Abstract
Lupus panniculitis is a chronic subtype of cutaneous lupus erythematosus. It typically presents as tender, firm subcutaneous nodules on the proximal extremities, face, and/or trunk and can leave behind disfiguring scarring or lipoatrophy in the post-inflammatory phase. When a patient presents with physical or emotional distress secondary to lupus panniculitis-induced lipoatrophy, there are variable data on treatment with autologous fat transfer and injectable fillers. We present the case of a 37-year-old female presenting with lipoatrophy of the right cheek, after 2 years of quiescent disease, undergoing successful volume restoration with injectable hyaluronic acid filler.
Keywords: Lupus panniculitis, lipoatrophy, facial lipoatrophy, injectables, filler, hyaluronic acid
Introduction
Lupus panniculitis (LP) is a chronic subtype of cutaneous lupus erythematosus (CLE). It has unique characteristics when compared to acute or subacute subtypes of CLE. LP affects 1%–3% of people with chronic CLE and typically presents as tender, firm subcutaneous nodules on the proximal extremities, face, and/or trunk. Histologically, it shows mainly lobular panniculitis with a mixed lymphocytic infiltrate in the dermis and subcutaneous fat. In the post-inflammatory phase, it can leave behind disfiguring scarring or lipoatrophy causing both physical and emotional distress.
Case presentation
We describe the case of a 37-year-old female from the Dominican Republic, who initially presented with localized lipoatrophy on the left arm. She described the site initially presenting as a tender, red nodule about 1 year prior. A biopsy performed, at that time, showed a lymphocytic panniculitis and interface change involving the hair follicle, along with increased interstitial mucin, favoring a diagnosis of LP. She did not have associated clinical symptoms suggestive of systemic lupus erythematosus.
Her laboratory investigations showed antinuclear antibodies of 1:80, elevated anti-cyclic citrullinated peptide of 42.6 U/mL, and an elevated rheumatoid factor of 202 kU/L. She had a normal C-reactive protein and urinalysis. Investigations for double-stranded DNA, cardiolipin antibodies, lupus inhibitors, and autoantibodies to ribonucleoprotein were negative.
One year after dermatology evaluation, a new inflamed subcutaneous nodule occurred on the right arm and right cheek, with a biopsy again showing lobular lymphocytic panniculitis with superficial and deep perivascular and periadnexal lymphoid infiltrate. She was started on hydroxychloroqine 200 mg twice daily, but this was discontinued after 3 weeks due to side effects of irritability and mood changes. She was then initiated on quinacrine 100 mg once daily and achieved a durable response with no new lesions and a reduction in inflammation in existing lesions.
With the patient’s concerns about cosmesis of the right cheek atrophy, after 2 years of quiescent disease, she elected to treat the area with injectable filler. Using a 25 gauge 50 mm cannula plus a 22 gauge 60 mm cannula, day 1 consisted of injecting 1.6 mL of resilient hyaluronic acid (RHA) 2 filler (TEOXANE, Geneva, Switzerland) into the right cheek. Immediately prior to injection, extensive subcision was performed with the blunt-tipped cannula, to break down scar tissue adhesions and yield space for the hyaluronic acid (HA) filler. Following subcision, the HA filler was then injected in an antegrade and retrograde fashion. Four weeks later, she received another 1 mL of RHA2, followed by 1 mL of RHA2 and 1 mL RedensityI 3 months later. Given the degree of volume loss, multiple treatment sessions were performed for repeat subcision with gradual tissue expansion and revolumization, to minimize hydrostatic effects on the surrounding vasculature. Again, at the 3-week mark, another 1 mL of RHA2 was injected, followed by her fifth injection 4 weeks later of 1 mL Redensity II with a 27 gauge/40 mm cannula. There were no complications and the patient had favorable volume-enhancing results, with high patient satisfaction.
Discussion
LP in the active phase can show lymphocytic lobular or septal panniculitis, progressing to fat necrosis or calcification if left untreated. These histologic findings can correlate with subcutaneous atrophy and subsequent cutaneous deformity. To reduce inflammation in the active phase of LP, hydroxychloroquine/chloroquine and oral corticosteroids are often the mainstay of treatment. Beyond this, reported options for second-line treatment are quite broad, including methotrexate, mycophenolate mofetil, azathioprine, cyclophosphamide, rituximab, and IVIG.1,2
When presenting with lipoatrophy secondary to LP, treatment options reported include injectable fillers and autologous fat transfer. Previous reports of injectable filler for the treatment of LP-induced facial lipoatrophy include the use of HA, poly-L-lactic acid (PLLA), polyacrylamide hydrogel, and polymethyl-methacrylate. 3 While there is a theoretical risk of disease exacerbation caused by antigenic stimulation of injectables, these cases show favorable results at follow-up between a 1-month and 9-year period. 3
Our patient was treated with injectable HA filler, which is amenable to reversal procedures. Previous reports of HA filler treatment of LP-induced lipoatrophy include Eastham et al. successfully using PLLA and HA, with stable results at 11 months and high patient satisfaction. 4 Gaón et al. also demonstrated improved subcutaneous volume loss in the malar and temporomandibular areas of a 47-year-old female, with 3 mL of HA filler. 5 While the injections were well tolerated by our patient, clinicians still need to counsel patients on possible side effects including ecchymoses, edema, hypersensitivity reaction, infection, nodular/granulomatous reaction, and vascular compromise.
Tissue augmentation with other non-permanent fillers such as PLLA showed significant volume restoration at 4 months when injected into the right cheek of a 26-year-old woman experiencing facial lipoatrophy secondary to LP. 6 Again, without complications of infection, tender nodules, skin, or systemic disease reactivation.
Although our patient did not elect to be treated with autologous fat transfer, the treatment option was still discussed during the initial consultation. Improvement of facial depression deformities specifically secondary to LP is previously documented with the use of autologous fat transfer.7,8 While rare, the patient must be counseled on possible serious complications such as non-reversible fat embolism, with one report even leading to death. 9
Overall, injectable HA filler to treat LP-induced facial lipoatrophy has shown effective and sustained volume restoration in our patients at 6 months. We highlight that our injection was during a quiescent period of disease, with a blunt cannula (Figures 1 and 2).
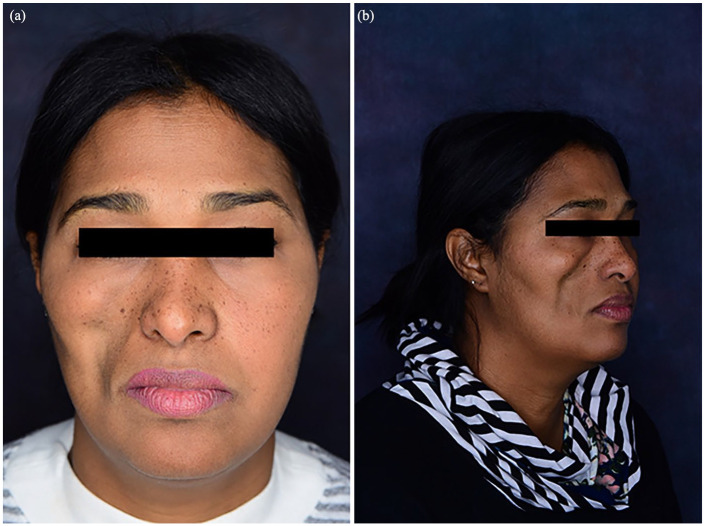
Figure 1.
(a, b) Right cheek lipoatrophy secondary to lupus panniculitis prior to injectable hyaluronic acid filler.
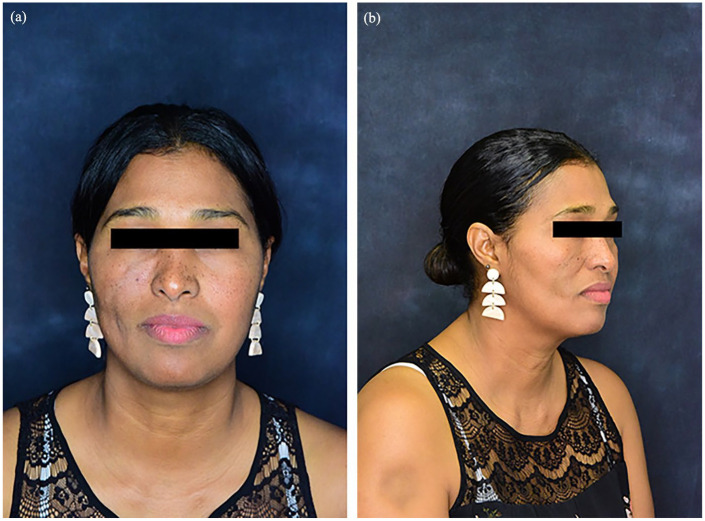
Figure 2.
(a, b) Results of the right cheek following five treatments of hyaluronic acid filler over a period of 6 months.
Conclusion
In conclusion, while larger controlled studies are needed to investigate injectable treatment options for LP-induced lipoatrophy, favorable results have been shown with filler. With limited reports on the use of HA filler in this patient population, we report the successful use of HA filler to treat facial lipoatrophy secondary to LP. We recommend clinicians consider a patient’s current immunosuppression regimen and whether the LP is in a post-inflammatory state when discussing treatment options for volume restoration of facial lipoatrophy in patients with a diagnosis of LP.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval and informed consent: A review from the Research Ethics Board (REB) was not required for this study.
Consent for publication: Informed consent for patient information and images to be published was obtained from the patient.
ORCID iD: Rebecca Green  https://orcid.org/0000-0002-0585-7199
https://orcid.org/0000-0002-0585-7199
References
- 1. Gupta P, Dhanawat A, Mohanty I, et al. Refractory lupus panniculitis treated successfully with rituximab: two cases. Ann Afr Med 2020; 19(3): 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. AlQadri NG, AlNooh B, AlTewerki MM, et al. Intravenous immunoglobulin in the management of lupus erythematosus panniculitis. Cureus 2020; 12(1): e6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Creadore A, Watchmaker J, Maymone MBC, et al. Cosmetic treatment in patients with autoimmune connective tissue diseases: best practices for patients with lupus erythematosus. J Am Acad Dermatol 2020; 83(2): 343–363. [DOI] [PubMed] [Google Scholar]
- 4. Eastham AB, Liang CA, Femia AN, et al. Lupus erythematosus panniculitis-induced facial atrophy: effective treatment with poly-L-lactic acid and hyaluronic acid dermal fillers. J Am Acad Dermatol 2013; 69(5): e260–e262. [DOI] [PubMed] [Google Scholar]
- 5. Gaón NQ, Vera Kellet C, Abarzúa-Araya A. Facial lipoatrophy secondary to lupus panniculitis corrected with hyaluronic acid—a case report. Surg Cosmet Dermatol 2015; 7(4): 352–355. [Google Scholar]
- 6. Wong I, Hanna E, Pon K. Poly-l-lactic acid for treating facial lipoatrophy secondary to lupus erythematosus panniculitis. Dermatol Surg 2021; 47(6): 848–849. [DOI] [PubMed] [Google Scholar]
- 7. Lei H, Ma GE, Liu Z. Evaluation of repairing facial depression deformities secondary to lupus erythematosus panniculitis with autologous fat grafting. J Craniofac Surg 2016; 17: 1765–1769. [DOI] [PubMed] [Google Scholar]
- 8. Kongkunnavat N, Prathyajuta J, Tonaree W. Autologous fat transfer in lupus panniculitis facial lipoatrophy. Arch Plast Surg 2022; 49(4): 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gleeson CM, Lucas S, Langrish CJ, et al. Acute fatal fat tissue embolism after autologous fat transfer in a patient with lupus profundus. Dermatol Surg 2011; 37: 111–115. [DOI] [PubMed] [Google Scholar]