Abstract
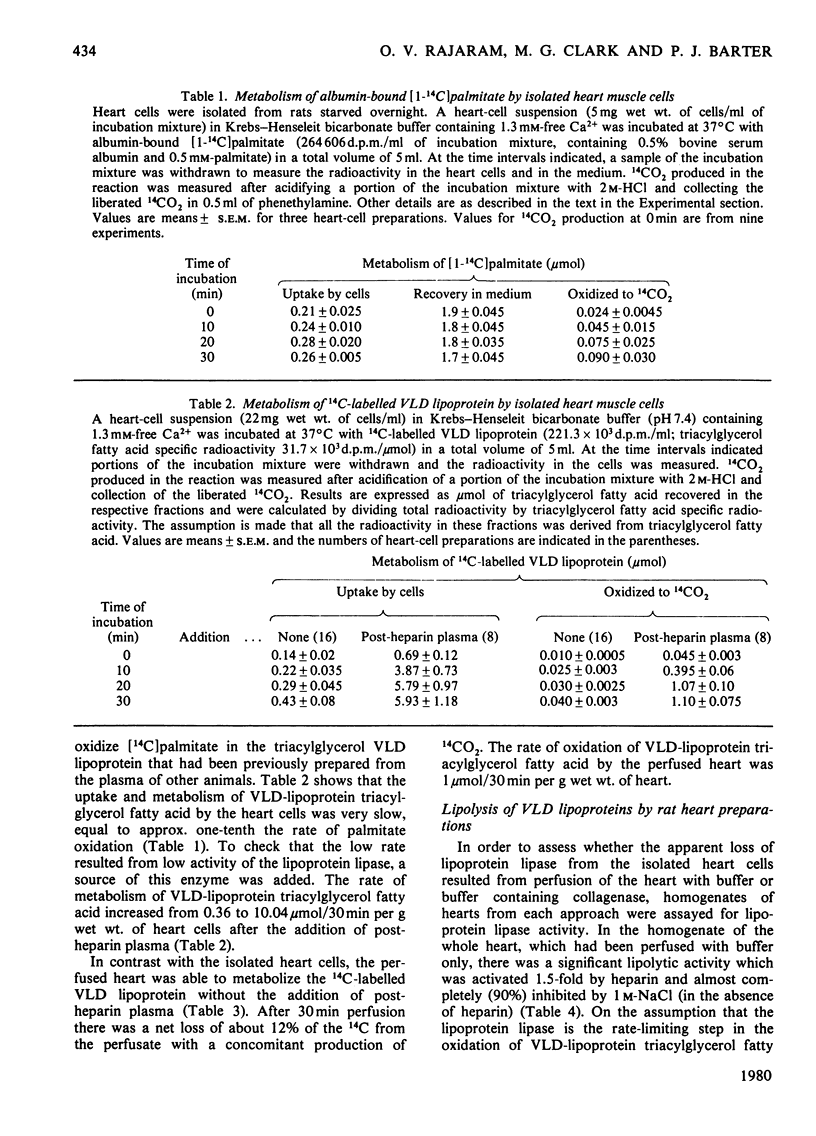
1. The metabolism of VLD lipoproteins (very-low-density lipoproteins) was studied in intact isolated beating-heart cells and isolated perfused rat heart from starved animals by using [14C]triacylglycerol fatty acid-labelled VLD lipoprotein prepared from rats previously injected with [1-14C]palmitate. 2. 14C-labelled VLD lipoprotein was metabolized by the isolated perfused heart, but was only minimally metabolized by the heart cells unless an exogenous source of lipoprotein lipase was added. 3. Measurements of lipoprotein lipase at pH 7.4 with the natural substrate 14C-labelled VLD lipoprotein indicated that during collagenase perfusion of the heart the enzyme was released into the perfusate, the activity released being proportional to the concentration of collagenase used. Lipoprotein lipase activity in homogenates of hearts that had been perfused with collagenase showed a corresponding loss of activity. 4. At high perfusate concentrations of collagenase, inactivation of the released lipoprotein lipase occurred. 5. Lipoprotein lipase activity was largely undetectable in the homogenate of the isolated heart cells. 6. It is concluded that the lipoprotein lipase responsible for the hydrolysis of VLD lipoprotein triacylglycerol is predominantly located externally to the heart muscle cells and that its release can be facilitated by perfusion of the heart with bacterial collagenase.
Full text
PDF



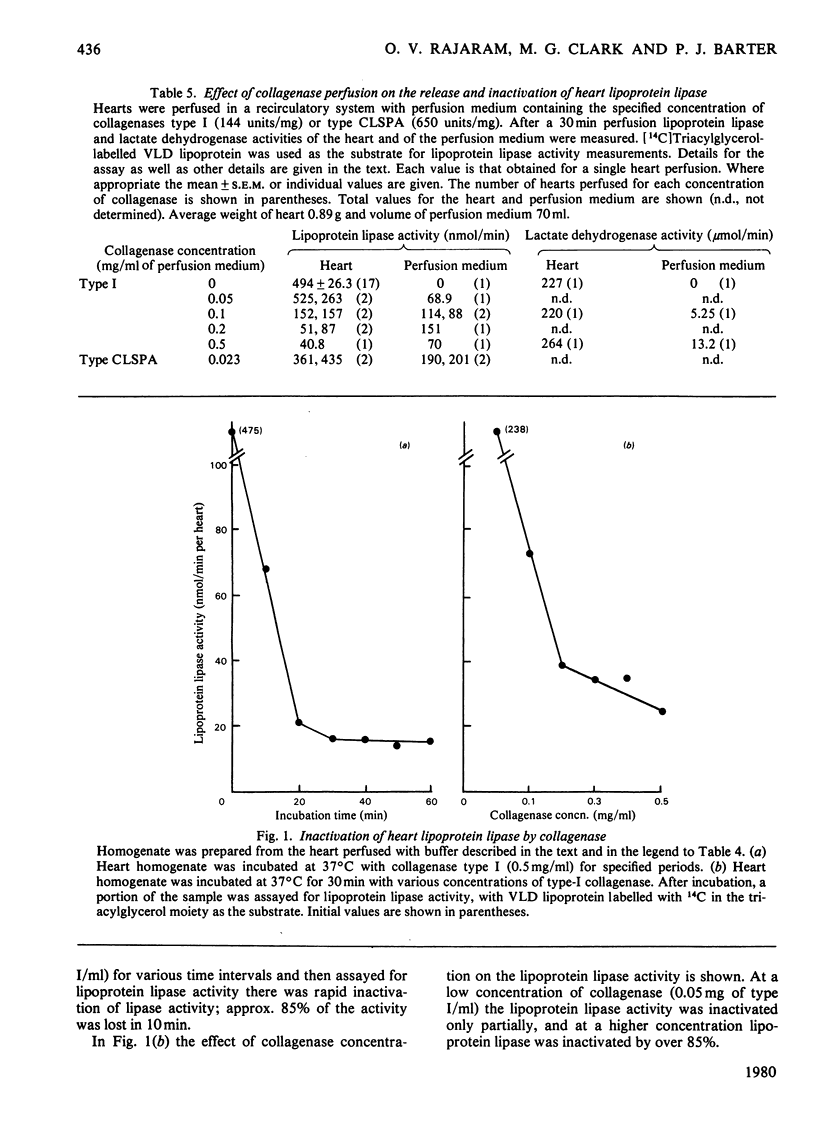


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abstracts for the Ninth Congress of the International Society for Heart Research, New Delhi, India, 28 September to 2 October, 1978. J Mol Cell Cardiol. 1978 Aug;10 (Suppl 1):1–137. [PubMed] [Google Scholar]
- Aktin E., Meng H. C. Release of clearing factor lipase (lipoprotein lipase) in vivo and from isolated perfused hearts of alloxan diabetic rats. Diabetes. 1972 Mar;21(3):149–156. doi: 10.2337/diab.21.3.149. [DOI] [PubMed] [Google Scholar]
- Bagby G. J., Liu M. S., Spitzer J. A. Lipoprotein lipase activity in rat heart myocytes. Life Sci. 1977 Aug 1;21(3):467–473. doi: 10.1016/0024-3205(77)90529-x. [DOI] [PubMed] [Google Scholar]
- Blanchette-Mackie E. J., Scow R. O. Sites of lipoprotein lipase activity in adipose tissue perfused with chylomicrons. Electron microscope cytochemical study. J Cell Biol. 1971 Oct;51(1):1–25. doi: 10.1083/jcb.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borensztajn J., Otway S., Robinson D. S. Effect of fasting on the clearing factor lipase (lipoprotein lipase) activity of fresh and defatted preparations of rat heart muscle. J Lipid Res. 1970 Mar;11(2):102–110. [PubMed] [Google Scholar]
- Borensztajn J., Rone M. S., Sandros T. Effects of colchicine and cycloheximide on the functional and non-functional lipoprotein lipase fractions of rat heart. Biochim Biophys Acta. 1975 Sep 19;398(3):394–400. doi: 10.1016/0005-2760(75)90190-3. [DOI] [PubMed] [Google Scholar]
- Chajek T., Stein O., Stein Y. Colchicine-induced inhibition of plasma lipoprotein lipase release in the intact rat. Biochim Biophys Acta. 1975 Jan 24;380(1):127–131. doi: 10.1016/0005-2760(75)90051-x. [DOI] [PubMed] [Google Scholar]
- Chajek T., Stein O., Stein Y. Rat heart in culture as a tool to elucidate the cellular origin of lipoprotein lipase. Biochim Biophys Acta. 1977 Jul 20;488(1):140–144. doi: 10.1016/0005-2760(77)90131-x. [DOI] [PubMed] [Google Scholar]
- Chohan P., Cryer A. The lipoprotein lipase (clearing-factor lipase) activity of cells isolated from rat cardiac muscle. Biochem J. 1978 Aug 15;174(2):663–666. doi: 10.1042/bj1740663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crass M. F., 3rd, McCaskill E. S., Shipp J. C., Murthy V. K. Metabolism of endogenous lipids in cardia muscle: effect of pressure development. Am J Physiol. 1971 Feb;220(2):428–435. doi: 10.1152/ajplegacy.1971.220.2.428. [DOI] [PubMed] [Google Scholar]
- Crass M. F., 3rd Regulation of triglyceride metabolism in the isotopically prelabeled perfused heart. Fed Proc. 1977 Jun;36(7):1995–1999. [PubMed] [Google Scholar]
- Delcher H. K., Fried M., Shipp J. C. Metabolism of lipoprotein lipid in the isolated perfused rat heart. Biochim Biophys Acta. 1965 Jul 7;106(1):10–18. doi: 10.1016/0005-2760(65)90090-1. [DOI] [PubMed] [Google Scholar]
- EVANS J. R., OPIE L. H., SHIPP J. C. METABOLISM OF PALMITIC ACID IN PERFUSED RAT HEART. Am J Physiol. 1963 Oct;205:766–770. doi: 10.1152/ajplegacy.1963.205.4.766. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Levy R. I. Lipoprotein metabolism. Adv Lipid Res. 1975;13:1–89. [PubMed] [Google Scholar]
- Eisenberg S., Rachmilewitz D. Interaction of rat plasma very low density lipoprotein with lipoprotein lipase-rich (postheparin) plasma. J Lipid Res. 1975 Sep;16(5):341–351. [PubMed] [Google Scholar]
- Enser M. B., Kunz F., Borensztajn J., Opie L. H., Robinson D. S. Metabolism of triglyceride fatty acid by the perfused rat heart. Biochem J. 1967 Jul;104(1):306–317. doi: 10.1042/bj1040306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fielding C. J., Higgins J. M. Lipoprotein lipase: comparative properties of the membrane-supported and solubilized enzyme species. Biochemistry. 1974 Oct 8;13(21):4324–4330. doi: 10.1021/bi00718a013. [DOI] [PubMed] [Google Scholar]
- Glangeaud M. C., Eisenberg S., Olivecrona T. Very low density lipoprotein. Dissociation of apolipoprotein C during lipoprotein lipase induced lipolysis. Biochim Biophys Acta. 1976 Jan 18;486(1):23–35. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson L. C., Schotz M. C., Harary I. Lipoprotein lipase in cultured heart cells: characteristics and cellular location. Biochim Biophys Acta. 1977 Apr 26;487(1):212–221. doi: 10.1016/0005-2760(77)90057-1. [DOI] [PubMed] [Google Scholar]
- Higgins J. M., Fielding C. J. Lipoprotein lipase. Mechanism of formation of triglyceride-rich remnant particles from very low density lipoproteins and chylomicrons. Biochemistry. 1975 Jun 3;14(11):2288–2293. doi: 10.1021/bi00682a002. [DOI] [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem. 1955 Jul;215(1):1–14. [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. II. Substrate specificity and activation of coconut oil. J Biol Chem. 1955 Jul;215(1):15–26. [PubMed] [Google Scholar]
- Lagunoff D., Pritzl P. Characterization of rat mast cell granule proteins. Arch Biochem Biophys. 1976 Apr;173(2):554–563. doi: 10.1016/0003-9861(76)90292-7. [DOI] [PubMed] [Google Scholar]
- Mallov S., Alousi A. A. Effect of altered cardiac metabolism and work on lipoprotein lipase activity of heart. Am J Physiol. 1967 May;212(5):1158–1164. doi: 10.1152/ajplegacy.1967.212.5.1158. [DOI] [PubMed] [Google Scholar]
- Mallov S., Cerra F. Effect of ethanol intoxication and catecholamines on cardiac lipoprotein lipase activity in rats. J Pharmacol Exp Ther. 1967 Jun;156(3):426–444. [PubMed] [Google Scholar]
- Mjos O. D., Faergeman O., Hamilton R. L., Havel R. J. Characterization of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest. 1975 Sep;56(3):603–615. doi: 10.1172/JCI108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J., Barter P. Metabolism of palmitic and linoleic acids in man: differences in turnover and conversion to glycerides. Clin Sci. 1971 Apr;40(4):345–350. doi: 10.1042/cs0400345. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Schotz M. C. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976 Sep;17(5):536–541. [PubMed] [Google Scholar]
- Olivecrona T., Bengtsson G., Marklund S. E., Lindahl U., Hök M. Heparin-lipoprotein lipase interactions. Fed Proc. 1977 Jan;36(1):60–65. [PubMed] [Google Scholar]
- Olson R. E., Hoeschen R. J. Utilization of endogenous lipid by the isolated perfused rat heart. Biochem J. 1967 Jun;103(3):796–801. doi: 10.1042/bj1030796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON D. S., JENNINGS M. A. RELEASE OF CLEARING FACTOR LIPASE BY THE PERFUSED RAT HEART. J Lipid Res. 1965 Apr;6:222–227. [PubMed] [Google Scholar]
- ROBINSON D. S. THE CLEARING FACTOR LIPASE AND ITS ACTION IN THE TRANSPORT OF FATTY ACIDS BETWEEN THE BLOOD AND TISSUES. Adv Lipid Res. 1963;1:133–182. doi: 10.1016/b978-1-4831-9937-5.50010-7. [DOI] [PubMed] [Google Scholar]
- SHONK C. E., BOXER G. E. ENZYME PATTERNS IN HUMAN TISSUES. I. METHODS FOR THE DETERMINATION OF GLYCOLYTIC ENZYMES. Cancer Res. 1964 May;24:709–721. [PubMed] [Google Scholar]
- Scheuer J., Berry M. N. Effect of alkalosis on glycolysis in the isolated rat heart. Am J Physiol. 1967 Nov;213(5):1143–1148. doi: 10.1152/ajplegacy.1967.213.5.1143. [DOI] [PubMed] [Google Scholar]
- Twu J. S., Garfinkel A. S., Schotz M. C. Rat heart lipoprotein lipase. Atherosclerosis. 1975 Nov-Dec;22(3):463–472. doi: 10.1016/0021-9150(75)90025-8. [DOI] [PubMed] [Google Scholar]


