Abstract
Background
Approximately 3.5% of pregnancies in the United Kingdom are complicated by gestational diabetes mellitus (GDM). Risk factors for this mirror those contributing to type 2 diabetes (T2DM). Though socioeconomic status (SES) is presumed to contribute to GDM, evidence in the United Kingdom is limited. In this unique study, we explored the impact of SES on GDM prevalence in a London suburb population.
Materials and Methods
Four thousand one hundred and sixty-three pregnant women who booked between July 2018 and March 2020 at Princess Royal University Hospital were retrospectively analyzed. Associations between GDM prevalence and SES trends (using multiple deprivation deciles (MDD)), and body mass index (BMI), age, ethnicity, screening uptake, birth-weights and birth outcomes, were analyzed.
Results
Patients with BMI >30 kg/m2, older than 35 years, and non-Caucasian ethnicity have an increased risk of developing GDM (p < 0.0001, p < 0.0001, p < 0.0001, respectively). No association existed between MDD and GDM prevalence (p-values over 0.05). Patients with risk factors for GDM were highest in the deprived areas p < 0.0001. MDD 1–4 (most deprived) had the highest percentage of missed screening (15% of patients with risk factors missed screening), compared to 8% in the least deprived group (p < 0.0001).
Discussion
Our data surprisingly suggest that low SES did not increase the incidence of GDM, despite a higher proportion of women with risk factors for GDM living in the most deprived postcodes. However this unclear finding may be due to low screening uptake of deprived populations, and therefore lack of GDM diagnosis, or indicate that GDM is a result of a different aetiology to T2DM. Further research is needed to explore if access to screening services, lack of health education or other health inequalities were responsible for the high proportion of missed screening opportunities in deprived areas.
Keywords: Gestational diabetes, socioeconomic deprivation, obesity, pregnancy, ethnicity
What is already known on this topic:
With recent coverage of inequalities in pregnancy, urgent intervention to improve the care of pregnant women with endocrinology disorders is necessary. While there is clear evidence for the inverse association between socioeconomic status (SES) and type 2 diabetes (T2DM) prevalence, with some research showing similar trends for gestational diabetes mellitus (GDM), data is lacking in the United Kingdom.
What this study adds:
While we aimed to show that deprivation predisposes to GDM in a British suburb population, our data proved otherwise. Despite populations with the most risk factors for GDM existing in deprived areas, surprisingly, GDM prevalence was higher in affluent populations. However, on exploring our data further, we saw a lower uptake in GDM screening in poorer areas. This may explain our unexpected results.
How this study might affect research, practice or policy:
We have exposed the clear need to develop strategies to improve screening attendance in deprived populations and decrease existing health inequalities in the United Kingdom. We need to target these communities in our practice, to avoid missing GDM diagnoses and potential pregnancy complications, while educating health professionals on clear inequalities and their impact on maternal and fetal health.
Introduction
With 3.9 million people currently living with diabetes in the United Kingdom, and its escalating incidence and burden on the NHS, ways of prevention are continually being sought. Of the diabetic population, 90% have T2DM and are 50% more likely to die prematurely. 1 The key risk factors for T2DM are age, ethnicity, obesity, and family history. However worldwide, an unbalanced number of affected individuals are from low and middle-income backgrounds, with SES reflecting access to resources including healthcare. 2 The American Diabetes Association recommends the inclusion of assessments of patients’ psychological and social background in the management of diabetes. 3 It is evident from previous studies that psychosocial deprivation is also associated with increased obesity, poor compliance with glycaemic control, increased incidence of T2DM, and associated complications, as well as higher morbidity and mortality rates. 3
Gestational diabetes mellitus (GDM) is diabetes that is first diagnosed in pregnancy and usually subsides after giving birth. It is prevalent in 3 to 5% of all pregnancies (i.e., 1 in 20 pregnant women will develop GDM) and poses a significant public health and clinical problem. 4 Both GDM and T2DM share common risk factors including age, ethnicity, family history and obesity while GDM itself is a strong predictor for developing future GDM, and T2DM later in life. Women who develop GDM are at risk of significant short-term and long-term adverse outcomes for both mother and fetus. Problems that may arise in women (and fetuses) with GDM include preeclampsia, premature birth, stillbirth, polyhydramnios, and macrosomia (with increased risks of shoulder dystocia and caesarean sections). These women are also six times more likely to develop T2DM, in comparison to women who are normoglycaemic in pregnancy. 5 Thus, the importance of preventing GDM is far greater than other forms of diabetes, due to the potential health implications for mother and child and the burden the disease puts on any country's National Health Service. 6
To prevent adverse outcomes for mother and child, screening has become paramount in detecting GDM. National guidelines advice screening all pregnant women at booking, and those with more than one risk factor for GDM are offered an oral glucose tolerance test between 24 and 28 weeks. A prospective cohort study nested within the LIMIT trial saw the prevalence of GDM increase with increasing maternal body mass index (BMI) (6.74% overweight vs 20.00% obese subclass 3). With adequate screening the study showed that the women who were diagnosed within the screening programme for GDM were significantly less likely to give birth to babies with birth weights above 4 kg. 7 A retrospective multivariate analysis on 16,838 women portrayed that the risk of GDM becomes significantly and progressively increased from 25 years onwards (p < 0.001). 8 Ethnicity is also a strong predictor of GDM. A prospective study looking at 11,203 women attending antenatal clinics where all were screened for GDM, showed that women from ethnic groups other than Caucasian had a higher frequency of GDM than Caucasian women (2.9% vs 0.4%, p < 0.001). 9 NICE guidelines outline that women with risk factors (all of which mirror risk factors for T2DM) of BMI more than 30, previous macrosomic babies (>4.5 kg) or GDM, family history of diabetes, and South Asian, African-Caribbean, or Middle Eastern origin (even if born in the United Kingdom) should be screened for GDM. 10 However, unlike T2DM where socioeconomic deprivation is extensively studied and increasingly included in the assessment of patients, it is poorly integrated and infrequently considered for GDM patients.
Recently there has been more media focus and increased awareness of socioeconomic deprivation and ethnicity contributing to adverse outcomes in pregnancy. A large cohort study on pregnancy outcomes using data compiled by the National Maternity and Perinatal Audit concluded that the largest inequalities were seen in Black and South Asian women in the most socioeconomically deprived quintile and that these specific high-risk groups should be targeted to address risk factors. 11 During pregnancy, psychosocial deprivation is associated with poor outcomes; increased rates of maternal and neonatal hospitalisation, stillbirth, postnatal death, preterm delivery and small for gestational age infants. 12 Social factors play an important role in patients’ compliance with medical guidance and recommendations. 13 Moreover, factors such as the levels of education, occupation, income, housing, nutrition, environment, and culture, have significant direct and indirect roles on the health status of individuals and communities. Those most deprived—the poor and least educated—experience the worst health outcomes, compared to those wealthier and more educated. 14 Despite the socioeconomic trends for major known risk factors for T2DM, there is little in the form of data to assess if similar trends exist with GDM prevalence in the United Kingdom.
Given the vast recent media coverage on pregnancy inequalities, and much evidence showing that health is influenced by socioeconomic factors in addition to genetic and biological factors, together with the strong association between GDM and T2DM, we aim to investigate if socioeconomic trends, along with already established risk factors, have an impact on GDM prevalence, which may lead to novel ways to reduce the incidence and potential complications of the condition. 15
Materials and methods
All women who booked between July 2018 and March 2020 inclusive at Princess Royal University Hospital (Kings College NHS Trust) were included in this retrospective analysis. Clinical data was obtained using BadgerNet (Clevermed Ltd Version 54.3.1) and EPR (Allscripts Sunrise Enterprise Release 18.4).
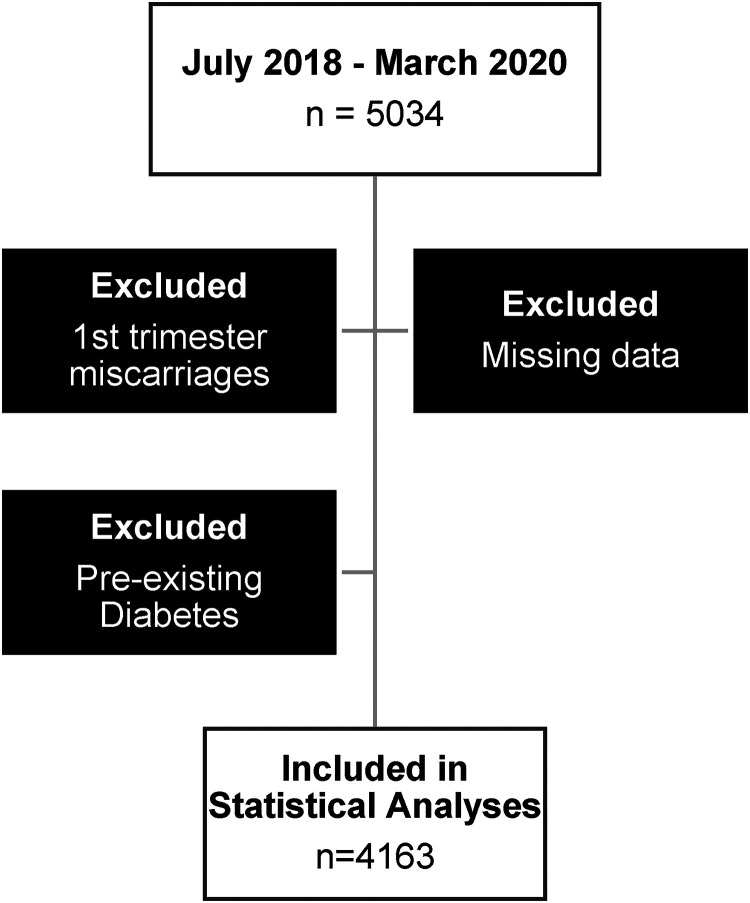
Women with first-trimester miscarriages, missing data on risk factors (BMI, family history, previous pregnancy details, and demographics), and those with pre-existing diabetes, were excluded (n = 4163) (Figure 1).
Figure 1.
Flowchart to represent exclusion criteria (n = number).
Postcodes of the addresses patients disclosed when booking, were used. Postcodes incorporate a small group of addresses in that post town, termed a small area. The postcodes were inputted into an open data tool to obtain Index of Multiple Deprivation (IMD) data for each postcode. 16 The IMD ranks every small area in England from 1 (most deprived area) to 32,844 (least deprived area). The IMD combines information from the seven domains to produce an overall relative measure of deprivation. The domains are combined using the following weights:
Income deprivation (22.5%)
Employment deprivation (22.5%)
Education, skills and training deprivation (13.5%)
Health deprivation and disability (13.5%)
Crime (9.3%)
Barriers to housing and services (9.3%)
Living environment deprivation (9.3%).
The output file lists the postcodes entered in Excel format, the Lower-layer Super Output Area (LSOA) that each postcode falls within, and the deprivation data for that LSOA. In this study, we looked at the deciles of IMD (multiple deprivation decile or MDD). The IMD is outlined into 10 ranked deciles, with decile 1 reflecting the most deprived postcodes and decile 10, the least deprived postcode areas. The data was then categorised into 3 groups for analysis purposes: MDD 1–4, MDD 5–7, and MDD 8–10.
Statistical analysis was completed using Statdirect software (Version 3.3.5 22/03/2021). The initial analysis explored differences between groups with regard to basic characteristics such as age, BMI, and ethnicity. Ethnicities were characterised as shown in Table 1. We limited the data on outcomes of pregnancies to the mode of delivery, outcome of births and preterm births, and birth weights, as the scope of this article was aimed at the correlation of GDM prevalence to SES.
Table 1.
Baseline characteristics.
| Multiple deprivation decile 1–4 N = 1085 | Multiple deprivation decile 5–7 N = 1204 | Multiple deprivation decile 8–10 N = 1874 | ||
|---|---|---|---|---|
| Age years M ± SD | 30.3 ± 5.7 | 31.8 ± 5 | 32.7 ± 4.8 | <0.0001 |
| BMI kg/m2 M ± SD | 27.2 ± 6 | 26.4 ± 5.6 | 25.4 ± 5.1 | <0.0001 |
| Ethnicity | ||||
| Caucasian | 779 (71.8) | 873 (72.5) | 1445 (77.1) | 0.001 |
| South Asian | 81 (7.5) | 104 (8.6) | 184 (9.8) | NS |
| East Asian | 13 (1.2) | 32 (2.7) | 54 (2.9) | 0.006 |
| Any other Asian background | 1 (0.1) | 5 (0.4) | 6 (0.3) | NS |
| Black or Afro-Caribbean | 159 (14.7) | 126 (10.5) | 92 (4.9) | <0.0001 |
| Any other ethnic background | 28 (2.6) | 33 (2.7) | 46 (2.5) | NS |
| Mixed | 24 (2.2) | 31 (2.6) | 47 (2.5) | NS |
BMI: body mass index.
Theory/calculation
One-way ANOVA was used for normally distributed continuous variables, Kruskal–Wallis test was used for non-parametric variables, and chi-square/Fisher tests were used for categorical variables. At booking, patients were screened for risk factors for GDM (previous GDM or baby of more than 4.5 kg, family history (first degree) of diabetes, ethnicity (Asian, African–Caribbean, or Middle Eastern), and BMI of more than 30 kg/m2. Patients identified as high risk at booking should then be offered an oral-glucose tolerance test between 24 and 28 weeks and given information about this. An appointment is then usually made by the midwife carrying out the risk assessment and the patient is subsequently contacted prior to the appointment with information on how the test is carried out. Multiple logistic regression analyses were performed to identify the effect of MDD on the risk of being diagnosed with GDM during pregnancy, after adjusting results for age, BMI and ethnicity as covariates. Results were presented as odds ratios (ORs) with 95% confidence interval (CI), and p-values. The data was analysed with regard to the presence of GDM risk factors, uptake of GDM screening (those with oral glucose tolerance test (OGTT) results), mode of delivery and outcome of birth. p-values of less than 0.05 were considered statistically significant. Preterm deliveries were classified as those babies born before 37 weeks' gestation. Birth centiles were calculated based on the World Health Organisation (WHO) gestational age and birth weight calculator. 17
Results
Out of the 4163 patients included in this analysis, 1085 were in the MDD 1–4 group, 1204 in the MDD 5–7 group and 1874 in the MDD 8–10 group. The baseline characteristics revealed younger patients with a higher BMI in the most deprived group. The most deprived group also had a higher proportion of non-Caucasian ethnic patients, with 28% in the MDD 1–4 compared to 27.5% in the MDD 5–7 group and 23% in the MDD 8–10 group (p < 0.001). The ethnic subgroup analysis revealed more Afro-Caribbean patients in the most deprived areas, with 14.7% in the MDD 1–4 group, compared to 10.5% in the MDD 5–7 group and 4.9% in the MDD 8–10 group (p < 0.0001). The least deprived area had significantly higher Caucasian and East-Asian patients (Table 1).
When looking into known risk factors and the association with GDM development, the results were as hypothesised. Patients with BMI > 30 have an increased risk of developing GDM during pregnancy (OR 3.9 with 95% CI (3.2–4.9) p < 0.0001). Women older than 35 years have an increased risk of developing GDM during pregnancy (OR 1.9 with 95% CI (1.5–2.4) p < 0.0001). Non-Caucasian ethnicity also showed an increased GDM risk with OR 2.1 and 95% CI (1.7–2.6) p < 0.0001 (Table 2). Multivariable logistic regression was performed to assess the effect of MDD on the risk of diagnosing GDM during pregnancy. After adjusting all other contributing risk factors, such as BMI, age and ethnicity, there was no association between the level of deprivation and the risk of being diagnosed with GDM during pregnancy (p-values over 0.05). Surprisingly, though it was not significant, the risk of diagnosing GDM was highest in the least deprived area OR 1.2 with 95% CI (0.97–1.50) p = 0.09.
Table 2.
Risk of being diagnosed with GDM.
| OR | Coefficient | 95% CI | p-value | |
|---|---|---|---|---|
| Index of multiple deprivation decile 1–4 | 0.85 | −0.16 | 0.67–1.09 | .19 |
| Index of multiple deprivation decile 5–7 | 0.94 | −0.06 | 0.75–1.18 | .59 |
| Index of multiple deprivation decile 8–10 | 1.2 | 0.18 | 0.97–1.49 | .09 |
| Age at booking/ > 35years | 1.9 | 0.64 | 1.54–2.35 | <.0001 |
| Ethnicity non-Caucasian | 2.1 | 0.74 | 1.69–2.62 | <.0001 |
| BMI>30 kg/m2 | 3.99 | 1.38 | 3.21–4.95 | <.0001 |
GDM: gestational diabetes mellitus; BMI: body mass index.
The background assessment of GDM risk per group revealed a higher number of patients with risk factors in the most deprived area 65% in the MDD 1–4, 57% in the MDD 5–7 group and 47% in the MDD 8–10 group (p < 0.001). With the lack of association between deprivation and GDM diagnosis, however, despite clear findings of a significant association of the deprived populations having the highest portion of risk factors to develop GDM, we crudely evaluated if the findings were due to screening uptake. We looked at all the women who had clear risk factors in each group to see if all of them had OGTT results, and if not, took this to imply they had no screening. Remarkably, the percentage of GDM screening uptake and patients diagnosed with GDM is similar between the groups. When looking at the missed screening opportunities in patients with risk factors for GDM however, the most deprived area had the highest percentage, as 15% of patients with risk factors did not go for screening compared to 11% in the MDD 5–7 group and 8% in the MDD 8–10 group (p < 0.0001). Furthermore, the analysis revealed a higher percentage of patients who went for GDM screening with no risk factors in the least deprived area. The overscreening rate was 19% in the MDD 8–10 group compared to 13% in the MDD 5–7 group and 11% in the MDD1–4 group (p < 0.0001) (Table 3).
Table 3.
GDM screening and risk factors.
| Multiple deprivation decile 1–4 N = 1085 | Multiple deprivation decile 5–7 N = 1204 | Multiple deprivation decile 8–10 N = 1874 | ||
|---|---|---|---|---|
| Patients with risk factors for GDM | 707 (65.2) | 691 (57.4) | 883 (47.1) | <.0001 |
| Patients screened for GDM | 663 (61.1) | 709 (58.9) | 1091 (58.2) | NS |
| Patients screened for GDM with no risk factor | 119 (11) | 155 (12.9) | 362 (19.3) | <.0001 |
| Patients missed screening | 163 (15) | 136 (11.3) | 154 (8.2) | <.0001 |
| GDM | 106 (9.8) | 123 (10.2) | 190 (10.1) | NS |
GDM: gestational diabetes mellitus.
The analysis revealed no significant differences in gestational age at delivery, birth gender, induction of labour rate or caesarean section rate between the groups. The incidence of assisted vaginal delivery and use of regional (epidural) anaesthetic was lower in the most deprived group (Table 4). There was a lower mean birth weight in the most deprived area, 3345 g compared to 3399 g in the MDD 5–7 and 3414 g in the MDD 8–10 (p = 0.003). This was matched with a lower percentage of birth weight over 4 kg and a higher percentage of less than 10th centile birth weight in the most deprived group. However, there was no difference in the rate of more than 90th centile birth weight between the groups. The analysis revealed no difference in the rate of non-live birth and preterm delivery between the groups (Table 5).
Table 4.
Delivery characteristics.
| Multiple deprivation decile 1–4 N = 1085 | Multiple deprivation decile 5–7 N = 1204 | Multiple deprivation decile 8–10 N = 1874 | ||
|---|---|---|---|---|
| Gestational age | ||||
| Mean weeks ± SD | 39.5 ± 1.7 | 39.6 ± 1.8 | 39.6 ± 1.6 | NS |
| Median (range) | 39.7 (24–43.4) | 39.7 (24.3–42.3) | 39.7 (25.1–42.9) | |
| Birth gender | ||||
| Female | 548 (50.5) | 585 (48.6) | 903 (48.2) | NS |
| Male | 537 (49.5) | 619 (51.4) | 971 (51.8) | |
| Induction of labour | 318 (29.3) | 317 (26.3) | 475 (25.4) | NS |
| Caesarean birth | 279 (25.7) | 358 (29.7) | 520 (27.8) | NS |
| Assisted vaginal delivery | 150 (18.6) | 218 (25.8) | 337 (24.9) | 0.0007 |
| Use of spinal/epidural | 545 (50.2) | 684 (56.8) | 1043 (55.7) | 0.003 |
| Epidural in labour | 278 (34.5) | 341 (40.3) | 544 (40.2) | 0.01 |
Table 5.
Effect of MDD groups on birth outcome.
| Multiple deprivation decile 1–4 N = 1085 | Multiple deprivation decile 5–7 N = 1204 | Multiple deprivation decile 8–10 N = 1874 | ||
|---|---|---|---|---|
| Mean birth weight ± SD | 3345 ± 532 | 3399 ± 553 | 3414 ± 510.4 | 0.003 |
| Mean birth centile ± SD | 60.7 ± 28.6 | 63.4 ± 28.5 | 64.2 ± 26.9 | 0.003 |
| Birth weight > 4kg | 101 (9.3) | 154 (12.8) | 217 (11.6) | 0.03 |
| Nonlive birth | 5 (0.5) | 6 (0.5) | 5 (0.3) | NS |
| Birth weight <10th centile | 71 (6.5) | 73 (6.1) | 67 (3.6) | 0.0003 |
| Birth weight >90th centile | 225 (20.7) | 283 (23.5) | 435 (23.2) | NS |
| Total preterm delivery | 53 (4.9) | 56 (4.7) | 84 (4.5) | NS |
| Extreme preterm | 1 (0.09) | 6 (0.5) | 4 (0.2) | NS |
| Very preterm | 4 (0.4) | 3 (0.3) | 6 (0.3) | NS |
| Late preterm | 48 (4.4) | 47 (3.9) | 74 (4) | NS |
MDD: multiple deprivation decile.
Discussion
Our findings show that the already known risk factors for developing GDM prevail and are rightly used to screen those most at risk. Obesity is one such risk factor that our findings have supported as a key cause of GDM development. The odds ratio of diagnosing GDM in those with a BMI of more than 30 was significantly high in all deprivation decile groups (OR 3.9). A prospective cohort study within the LIMIT randomised controlled trial recruited 1030 women between 10 and 20 weeks' gestation, with a BMI ≥ 25 kg/m grouped into subclasses using the WHO criteria. 18 The prevalence of GDM increased with increasing maternal BMI (6.74% overweight vs 13.42% obese subclass 1 vs 12.79% obese subclass 2 vs 20.00% obese subclass 3). Communities that are socioeconomically deprived display many features linked to higher incidences of obesity, 19 such as inadequate access to healthy foods and higher exposure to fast food restaurants, 20 as well as an absence of recreational facilities with an inability to afford exercise or gym memberships. 21 We know that individual lifestyle modification strategies (e.g. with dietitian input) have been tried in primary care settings, as well as in secondary care, but often have little impact. Addressing this, the American Dietetic Association has called for community interventions to facilitate group behavioural change. 22 One suggested change that could be implemented from our findings is addressing the community where deprivation exists and within which individual unhealthy behaviour unfolds, hopefully generating more efficient results.
Ethnicity also remains a clear risk factor for GDM prevalence, with our non-Caucasian group having a significant association with diagnosing GDM. Ethnicity has long been described as a key risk factor for the development of diabetes (type 2 and GDM). Women from ethnicities other than white, Caucasian or Europids, are at the most risk of developing GDM for reasons that are multifactorial; genes associated with specific ethnicities, obesity, and body composition. 23 Our highest population of non-Caucasian communities resided in area postcodes that were most deprived, with a higher incidence of the black community based in MDD 1 to 4. The ethnic subgroup analysis revealed more Afro-Caribbean patients in the most deprived areas, with 14.7% in the MDD 1–4 group, compared to 10.5% in the MDD 5–7 group and 4.9% in the MDD 8–10 group. These findings are in keeping with Ethnicity Facts and Figures 24 on people living in deprived neighbourhoods, which showed that Black people (15.2%) were second most likely out of all ethnic groups to live in deprived neighbourhoods, after Asian people (15.7%). The National Maternity and Perinatal audit of births between April 2015 and March 2018 in England, Scotland, and Wales, highlighted a clear disparity in outcomes between black and South Asian communities, and Caucasian populations. While overall stillbirth rates in the audit were low, rates were higher for babies born to women from South Asian (6 in 1000) and Black (7 in 1000) ethnic groups when compared with Caucasian (4 in 1000) ethnic groups; and for those in the most deprived areas. 25 We are more aware since the COVID-19 pandemic that black and Asian populations are at higher risk of adverse health outcomes, with growing evidence, supported by our own findings, that these communities reside in areas with the lowest deprivation indices. This enforces the need for healthcare providers to be trained to enhance their knowledge of equality and diversity, thus improving patients’ experience of maternity services.
Our results also showed that the least deprived areas had the highest average age of mothers compared to the most deprived (32.7 and 30.3 years old, respectively). Increased maternal age is an independent risk factor for GDM. As we have seen the average maternal age increasing over the past four decades, it is important to clearly understand the extent of age-based risk for GDM. The exact mechanism behind this link has not been clearly demonstrated, however high levels of insulin resistance, adipokines and inflammatory markers, as well as oxidative stress may partly explain this trend. 26 This independent risk factor apparent in our more affluent deciles, with risk factors of BMI and ethnicity seen more in the most deprived areas, may explain our balanced findings of a similar level of GDM incidence across the three MDD categories.
Interestingly our findings demonstrated that despite having patients at the highest risk of developing GDM significantly associated with deprivation deciles 1 to 4 (i.e. the highest proportion of women in MDD 1–4 that have risk-factors of high BMI, and non-Caucasian ethnicity which are recognised to have an association with GDM diagnosis), the incidence of GDM in these deprived areas showed a non-significant association. When reviewing studies on affluent communities and the development of diabetes, only one cross-sectional survey completed in New Zealand 27 looking at the influence of ethnicity and social deprivation, showed that while most communities followed the trend of deprivation inversely influencing diabetes incidence, the trend among Māori groups emphasised that the least deprived are equally at risk of diabetes. However, this does not fully explain our unexpected findings of an equal prevalence between the groups.
One theory from other studies demonstrating higher rates of GDM in more affluent areas is the idea that these women are likely to have occupations that are busy, high-end and time-consuming. This suggests that perhaps they have less time to comply with management advice and control their diabetes. A qualitative study exploring the ideas, concerns, and expectations of women faced with a diagnosis of GDM, outlined that self-management was difficult, particularly due to the time required to learn food values and dietary advice, and the time it takes to cook healthy food. 28 Women in deprived areas may have more time to pay attention to their health despite having risk factors of higher BMI and non-Caucasian ethnicity. This of course would need further data evaluation to look at the occupations of women in the different deprivation sectors. Another hypothesis is that pregnant women with low economic status have a higher perception of risk and therefore adhere to medical advice, to avoid unfavourable physical and economic consequences. 29 However, further research into this theory is required.
On exploring our outcomes further, a recent review reported that true GDM prevalence rates could be understated due to access and attitudes to GDM diagnosis and screening in low-resource settings with foreign-born women. 23 This notion is further justified by our results, which showed a higher proportion of women at risk of developing GDM in the lower deprivation deciles (1–4), not undergoing the screening (p < 0.0001). A qualitative study looking at the influences on the uptake of diabetes screening for T2DM highlighted that those in deprived areas, who were also non-attendees to screening tests, considered the condition as lacking in severity and easy to manage. 30 This is coupled with practical barriers to attending; the timing and length of appointment for the OGTTs. This barrier is probably more evident in those who will lose pay by taking a morning off to attend the screening, so will disadvantage those from lower socioeconomic backgrounds.
Our initial aim did not involve analysing data on screening, however, with GDM prevalence not correlating with socioeconomic deprivation, we crudely analysed data on whether screening was performed on high-risk women. Interestingly our findings showed that a higher proportion of patients at risk of developing GDM in pregnancy, missed or were not offered screening (assumed from lack of GDM screening results available), which may account for the insignificant incidence of GDM in the most deprived centiles, contrary to our predictions. On the assumption that all women at risk were offered GDM screening, it is known that screening uptake for diseases overall is lower in deprived communities. Given that the OGTT is a screening program, when reviewing other screening programmes offered nationally, it was found that socio-economically deprived communities have a reduced uptake of screening programmes offered for diseases such as colorectal cancer, and hence have a higher risk of presenting later with complications. 31 A systematic review of the association between area-level deprivation and breast cancer screening uptake in Europe concluded that women living in areas of high deprivation had limited knowledge about screening programmes or had misconceptions regarding the disease and screening. 32 Embarrassment, fear, and inconvenience have also been cited as possible reasons for low screening rates in these communities, with practical barriers again highlighted by an Australian Government Report suggesting women in deprived communities encounter difficulties attending appointments, due to transport (cost and availability), family commitments, or loss of pay. Transport is an important facilitator of social well-being, which can affect economic and social outcomes, and therefore inequality. While in Australia, distance plays the biggest role in barriers to healthcare access, in the United Kingdom, transport costs are an obstacle to its use. Difficulty accessing transport limits access to health services, such as medical appointments. An evidence review using systematic search, inclusion and synthesis process to understand the current knowledge base on the relationships between transport and health and wellbeing, showed that there is a relationship between transport disadvantage and poverty and the inaccessibility of health services. 33 Targeting communities that are most deprived by offering solely HbA1c or random glucose tests which are less time consuming, in local community settings such as GP surgeries, may improve uptake of screening, early detection, and efficient management of GDM.
Migrant women also have shown reduced levels of engagement with healthcare services. The reasons behind this include a lack of familiarity with NHS systems, significant language barriers, uncertainty about whether they will be asked for payment for care received and worries that using the services might affect their immigration status. Formulating system-wide approaches to the barriers these women face due to a lack of understanding, is not the responsibility of obstetricians and midwives alone, but also other healthcare providers from primary care to other specialities in the secondary care setting. The misunderstandings listed above can be tackled by simply exploring this group's ideas, concerns, and expectations, and targeting these patients with appropriate education (including the use of interpreters and language-specific information, to overcome these misguided ideas. 34 One such way is by mirroring World Diabetes Foundation-funded projects in India and Cameroon which have used programmes that involve community outreach workers to give continued support to women less likely to engage in healthcare services. 35
The birth outcomes in our analysis showed no correlation between GDM diagnoses and MDD background. This may be due to the improved GDM care pathways within the NHS, more frequent reviews and growth scans, digital methods to review glycaemic control and the multidisciplinary approach adopted by most obstetric departments. By offering induction early for our insulin-dependent GDM patients, we are avoiding the risk of macrosomia and the potential risks associated. 10
A limitation in our analysis is assessing whether GDM screening was offered to women at risk when assessed at booking, as per NICE guidelines. An extension of the study would be to retrospectively analyse if there is documented evidence of the screening offer, and if accepted. With the lack of this data for this study, we assumed that all women with risk factors should have been offered the screening. If no OGTT results were present, we assumed this offer was declined, the patient did not attend, or the screening offer was not made. With the increasing disparity and health inequality data on BAME (Black, Asian, minority ethnicity) groups, we must wonder if our results were due to the non-Caucasian women (more significantly abiding in deprived areas) not being offered screening in the first instance. While ethnic inequalities can be due to socio-economic factors with possible language barriers, these disparities persist despite considering these variables. Given this, we are inclined to consider unintentional racial bias, which can occur even though most healthcare professionals neither hold nor support racial stereotypes.36,37 Currently national guidance does not consider being BAME as a risk factor for pregnancy. This has led to the proposal of various strategies to tackle ethnic health disparities including induction of labour at term for all ethnic minority women. 38 Further analysis of data is required to determine the true cause for lack of screening in the most deprived group while looking into reasons behind higher screening in the affluent group, even if women did not disclose risk factors for GDM. This finding of over-screening needs to be explored further for gender bias, as the least deprived group also had a higher proportion of Caucasian women.
Other limitations of our study include the analysis of specific data that constitutes IMD, particularly education and job status, to evaluate if these disparities in particular are associated with reduced screening uptake. While the scope of the study was to align SES with the incidence of GDM, an extension could involve neonatal intensive care admission, neonatal hypoglycaemia, shoulder dystocia, other birth trauma, Apgar scores, perinatal depression, neonatal infection, and late stillbirth. These are all potential outcomes of GDM women that could be explored.
Conclusion
This study, while being the first of its kind exploring GDM incidence and socioeconomic deprivation in a London suburb population, with very limited data on this risk association in the United Kingdom overall, highlights that our previous theories that GDM and T2DM are closely related may be misconceived. While we did not find that deprivation is associated with GDM diagnosis in pregnancy, despite the low SES populations having a greater number of women with risk factors for GDM, our findings did expose the urgent need to develop strategies to improve screening attendance aimed at deprived areas, to increase individual participation and tackle health-inequalities in the population. Healthcare providers should be offered training to enhance their knowledge of ethnic diversity, to help improve service experience for those less likely to comply with health improvement suggestions. Exploring misconceptions and addressing these early in the pregnancy, through outreach programmes in these high-risk communities, may help engage these women, thus improving health outcomes. There is also a role for primary-care sectors to implement community-based strategies aimed at lifestyle improvement and prevention of GDM. Possible future GP incentive schemes are likely to improve GDM screening uptake, like the cervical screening programme, which is conducted in primary care and therefore more accessible. Our study was limited as we did not explore the reasons behind lower GDM screening in women most at risk. Further research is needed to pinpoint the exact reasons behind why those from less affluent areas are less likely to undergo GDM screening.
Acknowledgements
We would like to thank Hameed for her assistance and guidance in this research.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval was not required for this research study. All data analysed was anonymised and retrospective. Data was drawn from our electronic records using dates of pregnancy booking, and no personal information was used.
Contributorship: Sai Gnanasambanthan researched literature and conceived the study, with the help of Salma Jabak. Nawal Dayoub together with all the authors were involved in data analysis. Sai Gnanasambanthan wrote the first submission, and revision of the manuscript. All authors reviewed the manuscript and approved the final version.
ORCID iD: Salma Jabak https://orcid.org/0000-0002-1868-9737
References
- 1.Number of people with diabetes reaches 4.8 million (2019) Diabetes UK, https://www.diabetes.org.uk/about_us/news/diabetes-prevalence-2019https://www.diabetes.org.uk/about_us/news/diabetes-prevalence-2019 (accessed 20 June 2022).
- 2.Banks LM, Kuper H, Polack S. Poverty and disability in low-and middle-income countries: a systematic review. PloS One 2017; 12: e0189996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young-Hyman D, De Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016; 39: 2126–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gestational Diabetes (2019) Diabetes.co.uk- the global diabetes community, http://www.diabetes.co.uk/gestational-diabetes.html (accessed 20 June 2022).
- 5.Carolan M, Gill GK, Steele C. Women's experiences of factors that facilitate or inhibit gestational diabetes self-management. BMC Pregnancy Childbirth 2012 Sep 18; 12: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 2012; 8: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin KE, Grivell RM, Yelland LNet al. et al. The influence of maternal BMI and gestational diabetes on pregnancy outcome. Diabetes Res Clin Pract 2015; 108: 508–513, https://www.sciencedirect.com/science/article/abs/pii/S0168822715000388 . [DOI] [PubMed] [Google Scholar]
- 8.Terence T, Lao L-FH, Chan BCPet al. et al. Maternal age and prevalence of gestational diabetes mellitus. Diabetes Care 2006; 29: 948–949. [DOI] [PubMed] [Google Scholar]
- 9.Dornhorst A, Paterson CM, Nicholls JS, et al. High prevalence of gestational diabetes in women from ethnic minority groups. Diabetic Med 1992; 9: 820–825. [DOI] [PubMed] [Google Scholar]
- 10.National Institute of Health and Care Excellence (NICE) (2015) Diabetes in pregnancy: management from preconception to the postnatal period. NG3. [PubMed]
- 11.Jardine J, Walker K, Gurol-Urganci I, et al. Adverse pregnancy outcomes attributable to socioeconomic and ethnic inequalities in England: a national cohort study. Lancet 2021; 398: 1905–1912. [DOI] [PubMed] [Google Scholar]
- 12.Ethnic and socio-economic inequalities in NHS maternity and perinatal care for women and their babies (2018) National perinatal and maternity audit, https://Maternityaudit.org.uk/filesuploaded/RCOG_Inequalities%20Report_Lay_Summary.pdf (accessed 20 June 2022).
- 13.Gonzalez JS, Tanenbaum ML, Commissariat PV. Psychosocial factors in medication adherence and diabetes self-management: implications for research and practice. Am Psychol 2016; 71: 539–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health 2010; 100: S186–S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marmot M, Wilkinson RG. Ourselves and others–for better or worse: social vulnerability and inequality. Soc Determ Health 2006; 2: 341–357. [Google Scholar]
- 16.English indices of deprivation (2015) Ministry of Housing, communities and local government, https://imd-by-postcode.opendatacommunities.org/imd/2015 (accessed 5 April 2019).
- 17.Villar J, Cheikh Ismail L, Victora CG, et al. International Fetal and Newborn Growth Consortium for the 21st century (INTERGROWTH-21st). International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014; 384: 857–868. [DOI] [PubMed] [Google Scholar]
- 18.Cox ED, Fritz KA, Hansen KW, et al. Development and validation of PRISM: a survey tool to identify diabetes self-management barriers. Diabetes Res Clin Pract 2014; 104: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nau C, Schwartz BS, Bandeen-Roche K, et al. Community socioeconomic deprivation and obesity trajectories in children using electronic health records. Obesity 2015; 23: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block JP, Scribner RA, DeSalvo KB. Fast food, race/ethnicity, and income: a geographic analysis. Am J Prev Med 2004; 27: 211–217. [DOI] [PubMed] [Google Scholar]
- 21.Gordon-Larsen P, Nelson MC, Page Pet al. et al. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics 2006; 117: 417–424. [DOI] [PubMed] [Google Scholar]
- 22.American Dietetic Association. Position of the American Dietetic Association: individual-, family-, school-, and community-based interventions for pediatric overweight. J Am Diet Assoc 2006; 106: 925–945. [DOI] [PubMed] [Google Scholar]
- 23.Lili Y, Wong VW, Simmons D. Ethnic disparities in gestational diabetes. Curr Diab Rep 2018; 18: 1–12. [DOI] [PubMed] [Google Scholar]
- 24.GOV.UK (2020). People living in deprived neighbourhoods. [online] www.ethnicity-facts-figures.service.gov.uk, https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/demographics/people-living-in-deprived-neighbourhoods/latest#data-sources.
- 25.Clinical Report (2017) National maternal and perinatal audit, https://maternityaudit.org.uk. (Accessed on 21 June 2020).
- 26.Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007; 56: 1010–1013. [DOI] [PubMed] [Google Scholar]
- 27.Joshy G, Porter T, Le Lievre C, et al. Prevalence of diabetes in New Zealand general practice: the influence of ethnicity and social deprivation. J Epidemiol Community Health 2009; 63: 386–390. [DOI] [PubMed] [Google Scholar]
- 28.Thara R, Padmavati R, Aynkran JRet al. et al. Community mental health in India: A rethink. Int J Ment Health Syst 2008; 2: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haghdoost AA, Baneshi MR, Razzaghi Aet al. et al. The impact of socio economic factors on the adherence of patients with gestational diabetes mellitus to medical recommendations. Iran J Public Health 2019; 48: 1690–1696. [PMC free article] [PubMed] [Google Scholar]
- 30.Eborall J. Influences on the uptake of diabetes screening: a qualitative study in primary care. Br J Gen Pract 2012; 62: 629–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lal N, Singh HK, Majeed Aet al. et al. The impact of socioeconomic deprivation on the uptake of colorectal cancer screening in London. J Med Screen 2021; 28: 114–121. [DOI] [PubMed] [Google Scholar]
- 32.Smith D, Thomson K, Bambra Cet al. et al. The breast cancer paradox: a systematic review of the association between area-level deprivation and breast cancer screening uptake in Europe. Cancer Epidemiol 2019; 60: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper E, Gates S, Grollman C, et al. Transport, health, and wellbeing: An evidence review for the Department for Transport Prepared for: The Department for Transport. [online], https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/847884/Transport__health_and_wellbeing.pdf (2019).
- 34.Womersley K, Ripullone K, Hirst JE. Tackling inequality in maternal health: beyond the postpartum. Fut Healthcare J 2021; 8: 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gestational diabetes prevention and control (2012-2015) World Diabetes Foundation, https://www.diabetes.org.uk/about_us/news/diabetes-prevalence-2019https://www.worlddiabetesfoundation.org/projects/india-wdf12-678 (accessed 20 June 2022).
- 36.Romero R. Introduction to the guest editors of the American Journal of Obstetrics & Gynecology supplement on preeclampsia 2022. J Obstet Gynaecol 2022; 226: S781–S785. [DOI] [PubMed] [Google Scholar]
- 37.Inquiry into racial injustice in maternity care (2022) Birthrights- protecting human rights in childbirth, https://www.birthrights.org.uk/wp-content/uploads/2022/05/Birthrights-inquiry-systemic-racism_exec-summary_May-22-web.pdf (accessed 20 June 2022).
- 38.National Institute of Health and Care Excellence (NICE) (2021) Inducing Labour. NG207. [PubMed]