Abstract
Introduction: Osteoprotegerin (OPG) inhibits vascular calcification which is central to pathogenesis of arterial stiffness. However, it promotes inflammation by upregulating expression of vascular cell adhesion molecule-1(VCAM-1), thereby contributing to arterial stiffness. We investigated longitudinal association between OPG and arterial stiffness in type 2 diabetes (T2D), causality of the association and mediation by VCAM-1. Methods: This was a prospective cohort study of T2D patients (N = 1877, mean age 57.0 ± 10.8) with 10 years’ follow-up. Baseline plasma OPG was measured using immunoassay. Pulse wave velocity (PWV) was assessed using applanation tonometry. We examined association between OPG and follow-up PWV using linear mixed model. One-sample Mendelian Randomization (MR) was conducted with rs1385492 as OPG-associated single nucleotide polymorphism (SNP). Results: Baseline natural log (Ln)-transformed OPG was positively associated with baseline and follow-up PWV with adjusted coefficients 0.43 (95%CI 0.05, 0.80; p = .026) and 0.51 (95%CI 0.06 to 0.97; p = .028) respectively. Genetically-predicted higher levels of plasma OPG was associated with higher last follow-up PWV with coefficient 10.81 (95%CI 2.97, 18.65; p = .007) per unit increase in LnOPG. Higher VCAM-1 accounted for 10.2% of association between LnOPG and follow-up PWV. Discussion: Baseline plasma OPG was associated with higher follow-up PWV in patients with T2D, with genetic evidence from MR. This association may be mediated, at least in part, by VCAM-1.
Keywords: osteoprotegerin, arterial stiffness, type 2 diabetes
Key messages
1. Baseline plasma Osteoprotegerin (OPG) was associated with higher follow-up pulse wave velocity in type 2 diabetes.
2. This association was mediated, at least in part, by vascular call adhesion molecule-1.
3. Mendelian Randomization suggested potential causal association between genetically influenced plasma OPG and PWV.
Introduction
The prevalence of type 2 diabetes (T2D) is growing rapidly worldwide. According to International Diabetes Federation, it is estimated that 537 million adults are living with diabetes and this number will reach 643 million by 2030. 1 Complications from T2D have contributed to an enormous burden of morbidity and mortality. 2 Of note, cardiovascular sequelae of T2D remains a major cause of morbidity and mortality in people with T2D. 2 Hence it is important to understand the pathophysiology of CVD in order to implement effective prevention and management strategies in patients with T2D.
Hyperglycemia and insulin resistance lead to an increase in chronic inflammation, oxidative stress, advanced glycation end-products, endothelial dysfunction and activation of protein kinase C, thereby contributing to CVD in patients with T2D. 3 Accumulating evidence has shown that arterial stiffness is a risk factor for CVD and mortality among patients with T2D.4–6 Carotid-femoral pulse wave velocity (PWV) is the gold-standard non-invasive measure of arterial stiffness. 7 Studies have reported that an increase in carotid-femoral PWV by 1 m/s was associated with an increase in frequency of CV events by 35% to 45%. 7 Arterial stiffness is characterized by a reduction in arterial distensibility and arterial compliance. 7 A key contributor to the pathogenesis of arterial stiffness is vascular calcification whereby vascular smooth muscle cells are trans-differentiated into chondrocyte-like or osteoblast-like cells. 7
Osteoprotegerin (OPG) is a member of the tumor necrosis factor (TNF) receptor superfamily and is produced by vascular endothelial and smooth muscle cells and osteoblasts. 8 It acts as a decoy receptor for receptor activator of nuclear factor kβ ligand (RANKL) and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL). 9 It plays a role in regulating the vascular calcification process by acting as a vascular calcification inhibitor. 7 Results from animal studies suggested that OPG conferred protection of the vasculature. 8 For example, vascular calcification, which developed in OPG knockout mice, could be prevented by re-introduction of the OPG protein.8,10 However, these findings contrasted with clinical studies which showed that serum OPG was positively associated with arterial medial calcification in patients on hemodialysis.8,11 It remains unclear if elevated OPG exerts a detrimental impact on the vasculature or merely reflects a compensatory increase which aims to mitigate further vascular calcification. 8
Clinical studies in patients with renal impairment, renal transplant, heart failure and hypertension have shown an association between OPG and arterial stiffness.7,8,12–18 Most of these studies had a cross-sectional design and hence could not establish a causal association between OPG and arterial stiffness.7,8,12–14 There were a few prospective cohort studies which demonstrated an association between OPG and arterial stiffness in patients with chronic kidney disease or on hemodialysis with sample sizes ranging 48 to 151.16–18 However, two cross-sectional studies showed no correlation or association between serum OPG and arterial stiffness in patients with ankylosing spondylitis 19 and hemodialysis. 20 Although a cross-sectional study by Jung C et al (2010) showed a positive correlation between serum OPG and arterial stiffness in patients with T2D, 21 our knowledge on the longitudinal association and the underlying pathophysiological mechanism remains limited.
Given the paucity of data, further longitudinal studies are needed to establish the causal relationship between OPG and arterial stiffness in patients with T2D. In recent years, Mendelian Randomization (MR) has been increasingly applied in research to evaluate causality and provide complementary insights into epidemiological relationships. 22 This offers a timely opportunity for us to examine the causal relationship between genetically predicted OPG and arterial stiffness in patients with T2D.
Interestingly, it was reported that OPG could upregulate production of endothelial adhesion molecules and promote leukocyte adhesion to endothelial cells. 23 Vascular cell adhesion molecule-1 (VCAM-1) was also shown to be associated with higher brachial-ankle PWV (baPWV) which reflected increased arterial stiffness. 24 VCAM-1 contributed to extracellular matrix remodeling resulting in increased fragmentation of elastin molecules which characterized arterial stiffness. 24 Hence it is plausible that VCAM-1 plays a mediatory role in the association between OPG and arterial stiffness.
In this study, we aimed to examine the longitudinal association between plasma OPG and arterial stiffness in patients with T2D. Our secondary aims were to establish the causal association with MR analysis and elucidate the pathophysiological mechanism with VCAM-1 as a potential mediator.
Patients and methods
Study population and design
We conducted a prospective cohort study of 2258 patients with T2D who were recruited by the Singapore Study of Macro-angiopathy and Micro-vascular Reactivity in Type 2 Diabetes (SMART2D) study in August 2011 to March 2014 (phase 1) in a public hospital and primary care polyclinics in the northern region of Singapore. The patients were followed up in September 2014 to May 2019 (phase 2) and again in July 2019 to July 2022 (phase 3). The patients were excluded from the study for the following criteria: active inflammation, active malignancy, use more than 7.5 mg/day of oral steroids and/ or use of non-steroidal anti-inflammatory drugs on the day of study. We further excluded patients with history of cardiovascular disease (including heart block, acute myocardial infarction, coronary artery bypass surgery and stroke; n = 161) and those without baseline measurement of OPG (n = 220) for the purpose of the analysis. There was a total of 1877 patients eligible for the study at baseline. Subsequently, there were 540 patients who had 2 follow-up readings of PWV for the longitudinal analysis. Out of these 540 patients, 528 patients had single-nucleotide polymorphism (SNP) data for MR analysis. Supplemental Figure 1 shows the patient flow-chart. The National Healthcare Group Domain Specific Review Board in Singapore granted the ethics approval. Written informed consent was given by all the participants.
Data collection
Data on demographics, smoking history, medical history and medications were collected via questionnaires administered to the patients. Clinical case notes were reviewed to verify the information obtained from the questionnaires. Our trained research coordinators measured blood pressure (BP) using a standard BP monitor for the patients at a sitting position after resting for 10 minutes. An average BP value was derived from two BP measurements.
We measured PWV using the SphygmoCor® (AtCor Medical, Sydney, Australia) which had high validity and reproducibility in patients, including those with diabetes. 25 Carotid-femoral PWV was quantified based on a foot-to-foot method. 25 An applanation tonometer detected pulse waveform at the carotid and femoral arteries. 25 The mean passage time for the waves between carotid and femoral arteries was calculated from 9 to 10 waves for every analysis. 25 PWV was expressed as the distance between these recording sites divided by passage time (metres per second). 25
We collected fasting blood samples which were tested for the following tests in the hospital laboratory: enzymatic colorimetric test for serum creatinine, low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG); and hexokinase method (COBAS-Roche, Mannheim, Germany) for fasting plasma glucose (FPG). Estimated glomerular filtration rate (eGFR) was calculated based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. 7
We measured fasting plasma level of OPG using immunoassay kits from BioVendor (Modrice, Czech Republic), and plasma level of VCAM-1 using multiplex immunoassay on a Luminex 200 platform (Affymetrix, Santa Clara, CA, USA).
Statistical analysis
The baseline characteristics were summarised as number (percentage) for categorical variables and mean (± standard deviation (SD)) or medians (interquartile range (IQR)) for continuous variables. The correlation between OPG and baseline characteristics was examined using spearman correlation. The association between natural log (Ln) transformed OPG and PWV at baseline was examined using linear regression, adjusting for age, sex, ethnicity, smoking history, FPG, systolic blood pressure (SBP), diastolic blood pressure (DBP), LDL-C, LnTG, eGFR and medications for diabetes (insulin, metformin, sulphonylurea and dipeptidyl peptidase IV inhibitor (DPP4i)), medications for hyperlipidemia (statins and fibrates) and renin-angiotensin system (RAS) antagonist. The adjusting covariates were selected in view of biological plausibility26–30 or p-value less than 0.1 31 in the univariable analysis. The longitudinal association between baseline OPG and follow-up PWV was examined using linear mixed model. We adjusted for follow-up period in Model 1, and additionally for the demographic and clinical covariates used in the cross-sectional analysis in Model 2. Model 3 included the medications in addition to the covariates included in Model 2.
Mendelian randomization analysis
MR is a well-established approach to examine causality using genotype as instrumental variable. 32 MR is considered valid when the following assumptions are fulfilled: (1) “relevance assumption” whereby there is an association between the genetic variant and the exposure of interest; (2) “independence assumption” whereby the genetic variant is independent of cofounders (measured or unmeasured) for the association between exposure of interest and outcome; and (3) “exclusion restriction” whereby the genetic variant is not related to the outcome except through the exposure of interest.33,34 We determined the causal relationship between plasma OPG and last PWV at follow-up using one-sample MR where the genetic variant, exposure of interest and outcome were measured in the same population. 35 We performed genome-wide association (GWAS) in the SMART2D cohort with genome-wide efficient mixed model association (GEMMA), adjusting for age, sex and population structure (principal component 1-3).36,37 We extracted the genotyped plasma OPG-associated SNP rs1385492 from our GWAS data. This SNP was previously reported in an earlier study. 38 The effect allele is G according to the NCBI database of genetic variation (dbSNP) 39 search results (26 February 2024). Genotypes AG and GG were combined and coded as 1, whereas AA was coded as 0. This is an intronic SNP with minor allele frequency (MAF) of 42.6% in East Asians based on the Genome Aggregation Database (gnomAD) in dbSNP and 41.3% in our study population. One-sample MR analysis was performed with a two-stage least squares (2SLS) method using a linear model and continuous outcomes. The first step involved estimating the effect on exposure to the genetic variant (plasma OPG-associated SNP), adjusting for age and sex. The second step involved estimating the effect on the outcome (last PWV at follow-up) through the estimated exposure derived from the first step, adjusting for age, sex, population structure and baseline PWV. We then expressed the causal estimate for the association as a regression coefficient for the outcome (last PWV at follow-up) due to unit change in exposure (plasma OPG) in linear regression (Supplemental Figure 2). We also examined the association between plasma OPG-associated SNP and clinical variables (SBP, DBP, FPG, LDL-C and eGFR) in order to determine if the genetic variant was independent of potential confounding factors.
Mediation analysis
We examined the mediation of the association between OPG and follow-up PWV by VCAM-1 based on Barron and Kenny’s framework 40 with the following required criteria: (1) the independent variable (OPG) was significantly associated with the mediating variable (VCAM-1) (pathway a); (2) the mediating variable (VCAM-1) was significantly associated with the dependent variable (follow-up PWV) (pathway b); (3) the independent variable (OPG) was significantly associated with the dependent variable (pathway c); and (4) the association between the independent variable (OPG) and the dependent variable (follow-up PWV) was attenuated when VCAM-1 was added to the model (pathway c’). The generalized structural equation model (GSEM) was used for the mediation analysis. Statistical analysis was done using STATA version 14.0 (STATA Corporation, College Station, Texas). Results with p-values less than 0.05 were deemed statistically significant.
Results
The baseline characteristics were shown in Table 1: mean age 57.0 ± 10.8 years; males 49.3%; Chinese 51.8%, Malay 21.9%, Indian 22.5% and Other 3.8%. The proportions of ex-smoker and current smoker were 6.6% and 8.3% respectively. The mean SBP was 140.0 ± 18.9 mmHg and mean DBP was 79.0 ± 9.3 mmHg. The mean PWV was 9.9 ± 3.5 m/s. The median plasma OPG was 6.1 pmol/L (IQR 4.6–8.0). Supplemental Table 1 showed that OPG was positively correlated with age, SBP, FPG, TG, PWV and VCAM-1 and negatively correlated with eGFR.
Table 1.
Baseline characteristics of patients (N = 1877).
| Variable | Mean ± SD or N (%) |
|---|---|
| Age (years) | 57.0 ± 10.8 |
| Male (%) | 926 (49.3) |
| Ethnicity (%) | |
| Chinese | 972 (51.8) |
| Malay | 411 (21.9) |
| Indian | 423 (22.5) |
| Other | 71 (3.8) |
| History of smoking (%) | |
| No | 1593 (85.1) |
| Ex | 124 (6.6) |
| Current | 156 (8.3) |
| BMI (kg/m2) | 27.8 ± 5.2 |
| SBP (mmHg) | 140.0 ± 18.9 |
| DBP (mmHg) | 79.0 ± 9.3 |
| FPG (mmol/l) | 8.1 ± 2.6 |
| LDL-C (mmol/l) | 2.8 ± 0.8 |
| TG (mmol/l) | 1.4 (1.1 – 2.0) |
| eGFR (ml/min/1.73 m2) | 85.5 ± 33.2 |
| PWV (m/s) | 9.9 ± 3.5 |
| OPG (pmol/L) | 6.1 (4.6 – 8.0) |
| VCAM-1 (ng/ml) | 81.8 (66.2 – 99.8) |
| Medications for diabetes (%) | |
| None | 138 (7.4) |
| Oral | 1210 (64.6) |
| Insulin ± Oral a | 524 (28.0) |
| Medications for hyperlipidemia (Statins +/ fibrates) (%) | 1549 (82.8) |
| RAS antagonist (%) | 1094 (58.4) |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, haemoglobin A1c; FPG, fasting plasma glucose; LDL-C, low density lipoprotein-cholesterol; TG, triglycerides; eGFR, estimated glomerular filtration rate; PWV, pulse wave velocity; OPG, osteoprotegerin; VCAM-1, vascular cell adhesion molecule; RAS, Renin-angiotensin system.
aOral medications include metformin, sulphonylurea and dipeptidyl peptidase IV (DPP IV) inhibitors.
In Table 2, the cross-sectional analysis showed that plasma LnOPG was positively associated with PWV at baseline with unadjusted coefficient 1.80 (95% Confidence Interval (CI) 1.44 to 2.17; p < .001), and with adjusted coefficient 0.43 (95%CI 0.05 to 0.80; p = .026) in Model 1. However, addition of medications attenuated the association with coefficient 0.31 (95%CI −0.06 to 0.68; p = .102) in Model 2.
Table 2.
Association between OPG and PWV at baseline (N = 1877).
| Coefficient (95% CI) p-value | |||
|---|---|---|---|
| Unadjusted | Model 1 b | Model 2 c | |
| OPG a | 1.80 (1.44 to 2.17) p < .001 | 0.43 (0.05 to 0.80) p = .026 | 0.31 (−0.06 to 0.68) p = .102 |
OPG, osteoprotegerin; PWV, pulse wave velocity; CI, confidence interval.
aNatural log-transformed.
bModel 1: Adjusted for age, sex, ethnicity, history of smoking, fasting plasma glucose, systolic blood pressure, diastolic blood pressure, low-density lipoprotein cholesterol, natural log-transformed triglycerides and estimated glomerular filtration rate.
cModel 2: Model 1 + medications for diabetes, medications for hyperlipidemia and renin-angiotensin system antagonist.
The proportions of SBP ≥140 mmHg and RAS antagonist use were 47.4% and 58.4% respectively at baseline. In Supplemental Figure 3, OPG was positively correlated with baseline PWV regardless of SBP (SBP <140 mmHg and ≥140 mmHg). Similarly, OPG was positively correlated with baseline PWV regardless of RAS antagonist use in Supplemental Figure 6.
In Supplemental Figure 5, OPG was positively correlated with baseline PWV regardless of glycemic control (HbA1c < 7.5% and ≥7.5%; the median HbA2c 7.5% was chosen as cut-off). Similarly, OPG was positively correlated with baseline PWV regardless of diabetes duration (duration <10 years and ≥10 years; the median duration 10 years was chosen as cut-off) in Supplemental Figure 6.
Over a mean period of 8.0 ± 0.9 years (maximum 10.5 years), there were 540 patients who had at least two follow-up PWV assessments. In Table 3, plasma LnOPG was positively associated with follow-up PWV with unadjusted coefficient 1.53 (95%CI 1.04 to 2.02; p < .001). The positive association persisted in the Model 1 with adjusted coefficient 0.51 (95%CI 0.06 to 0.97; p = .028) and Model 2 with adjusted coefficient 0.56 (0.30 to 0.82; p < .001).
Table 3.
Association between baseline OPG and follow-up PWV (N = 540).
| Coefficient (95% CI) p-value | |||
|---|---|---|---|
| Model 1 b | Model 2 c | Model 3 d | |
| OPG a | 1.53 (1.04 to 2.02) p < .001 | 0.51 (0.06 to 0.97) p = .028 | 0.56 (0.30 to 0.82) p < .001 |
OPG, osteoprotegerin; PWV, pulse wave velocity; CI, confidence interval.
aNatural log-transformed.
bModel 1: Follow-up period.
cModel 2: Model 1 + age, sex, ethnicity, history of smoking, fasting plasma glucose, systolic blood pressure, diastolic blood pressure, low-density lipoprotein cholesterol, natural log-transformed triglycerides and estimated glomerular filtration rate.
dModel 3: Model 2 + medications for diabetes, medications for hyperlipidemia and renin-angiotensin system antagonist.
Out of 540 patients, 528 had SNP data for the MR analysis. Supplemental Table 2 showed that the proportion of genotypes AG and GG increased with OPG tertile augmentation. Compared to patients with genotype AA, those with genotypes AG and GG had higher PWV at last follow-up with coefficient 0.78 (95%CI 0.21 to 1.35; p = .007). The result suggested an increased risk of arterial stiffness in homozygous and heterozygous carriers of G allele. We also observed that rs1385492 was significantly associated with plasma OPG (β = 0.07, SE = 0.03, p = .048) and explained 12.4% of variance (F statistic = 26.1). Genetically determined OPG was associated with higher PWV at last follow-up, with coefficient 10.81 (95%CI 2.97 to 18.65; p = .007) per unit increase in plasma LnOPG (Table 4). There was no statistically significant association between rs1385492 and clinical variables in Supplemental Table 3, suggesting the absence of major horizontal pleiotropy.
Table 4.
MR analysis on association between OPG and follow-up PWV.
| Instrumental variable | Effect allele | Other allele | Effect allele frequency | Coefficient of OPG per allele (SE) | p-value | Coefficient (95%CI) for follow-up PWV per allele | p-value | MR estimate of follow-up PWV per unit increase in OPG (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|---|
| rs1385492 | G | A | 0.413 | 0.07 (0.033) | 0.029 | 0.78 (0.21 to 1.35) | 0.007 | 10.81 (2.97 to 18.65) | 0.007 |
OPG, osteoprotegerin; PWV, pulse wave velocity; SE, standard error; CI, confidence interval.
Adjusted for age, sex, population structure and baseline PWV.
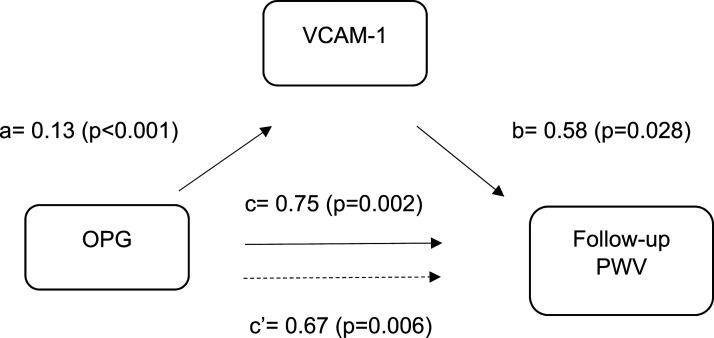
Figure 1 showed that (1) per unit increase in baseline plasma LnOPG was associated with higher baseline VCAM-1 with coefficient for pathway a 0.13 (p < .001); (2) per unit increase in baseline VCAM-1 was associated with higher follow-up PWV with coefficient for pathway b 0.58 (p = .028); (3) per unit increase in baseline plasma LnOPG was associated with higher follow-up PWV with coefficient for pathway c 0.75 (p = .002); and (4) the association between baseline plasma LnOPG and follow-up PWV was attenuated when adjusted for VCAM-1 with coefficient for pathway c’ 0.67 (p = .006). VCAM-1 accounted for 10.2% of the association between plasma LnOPG and follow-up PWV (p = .041).
Figure 1.
Mediation of baseline VCAM-1 between OPG and PWV at follow-up. OPG, osteoprotegerin; PWV, pulse wave velocity; VCAM-1, vascular cell adhesion molecule. Adjusted for age, sex, ethnicity, systolic blood pressure, diastolic blood pressure and low-density lipoprotein cholesterol and follow-up period.
Discussion
In the present study, plasma OPG was associated with higher PWV at baseline and follow-up in patients with T2D. For the first time, the longitudinal association was supported by genetic evidence which demonstrated that genetically predicted higher plasma OPG level was associated with higher last PWV at follow-up. Another previously unobserved finding was that VCAM-1 played a mediating role in the association between OPG and follow-up PWV.
Although previous studies showed a cross-sectional association between OPG and arterial stiffness,7,8,12–14 there was limited knowledge on the longitudinal association especially in patients with T2D. Our results supported these studies by demonstrating the cross-sectional and longitudinal association between plasma OPG and PWV in patients with T2D independent of conventional metabolic risk factors.
The pathophysiological mechanism underlying the association between OPG and PWV is unclear. However, OPG was correlated with traditional metabolic risk factors (e.g. hyperglycemia, hypertension and hypertriglyceridemia) which themselves confer high risk of arterial stiffness.41–43 This corroborates findings from earlier studies showing that OPG conferred elevated risk for incident CVD and mortality. 44
Furthermore, OPG binds to TRAIL which is an activator of apoptosis. Hence OPG increases survival of endothelial cells by neutralising TRAIL and inhibiting apoptosis.9,44 It was also observed that inflammatory cytokines stimulated the release of OPG in endothelial cell culture 45 which then enhanced the expression of E-selectin, VCAM-1 and intercellular adhesion molecule-1 by endothelial cells.44,46 This contributed to influx of monocytes and lymphocytes into the intima of blood vessel walls.44,46 Hence OPG plays a role in promoting inflammation which is a substrate for endothelial dysfunction. 44 The mediation analysis results in our study provided further mechanistic insights into the association between plasma OPG and follow-up PWV by demonstrating that VCAM-1 played a potential mediating role in the association between OPG and follow-up PWV.
OPG was correlated with PWV at baseline in patients regardless of glycemic control and diabetes duration in Supplemental Figures 5 and 6 respectively. The findings suggest that the association between OPG and PWV at baseline was evident for patients with patients with T2D; it does not merely represent the underlying cardiovascular disease that is associated with long-standing and/or sub-optimally controlled T2D.
The results obtained from Mendelian Randomization (MR) provide genetic evidence to support a plausible causal relationship between OPG and PWV. MR approach is based on the framework of inference by instrumental variable (IV), in this case, the SNP rs1385492 (which influences circulating OPG concentration) represents the IV. Given that allele assignment is random at conception and will temporally precedes any outcome later in life, MR may be analogous to nature’s conducted randomized controlled experiment, thereby facilitating causal inference. 47 We have demonstrated that rs1385492 was significantly associated with plasma OPG and the association between genetic variant and last PWV at follow-up was primarily through increased plasma OPG. In addition, the genetic variant was not associated with the conventional metabolic risk factors although it is possible for pleotropic effect to occur with other unmeasured confounding factors.
To the best of our knowledge, this is the first study to examine the longitudinal association between plasma OPG and PWV in patients with T2D with genetic support from MR and mechanistic insights from mediation analysis. We also assessed PWV with at least two follow-up measurements over fairly long follow-up period. However, some limitations remain in our study. First, the study was performed on patients with T2D, thereby limiting the generalisability of the findings to the general population. Second, we did not have complete information on medications, such as anti-hypertensive medications, which might affect PWV. We only had data on RAS antagonist for anti-hypertensive medications. Hence we do not have complete understanding on the potential confounding effect of other anti-hypertensive medications on the association between OPG and PWV. Third, we lacked the follow-up measurement of plasma OPG and hence could not assess if the change in OPG was linked to follow-up PWV. Furthermore, the one-sample MR approach may lead to upward biases for genetic instrument and exposure estimates. 33
Plasma OPG could be a potential biomarker for progression of arterial stiffness in patients with T2D. Our results may pave the way for future interventions which target OPG inhibition as a therapeutic target to ameliorate progression of arterial stiffness. An vitro study showed that Irbesartan down-regulated OPG expression via angiotensin II blockade. Intensive lipid-lowering treatment also reduced OPG levels in patients with carotid stenosis. Further studies are warranted to confirm the effect of these therapeutic agents on OPG levels as a monitoring target in prevention of arterial stiffness.
In conclusion, plasma OPG was associated with higher follow-up PWV in patients with T2D. This association may be mediated, at least in part, by VCAM-1. The MR analysis provided genetic support for an association between genetically predicted plasma OPG and higher last PWV at follow-up.
Supplemental Material
Supplemental Material for When Work Doesn’t Work: An Alternative Framework to Examine Cognitive Factors in Employment-Entrepreneurship Transitions by Serena Low, Sharon Pek, Angela Moh, Liu Jian-Jun, Bhuvaneswari Pandian, Keven Ang, Wern Ee Tang, Ziliang Lim, Tavintharan Subramaniam, Chee Fang Sum, and Su Chi Lim in Diabetes & Vascular Disease Research.
Author contributions: Serena Low: Conceptualisation, Investigation, Methodology, Writing – original draft. Sharon Pek: Investigation, Methodology, and Writing – original draft. Angela Moh: Investigation, Methodology, and Writing – original draft. Liu Jian-Jun: Investigation, Writing – review and editing. Bhuvaneswari Pandian: Data curation, Writing – review and editing. Keven Ang: Data curation. Wern Ee Tang: Writing – review and editing. Ziliang Lim: Writing – review and editing. Tavintharan Subramaniam: Writing – review and editing. Chee Fang Sum: Writing – review and editing. Su Chi Lim: Funding acquisition, Supervision, Writing – review and editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Singapore National Medical Research Council (MOH-000066, MOH-0000714, MOH-001327-02 and NMRC/CSA-INV/0020/2017).
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Tavintharan Subramaniam https://orcid.org/0000-0002-5365-8899
Su Chi Lim https://orcid.org/0000-0003-1742-5817
References
- 1.International Diabetes Federation . IDF diabetes atlas. 10th ed. Brussels, Belgium: International Diabetes Federation, 2021. [Google Scholar]
- 2.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018; 14: 88–98. [DOI] [PubMed] [Google Scholar]
- 3.Bansal S, Burman A, Tripathi AK. Advanced glycation end products: key mediator and therapeutic target of cardiovascular complications in diabetes. World J Diabetes 2023; 14: 1146–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonopoulos AS, Siasos G, Oikonomou E, et al. Arterial stiffness and microvascular disease in type 2 diabetes. Eur J Clin Invest 2021; 51: e13380. [DOI] [PubMed] [Google Scholar]
- 5.Kim JM, Kim SS, Kim IJ, et al. Arterial stiffness is an independent predictor for risk of mortality in patients with type 2 diabetes mellitus: the REBOUND study. Cardiovasc Diabetol 2020; 19: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharif S, Visseren FLJ, Spiering W, et al. Arterial stiffness as a risk factor for cardiovascular events and all-cause mortality in people with Type 2 diabetes. Diabet Med 2019; 36: 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu BG, Shih MH, Chen YC, et al. High serum osteoprotegerin is associated with arterial stiffness in kidney transplant patients. Tohoku J Exp Med 2015; 236: 247–253. [DOI] [PubMed] [Google Scholar]
- 8.Pateinakis P, Papagianni A, Douma S, et al. Associations of fetuin-A and osteoprotegerin with arterial stiffness and early atherosclerosis in chronic hemodialysis patients. BMC Nephrol 2013; 14: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoppet M, Al-Fakhri N, Franke FE, et al. Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-kappaB ligand in Mönckeberg's sclerosis and atherosclerosis. J Clin Endocrinol Metab 2004; 89: 4104–4112. [DOI] [PubMed] [Google Scholar]
- 10.Min H, Morony S, Sarosi I, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med 2000; 192: 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CT, Chua S, Hsu CY, et al. Biomarkers associated with vascular and valvular calcification in chronic hemodialysis patients. Dis Markers 2013; 34: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chae SY, Chung W, Kim YH, et al. The correlation of serum osteoprotegerin with non-traditional cardiovascular risk factors and arterial stiffness in patients with pre-dialysis chronic kidney disease: results from the KNOW-CKD study. J Kor Med Sci 2018; 33: e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehérvári L, Frigy A, Kocsis L, et al. Serum osteoprotegerin and carotid intima-media thickness are related to high arterial stiffness in heart failure with reduced ejection fraction. Diagnostics 2021; 11: 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CJ, Wang JH, Chen ML, et al. Serum osteoprotegerin is associated with arterial stiffness assessed according to the cardio-ankle vascular index in hypertensive patients. J Atherosclerosis Thromb 2015; 22: 304–312. [DOI] [PubMed] [Google Scholar]
- 15.Hou JS, Lin YL, Wang CH, et al. Serum osteoprotegerin is an independent marker of central arterial stiffness as assessed using carotid-femoral pulse wave velocity in hemodialysis patients: a cross sectional study. BMC Nephrol 2019; 20: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JE, Kim HJ, Moon SJ, et al. Serum osteoprotegerin is associated with vascular stiffness and the onset of new cardiovascular events in hemodialysis patients. Korean J Intern Med 2013; 28: 668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashima A, Carrero JJ, Qureshi AR, et al. Plasma osteoprotegerin, arterial stiffness, and mortality in normoalbuminemic Japanese hemodialysis patients. Osteoporos Int 2011; 22: 1695–1701. [DOI] [PubMed] [Google Scholar]
- 18.Sendic S, Mansouri L, Hong MG, et al. Soluble CD14 and osteoprotegerin associate with ankle-brachial index as a measure of arterial stiffness in patients with mild-to-moderate chronic kidney disease in a five-year prospective study. Cardiorenal Med 2023; 13: 189–201. [DOI] [PubMed] [Google Scholar]
- 19.Beyazal MS, Erdoǧan T, Devrimsel G, et al. Relationship of osteoprotegerin to pulse wave velocity and carotid intima-media thickness in rheumatoid arthritis patients. Z Rhematol 2016; 75: 723–728. [DOI] [PubMed] [Google Scholar]
- 20.Csiky B, Sági B, Peti A, et al. The impact of osteocalcin, osteoprotegerin and osteopontin on arterial stiffness in chronic renal failure patients on hemodialysis. Kidney Blood Press Res 2017; 42: 1312–1321. [DOI] [PubMed] [Google Scholar]
- 21.Jung CH, Lee WY, Kim SY, et al. The relationship between coronary artery calcification score, plasma osteoprotegerin level and arterial stiffness in asymptomatic type 2 DM. Acta Diabetol 2010; 47(Suppl 1): 145–152. [DOI] [PubMed] [Google Scholar]
- 22.Kim MS, Kim WJ, Khera AV, et al. Association between adiposity and cardiovascular outcomes: an umbrella review and meta-analysis of observational and Mendelian randomization studies. Eur Heart J 2021; 42: 3388–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dessein PH, López-Mejias R, González-Juanatey C, et al. Independent relationship of osteoprotegerin concentrations with endothelial activation and carotid atherosclerosis in patients with severe rheumatoid arthritis. J Rheumatol 2014; 41: 429–436. [DOI] [PubMed] [Google Scholar]
- 24.Deng H, Song Z, Xu H, et al. MicroRNA-1185 promotes arterial stiffness though modulating VCAM-1 and E-selectin expression. Cell Physiol Biochem 2017; 41: 2183–2193. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Liu JJ, Sum CF, et al. Ethnic disparity in central arterial stiffness and its determinants among Asians with type 2 diabetes. Atherosclerosis 2015; 242: 22–28. [DOI] [PubMed] [Google Scholar]
- 26.Fujii M, Tomiyama H, Nakano H, et al. Differences in longitudinal associations of cardiovascular risk factors with arterial stiffness and pressure wave reflection in middle-aged Japanese men. Hypertens Res 2021; 44: 98–106. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto R, Tabara Y, Kusunoki T, et al. A slightly high-normal glucose level is associated with increased arterial stiffness in Japanese community-dwelling persons with pre-diabetes. Vasc Med 2013; 18: 251–256. [DOI] [PubMed] [Google Scholar]
- 28.Leto G, Tartaglione L, Rotondi S, et al. Diastolic pressure and ACR are modifiable risk factors of arterial stiffness in T2DM without cardiovascular disease. J Clin Endocrinol Metab 2022; 107: e3857–e3865. [DOI] [PubMed] [Google Scholar]
- 29.Wilson J, Webb AJS. Systolic blood pressure and longitudinal progression of arterial stiffness: a quantitative meta-analysis. J Am Heart Assoc 2020; 9: e017804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie X, Ma YT, Yang YN, et al. Decreased estimated glomerular filtration rate (eGFR) is not an independent risk factor of arterial stiffness in Chinese women. Blood Pres 2013; 22: 73–79. [DOI] [PubMed] [Google Scholar]
- 31.Newcombe PJ, Connolly S, Seaman S, et al. A two-step method for variable selection in the analysis of a case-cohort study. Int J Epidemiol 2018; 47: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods 2019; 10: 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014; 23: R89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howey R, Shin SY, Relton C, et al. Bayesian network analysis incorporating genetic anchors complements conventional Mendelian randomization approaches for exploratory analysis of causal relationships in complex data. PLoS Genet 2020; 16: e1008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgess S, Davey Smith G, Davies NM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res 2019; 4: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurung RL, Dorajoo R, Yiamunaa M., et al. Association of genetic variants for plasma LRG1 with rapid decline in kidney function in patients with type 2 diabetes. J Clin Endocrinol Metab 2021; 106: 2384–2394. [DOI] [PubMed] [Google Scholar]
- 37.Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat Genet 2012; 44: 821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao P, Zhang X, Zhang L, et al. Causal effects for genetic variants of osteoprotegerin on the risk of acute myocardial infarction and coronary heart disease: a two-sample Mendelian randomization study. Front Cardiovasc Med 2023; 10: 1041231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherry ST, Ward MH, Kholodov M, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 2001; 29: 308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 41.Pavlovska I, Kunzova S, Jakubik J, et al. Associations between high triglycerides and arterial stiffness in a population-based sample: kardiovize Brno 2030 study. Lipids Health Dis 2020; 19: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian X, Zuo Y, Chen S, et al. Hypertension, arterial stiffness, and diabetes: a prospective cohort study. Hypertension 2022; 79: 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Liu L, Zhou Y, et al. Increased fasting glucose and the prevalence of arterial stiffness: a cross-sectional study in Chinese adults. Neurol Res 2014; 36: 427–433. [DOI] [PubMed] [Google Scholar]
- 44.Montagnana M, Lippi G, Danese E, et al. The role of osteoprotegerin in cardiovascular disease. Ann Med 2013; 45: 254–264. [DOI] [PubMed] [Google Scholar]
- 45.Secchiero P, Corallini F, Pandolfi A, et al. An increased osteoprotegerin serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction. Am J Pathol 2006; 169: 2236–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangan SH, Van Campenhout A, Rush C, et al. Osteoprotegerin upregulates endothelial cell adhesion molecule response to tumor necrosis factor-alpha associated with induction of angiopoietin-2. Cardiovasc Res 2007; 76: 494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgakis MK, Gill D. Mendelian randomization studies in stroke: exploration of risk factors and drug targets with human genetic data. Stroke 2021; 52: 2992–3003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for When Work Doesn’t Work: An Alternative Framework to Examine Cognitive Factors in Employment-Entrepreneurship Transitions by Serena Low, Sharon Pek, Angela Moh, Liu Jian-Jun, Bhuvaneswari Pandian, Keven Ang, Wern Ee Tang, Ziliang Lim, Tavintharan Subramaniam, Chee Fang Sum, and Su Chi Lim in Diabetes & Vascular Disease Research.