsychires.2021.08.021. [DOI] [PubMed] [Google Scholar]
- Llorca-Bofí V., Petersen L.V., Mortensen P.B., Benros M.E. White blood cell counts, ratios, and C-reactive protein among individuals with schizophrenia spectrum disorder and associations with long-term outcomes: a population-based study. Brain Behav. Immun. 2024;122:18–26. doi: 10.1016/j.bbi.2024.07.041. [DOI] [PubMed] [Google Scholar]
- López-Villatoro J.M., Díaz-Marsá M., De la Torre-Luque A., MacDowell K.S., Prittwitz C., Leza J.C., et al. Inflammatory and oxidative endophenotypes in borderline personality disorder: a biomarker cluster analysis. World J. Biol. Psychiatr. 2023;24:587–594. doi: 10.1080/15622975.2023.2183254. [DOI] [PubMed] [Google Scholar]
- Lorenz D.R., Dutta A., Mukerji S.S., Holman A., Uno H., Gabuzda D. Marijuana Use impacts Midlife cardiovascular Events in HIV-infected men. Clin. Infect. Dis. 2017;65:626–635. doi: 10.1093/CID/CIX391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.C., Mackie K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2021;6:607–615. doi: 10.1016/j.bpsc.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait A.M., Gallily R., Sumariwalla P.F., Malik A.S., Andreakos E., Mechoulam R., et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9561–9566. doi: 10.1073/PNAS.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle D.R., Watson J.E., Abdeen A.A., Adili R., Caputo M.P., Krapf J.E., et al. Anti-inflammatory ω-3 endocannabinoid epoxides. Proc.Natl. Acad. Sci. U. S. A. 2017;114:E6034–E6043. doi: 10.1073/PNAS.1610325114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmedic Z., Chandra S., Slade D., Denham H., Foster S., Patel A.S., et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci. 2010;55:1209–1217. doi: 10.1111/J.1556-4029.2010.01441.X. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y., Lamers F., Berk M., Penninx B.W.J.H. Depression Heterogeneity and its biological Underpinnings: toward Immunometabolic depression. Biol. Psychiatry. 2020;88:369–380. doi: 10.1016/J.BIOPSYCH.2020.01.014. [DOI] [PubMed] [Google Scholar]
- Moore T.H., Zammit S., Lingford-Hughes A., Barnes T.R., Jones P.B., Burke M., et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Olabiyi B.F., Schmoele A.C., Beins E.C., Zimmer A. Pharmacological blockade of cannabinoid receptor 2 signaling does not affect LPS/IFN-γ-induced microglial activation. Sci. Rep. 2023;13:11105. doi: 10.1038/S41598-023-37702-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orum M.H., Kara M.Z., Egilmez O.B., Kalenderoglu A. In: Complete Blood Count Alterations Due to the Opioid Use: what about the Lymphocyte-Related Ratios, Especially in Monocyte to Lymphocyte Ratio and Platelet to Lymphocyte Ratio? J. Immunoassay Immunochem. 2018;39:365–376. doi: 10.1080/15321819.2018.1460272. [DOI] [PubMed] [Google Scholar]
- Pangrazzi L., Naismith E., Miggitsch C., Carmona Arana J.A., Keller M., Grubeck-Loebenstein B., et al. The impact of body mass index on adaptive immune cells in the human bone marrow. Immun. Ageing. 2020;17:15. doi: 10.1186/S12979-020-00186-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger T., Osimo E.F., Brugger S., Mondelli V., McCutcheon R.A., Howes O.D. A meta-analysis of immune parameters, Variability, and Assessment of modal distribution in psychosis and test of the immune subgroup hypothesis. Schizophr. Bull. 2019;45:1120–1133. doi: 10.1093/SCHBUL/SBY160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter D.J., Clark P., Brown M.B. Potency of delta 9-THC and other cannabinoids in cannabis in England in 2005: implications for psychoactivity and pharmacology. J. Forensic Sci. 2008;53:90–94. doi: 10.1111/J.1556-4029.2007.00603.X. [DOI] [PubMed] [Google Scholar]
- Raouf M., Bettinger J.J., Fudin J. A Practical Guide to urine drug Monitoring. Fed. Pract. 2018;35:38–44. [PMC free article] [PubMed] [Google Scholar]
- Reyes F., Vaitkus V., Al-Ajam M. A case of cocaine-induced eosinophilic pneumonia: case report and review of the literature. Respir. Med. Case. Rep. 2018;23:98–102. doi: 10.1016/J.RMCR.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo B., Lestra V., Martelli C., Amirouche A., Benyamina A., Hamdani N. Increased markers of inflammation after cannabis cessation and their association with psychotic symptoms. Acta Neuropsychiatr. 2023;36:118–127. doi: 10.1017/NEU.2023.24. [DOI] [PubMed] [Google Scholar]
- Ross S., Peselow E. Co-occurring psychotic and addictive disorders: neurobiology and diagnosis. Clin. Neuropharmacol. 2012;35:235–243. doi: 10.1097/WNF.0B013E318261E193. [DOI] [PubMed] [Google Scholar]
- SAMHSA Proposed revisions to Mandatory Guidelines for Federal Workplace drug testing programs. Fed. Regist. 2004;60:19673–19732. [Google Scholar]
- Santos-Álvarez I., Pérez-Lloret P., González-Soriano J., Pérez-Moreno M. An approach to the evaluation of the potency of cannabis resin in Madrid: a health hazard? Adicciones. 2021;12:279–288. doi: 10.20882/adicciones.1630. [DOI] [PubMed] [Google Scholar]
- Schiweck C., Claes S., Van Oudenhove L., Lafit G., Vaessen T., de Beeck G.O., et al. Childhood trauma, suicide risk and inflammatory phenotypes of depression: insights from monocyte gene expression. Transl. Psychiatry. 2020;10:296. doi: 10.1038/S41398-020-00979-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott M. Insights on cannabidiol’s antiallodynic and anxiolytic mechanisms of action in a model of neuropathic pain. Pain Rep. 2019;4:e774. doi: 10.1097/PR9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E., Higgins M., Hammer B., Stephenson P. Clozapine rechallenge and initiation despite neutropenia- a practical, step-by-step guide. BMC Psychiatry. 2020;20:279. doi: 10.1186/s12888-020-02592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M.S., Ioannou M., Arteaga-Henríquez G., Wijkhuijs A., Berghmans R., Musil R., et al. Premature T cell aging in major depression: a double hit by the state of disease and cytomegalovirus infection. Brain Behav. Immun. Health. 2023;29:100608. doi: 10.1016/J.BBIH.2023.100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehnlein O., Steffens S., Hidalgo A., Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017;17:248–261. doi: 10.1038/nri.2017.10. [DOI] [PubMed] [Google Scholar]
- Sørensen N.V., Frandsen B.H., Orlovska-Waast S., Buus T.B., Ødum N., Christensen R.H., et al. Immune cell composition in unipolar depression: a comprehensive systematic review and meta-analysis. Mol. Psychiatry. 2023;28:391–401. doi: 10.1038/S41380-022-01905-Z. [DOI] [PubMed] [Google Scholar]
- Tanasescu R., Constantinescu C.S. Cannabinoids and the immune system: an overview. Immunobiology. 2010;215:588–597. doi: 10.1016/J.IMBIO.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Tang Y., Horvitz-Lennon M., Gellad W.F., Lave J.R., Chang C.C.H., Normand S.L., et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr. Serv. 2017;68:579–586. doi: 10.1176/APPI.PS.201600041. [DOI] [PubMed] [Google Scholar]
- Toftdahl N.G., Nordentoft M., Hjorthøj C. Prevalence of substance use disorders in psychiatric patients: a nationwide Danish population-based study. Soc. Psychiatry Psychiatr. Epidemiol. 2016;51:129–140. doi: 10.1007/S00127-015-1104-4. [DOI] [PubMed] [Google Scholar]
- Verstraete A.G., Pierce A. Workplace drug testing in Europe. Forensic Sci. Int. 2001;121:2–6. doi: 10.1016/S0379-0738(01)00445-5. [DOI] [PubMed] [Google Scholar]
- Vogels R.J., Koenders M.A., van Rossum E.F.C., Spijker A.T., Drexhage H.A. T cell Deficits and Overexpression of Hepatocyte Growth factor in anti-inflammatory circulating monocytes of Middle-aged patients with bipolar disorder characterized by a high Prevalence of the metabolic Syndrome. Front. Psychiatry. 2017;8:34. doi: 10.3389/FPSYT.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.