Abstract
Despite marked advancements in the recognition and diagnosis of spontaneous coronary artery dissection (SCAD) over the past decade, knowledge of the basic pathophysiologic mechanisms of disease, contributing factors, and treatment continue to be poorly understood. We describe significant research gaps in our knowledge of SCAD and introduce strategies including the role of patient advocacy, independent registries, and creation of diverse centers of excellence to bridge the gap in clinical care, research, and outcomes. Lastly, we introduce an innovative patient-centered clinical care and research framework established through the SCAD Alliance and International Spontaneous Coronary Artery Dissection registry as a model for advancing knowledge of SCAD.
Key words: SCAD, health inequity, sex differences
Central Illustration
Highlights
-
•
Despite its increasing recognition, spontaneous coronary artery dissection remains poorly understood.
-
•
Significant knowledge gaps exist in our understanding of pathophysiology, genetics, treatment, and outcomes for spontaneous coronary artery dissection.
-
•
Multicenter collaborations such as the International Spontaneous Coronary Artery Dissection registry and patient inclusivity are critical to the advancement of spontaneous coronary artery dissection.
Women remain underrepresented in cardiovascular research, limiting our understanding of sex-specific differences and contributing to inequities in care.1 This underrepresentation is perpetuated by low perceived risk of disease among patients and clinicians, exclusion of pregnant and childbearing age people from research, and disparities in referrals of women to centers of excellence.1 Spontaneous coronary artery dissection (SCAD) more frequently affects women and is associated with sex and gender-specific factors. However, our understanding of the disease’s genetic underpinnings, sex predisposition, and pathophysiological mechanisms remains poorly understood.2 Previously an underrecognized cause of acute myocardial infarction (MI) among women, the increasing recognition and diagnosis of SCAD has far exceeded the pace of our understanding of the disease.3 This manuscript highlights knowledge gaps in our understanding of SCAD and future directions to tailor research initiatives to advance knowledge.
While the work of several single-center and multicenter registries throughout the world, including large registries in the United States, Canada, the United Kingdom, and Spain, among others, has advanced our current knowledge of SCAD,2,3,4, 5, 6, 7, 8, 9 in this manuscript, we highlight the unique features of the iSCAD (International Spontaneous Coronary Artery Dissection) registry (NCT04496687). As a prospective, multicenter, patient-centered research model established to advance critical understanding of a condition that disproportionately impacts women, iSCAD provides a potential framework for the study of rare cardiovascular diseases that may affect specific subpopulations, particularly those who have been underrepresented in clinical research. This manuscript aims to highlight the unique approach to changing the landscape of cardiovascular research to include more women, and in particular, women of color, as investigators and participants by including sites with diversity in geography and patient population.
The iSCAD Registry was borne out of the efforts of the SCAD Alliance, a nonprofit advocacy organization of SCAD survivors and their families that engaged women’s cardiovascular disease experts in creating an independent data repository to advance the science of SCAD. Our panel of authors represents diverse experts on SCAD from the lens of expertise in vascular medicine (in particular, fibromuscular dysplasia [FMD]), cardiovascular genetics, behavioral health, exercise physiology, cardio-obstetrics, cardiac imaging, and interventional cardiology. Authors are iSCAD investigators who were invited to participate on the basis of clinical expertise (often Directors of Women’s Heart Centers with dedicated SCAD clinics), authorship on publications related to SCAD or arteriopathies, or national reputation as leaders in the field of interventional cardiology, imaging, or sports cardiology. The panel is also comprised of patients and patient advocates who lead SCAD Alliance, which has been instrumental to the creation of registries and patient outreach programs.
Research gaps in current knowledge of SCAD
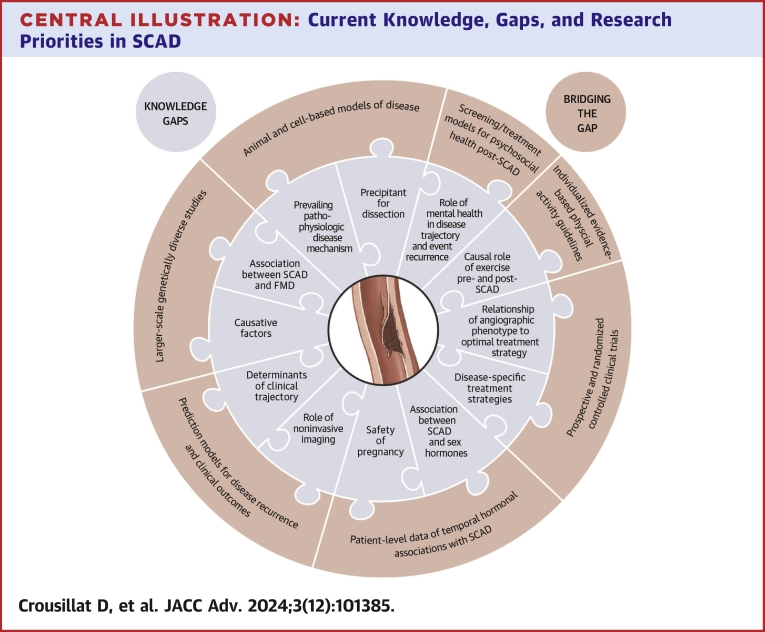
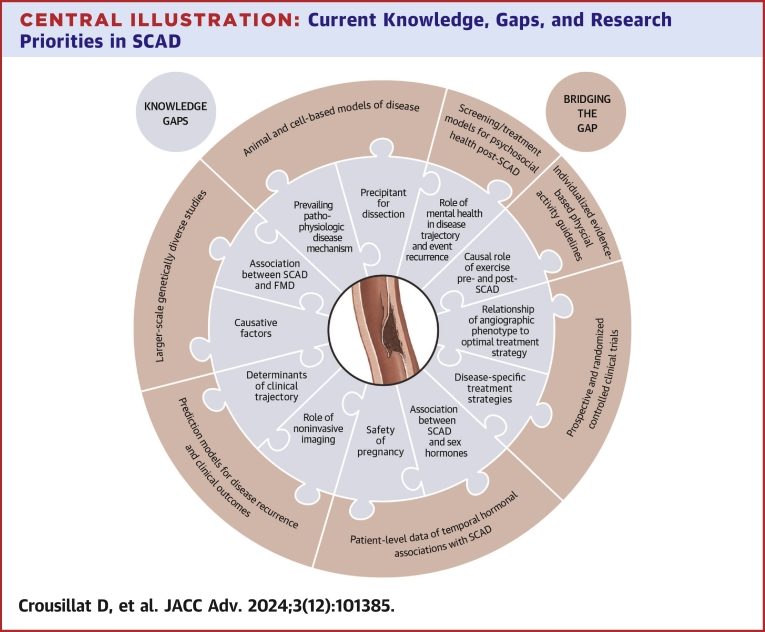
Despite marked advancements in the recognition and diagnosis of SCAD over the past decade, knowledge of the basic pathophysiologic mechanisms of disease, contributing factors, and treatment continue to be poorly understood (Table 1, Table 2). Significant heterogeneity in outcomes following SCAD is observed, but risk-stratification methods and strategies to reduce recurrence are lacking (Central Illustration).
Table 1.
Current Knowledge, Gaps, and Research Priorities in SCAD
| Domain | Current Knowledge | Knowledge Gaps | Research Priorities |
|---|---|---|---|
| Pathophysiology and disease mechanisms | Female, various genetic associations and different triggering events are now linked to SCAD Imaging studies have shown that a primary intramural hematoma is likely the predominant mechanism in SCAD |
We do not yet understand how these predisposing factors lead to SCAD and what exactly happens at the levels of the arterial wall (eg, why does an intramural hematoma occur?) What is the prevalence and relationship between post-SCAD chest pain and coronary microvascular dysfunction (CMD)? |
Deeper understanding of genetic mechanisms, including how known SCAD gene associations are causal Develop animal and cell-based model systems for SCAD and FMD Continued human imaging and other studies to delineate precise pathobiological mechanisms |
| Fibromuscular dysplasia | A high proportion of patients with SCAD also have FMD, with possible shared genetic mechanisms | How do SCAD and FMD relate, and does FMD alter the clinical trajectory of SCAD? Which FMD patients are at risk for SCAD? |
Further genetic study into the mechanisms relating FMD and SCAD |
| Medical therapy | Beta-blockers associated with reduction in recurrent SCAD7 DAPT is associated with increased MACE among conservatively managed SCAD37 |
Efficacy and optimal duration of SAPT vs DAPT for conservatively managed SCAD Beta-blockers use and optimal duration post-SCAD Novel, disease-specific therapies are lacking |
Randomized controlled trials of differing medical therapy strategies to assess short- and long-term clinical outcomes Further basic and translational research into pathophysiologic mechanisms of disease to enable development of disease-specific therapeutics |
| Angiography for diagnosis | Yip Saw SCAD Angiographic Classification29 Isolated intramural hematoma may have higher risk of progression4 |
Optimal diagnostic technique (invasive vs noninvasive) for initial diagnosis vs follow-up | Optimal invasive strategies for management of unstable patients with high-risk anatomy |
| Percutaneous coronary intervention | High rates of SCAD PCI complications32 | Optimal intervention techniques | Comparative evaluation of suggested techniques to limit PCI complications Improved risk-prediction models to identify patients at high risk for failure of medical therapy |
| Coronary artery bypass surgery | Higher than expected early short-term mortality and poor graft patency long-term30 | Optimal bypass techniques; indications for bypass | Comparative evaluation of PCI vs CABG for certain lesions considered high risk (LMCA) |
| Psychosocial Health | Acute emotional stress is a commonly identified SCAD trigger, particularly among women43 SCAD events are potentially traumatic experiences that can lead to depression, anxiety, and PTSD symptoms44,45 |
Mechanisms linking experiences of acute emotional stress with SCAD onset are unknown. Whether and how pre-SCAD mental health is relevant to SCAD onset is unclear. How depression, anxiety, and PTSD symptoms manifest over time after SCAD and whether they predict course of illness and event recurrence is unknown. Psychosocial treatments to address mental health outcomes IN SCAD ARE lacking. |
Longitudinal studies of mental health outcomes after SCAD and how they relate to course of illness Evaluating psychosocial interventions specifically for addressing mental health in patients with SCAD Developing models for screening and treating SCAD-related mental health consequences in specialized cardiology care clinics |
| Reproductive health considerations | ∼40% of pregnancy-associated MI are due to SCAD8 Pregnancy-associated SCAD is associated with higher-risk presentations as compared with nonpregnancy related SCAD (left main or multivessel coronary involvement, greater risk of left ventricular systolic dysfunction, cardiogenic shock) |
Clearer understanding of pathophysiology of SCAD, to include role of estrogen, progesterone, and prolactin in the pathogenesis of SCAD A clearer understanding of relative contribution of predisposing factors, including hypertensive disorders of pregnancy, as they relate to better risk personalization for patients desiring pregnancy after SCAD |
Patient level data of dose/temporal exposure of hormonal exposure as it relates to SCAD presentation including changes throughout the reproductive lifespan (eg, onset of premature menopause, diagnosis of cervical incompetence, uterine ablation, tubal ligation, and/or caesarean section) and impact of exogenous hormone use (including contraception, hormone replacement therapy, and hormone-based breast cancer therapy). Further risk stratification of patients desiring future pregnancy |
| Exercise | Repetitive Valsalva can increase intravascular pressure with theoretical increased risk for vascular dissection | How and to what degree is high-intensity exercise and/or repetitive Valsalva causal in the pathogenesis of SCAD. | Evidence-based guidance to give individual-level exercise recommendations to patients following SCAD to avoid overly restrictive recommendations that may have downstream negative physical and mental health consequences |
CABG = coronary artery bypass grafting; DAPT = dual antiplatelet therapy; FMD = fibromuscular dysplasia; LMCA = left main coronary artery; PCI = percutaneous coronary intervention; SCAD = spontaneous coronary artery dissection.
Table 2.
Summary of Published Research in SCAD
| First Author, Year | Study Design | Outcome | Findings |
|---|---|---|---|
| Saw et al, 20177 | Prospective single-center (Vancouver General Hospital) longitudinal cohort study | 327 patients Assess clinical predictors of recurrent SCAD |
Multivariate analysis demonstrated reduction in recurrent SCAD with use of beta blockers (HR: 0.36, P = 0.004) |
| Edwards et al, 201961 | Cross-sectional pilot study | 14 female patients Assessed psychosocial symptoms |
93% of patients reported symptoms of stress, 57% insomnia, 71% anxiety, 36% depression, and 43% PTSD; majority linked these symptoms to SCAD |
| Johnson et al, 202045 | Cross-sectional study of patients in the Mayo Clinic “Virtual” Multicenter SCAD Registry | 512 patients Assessed psychosocial symptoms |
28% of patients reported at least mild symptoms of PTSD, 41% at least mild symptoms of anxiety, and 32% at least mild symptoms of depression after SCAD |
| Liang et al, 201462 | Cross-sectional study | 158 patients Assessed psychosocial symptoms and related treatment |
33%-37% of patients reported receiving medication or counseling for depression or anxiety post-SCAD Patients with (vs without) peripartum SCAD had higher average depression and anxiety scores |
| Murphy et al, 202463 | Cross-sectional study of patients in the Victor Change Cardiac Research Institute genetics study | 310 patients Assessed psychosocial symptoms and lifestyle impacts of SCAD |
21% of patients reported current anxiety and depression 60% of patients indicated at least 1 SCAD-related impact on work, and 45% reported stopping doing a favorite sport or exercise |
| Sumner et al, 202444 | Cross-sectional study of patients in the iSCAD Registry | 859 patients Assessed SCAD-induced PTSD symptoms, related treatment seeking, and associations with functioning |
Nearly 35% of patients reported symptoms consistent with lifetime probable PTSD; 6.4% met criteria for past-month probable PTSD 34.8% of patients reporting any symptoms of SCAD-induced PTSD sought treatment, yet nearly half of patients with probable PTSD never received treatment for this distress Greater past-month PTSD symptoms were associated with worse past-week sleep disturbance and disease-specific health status |
| Saw et al, 201429 | Review paper | Description of SCAD angiographic classification | |
| Tweet et al, 201430 | Retrospective single-center cohort of patients with first SCAD | 189 patients Assess outcomes of management strategies (conservative, PCI) |
PCI for SCAD has high risk of failure; initial conservative therapy may be preferable CABG for SCAD carries higher early risk and poor graft patency Revascularization for SCAD does not protect against target vessel revascularization or repeat SCAD. |
| Waterbury et al, 20184 | Retrospective registry study of patients with SCAD managed with an initial conservative strategy | 240 patients Evaluation of predictors of SCAD progression |
Isolated IMH may predict progression of SCAD after initial conservative therapy |
| Wells et al, 202458 | Cross-sectional study of patients in the iSCAD Registry | 773 patients Evaluation of migraine-related disability using MIDAS (Migraine Disability Assessment) |
46% current or previous migraines, more common in women and FMD 14.4% mild, 12.7% moderate, and 12.7% severe disability |
CABG = coronary artery bypass grafting; FMD = fibromuscular dysplasia; IMH = intramural hematoma; iSCAD = International Spontaneous Coronary Artery Dissection; PCI = percutaneous coronary intervention; PTSD = post-traumatic stress disorder; SCAD = spontaneous coronary artery dissection.
Central Illustration.
Current Knowledge, Gaps, and Research Priorities in SCAD
Significant knowledge gaps in our understanding of spontaneous coronary artery dissection (SCAD) and suggested future research initiatives to bridge the gaps.
Pathophysiology and disease mechanisms
The primary mechanism of SCAD remains unresolved, a fundamental knowledge gap central to uncertainty surrounding SCAD treatment and prevention. The prevailing hypotheses for the inciting pathophysiological mechanism in SCAD are the “outside-in” model, which involves a primary intramural bleeding event leading to an intramural hematoma (IMH). It is hypothesized that this is due to primary rupture of the vasa vasorum, but there is no direct evidence to support this theory. A proportion of these primary IMH may rupture into the arterial lumen, creating an intimal breech or tear.4 Intracoronary imaging studies support this model as there is no identifiable breech in the arterial wall (ie, no intimal flap or tear) in approximately two-thirds of SCAD cases, suggesting that the inciting event arose within the vessel wall.10 Alternatively, the competing “inside-out” model proposes a primary tear of the arterial intima. While limited data suggest the outside-in mechanism is predominant, the totality of evidence suggests that both mechanisms can occur, in which case SCAD may represent 2 similar but distinct diseases with somewhat different causal factors and triggers.
It is widely assumed that sex hormones are causal in SCAD, but it is not known if the female predisposition to SCAD is secondary to differences in hormones or other sex differences including nonhormonal factors, psychological stress, and comorbid conditions including FMD. Recent studies have demonstrated intrinsic differences in the arterial wall between males and females, and it is possible that sex-based differences in the arterial architecture may be causal for SCAD.11 Unlike for large vessel arteriopathies, there are no published animal models for SCAD or FMD, further limiting our ability to understand the pathophysiological underpinnings of this disease. However, in vitro cell model systems are currently being used to study key genes implicated in SCAD. For example, Liu et al12 created smooth muscle cells with knockout of the gene LRP1 (low-density lipoprotein receptor-related protein 1), a gene that is implicated in SCAD, FMD, and sporadic thoracic aortic dissection. By studying these cells in vitro, they were able to conclude that decreased LRP1 expression in smooth muscle cells is involved in the remodeling of extracellular matrix via the transforming growth factor (TGF)-β pathway as a potential mechanism for the involvement of LRP1 in these vascular disorders.12 An important caveat is that while these in vitro systems are already providing important insights, they cannot recapitulate the complex in vivo milieu involved in SCAD and other vascular disorders. Future basic and translational research should aim to understand the primary precipitant of intramural bleeding and/or dissection, disease mechanisms and potential variations based on causal factors, and the physiological and hormonal milieu associated with arterial wall vulnerability and dissection.
Fibromuscular dysplasia/arteriopathy
Individuals with SCAD have several clinical, phenotypic, and genetic similarities to those with FMD, including typical age of onset, female predominance, and frequent presence of extra-coronary vascular abnormalities including aneurysms, dissections, pseudoaneurysms, and vessel tortuosity.13 FMD and SCAD share many histopathologic processes that primarily involve the vascular smooth muscle cells and extracellular matrix.14, 15, 16, 17 While extra coronary aneurysms and dissections may warrant repair, it is not clear beyond this whether and how concomitant FMD alters the clinical trajectory of SCAD patients.13
FMD is reported among a high proportion of SCAD patients (50%). Interestingly, however, among patients enrolled in the United States FMD registry, SCAD is present in only 2.7% of patients, though prevalence may be higher with longer follow-up and increased awareness and detection of SCAD.13,18 There are insufficient data to help predict which patients with FMD may ultimately suffer SCAD, although it is possible that tools such as polygenic risk scores may help risk stratify patients in the future.16,19
Genetics
Evidence suggests a mild familial predisposition to SCAD, with a higher incidence of the condition and other associated arteriopathies observed among first-degree relatives of affected individuals. However, familial clustering is rare (∼1% of cases), and large pedigrees with multigeneration inheritance of SCAD have not been described, implying that SCAD risk is largely polygenic.20 Patients with a family history of aneurysm or dissection, thoracic aortic aneurysm (TAA), and extra coronary dissections on imaging should be considered for genetic evaluation, which typically consists of a gene panel for associated TAA as no genetic panel is commercially available for SCAD.
Although no SCAD-specific genetic testing exists, SCAD has been associated with multiple Mendelian disorders, most frequently in the context of collage 3 deficiency (COL3A1). Cases of SCAD have been described in patients with Marfan syndrome (FBN1) and Loeys-Dietz syndrome (TGFBR1, TGFBR2, SMAD2, SMAD3, TGFB2, and TGFB3), usually with advanced vascular disease.21,22 Rare pathogenic variants in the TAA-associated genes, LOX and FLNA, and 23monogenic polycystic kidney (PKD1) disease have also been associated with SCAD.21
Research studies to identify novel rare variants using whole genome sequencing identified pathogenic or likely pathogenic mutations in 7 genes, including PKD1, COL3A1, SMAD3, TGFB2, LOX, MYLK, and YY1AP1, although no gene reached genome-wide significance, illustrating the significant genetic heterogeneity of the disorder.23 Using exome sequencing, enrichment of rare variants in fibrillar collagen genes including COL3A1, COL4A1, and COL5A1, were discovered in SCAD cases vs MI-free controls.24 Additionally, both TSR1 and TLN1 have been associated with SCAD, although the clinical implications of these genes are unclear.25,26
Genetic research into SCAD is in its infancy; however, genotyping of patients without monogenic disease has revealed interesting and meaningful associations. An initial report localized a single nucleotide polymorphism near the PHACTR1 locus associated with SCAD.14 This association has been replicated with additional identification of loci near LRP1, ADAMTSL4, and on chromosome 21q22.11 near LINC00310.16,17 These studies highlighted the primacy of genetic loci related to extracoronary vascular function, consistent with the clinical association with FMD. Interestingly, a polygenic risk score constructed from these loci was significantly related to atherosclerotic coronary artery disease in the UK Biobank, but the risk was in the opposite direction from SCAD risk.16 One locus, AFAP1, has been implicated to specifically be associated with pregnancy-associated SCAD (PSCAD), although it has also been previously identified in aortic disease.27,28 Most recently, a meta-analysis of 1917 cases of SCAD with 9,292 controls uncovered 16 novel loci associated with the phenotype adjacent to many known extracoronary vascular targets including FBN1, PHACTR1, LRP1, COL4A1/COL4A2, THSD4, and AFAP1.5
The genetic heterogeneity of SCAD and the relative rarity of the condition pose obstacles to large-scale genetic studies. Another challenge relates to the inability to access vascular tissue associated with SCAD, as there is no surgical resection associated with these events. Future research endeavors will focus on both increasing the number of samples for analysis and, importantly, broadening and diversifying the ethnic makeup of collections. Genetic variants associated with SCAD will require interpreting these signals in the light of accessible biologic readouts such as circulating biomarkers, cellular assays, and animal models of arterial dysfunction.
Diagnostic coronary angiography and percutaneous and surgical management
Invasive coronary angiography remains the gold standard for the appropriate identification and diagnosis of suspected SCAD. The Yip-Saw angiographic classification29 is used for the phenotypic recognition of angiographic SCAD patterns with the use of additional intracoronary imaging only in the context of angiographic uncertainty given the increased risk of iatrogenic extension of the IMH and dissection propagation. However, angiographic classification has neither been shown to assist in decision-making nor to be predictive of outcomes.2 The high rates of procedural complications associated with percutaneous coronary interventions (PCIs) in SCAD, such as extension of dissection, technical failure, and long-term risk of stent restenosis and thrombosis, have led to low rates of revascularization among patients with identified SCAD.6,30,31 Additionally, given the high rates of short-term spontaneous angiographic healing and no association between PCI and reduction in the risk of recurrent SCAD, the majority of SCAD lesions are primarily medically managed.30 Limited data exist to guide appropriate risk stratification to predict which patients will demonstrate SCAD progression and/or failure of medical management upfront.2,4,32,33
Surgical management using coronary artery bypass grafting (CABG) has been described in case series, observational studies, and case reports. Data support initial technical success but a high rate of graft failure due to the natural history of SCAD lesion healing. Given that CABG has usually been reserved for unstable patients with left main lesions, PCI complications, or failures, there is neither prospective comparison of PCI to CABG nor of CABG conduit type in the setting of SCAD.2 Use of venous bypass grafts and preservation of arterial conduits for future revascularization should be considered and further investigated.
Optimal strategies for invasive management of high-risk anatomy for which neither PCI nor surgical strategies have been optimized for SCAD are currently unknown. Prospective studies should focus on innovation in invasive options, role of temporary mechanical circulatory support devices, and understanding mechanisms of stent restenosis and thrombosis in SCAD PCI.
Noninvasive diagnostic imaging
Coronary computed tomography angiography (CCTA) offers a noninvasive option for coronary imaging that avoids the risks of iatrogenic dissection. However, SCAD findings can be subtle, particularly in smaller, distal arteries, and may be obscured by patient motion or complex cardiac dynamics. The spatial resolution of most current generation computed tomography (CT) scanners is approximately 0.5 mm. In comparison, invasive coronary angiography has a resolution of 0.1 to 0.2 mm, with higher levels of resolution afforded by intravascular ultrasound and optimal coherence tomography. Given these limitations, a negative CCTA or invasive coronary angiography does not exclude the diagnosis of SCAD.34 However, since most patients with SCAD demonstrate vessel healing, follow-up imaging with CCTA offers an opportunity to demonstrate restoration of vessel wall and lumen integrity to support a diagnosis of SCAD or assist in clinical monitoring. The recent addition of photon-counting CT technology represents a significant advancement for noninvasive coronary imaging. photon-counting CT, with its superior resolution, material differentiation, and potentially lower radiation exposure versus conventional CT35 may improve the detection of subtle vascular changes characteristic of SCAD.
Post-SCAD chest pain occurs in a third of patients and can be due to recurrent SCAD, coronary microvascular and endothelial dysfunction, and nonischemic mechanisms.36 When chest pain persists in the absence of recurrent SCAD, quantitative perfusion cardiac magnetic resonance imaging and positron emission tomography can be helpful noninvasive strategies for detecting coronary microvascular disease following SCAD. Research is required to understand the relationship and prevalence of coronary microvascular disease following SCAD, as well as potential nonischemic mechanisms underlying the high burden of post-SCAD chest pain.
Medical therapy
Limited observational data exist to guide the medical management to promote coronary artery healing and reduce dissection recurrence. Expert consensus guidelines recommend the empiric use of single antiplatelet therapy (SAPT) with consideration of short duration dual antiplatelet therapy (DAPT) strategy in conservatively managed patients with SCAD based on presumed pathophysiological benefits.2 In particular, antiplatelet agents are currently utilized based on concerns regarding shear-mediated platelet activation in the context of endothelial exposure in dissected vessels.37 However, data from conservatively managed SCAD patients in the multicenter, retrospective DISCO (DIssezioni Spontanee COronariche) registry found an increased risk of major adverse cardiovascular events at 12 months among the patients treated with DAPT compared with SAPT driven primarily by nonfatal MI and unplanned revascularization, though these data should be considered within the limitations of a small registry-based cohort.37 Single-center retrospective registry data have shown the use of beta-blockers post-SCAD to be associated with a decreased risk of recurrent SCAD. Beyond this, targeted medical therapy to reduce SCAD recurrence is unknown.7 The reliance of current treatment strategies on purely observational, retrospective registry data are limiting, and future prospective and randomized studies should be designed to disaggregate the medical treatment of SCAD based on presenting clinical phenotype, presence of underlying genetic vasculopathy, and initial treatment strategy, given the unified angiographic findings but marked heterogeneity in clinical context and patient substrate. The BA-SCAD (Beta-blockers and Antiplatelet agents in patients with Spontaneous Coronary Artery Dissection) randomized clinical trial (NCT04850417) led by the Spanish Society of Cardiology is designed to evaluate the potential benefit and duration of SAPT vs DAPT among SCAD patients without coronary revascularization and the use of beta blockers post-SCAD in the absence of left ventricular dysfunction.38 Future studies should aim to incorporate insights from genetic and mechanistic studies of pathophysiological mechanisms of SCAD to tailor treatment strategies.
Exercise
A proposed pathophysiological mechanism of SCAD suggests that hemodynamic effects of extreme exercise that surpass the dissection threshold of susceptible coronary arteries may lead to dissection. The increased arterial tortuosity often seen in SCAD patients may increase this risk due to increased shear stress. Observational data has indicated that extreme or unconventional physical activity triggers SCAD in 15% to 29% of cases.2,39 However, such data may be limited by recall bias and lack of control populations. Post-SCAD, patients are generally counseled to avoid high-intensity interval training, competitive sports, extreme head positions, and heavy isometric exercise involving repeated Valsalva to avoid shear stress. However, the data to support these recommendations is limited. A SCAD-specific cardiac rehabilitation program has demonstrated the safety of exercise and its benefits, including an impact on psychosocial well-being and a reduction in recurrent chest pain and adverse cardiovascular events.40,41 This program, however, was not intended to serve as long-term guidance for physical activity, and overly restrictive recommendations are likely a disservice to patients given the myriad of benefits that moderate-intensity exercise affords.42
Although sex-specific weight restrictions for SCAD patients have previously been proposed, in general, resistance training limitations should be individualized to avoid unnecessary restrictions, but rigorous methods to provide such individualization are lacking.42
Further investigation is necessary to precisely determine the optimal frequency, intensity, duration, and type of exercise to mitigate risk while enhancing outcomes post-SCAD, both in the acute post-SCAD period and also longer term. Overly restrictive recommendations that are not grounded in firm data run the risk of discouraging patients from exercise, with significant adverse effects regarding physical (eg, risk of cardiometabolic disease) and mental health, particularly for previously highly active individuals.42 Utilizing hemodynamic information from cardiopulmonary exercise testing holds promise for crafting personalized, structured exercise plans postrehabilitation.
Psychosocial health
Growing evidence suggests that psychosocial stress and mental health are relevant before and after SCAD. Intense emotional stressors are commonly identified as triggering SCAD events—particularly in women.2,43 However, underlying processes are unknown. Stressor-related catecholamine increases leading to coronary artery shear stress have been postulated, but this and other biopsychosocial pathways have not been tested in patients with SCAD.2 Although 20% to 30% of SCAD patients report prior mental health conditions, research is also needed to understand how this history may impact SCAD onset.44 Additionally, SCAD events can be potentially traumatic experiences. Cross-sectional research has increasingly documented elevated prevalence of depression, anxiety, and post-traumatic stress disorder symptoms after SCAD—highlighting important psychosocial consequences of these events.44,45 However, longitudinal research is needed to: 1) delineate mental health trajectories post-SCAD; 2) identify predictors of symptom change; and 3) examine how mental health post-SCAD relates to illness course and event recurrence.
Despite evidence-based treatments for depression, anxiety, and post-traumatic stress disorder, little research has examined psychosocial interventions for patients with SCAD.46,47 Key questions to address include what interventions and modalities work best for these patients, if adaptations are needed for this unique patient population, and if successful treatment improves illness course. Additionally, antidepressants are commonly prescribed for these mental health conditions, and they can have off-target cardiovascular effects that merit consideration in the context of SCAD.48,49 As many patients with SCAD are ultimately seen in specialized cardiology clinics, delivering psychosocial interventions through integrated care models within these settings may be a promising avenue, including potential integration into SCAD-specific cardiac rehabilitation programs. Roadmaps for such integration are available and can guide future efforts.50
Reproductive health considerations
Approximately 40% of pregnancy-associated MI (PSCAD) is due to SCAD.8 Registry data highlight that PSCAD is associated with higher-risk presentations as compared with nonpregnancy-related SCAD (NP-SCAD) including left main or multivessel coronary involvement, and greater risk of left ventricular systolic dysfunction and cardiogenic shock. There are currently insufficient data to guide PSCAD-specific management including strategies for medical therapy, revascularization, associated cardiogenic shock, and mode of delivery.51 Expert recommendations for management therefore stem from experience with related cardiovascular diseases.52 In the context of these data, expert consensus currently recommends against pregnancy after SCAD.2,53,54 However, registries have reported that pregnancy can occur safely after SCAD, though data have been limited by small numbers, heterogeneity in index presentation, and the selection bias inherent to these retrospective cohorts, which limits generalizability.53,55 With concerns regarding the higher-risk presentation of PSCAD, there are limitations to real-world investigation, and registry-based data are likely to remain limited, with a bias potentially toward those with lower-risk index presentations. As such, development of animal models of SCAD may enable advancements in our understanding of both PSCAD and pregnancy following SCAD. In addition, genome-wide association studies hold promise to advance our understanding of SCAD risk in general, as well as differences between PSCAD and NP-SCAD.27
Beyond pregnancy, our nascent understanding of the association of SCAD and female sex hormones limits our understanding of the potential impact of exogenous hormone use, particularly in the context of contraception, assisted reproductive technologies, breast cancer therapies, pregnancy termination, and perimenopausal hormone replacement therapy. It remains unknown whether absolute hormone levels or their fluctuations affect risk. PSCAD occurs most commonly postpartum, when hormone levels are rapidly declining. In the context of expert consensus opinion to avoid (yet, to personalize) oral exogenous hormone use, alternative strategies are typically recommended, but this significantly limits treatment options for patients. Further mechanistic study into the pathophysiology of SCAD should include an understanding of the role of estrogen, progesterone, and prolactin in the pathogenesis of SCAD. This may help inform future clinical studies of exogenous use among SCAD survivors seeking hormone-based treatments.
iSCAD: innovative patient-centered research framework
The patient voice
A unique feature of SCAD research to date has been the driving force of patients as intellectual partners. Patients have been central to the creation of registries upon which most of the current data regarding SCAD are based. SCAD Alliance, a pioneer in the patient-initiated research movement,56 is a 501(c)(3) nonprofit founded by patients to provide an integrated, collaborative approach to patient and caregiver support, expand general and professional awareness, and enhance the diversity and quality of data. The Board of SCAD Alliance consists of SCAD patients, family, and allies who ensure that research prioritizes a comprehensive understanding of the SCAD experience and focuses on the questions that are most pressing for the patients. SCAD Alliance has aimed to increase patient access to vetted and reliable medical information regarding SCAD and created supportive patient communities both virtually and through in-person patient symposiums.
iSCAD registry
The iSCAD Registry (NCT04496687) is sponsored by SCAD Alliance, with a mission to develop and maintain an independent, multicenter data repository to accelerate international SCAD research (Figure 1).57 Data are decentralized and owned by SCAD Alliance. Because the data are not owned by a single academic institution but stewarded at a nonenrolling coordinating center, all enrolling investigators have the opportunity to lead resulting research investigations. This democratization of study data has fostered meaningful collaboration among investigators and has encouraged rapid enrollment of patients. Since the first patient was enrolled in March 2019, more than 1,700 patients from 26 sites across the United States and from Sydney, Australia, have been enrolled. Since 2022, the data from the registry have been presented in multiple abstracts and in published manuscripts.44,58
Figure 1.
Founding Principles of the iSCAD Registry
iSCAD = International Spontaneous Coronary Artery Dissection.
SCAD centers of excellence
The iSCAD study design arose out of the need for increased diversity both among patients and investigators. Historically, SCAD registries have included populations that are less diverse than reference populations with regard to racial, ethnic, and socioeconomic variables.32 iSCAD represents a collaboration of 27 centers, including academic, hybrid, and community sites, with principal investigators expert in the key areas of vascular medicine, women’s cardiovascular health, cardiac imaging, and interventional cardiology. By including sites outside of traditional academic medical centers, which have not previously been represented within SCAD registries, we aim to increase the diversity of the included population as well as our understanding of SCAD outcomes among the diversity of care settings represented.55,59,60
Given the sex and gender disparities in cardiovascular research, a particularly powerful aspect of this registry is the focus on a disease process that disproportionately impacts females. The study also highlights a rare finding in cardiovascular research, with 19 site principal investigators being women. In addition to serving as a research network, iSCAD has created a diverse network of SCAD “Centers of Excellence” with the goal that patients of all socioeconomic groups have access to SCAD expertise. This would not only prove to be a model for patient access but will also allow us to better study an inclusive population of patients with SCAD.
Patients affected by SCAD are clinically complex and require access to multiple specialists familiar with SCAD and access to appropriate diagnostic testing and procedures. Understanding that participation in the iSCAD Registry would lead to heightened recognition of sites as centers with experience in treating patients with SCAD, the leadership of the study developed criteria for SCAD “Centers of Excellence.” The key component for a SCAD “Center of Excellence” is the presence of at least 1 clinician champion who has, or is willing to, develop and grow referral pathways to ensure standardized care of patients with SCAD at their institution. Other core criteria include the access to a multidisciplinary team of specialists including those with expertise in vascular disease, headache and vascular neurology, interventional cardiology, maternal fetal medicine, behavioral health, cardiac rehabilitation, and medical genetics, among others. SCAD “Centers of Excellence” are also capable of committing needed time and resources to support participation in multicenter research and have a commitment to connecting patients to patient advocacy groups and encouraging, participating in, and/or organizing patient-centered educational events (Table 3). Through the iSCAD registry, there is an annual conference for clinician education as well as an in-person patient educational conference, led by SCAD Alliance. In addition, as knowledge of SCAD management advances through research, it is a priority to disseminate this to all cardiovascular clinicians who care for patients both within and outside of specific SCAD “Centers of Excellence” through publications in high-impact journals and presentations at highly attended medical conferences.
Table 3.
Suggested Components of a SCAD “Center of Excellence”
| Clinician champion |
|
| Specialist care |
|
| Diagnostic imaging |
|
| Research |
|
Call to action through the lens of iSCAD
While significant advances have been made in the accurate diagnosis of SCAD, evidence-based strategies for treatment and risk stratification of patients are lacking. Multipronged strategies are essential to address the current gaps in knowledge and further the clinical care and outcomes of individuals with SCAD (Table 4). A central knowledge gap remains that the precise pathophysiology of the disease remains unknown; therefore, targeted therapies are not available. As such, supporting the rapid advancement of knowledge is critical to improving clinical care. Multicenter collaborations like the iSCAD registry are critical to the advancement of rarer diseases, particularly those that disproportionately affect patient groups traditionally underrepresented in cardiovascular studies. In addition, collaborative research networks inclusive of investigators from a variety of practice settings and areas of expertise and the patients themselves enable more rapid research inquiry into the questions that are most relevant to the patients affected by the disease.
Table 4.
Strategies to Bridge Gaps in Clinical Care, Research, and Outcomes in SCAD
| Clinical care | Encourage the development of expert consensus statements to continually update current practice in the absence of randomized-controlled trial data. |
| Collaborate with patient advocacy groups to disseminate the newest research and recommendations. | |
| Longitudinal and continued clinician education through SCAD presentations at national meetings and SCAD-specific meetings | |
| Development of SCAD Centers of Excellence (COEs) | |
| Research | Enable regular meetings of experts representing multiple specialties to identify ongoing gaps in clinical care, research, and outcomes and spur research collaborations. |
| Multicenter registries (eg, iSCAD) are the current mainstay of data. Multicenter randomized trials are needed. | |
| Collaborative efforts between diverse specialties (eg, translational scientists, genetic experts, interventionalists, imaging specialists, vascular medicine specialists, sports cardiology specialists, and women’s health specialists) | |
| Outcomes | Dissemination of vetted patient information and resources through online and in-person patient educational conferences |
| Advocacy is required to increase awareness of SCAD among clinicians and assist in connecting SCAD patients with clinicians who have SCAD expertise and/or SCAD COE. | |
| Diversification of geographic location and practice setting of SCAD Centers of Excellence to maximize accessibility to specialty care | |
| Recruitment and diversification of SCAD clinical providers and investigators | |
| Inclusivity and retention of women and underrepresented racial and ethnic minority groups in research initiatives |
iSCAD = International Spontaneous Coronary Artery Dissection; SCAD = spontaneous coronary artery dissection.
Funding support and author disclosures
Dr Sarma has received grants from CRICO and the American Heart Association; and is a consultant at Pfizer. Dr Wood is a member of the Scientific Advisory Board of the SCAD Alliance. Dr Naderi is a member of the Scientific Advisory Board of the SCAD Alliance. Dr Gibson has received research support from the SCAD Alliance. Dr Aday has consulted for Merck and Janssen. Dr Lindsay is a member of the professional advisory board of the Marfan Foundation and a member of the scientific advisory board of the Myhre syndrome foundation and the ACTA2 alliance; and has received research funding from Angea Biotherapeutics. Dr Rodriguez Lozano is an iTHRIV Scholar. The iTHRIV Scholars Program is supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR003015 and KL2TR003016. Dr Sharma is a consultant for enVVeno Corporation; is on the Speakers Bureau for Boston Scientific Corporation; and has received institutional research funding from Boston Scientific Corporation and Vascular Medcure. Dr Scott is a consultant for Mediflix. Dr Scherer is on the Speakers Bureau for Abbott, Boston Scientific, Edwards Lifesciences, HeartFlow, Philips, and Siemens. Dr Sweis is on the Speakers Bureau for Edwards Lifesciences and Medtronic; and is an editor of the Merck Manual. Dr Kim is the Chair of the Scientific Advisory Board of the SCAD Alliance. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Jin X., Chandramouli C., Allocco B., Gong E., Lam C.S.P., Yan L.L. Women's participation in cardiovascular clinical trials from 2010 to 2017. Circulation. 2020;141:540–548. doi: 10.1161/CIRCULATIONAHA.119.043594. [DOI] [PubMed] [Google Scholar]
- 2.Hayes S.N., Kim E.S.H., Saw J., et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart association. Circulation. 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim E.S.H. Spontaneous coronary-artery dissection. N Engl J Med. 2020;383:2358–2370. doi: 10.1056/NEJMra2001524. [DOI] [PubMed] [Google Scholar]
- 4.Waterbury T.M., Tweet M.S., Hayes S.N., et al. Early natural history of spontaneous coronary artery dissection. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.118.006772. [DOI] [PubMed] [Google Scholar]
- 5.Adlam D., Berrandou T.E., Georges A., et al. Genome-wide association meta-analysis of spontaneous coronary artery dissection identifies risk variants and genes related to artery integrity and tissue-mediated coagulation. Nat Genet. 2023;55:964–972. doi: 10.1038/s41588-023-01410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan S., Samuel R., Starovoytov A., Lee C., Aymong E., Saw J. Outcomes of percutaneous coronary intervention in patients with spontaneous coronary artery dissection. J Interv Cardiol. 2021;2021 doi: 10.1155/2021/6686230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saw J., Humphries K., Aymong E., et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148–1158. doi: 10.1016/j.jacc.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 8.Elkayam U., Jalnapurkar S., Barakkat M.N., et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129:1695–1702. doi: 10.1161/CIRCULATIONAHA.113.002054. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Guimaraes M., Fuertes-Ferre G., Jimenez-Valero S., et al. Characteristics, acute results, and prognostic impact of percutaneous coronary interventions in spontaneous coronary artery dissection (from the prospective Spanish registry on SCAD [SR-SCAD]) Am J Cardiol. 2022;171:177–178. doi: 10.1016/j.amjcard.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Jackson R., Al-Hussaini A., Joseph S., et al. Spontaneous coronary artery dissection: pathophysiological insights from optical coherence tomography. JACC Cardiovasc Imaging. 2019;12:2475–2488. doi: 10.1016/j.jcmg.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Hartman R.J.G., Owsiany K., Ma L., et al. Sex-stratified gene regulatory networks reveal female key driver genes of atherosclerosis involved in smooth muscle cell phenotype switching. Circulation. 2021;143:713–726. doi: 10.1161/CIRCULATIONAHA.120.051231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L., Henry J., Liu Y., Jouve C., Georges A., Bouatia-Naji N. Regulatory mechanisms in multiple vascular diseases locus LRP1 involve repression by SNAIL and extracellular matrix remodeling. bioRxiv. Posted May 9, 2023 doi: 10.1101/2023.05.09.539992. [DOI] [Google Scholar]
- 13.Kim E.S.H., Saw J., Kadian-Dodov D., Wood M., Ganesh S.K. FMD and SCAD: sex-biased arterial diseases with clinical and genetic pleiotropy. Circ Res. 2021;128:1958–1972. doi: 10.1161/CIRCRESAHA.121.318300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adlam D., Olson T.M., Combaret N., et al. Association of the PHACTR1/EDN1 genetic locus with spontaneous coronary artery dissection. J Am Coll Cardiol. 2019;73:58–66. doi: 10.1016/j.jacc.2018.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiando S.R., Tucker N.R., Castro-Vega L.J., et al. PHACTR1 is a genetic susceptibility locus for fibromuscular dysplasia supporting its complex genetic pattern of inheritance. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saw J., Yang M.L., Trinder M., et al. Chromosome 1q21.2 and additional loci influence risk of spontaneous coronary artery dissection and myocardial infarction. Nat Commun. 2020;11:4432. doi: 10.1038/s41467-020-17558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turley T.N., O'Byrne M.M., Kosel M.L., et al. Identification of susceptibility loci for spontaneous coronary artery dissection. JAMA Cardiol. 2020;5:929–938. doi: 10.1001/jamacardio.2020.0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadian-Dodov D., Gornik H.L., Gu X., et al. Dissection and aneurysm in patients with fibromuscular dysplasia: findings from the U.S. Registry for FMD. J Am Coll Cardiol. 2016;68:176–185. doi: 10.1016/j.jacc.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 19.Tarr I., Hesselson S., Troup M., et al. Polygenic risk in families with spontaneous coronary artery dissection. JAMA Cardiol. 2024;9:254–261. doi: 10.1001/jamacardio.2023.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel K., Tweet M., Olson T.M., Maleszewski J.J., Gulati R., Hayes S.N. Familial spontaneous coronary artery dissection: evidence for genetic susceptibility. JAMA Intern Med. 2015;175:821–826. doi: 10.1001/jamainternmed.2014.8307. [DOI] [PubMed] [Google Scholar]
- 21.Kaadan M.I., MacDonald C., Ponzini F., et al. Prospective cardiovascular genetics evaluation in spontaneous coronary artery dissection. Circulation Genomic and precision medicine. 2018;11 doi: 10.1161/CIRCGENETICS.117.001933. [DOI] [PubMed] [Google Scholar]
- 22.Verstraeten A., Perik M., Baranowska A.A., et al. Enrichment of rare variants in loeys-dietz syndrome genes in spontaneous coronary artery dissection but not in severe fibromuscular dysplasia. Circulation. 2020;142:1021–1024. doi: 10.1161/CIRCULATIONAHA.120.045946. [DOI] [PubMed] [Google Scholar]
- 23.Carss K.J., Baranowska A.A., Armisen J., et al. Spontaneous coronary artery dissection: insights on rare genetic variation from genome sequencing. Circulation Genomic and precision medicine. 2020;13 doi: 10.1161/CIRCGEN.120.003030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zekavat S.M., Chou E.L., Zekavat M., et al. Fibrillar collagen variants in spontaneous coronary artery dissection. JAMA Cardiol. 2022;7:396–406. doi: 10.1001/jamacardio.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y., Chen Y., Li Y., et al. Association of TSR1 variants and spontaneous coronary artery dissection. J Am Coll Cardiol. 2019;74:167–176. doi: 10.1016/j.jacc.2019.04.062. [DOI] [PubMed] [Google Scholar]
- 26.Turley T.N., Theis J.L., Sundsbak R.S., et al. Rare missense variants in TLN1 are associated with familial and sporadic spontaneous coronary artery dissection. Circulation Genomic and precision medicine. 2019;12 doi: 10.1161/CIRCGEN.118.002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turley T.N., Kosel M.L., Bamlet W.R., et al. Susceptibility locus for pregnancy-associated spontaneous coronary artery dissection. Circulation Genomic and precision medicine. 2021;14 doi: 10.1161/CIRCGEN.121.003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirruccello J.P., Chaffin M.D., Chou E.L., et al. Deep learning enables genetic analysis of the human thoracic aorta. Nat Genet. 2022;54:40–51. doi: 10.1038/s41588-021-00962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84:1115–1122. doi: 10.1002/ccd.25293. [DOI] [PubMed] [Google Scholar]
- 30.Tweet M.S., Eleid M.F., Best P.J., et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–786. doi: 10.1161/CIRCINTERVENTIONS.114.001659. [DOI] [PubMed] [Google Scholar]
- 31.Hassan S., Prakash R., Starovoytov A., Saw J. Natural history of spontaneous coronary artery dissection with spontaneous angiographic healing. JACC Cardiovasc Interv. 2019;12:518–527. doi: 10.1016/j.jcin.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Hayes S.N., Tweet M.S., Adlam D., et al. Spontaneous coronary artery dissection: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:961–984. doi: 10.1016/j.jacc.2020.05.084. [DOI] [PubMed] [Google Scholar]
- 33.Krittanawong C., Gulati R., Eitzman D., Jneid H. Revascularization in patients with spontaneous coronary artery dissection: where are we now? J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tweet M.S., Akhtar N.J., Hayes S.N., Best P.J., Gulati R., Araoz P.A. Spontaneous coronary artery dissection: acute findings on coronary computed tomography angiography. Eur Heart J Acute Cardiovasc Care. 2019;8:467–475. doi: 10.1177/2048872617753799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagar M.T., Soschynski M., Saffar R., et al. Accuracy of ultrahigh-resolution photon-counting CT for detecting coronary artery disease in a high-risk population. Radiology. 2023;307 doi: 10.1148/radiol.223305. [DOI] [PubMed] [Google Scholar]
- 36.Sedlak T., Starovoytov A., Humphries K., Saw J. Coronary flow reserve in patients with prior spontaneous coronary artery dissection and recurrent angina. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerrato E., Giacobbe F., Quadri G., et al. Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry. Eur Heart J. 2021;42:3161–3171. doi: 10.1093/eurheartj/ehab372. [DOI] [PubMed] [Google Scholar]
- 38.Alfonso F., de la Torre Hernandez J.M., Ibanez B., et al. Rationale and design of the BA-SCAD (Beta-blockers and antiplatelet agents in patients with spontaneous coronary artery dissection) randomized clinical trial. Revista espanola de cardiologia. 2022;75:515–522. doi: 10.1016/j.rec.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Saw J., Starovoytov A., Humphries K., et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J. 2019;40:1188–1197. doi: 10.1093/eurheartj/ehz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chou A.Y., Prakash R., Rajala J., et al. The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol. 2016;32:554–560. doi: 10.1016/j.cjca.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Samuel R., Alfadhel M., McAlister C., Nestelberger T., Saw J. Cardiac rehabilitation following coronary artery dissection: recommendations and patient considerations. Expert Rev Cardiovasc Ther. 2021;19:1005–1012. doi: 10.1080/14779072.2021.2013812. [DOI] [PubMed] [Google Scholar]
- 42.Tweet M.S., Olin J.W., Bonikowske A.R., Adlam D., Hayes S.N. Physical activity and exercise in patients with spontaneous coronary artery dissection and fibromuscular dysplasia. Eur Heart J. 2021;42:3825–3828. doi: 10.1093/eurheartj/ehab307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saw J., Aymong E., Sedlak T., et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645–655. doi: 10.1161/CIRCINTERVENTIONS.114.001760. [DOI] [PubMed] [Google Scholar]
- 44.Sumner J.A., Kim E.S.H., Wood M.J., et al. Posttraumatic stress disorder after spontaneous coronary artery dissection: a report of the international spontaneous coronary artery dissection registry. J Am Heart Assoc. 2024;13 doi: 10.1161/JAHA.123.032819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson A.K., Hayes S.N., Sawchuk C., et al. Analysis of posttraumatic stress disorder, depression, anxiety, and resiliency within the unique population of spontaneous coronary artery dissection survivors. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaca K.C., Tremmel J.A., Edwards K.S. Preliminary support for group cognitive behavioral therapy (CBT) to reduce psychological distress in patients with spontaneous coronary artery dissection (SCAD) J Clin Psychol Med Settings. 2021;28:826–832. doi: 10.1007/s10880-021-09803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Damme A., McDermott S., McMurtry S., Kung J.Y., Gyenes G., Norris C. Secondary prevention and rehabilitation for spontaneous coronary artery dissection: a systematic review. Can J Cardiol. 2023;39:S395–S411. doi: 10.1016/j.cjca.2023.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Kahl K.G., Stapel B., Correll C.U. Psychological and psychopharmacological interventions in psychocardiology. Front Psychiatry. 2022;13 doi: 10.3389/fpsyt.2022.831359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pina I.L., Di Palo K.E., Ventura H.O. Psychopharmacology and cardiovascular disease. J Am Coll Cardiol. 2018;71:2346–2359. doi: 10.1016/j.jacc.2018.03.458. [DOI] [PubMed] [Google Scholar]
- 50.Gaffey A.E., Harris K.M., Mena-Hurtado C., Sinha R., Jacoby D.L., Smolderen K.G. The yale roadmap for health psychology and integrated cardiovascular care. Health Psychol. 2022;41:779–791. doi: 10.1037/hea0001152. [DOI] [PubMed] [Google Scholar]
- 51.Tweet M.S., Hayes S.N., Codsi E., Gulati R., Rose C.H., Best P.J.M. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70:426–435. doi: 10.1016/j.jacc.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 52.Havakuk O., Goland S., Mehra A., Elkayam U. Pregnancy and the risk of spontaneous coronary artery dissection: an analysis of 120 contemporary cases. Circ Cardiovasc Interv. 2017;10 doi: 10.1161/CIRCINTERVENTIONS.117.004941. [DOI] [PubMed] [Google Scholar]
- 53.Tweet M.S., Young K.A., Best P.J.M., et al. Association of pregnancy with recurrence of spontaneous coronary artery dissection among women with prior coronary artery dissection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.18170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan N., Premawardhana D., Al-Hussaini A., et al. Pregnancy and spontaneous coronary artery dissection: lessons from survivors and nonsurvivors. Circulation. 2022;146:69–72. doi: 10.1161/CIRCULATIONAHA.122.059635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen S., Merchant M., Mahrer K.N., Ambrosy A.P., Lundstrom R.J., Naderi S. Pregnancy-associated spontaneous coronary artery dissection: clinical characteristics, outcomes, and risk during subsequent pregnancy. J Invasive Cardiol. 2021;33:E457–E466. doi: 10.25270/jic/20.00529. [DOI] [PubMed] [Google Scholar]
- 56.Wood M.J., Wen L., Adlam D., Kim E.S.H. 2014. The vital role of the emergency department in improving outcomes of spontaneous coronary artery dissection (SCAD): a SCAD alliance white paper based on the 2014 SCAD alliance scientific advisory board roundtable.https://scadalliance.org/wp-content/uploads/The-Vital-Role-of-the-ER-in-Improving-Outcomes-of-SCAD.pdf [Google Scholar]
- 57.iSCAD registry study page. www.iscadregistry.bidmc.org
- 58.Wells B.J., Wood M.J., O'Duffy A.E., et al. Migraine headache in patients with spontaneous coronary artery dissection: a report of the iSCAD Registry. Vasc Med. 2024;29:286–295. doi: 10.1177/1358863X241252444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clare R., Duan L., Phan D., et al. Characteristics and clinical outcomes of patients with spontaneous coronary artery dissection. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen S., Ambrosy A.P., Mahrer K.N., Lundstrom R.J., Naderi S. Spontaneous coronary artery dissection and incident ventricular arrhythmias: frequency, clinical characteristics, and outcomes. JACC Cardiovasc Interv. 2020;13:539–541. doi: 10.1016/j.jcin.2019.10.041. [DOI] [PubMed] [Google Scholar]
- 61.Edwards K.S., Vaca K.C., Naderi S., Tremmel J.A. Patient-reported psychological distress after spontaneous coronary artery dissection: evidence for post-traumatic stress. J Cardiopulm Rehabil Prev. 2019;39:E20–E23. doi: 10.1097/HCR.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 62.Liang J.J., Tweet M.S., Hayes S.E., Gulati R., Hayes S.N. Prevalence and predictors of depression and anxiety among survivors of myocardial infarction due to spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 2014;34:138–142. doi: 10.1097/HCR.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 63.Murphy B.M., Rogerson M.C., Le Grande M.R., et al. Psychosocial and lifestyle impacts of spontaneous coronary artery dissection: a quantitative study. PLoS One. 2024;19 doi: 10.1371/journal.pone.0296224. [DOI] [PMC free article] [PubMed] [Google Scholar]