Abstract
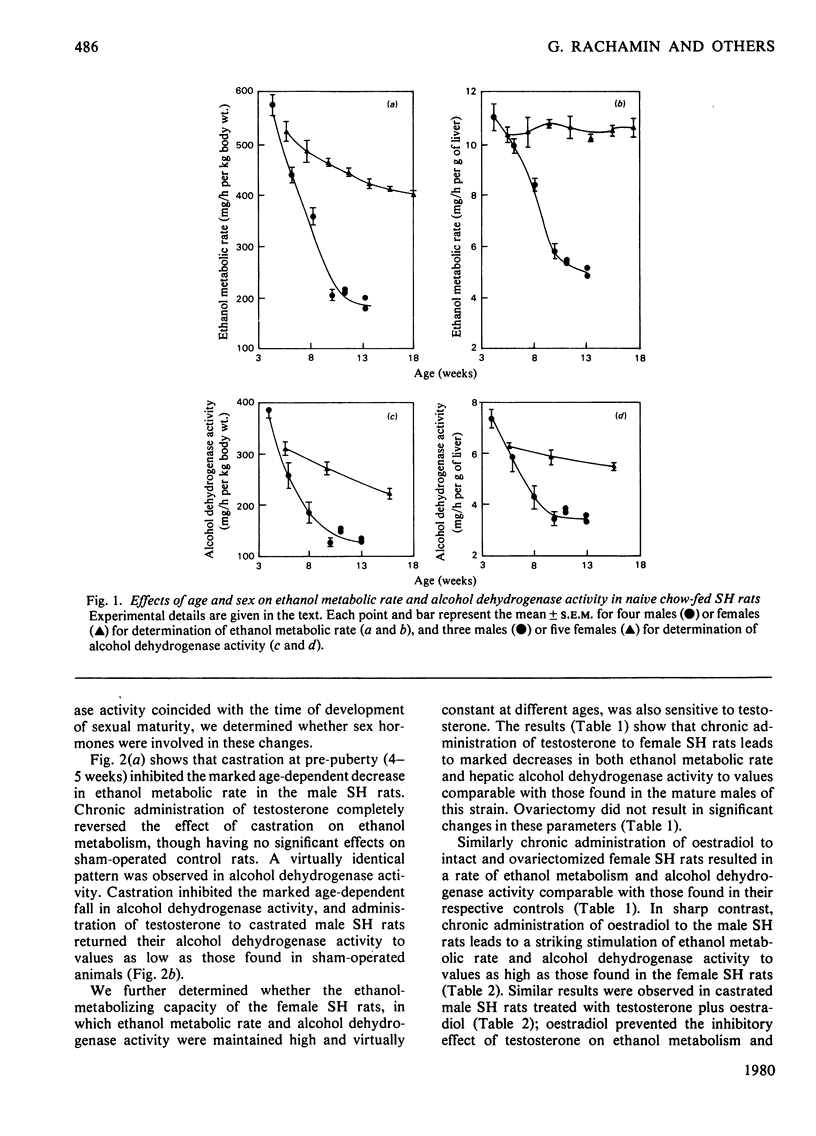
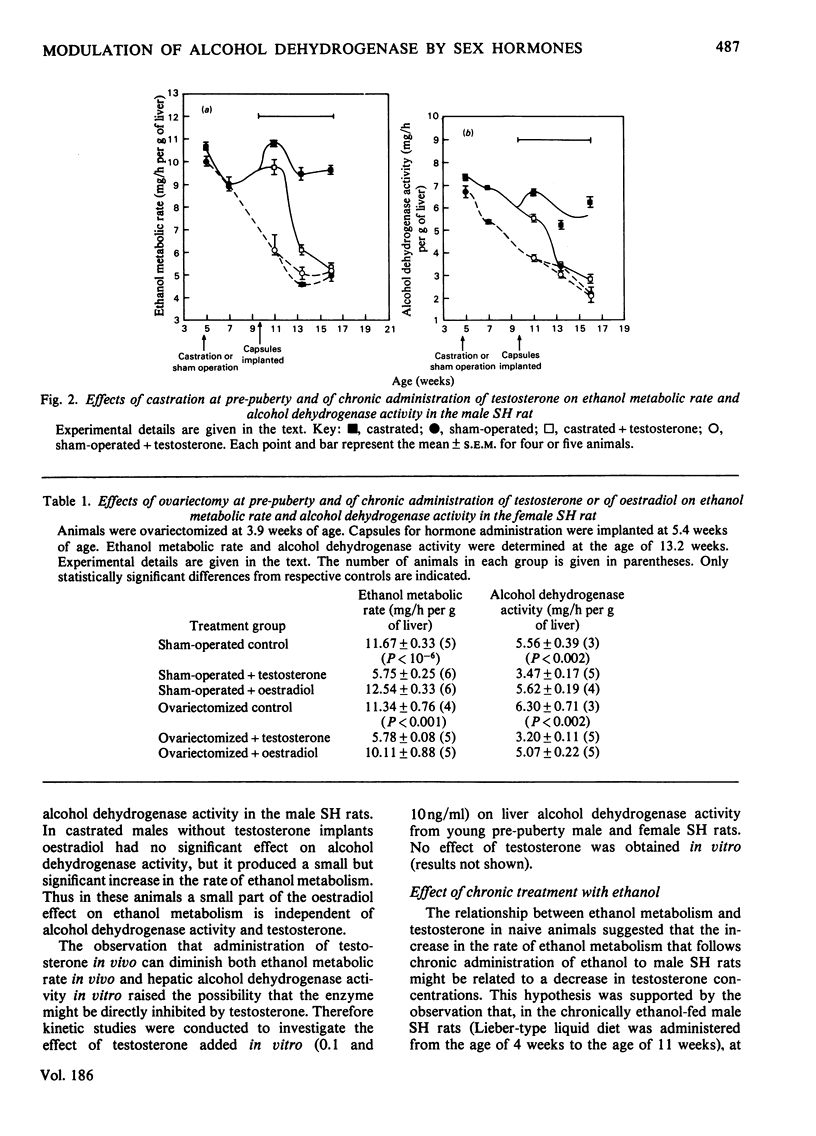
In young (4-week-old) male and female spontaneously hypertensive (SH) rats, ethanol metabolic rate in vivo and hepatic alcohol dehydrogenase activity in vitro are high and not different in the two sexes. In males, ethanol metabolic rate falls markedly between 4 and 10 weeks of age, which coincides with the time of development of sexual maturity in the rat. Alcohol dehydrogenase activity is also markedly diminished in the male SH rat and correlates well with the changes in ethanol metabolism. There is virtually no influence of age on ethanol metabolic rate and alcohol dehydrogenase activity in the female SH rat. Castration of male SH rats prevents the marked decrease in ethanol metabolic rate and alcohol dehydrogenase activity, whereas ovariectomy has no effect on these parameters in female SH rats. Chronic administration of testosterone to castrated male SH rats and to female SH rats decreases ethanol metabolic rate and alcohol dehydrogenase activity to values similar to those found in mature males. Chronic administration of oestradiol-17β to male SH rats results in marked stimulation of ethanol metabolic rate and alcohol dehydrogenase activity to values similar to those found in female SH rats. Chronic administration of ethanol to male SH rats from 4 to 11 weeks of age prevents the marked age-dependent decreases in ethanol metabolic rate and alcohol dehydrogenase activity, but has virtually no effect in castrated rats. In the intoxicated chronically ethanol-fed male SH rats, serum testosterone concentrations are significantly depressed. In vitro, testosterone has no effect on hepatic alcohol dehydrogenase activity of young male and female SH rats. In conclusion, in the male SH rat, ethanol metabolic rate appears to be limited by alcohol dehydrogenase activity and is modulated by testosterone. Testosterone has an inhibitory effect and oestradiol has a testosterone-dependent stimulatory effect on alcohol dehydrogenase activity and ethanol metabolic rate in these animals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baraona E., Leo M. A., Borowsky S. A., Lieber C. S. Pathogenesis of alcohol-induced accumulation of protein in the liver. J Clin Invest. 1977 Sep;60(3):546–554. doi: 10.1172/JCI108806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero T. J., Badger T. M. Effects of alcohol on the hypothalamic-pituitary-gonadal axis in the male rat. J Pharmacol Exp Ther. 1977 May;201(2):427–433. [PubMed] [Google Scholar]
- Collins A. C., Lebsack M. E., Yeager T. N. Mechanisms that underlie sex-linked and genotypically determined differences in the depressant actions of alcohol. Ann N Y Acad Sci. 1976;273:303–316. doi: 10.1111/j.1749-6632.1976.tb52893.x. [DOI] [PubMed] [Google Scholar]
- Collins A. C., Yeager T. N., Lebsack M. E., Panter S. S. Variations in alcohol metabolism: influence of sex and age. Pharmacol Biochem Behav. 1975 Nov-Dec;3(6):973–978. doi: 10.1016/0091-3057(75)90004-0. [DOI] [PubMed] [Google Scholar]
- Crow K. E., Cornell N. W., Veech R. L. The rate of ethanol metabolism in isolated rat hepatocytes. Alcohol Clin Exp Res. 1977 Jan;1(1):43–50. doi: 10.1111/j.1530-0277.1977.tb05765.x. [DOI] [PubMed] [Google Scholar]
- Damassa D. A., Kobashigawa D., Smith E. R., Davidson J. M. Negative feedback control of LH by testosterone: a quantitative study in male rats. Endocrinology. 1976 Sep;99(3):736–742. doi: 10.1210/endo-99-3-736. [DOI] [PubMed] [Google Scholar]
- Eriksson C. J. Ethanol and acetaldehyde metabolism in rat strains genetically selected for their ethanol preference. Biochem Pharmacol. 1973 Sep 15;22(18):2283–2292. doi: 10.1016/0006-2952(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Eriksson K., Malmström K. K. Sex differencds in consumption and elimination of alcohol in albino rats. Ann Med Exp Biol Fenn. 1967;45(4):389–392. [PubMed] [Google Scholar]
- Eriksson K., Pikkarainen P. H. Differences between the sexes in voluntary alcohol consumption and liver ADH-activity in inbred strains of mice. Metabolism. 1968 Nov;17(11):1037–1042. doi: 10.1016/0026-0495(68)90011-5. [DOI] [PubMed] [Google Scholar]
- Gordon G. G., Olivo J., Rafil F., Southren A. L. Conversion of androgens to estrogens in cirrhosis of the liver. J Clin Endocrinol Metab. 1975 Jun;40(6):1018–1026. doi: 10.1210/jcem-40-6-1018. [DOI] [PubMed] [Google Scholar]
- Hollstedt C., Rydberg U. S. Ethanol metabolism in the growing rat. Arch Int Pharmacodyn Ther. 1970 Dec;188(2):341–348. [PubMed] [Google Scholar]
- Israel Y., Khanna J. M., Kalant H., Stewart D. J., Macdonald J. A., Rachamim G., Wahid S., Orrego H. The spontaneously hypertensive rat as a model for studies on metabolic tolerance to ethanol. Alcohol Clin Exp Res. 1977 Jan;1(1):39–42. doi: 10.1111/j.1530-0277.1977.tb05764.x. [DOI] [PubMed] [Google Scholar]
- Israel Y., Videla L., Bernstein J. Liver hypermetabolic state after chronic ethanol consumption: hormonal interrelations and pathogenic implications. Fed Proc. 1975 Oct;34(11):2052–2059. [PubMed] [Google Scholar]
- Khanna J. M., Kalant H., Bustos G. Effects of chronic intake of ethanol on rate of ethanol metabolism. II. Influence of sex and of schedule of ethanol administration. Can J Physiol Pharmacol. 1967 Sep;45(5):777–785. doi: 10.1139/y67-092. [DOI] [PubMed] [Google Scholar]
- Legan S. J., Coon G. A., Karsch F. J. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975 Jan;96(1):50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- Mendelson J. H., Mello N. K. Alcohol, aggression and androgens. Res Publ Assoc Res Nerv Ment Dis. 1974;52:225–247. [PubMed] [Google Scholar]
- OKAMOTO K., AOKI K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963 Mar;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- Odell W. D., Swerdloff R. S., Bain J., Wollesen F., Grover P. K. The effect of sexual maturation on testicular response to LH stimulation of testosterone secretion in the intact rat. Endocrinology. 1974 Nov;95(5):1380–1384. doi: 10.1210/endo-95-5-1380. [DOI] [PubMed] [Google Scholar]
- Ono S., Stenius C., Christian L., Harris C., Ivey C. More about the testosterone induction of kidney alcohol dehydrogenase activity in the mouse. Biochem Genet. 1970 Oct;4(5):565–577. doi: 10.1007/BF00486095. [DOI] [PubMed] [Google Scholar]
- Orrego H., Kalant H., Israel Y., Blake J., Medline A., Rankin J. G., Armstrong A., Kapur B. Effect of short-term therapy with propylthiouracil in patients with alcoholic liver disease. Gastroenterology. 1979 Jan;76(1):105–115. [PubMed] [Google Scholar]
- Plapp B. V. Rate-limiting steps in ethanol metabolism and approaches to changing these rates biochemically. Adv Exp Med Biol. 1975;56:77–109. doi: 10.1007/978-1-4684-7529-6_4. [DOI] [PubMed] [Google Scholar]
- RAEIHAE N. C., KOSKINEN M. S. EFFECT OF NON-IONIC SURFACE-ACTIVE SUBSTANCE ON THE ACTIVATION OF ALCOHOL DEHYDROGENASE OF RAT LIVER HOMOGENATES. Life Sci. 1964 Oct;3:1091–1095. doi: 10.1016/0024-3205(64)90123-7. [DOI] [PubMed] [Google Scholar]
- Reynier M., Theorell H. Studies on the stereospecificity of liver alcohol dehydrogenase (LADH) for 3beta-hydroxy-5beta-steroids. Inhibition effect of pyrazole and of a 3alpha-hydroxycholanoic acid. Acta Chem Scand. 1969;23(4):1130–1136. doi: 10.3891/acta.chem.scand.23-1130. [DOI] [PubMed] [Google Scholar]
- Schlesinger K. Genetic and biochemical correlates of alcohol preference in mice. Am J Psychiatry. 1966 Jan;122(7):767–773. doi: 10.1176/ajp.122.7.767. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., McKenna W. R., McCaffrey T. B. Pathways responsible for the adaptive increase in ethanol utilization following chronic treatment with ethanol: inhibitor studies with the hemoglobin-free perfused rat liver. Mol Pharmacol. 1976 Jan;12(1):156–166. [PubMed] [Google Scholar]
- Van Thiel D. H., Gavaler J. S., Lester R., Goodman M. D. Alcohol-induced testicular atrophy. An experimental model for hypogonadism occurring in chronic alcoholic men. Gastroenterology. 1975 Aug;69(2):326–332. [PubMed] [Google Scholar]
- Van Thiel D. H., Lester R., Sherins R. J. Hypogonadism in alcoholic liver disease: evidence for a double defect. Gastroenterology. 1974 Dec;67(6):1188–1199. [PubMed] [Google Scholar]
- Videla L., Flattery K. V., Sellers E. A., Israel Y. Ethanol metabolism and liver oxidative capacity in cold acclimation. J Pharmacol Exp Ther. 1975 Mar;192(3):575–582. [PubMed] [Google Scholar]
- Videla L., Israel Y. Factors that modify the metabolism of ethanol in rat liver and adaptive changes produced by its chronic administration. Biochem J. 1970 Jun;118(2):275–281. doi: 10.1042/bj1180275. [DOI] [PMC free article] [PubMed] [Google Scholar]


