Reason for posting: NSAIDs are useful in the treatment of several musculoskeletal conditions1 and primary dysmenorrhea,2 but they are associated with significant gastrointestinal events.1 Selective cyclooxygenase-2 (COX-2) inhibitors such as rofecoxib (Vioxx) and celecoxib (Celebrex) were developed and promoted as safer alternatives to traditional NSAIDs. This claim was tested in the VIGOR (rofecoxib)3 and CLASS (celecoxib)4 trials, but concerns have been raised about the gastrointestinal and cardiovascular effects of these agents.5,6 Unpublished safety data from the VIGOR and CLASS trials obtained from regulatory agencies such as the US Food and Drug Administraiton (see page 1649)7 and found in recent communications from the manufacturers2,8 may be useful to physicians wishing to prescribe these drugs appropriately.
The CLASS (Celecoxib Long-term Arthritis Safety Study) study was a randomized controlled trial designed primarily to compare the gastrointestinal safety of celecoxib (400 mg twice daily, a dose 4 times that recommended for osteoarthritis and twice that for rheumatoid arthritis) with 2 other NSAIDs: diclofenac (75 mg twice daily; 12-month trial, median exposure 9 months) and ibuprofen (800 mg 3 times daily; 15-month trial, median exposure 6 months).8,9 Patients with cardiovascular risk factors could enter the trial, and patients were permitted to take ASA (< 325 mg/d). A total of 8059 patients (with both osteoarthritis and rheumatoid arthritis) were randomly assigned to receive either celecoxib (n = 3987, mean age 60.6 years) or either one of the NSAIDs (n = 3981, mean age 59.8); 22% of the patients were taking ASA. The 3 treatment groups did not differ significantly in terms of the incidence of complicated ulcers.8 However, when the annualized incidence of complicated and symptomatic ulcers was combined, the rate in the celecoxib group (1.2%) was similar to the rate in the diclofenac group (1.3%), but it was significantly lower than the rate in the ibuprofen group (1.9%).7 Patients taking low-dose ASA in the celecoxib group had a combined rate of complicated and symptomatic ulcers 4 times that of patients not taking ASA.8 The annualized incidence of myocardial infarction (fatal and nonfatal) among all patients (including those taking ASA) was 0.5% in the celecoxib group (0.2% among patients not taking ASA), 0.2% in the diclofenac group (0.1% among those not taking ASA) and 0.5% in the ibuprofen group (0.1% among those not taking ASA).8 The rates of hypertension and edema were significantly higher in the ibuprofen group than in the celecoxib and diclofenac groups; the rates of heart failure were similar in the 3 groups.8
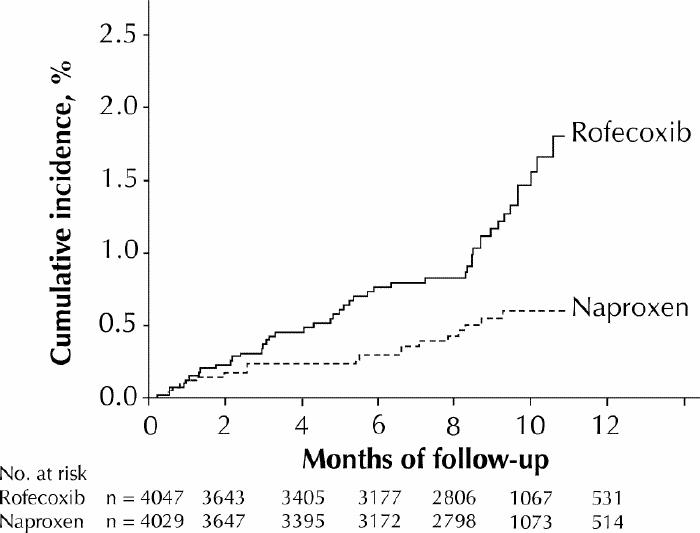
The VIGOR (Vioxx Gastrointestinal Outcomes Research) study was a randomized controlled trial designed primarily to compare the gastrointestinal safety of rofecoxib (50 mg once daily, a dose twice that recommended for long-term use in osteoarthritis) with that of naproxen (500 mg twice daily) in patients with rheumatoid arthritis.3 Patients were not permitted to take ASA or any other antiplatelet drug. The main outcomes studied were confirmed upper gastrointestinal tract events (gastroduodenal ulcers, perforations or obstructions, or bleeding); data on other adverse events, including cardiovascular outcomes, were also collected. A total of 8076 patients were randomly assigned to receive either rofecoxib (n = 4047, mean age 58) or naproxen (n = 4029, mean age 58) and were followed up for a median of 9 months. The rofecoxib group had a lower rate of gastrointestinal events than the naproxen group did (2.1 v. 4.5 events per 100 patient-years of treatment).3 However, the published annualized incidence of myocardial infarction in the rofecoxib group was 4 times that in the naproxen group (0.4 v. 0.1 %).3 The incidence of all thrombotic cardiovascular adverse events (demonstrated as a time-to-event plot in Fig. 1) was significantly higher in the rofecoxib group than in the naproxen group (1.7% v. 0.7%).2,8 The 2 groups had similar rates of death from cardiovascular causes.2 The incidence of hypertension was higher in the rofecoxib group than in the naproxen group (8.5% v. 4%).2
In the wake of cardiovascular concerns arising from the VIGOR trial, a separate study6 was conducted to compare the overall rate of cardiovascular events in COX-2 inhibitor trials (including the VIGOR and CLASS studies and 2 smaller studies) with the rate observed in a large placebo group of a meta-analysis. Although the annualized rate of myocardial infarction appeared to be higher in the COX-2 inhibitor group, baseline differences between the groups in terms of age and disease state make the methodology of this study suspect.10
Was the relative difference in myocardial infarction incidence seen in the VIGOR trial due to a harmful effect of the rofecoxib or a beneficial effect of the comparison drug naproxen? The results of an 11-year observational study involving 181 441 patients suggest that the use of non-ASA-containing NSAIDs, including naproxen, is not cardioprotective.11 However, an analysis of all thrombotic events by the manufacturer of rofecoxib from 23 clinical trials involving over 28 000 patients showed an increased risk of thrombotic events among patients taking rofecoxib when compared with those given naproxen (relative risk [RR] 1.69, 95% confidence interval [CI] 1.07–2.69) but no excess when compared with patients given placebo (RR 0.84, 95% CI 0.51–1.38) or non-naproxen NSAIDS (RR 0.79, 95% CI 0.40–1.55).12 In addition, 3 recent large case–control studies published simultaneously from the United States, Canada and the United Kingdom indicated that the rates of myocardial infarction among patients taking naproxen may be lower than the rates among patients not taking NSAIDs13,14 and those taking other NSAIDs.15 Thus, a previously unrecognized cardioprotective effect of naproxen may account, at least in part, for some of the discrepancies in cardiovascular thrombotic events observed in the VIGOR trial.
The number of reports in Canada of cardiovascular adverse events involving celecoxib, rofecoxib and a third drug, meloxicam (Mobicox), was recently reported.5 The drugs have never been compared head to head in a clinical trial.
What to do: Treatment of musculoskeletal pain should focus on the underlying cause, and in many cases the use of any anti-inflammatory drug is inappropriate. For non-inflammatory musculoskeletal pain, acetaminophen remains the drug of choice,1 and nonpharmacologic treatments including strengthening and stretching exercises, ice or heat are often underused. If COX-2 inhibitors are indicated, patients should be informed of the risks and benefits specific to each drug.
Celecoxib, in high doses and used chronically, does not appear to be better than nonselective NSAIDs at preventing complicated ulcers. However, if all ulcers are considered, it appears to be better than some NSAIDs (ibuprofen) and as effective as others (diclofenac). Celecoxib is not a substitute for ASA, and when used in combination with ASA the risk of ulcer is increased 4-fold. Patients taking such a combination should be appropriately warned and monitored.
Rofecoxib appears to afford some benefit in terms of preventing ulcers compared with naproxen;3 however, caution should be exercised when prescribing it to patients with a history of ischemic heart disease, especially at the high doses used in the VIGOR trial. The cardiovascular safety of rofecoxib will ultimately need to be better assessed in long-term clinical trials designed specifically with cardiovascular end points in mind, appropriate control groups, normal doses of the drug and concomitant administration of low-dose ASA. Rofecoxib is not a substitute for ASA and has not been evaluated in combination with ASA. If the drugs are used concomitantly, patients should be warned of the possible increased risk of gastrointestinal ulcers.
The product monographs of both celecoxib and rofecoxib are being updated to state that, as with nonselective NSAIDs, they are contraindicated in patients with active peptic ulcer disease, active gastrointestinal bleeding, active inflammatory bowel disease or liver disease. Both drugs can cause hypertension and fluid retention in rates similar to those of other NSAIDs and are contraindicated in people with severe renal impairment (creatinine clearance < 0.5 mL/s [< 30 mL/min]). People with lesser degrees of renal failure or a history of heart failure, hypertension or edema from any cause should be monitored carefully when prescribed these drugs. If the drugs are indicated, patients over the age of 65 and with a low body weight (< 50 kg) should be started on the lowest recommended dose (see the complete product monographs in the Compendium of Pharmaceuticals and Specialties for prescribing details) and monitored carefully for adverse effects.
The safety of rofecoxib or celecoxib used intermittently and in combination with cytoprotectants such as misoprostol, H2-receptor antagonists or proton pump inhibitors is unknown, but such use seems logical while we await more comprehensive safety data.
Eric Wooltorton Editorial Fellow, CMAJ
References
- 1.Huang SHK. Rheumatology: 7. Basics of therapy. CMAJ 2000;163(4):417-23. [PMC free article] [PubMed]
- 2.Important drug safety information — Vioxx [Dear Healthcare Professional Letter]. Pointe-Claire– Dorval (QC): Merck Frosst Canada; 2002 Apr 15. Available: www.hc-sc.gc.ca/hpb-dgps/therapeut/zfiles/english/advisory/industry/vioxx_e.html (accessed 2002 May 30).
- 3.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 2000;343:1520-8. [DOI] [PubMed]
- 4.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000;284:1247-55. [DOI] [PubMed]
- 5.Vu D, Murty M, McMorran M. Selective COX-2 inhibitors: suspected cardiovascular/cerebrovascular adverse reactions. Can Adverse Drug Reaction Newsl 2002;12(2):1-3. Available: www.hc-sc.gc.ca/hpb-dgps/therapeut/zfiles/english/publicat/adrv12n2_e.html (accessed 2002 May 30).
- 6.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954-9. [DOI] [PubMed]
- 7.McCormack JP, Rangno R. Digging for data from the COX-2 trials [letter]. CMAJ 2002;166 (13):1649-50. [PMC free article] [PubMed]
- 8.Important drug safety information — Celebrex [Dear Healthcare Professional Letter]. Mississauga (ON): Pharmacia Canada Inc; 2002 May 13. Available: www.hc-sc.gc.ca/hpb-dgps/therapeut/zfiles/english/advisory/industry/celebrex_e.html (accessed 2002 May 30).
- 9.COX-2 inhibitors update: Do journal publications tell the full story? Ther Letter 2001–2002;43(Nov-Dec/Jan):1-2. Available: www.ti.ubc.ca/pages/letter43.htm (accessed 2002 May 31).
- 10.Cleland JGF. No reduction in cardiovascular risk with NSAIDs – including aspirin? Lancet 2002;359:92-3. [DOI] [PubMed]
- 11.Ray WA, Stein CM, Hall K, Daugherty JR, Griffin MR. Non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease: an observational cohort study. Lancet 2002;359:118-23. [DOI] [PubMed]
- 12.Konstam MA, Weir MR, Reicin A, Shapiro D, Sperling RS, Barr E, et al. Cardiovascular thrombotic events in controlled clinical trials of rofecoxib. Circulation 2001;104:2280-8. [DOI] [PubMed]
- 13.Watson DJ, Rhodes T, Cai B, Guess HA. Lower risk of thromboembolic cardiovascular events with naproxen among patients with rheumatoid arthritis. Arch Intern Med 2002;162:1105-10. [DOI] [PubMed]
- 14.Solomon DH, Glynn RJ, Levin R, Avorn J. Nonsteroidal anti-inflammatory drug use and acute myocardial infarction. Arch Intern Med 2002; 162:1099-104. [DOI] [PubMed]
- 15.Rahme E, Pilote L, LeLorier J. Association between naproxen use and protection against acute myocardial infarction. Arch Intern Med 2002;162: 1111-5. [DOI] [PubMed]


