Abstract
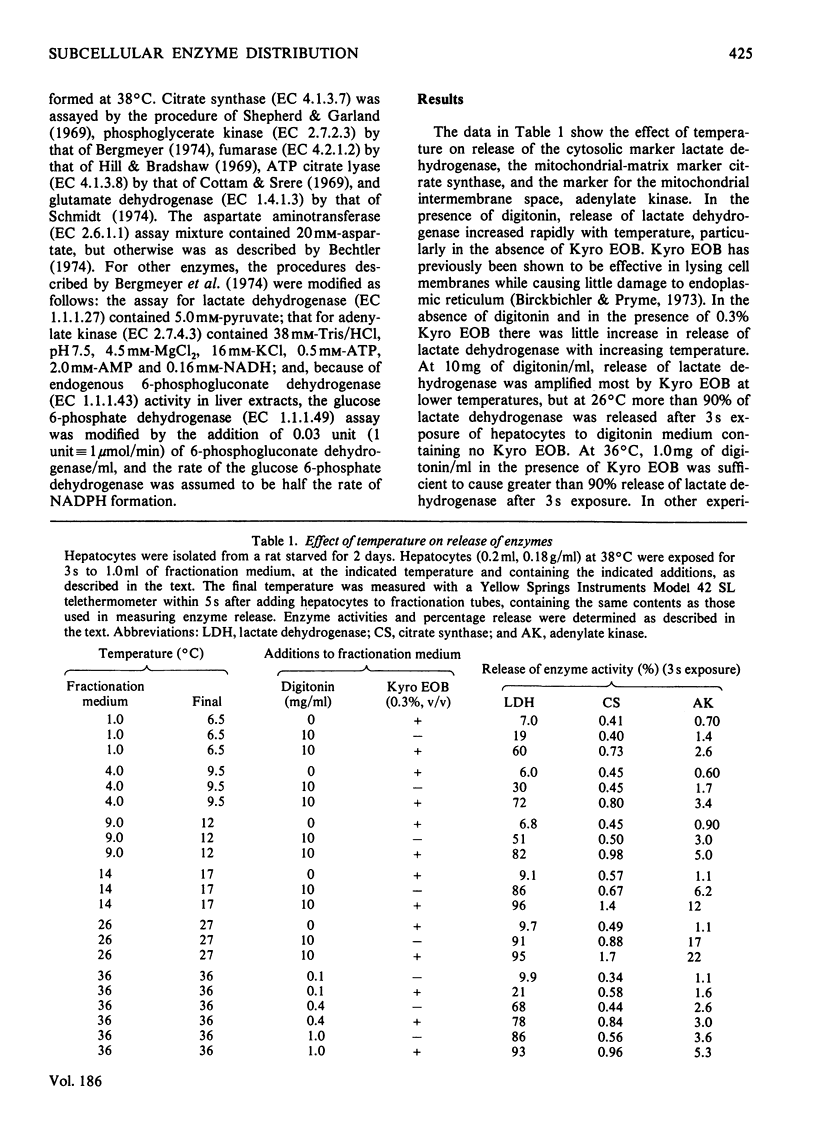
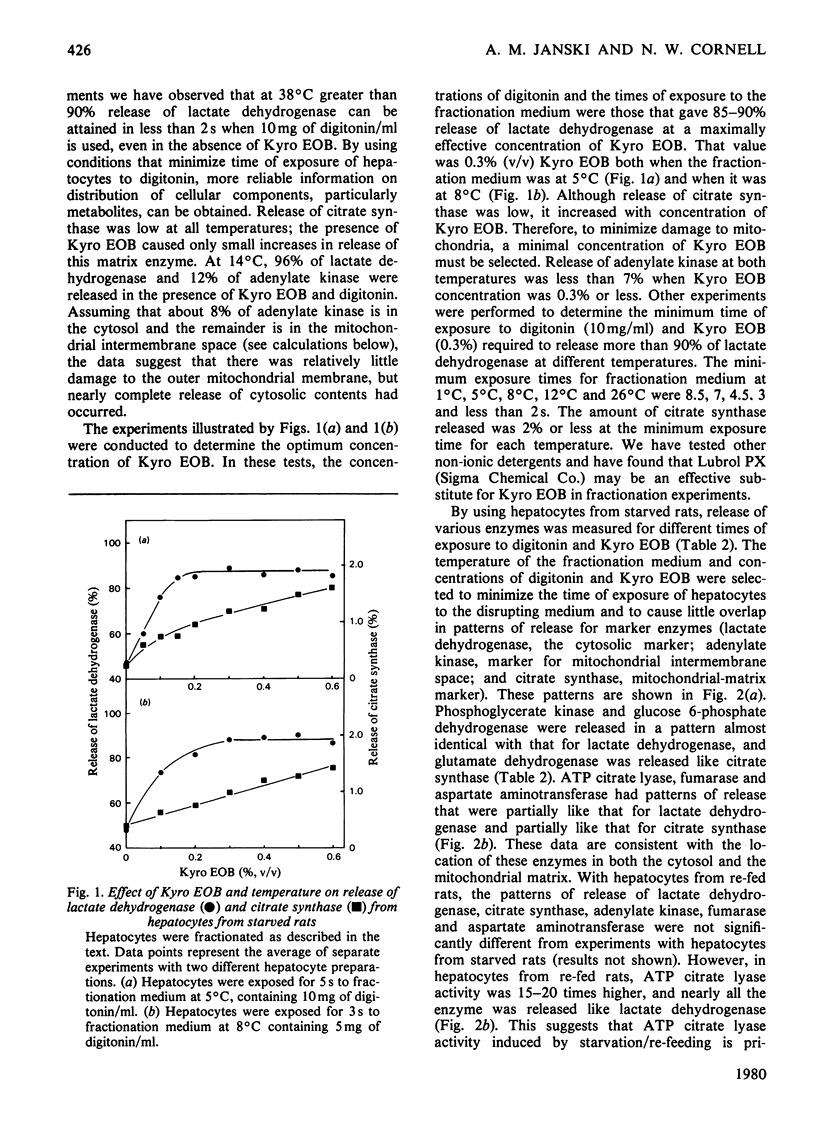
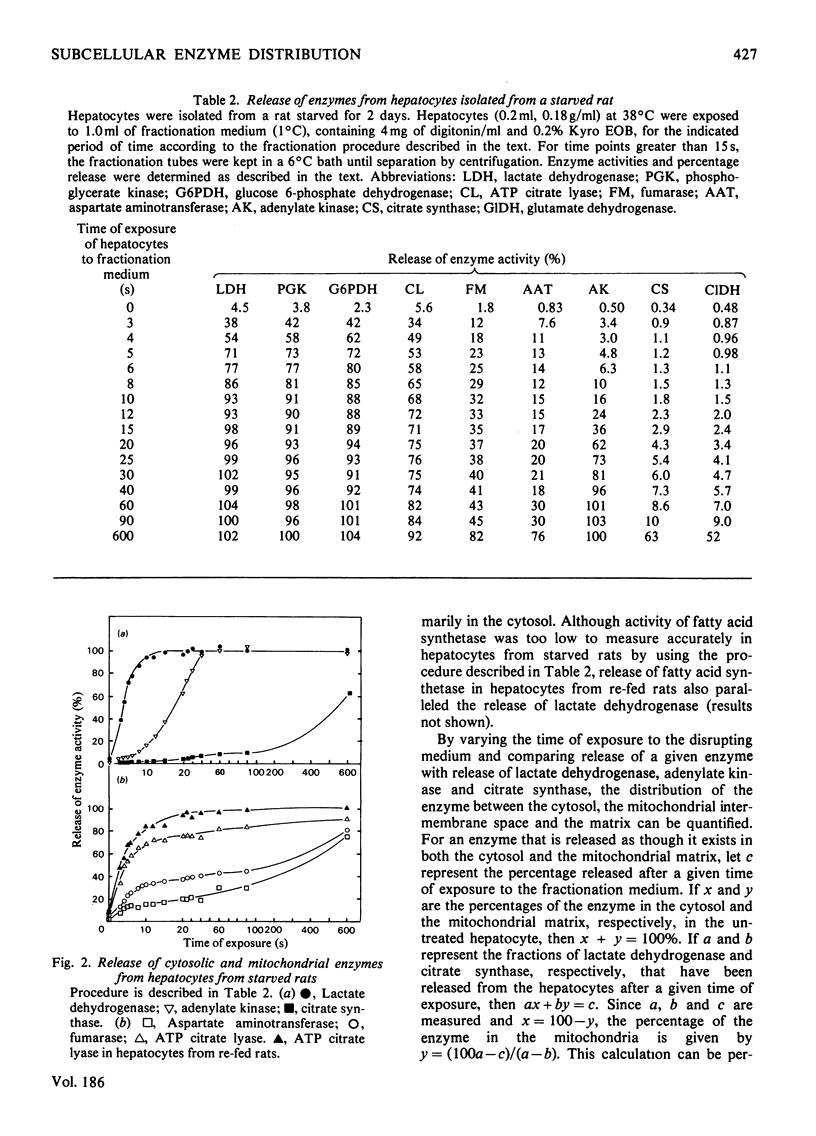
Conditions were determined for rapid separation of cytosolic and mitochondrial compartments by digitonin fractionation of rat hepatocytes. The minimum time required for separation of mitochondrial and cytosolic enzyme markers decreased rapidly with increasing temperature. Kyro EOB, a non-ionic detergent, increases the release of cytosolic enzymes, particularly at lower temperatures. Experimental procedures are described for greater than 90% release of cytosolic enzymes and less than 2% release of mitochondrial enzymes in 3s. By using appropriate concentrations of digitonin and Kyro EOB in a fractionation medium maintained at 1°C and a minimum time of exposure to the medium, nearly separate patterns of release were obtained for enzyme markers for the cytosol, mitochondrial matrix and mitochondrial intermembrane space. The distribution of enzymes that exist in more than one of these compartments was quantified by comparing their rates of release with those of marker enzymes. The cytosol/mitochondrial-matrix distributions for such enzymes in hepatocytes from starved rats were 16%/84% for aspartate aminotransferase, 34%/66% for fumarase and 77%/23% for ATP citrate lyase. In hepatocytes from rats that were induced to synthesize ATP citrate lyase by starvation and re-feeding, the ratio had increased to 95%/5%. The maximum cytosol/intermembrane-space ratio for adenylate kinase was 8%/92%. A procedure is also described for treating commercial digitonin that increases its solubility in water from about 1mg/ml to more than 800mg/ml.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birckbichler P. J., Pryme I. F. Fractionation of membrane-bound polysomes, free polysomes, and nuclei from tissue-cultured cells. Eur J Biochem. 1973 Mar 1;33(2):368–373. doi: 10.1111/j.1432-1033.1973.tb02692.x. [DOI] [PubMed] [Google Scholar]
- Brdiczka D., Pette D., Brunner G., Miller F. Kompartimentierte Verteilung von Enzymen in Rattenlebermitochondrien. Eur J Biochem. 1968 Jul;5(2):294–304. doi: 10.1111/j.1432-1033.1968.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Hems R., Krebs H. A. Acceleration of gluconeogenesis from lactate by lysine (Short Communication). Biochem J. 1973 Jun;134(2):671–672. doi: 10.1042/bj1340671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam G. L., Srere P. A. The sulfhydryl groups of citrate cleavage enzyme. Arch Biochem Biophys. 1969 Mar;130(1):304–311. doi: 10.1016/0003-9861(69)90037-x. [DOI] [PubMed] [Google Scholar]
- Criss W. E. Rat liver adenosine triphosphate: adenosine monophosphate phosphotransferase activity. II. Subcellular localization of adenylate kinase isozymes. J Biol Chem. 1970 Dec 10;245(23):6352–6356. [PubMed] [Google Scholar]
- DeRosa G., Swick R. W. Metabolic implications of the distribution of the alanine aminotransferase isoenzymes. J Biol Chem. 1975 Oct 25;250(20):7961–7967. [PubMed] [Google Scholar]
- EICHEL H. J., BUKOVSKY J. Intracellular distribution pattern of rat liver glutamic-oxalacetic transaminase. Nature. 1961 Jul 15;191:243–245. doi: 10.1038/191243a0. [DOI] [PubMed] [Google Scholar]
- Matlib M. A., O'Brien P. J. Compartmentation of enzymes in the rat liver mitochondrial matrix. Arch Biochem Biophys. 1975 Mar;167(1):193–202. doi: 10.1016/0003-9861(75)90456-7. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Gimpel J. A., Deleeuw G., Tischler M. E., Tager J. M., Williamson J. R. Interrelationships between gluconeogenesis and ureogenesis in isolated hepatocytes. J Biol Chem. 1978 Apr 10;253(7):2308–2320. [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Phosphorylation state of cytosolic and mitochondrial adenine nucleotides and of pyruvate dehydrogenase in isolated rat liver cells. Biochem J. 1976 Apr 15;156(1):91–102. doi: 10.1042/bj1560091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere P. A. The enzymology of the formation and breakdown of citrate. Adv Enzymol Relat Areas Mol Biol. 1975;43:57–101. doi: 10.1002/9780470122884.ch2. [DOI] [PubMed] [Google Scholar]
- Tolley E., Craig I. Presence of two forms of fumarase (fumarate hydratase E.C. 4.2.1.2) in mammalian cells: immunological characterization and genetic analysis in somatic cell hybrids. Confirmation of the assignment of a gene necessary for the enzyme expression to human chromosome 1. Biochem Genet. 1975 Dec;13(11-12):867–883. doi: 10.1007/BF00484417. [DOI] [PubMed] [Google Scholar]



