Abstract
Schizophrenia (SCZ) is a common psychiatric disorder that has a complex pathological mechanism. During the Coronavirus disease 2019 (COVID-19) epidemic, patients with SCZ had substantially higher rates of infection with SARS-CoV-2, the virus that causes COVID-19, as well as higher COVID-19 mortality relative to patients with other mental disorders. However, the reasons for these increased rates in patients with SCZ remain unknown. In this review, we hypothesize that certain molecular pathways exhibit abnormal function in both COVID-19 and SCZ, with a focus on those related to energy metabolism dysregulation, immune system disruption, and abnormalities of the central nervous system. We review that dysregulation of energy metabolism can result in disruptions to the immune system and abnormalities within the central nervous system (CNS). Furthermore, immune system disturbances may also contribute to CNS abnormalities in both SCZ and COVID-19. We also discuss macro-factors associated with the high infection and mortality rates of COVID-19 in patients with SCZ, including sociodemographic factors, reduced access to psychiatric healthcare, structural barriers to COVID-19 vaccination, and proposed approaches to mitigate these macro-factors.
Keywords: Schizophrenia, COVID-19, Molecular mechanism, Macro-factors, Solutions
1. Introduction
Schizophrenia (SCZ) carries a lifetime risk of approximately 1 % and is a frequently disabling psychiatric condition distinguished by positive symptoms, negative symptoms, and cognitive impairment (Lu, Wang et al. 2022). SCZ confers substantial mortality and morbidity, with a mean reduction in life expectancy of 15–30 years (Olfson, Gerhard et al. 2015). Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appeared at the end of 2019 (Zhang, He et al. 2023) (Fig. 1). COVID-19 increases the risk of mental disorders (Baranova, Cao et al. 2022), and patients with SCZ in particular have high rates of infection and mortality from SARS-CoV-2 (Papadopoulos et al., 2022, Sanchez-Rico et al., 2022). Meanwhile, psychiatric disorders (e.g., SCZ) can increase the risk of COVID-19 (Papadopoulos, Sutheesophon et al. 2022). However, the molecular mechanisms associated with these increased rates are unclear. This paper hypothesizes that energy metabolism dysregulation, immune system disruption, and abnormalities of the central nervous system (CNS) in both COVID-19 and SCZ may aggravate infection rates and mortality among patients with SCZ due to COVID-19. These pathways are micro-factors. There are also macro-factors that can lead to an increase in the SARS-CoV-2 infection and COVID-19 mortality rates of patients with SCZ. We discuss possible measures to address these controllable macro-factors.
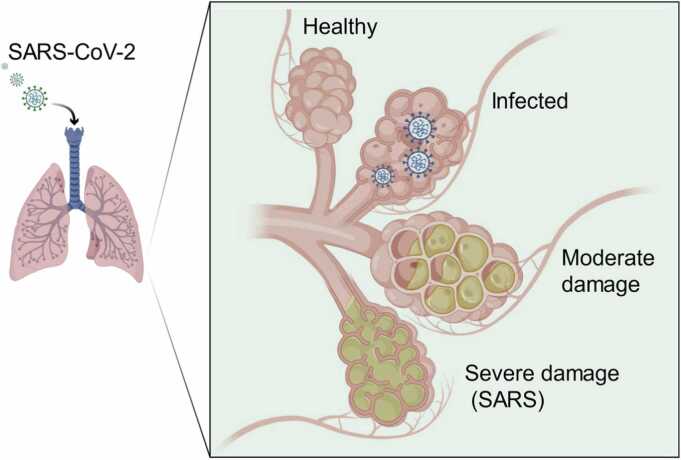
Fig. 1.
SARS-CoV-2 progression in the lungs of COVID-19 patients. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019.
2. Molecular mechanisms associated with increased COVID-19 infection and mortality rates in patients with SCZ
2.1. Dysregulation of energy metabolism and associated signaling pathways in SCZ and COVID-19
The brain is one of the most energy-consuming organs in the human body (Faria-Pereira and Morais, 2022). The function of the CNS depends on synaptic transmission processes, including postsynaptic currents, neurotransmitter recycling, and presynaptic Ca²⁺ flux, which are considered the primary energy-consuming processes (Faria-Pereira and Morais, 2022). Energy metabolic disturbances are associated with CNS related disorders. Mitochondria are responsible for energy supply, and mitochondrial dysfunction has emerged as one of the main triggers for these disorders (Chen, Zhao et al. 2023). The CNS primarily utilizes glucose to meet its energetic needs, including those of synapses, which can be metabolized through glycolysis and/or mitochondrial oxidative phosphorylation (Faria-Pereira and Morais, 2022).
Dysregulated cerebral glucose metabolism is considered a fundamental factor in the pathogenesis of SCZ, leading to secondary alterations in peripheral glucose metabolism (Yu, Liao et al. 2021). Abnormalities in glucose metabolism represent a crucial indicator in the subacute stage of COVID-19 (Yu, Liao et al. 2021) and in Long COVID (Dressing, Bormann et al. 2022). Therefore, disorders of carbon and energy metabolism are common features of both SCZ and COVID-19 (Antunes, Reis-de-Oliveira et al. 2024). These significant abnormalities involve many processes, such as glycolysis, the tricarboxylic acid cycle, the pentose phosphate pathway, and oxidative phosphorylation (Antunes, Reis-de-Oliveira et al. 2024).
Disturbances in energy metabolism will inevitably lead to changes in the pathways involved in sensing and transmitting energy signals, such as glucagon signaling, insulin signaling, and cyclic guanosine monophosphate (cGMP)-dependent protein kinase (PKG) signaling. Their disruptions have been observed in both SCZ and COVID-19 (Antunes, Reis-de-Oliveira et al. 2024). Both the glucagon signaling and insulin signaling pathway play crucial roles in sensing and regulating glucose metabolism in the body (Tengholm and Gylfe, 2017). These pathways maintain glucose homeostasis, balancing glucose levels by facilitating uptake when insulin is high and promoting glucose release when glucagon levels are elevated (Tengholm and Gylfe, 2017). cGMP-PKG signaling plays a role in regulating energy homeostasis and glucose metabolism (Taoro-González, Cabrera-Pastor et al. 2022). Disruption of cGMP-PKG signaling has been implicated in SCZ (Shim, Shuman et al. 2016) and serves as a potential target for therapeutic interventions (Sadeghi, Nassireslami et al. 2023). These signaling pathways disturbances may influence various processes in the brain, including synaptic plasticity, neuronal survival, mood regulation, and immune responses (Nemani et al., 2021, Yu et al., 2021, Antunes et al., 2024)
These abnormalities in energy metabolism and associated signaling pathways are common to both SCZ and COVID-19, influencing various processes in the brain, including synaptic plasticity, neuronal survival, mood regulation, and immune responses. This raises the question of whether these disturbances are connected to the increased mortality rates observed in patients with SCZ who are affected by COVID-19.
2.2. Abnormalities of the CNS in SCZ and COVID-19
Disruptions in energy metabolism can lead to disturbances in the metabolism of other substances, such as amino acids. Disruptions in amino acid metabolism, including that of alanine, aspartate, and glutamate, have been identified in both SCZ and COVID-19 (Antunes, Reis-de-Oliveira et al. 2024). Glycine, glutamate, and aspartate are all important neurotransmitters, with glutamate and aspartate functioning as excitatory neurotransmitters. Glutamate is crucial for the function of glutamatergic synapses. Glutamate ion channels have three subtypes, that is, α-amino-3-hydroxy-5-methyl-4-isoxazole (AMPA), kainate, and N-methyl-d-aspartate (NMDA) receptors. In COVID-19, there is a downregulation of the NMDA receptor subunits 1 and 2 A, which are essential components of the NMDA receptor (Zhang, He et al. 2023). The hypofunction of the NMDA receptor represents a significant factor in the pathophysiology of SCZ (Singh, Poterba et al. 2022). The postsynaptic membrane of neurons is rich in NMDA and AMPA receptor channels, with NMDA receptor channels playing a crucial role in long-term potentiation (LTP) and long-term depression (LTD) (Lüscher and Malenka, 2012). Disorder of glutamate metabolism can also cause disorder of GABAergic systems. GABA is mainly synthesized by the decarboxylation of glutamate who is mediated by glutamate decarboxylase 1 (GAD1) and GAD2, respectively. GAD1 and GAD2 can be expressed in the same inhibitory neuron at different locations and can have different functions (Magri, Giacopuzzi et al. 2018). Reduced levels of GAD1 mRNA and protein and the mutation in GAD1 gene, as well as reduced GABA concentrations, have been consistently observed in multiple regions in post-mortem brains from SCZ cases (Magri, Giacopuzzi et al. 2018). Therefore, the disturbance of glutamate metabolism can cause the disturbance of GABAergic systems, LTP, and LTD in both SCZ and COVID-19 (Antunes, Reis-de-Oliveira et al. 2024).
In addition, dysregulation of retrograde endocannabinoid signaling is shared in SCZ and COVID-19 (Antunes, Reis-de-Oliveira et al. 2024)。Retrograde endocannabinoids are a class of molecules that regulate signal transmission between neurons in the nervous system, playing a vital role in modulating synaptic strength and maintaining equilibrium (Scheyer, Yasmin et al. 2023). Endocannabinoids (eCBs) are well known to act as retrograde synaptic messengers capable of participating in several forms of short-term plasticity such as DSI/DSE, eCBs participate in several forms of long-term synaptic plasticity, including LTP and LTD, metaplasticity, and synaptic scaling (Scheyer, Yasmin et al. 2023). These processes involve Ca²⁺, GABA receptors and a variety of glutamate receptors (Scheyer, Yasmin et al. 2023). The main general role of the endocannabinoid system is likely to favour body energy accumulation. Type-1 cannabinoid receptors (CB1) receptor signalling represents an efficient system to adjust body metabolism towards the accumulation of energy reserves. the CB1 receptor blocker rimonabant was commercialized against obesity and associated metabolic disorders (Busquets-García, Bolaños et al. 2022). Dysregulation of the endocannabinoid system has been observed in SCZ (D'Addario, Micale et al. 2017). Moreover, dysregulation of retrograde endocannabinoids has also been identified in COVID-19 (D'Addario, Micale et al. 2017).
In summary, disruptions in energy metabolism may influence the metabolic disturbances of neurotransmitters such as glutamate, which in turn can lead to abnormalities in glutamatergic and GABAergic synapses, ultimately resulting in irregularities in LTP and LTD. Moreover, retrograde endocannabinoid signaling is not only related to synaptic plasticity but is also tied to energy metabolism. Its disruption intertwines with neurotransmitter metabolic disorders, exacerbating the complexity of SCZ and COVID-19. This contributes to the elevated infection and mortality rates linked with COVID-19 among individuals with schizophrenia. Future studies exploring the underlying mechanisms linking dysregulation of CNS function may yield valuable insights into the complex pathophysiology of these conditions and the heightened vulnerability of patients with SCZ to severe outcomes.
2.3. Immunization disruption in SCZ and COVID-19
The preceding content indicates that energy is not only crucial for the CNS but also plays an important role in the immune system (Straub, 2017). The immune system is sensitive to energy levels, and a disruption in energy metabolism can likely diminish the CNS's immune response to adverse environmental conditions (Bielanin and Sun, 2023).
Immunological disruptions have been observed in individuals affected by COVID-19. In peripheral tissues, levels of interleukin-6 (IL-6) were found to be higher in patients with SARS-CoV-2 infection compared to those without the infection (Thomas, Stefanoni et al. 2020). Among SARS-CoV-2-positive patients, those who died exhibited significantly higher levels of IL-6 compared to those who survived (Michaelis, Zelzer et al. 2022). In brain tissue, patients with COVID-19 presented with monocytic encephalitis, which is characterized by monocyte infiltration, activation of microglia and astrocytes, and the release of IL-4, IL-6, and IL-12 (Zhang, He et al. 2023).
Some evidence indicates the presence of immune dysfunction and inflammation in patients with SCZ (Comer, Carrier et al. 2020). Levels of proinflammatory cytokines, such as IFN-γ, IL-1, and tumor necrosis factor α, are elevated in the plasma of SCZ patients (Buki, Kekesi et al. 2021). Increased IL-6 levels have also been detected in the cerebrospinal fluid (CSF) of individuals with SCZ (Schwieler, Larsson et al. 2015). Furthermore, minocycline, an anti-inflammatory antibiotic, may improve symptom severity in patients with SCZ (Comer, Carrier et al. 2020). In patients with SCZ, reduced IL-3 activity has been linked to more severe illness, higher viral loads, and an increased likelihood of death in patients with SCZ who become infected with SARS-CoV-2 (Papadopoulos, Sutheesophon et al. 2022).
The depletion of lymphocyte subsets, such as natural killer (NK) cells that play a crucial role in early antiviral immunity, is commonly observed in both patients with SCZ and those with severe COVID-19 (Mohan, Perry et al. 2021). The reduced activity of NK cells can lead to a higher viral load, compromising the immune response in individuals with schizophrenia and making them more vulnerable to SARS-CoV-2 infection (Mohan, Perry et al. 2021). Therefore, the immune dysregulation associated with the progression of fatal SARS-CoV-2 infection, combined with the subclinical inflammation that characterizes schizophrenia, may amplify the systemic hyperinflammation caused by SARS-CoV-2 infection in these patients. These heightened pro-inflammatory responses can result in systemic tissue damage and multiple organ failure. This could be a significant factor contributing to the elevated infection and mortality rates associated with COVID-19 among individuals with schizophrenia. Vaccination can reduce the SARS-CoV-2 load in the body, thereby alleviating inflammation and easing the burden on the immune system, ultimately lowering the infection and mortality rates of COVID-19 in patients with SCZ.
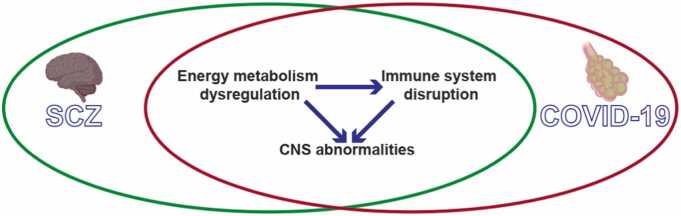
In conclusion, we propose the hypothesis that energy metabolism dysregulation links COVID-19 and schizophrenia, along with abnormalities of the CNS and immune system disruption (Fig. 2).
Fig. 2.
Dysfunctions shared by COVID-19 and SCZ. Dysregulation of energy metabolism can result in disruptions to the immune system and abnormalities within the CNS. Furthermore, immune system disturbances may also contribute to CNS abnormalities in both SCZ and COVID-19. COVID-19, coronavirus disease 2019; SCZ, schizophrenia; CNS, central nervous system.
3. Macro-factors that could increase the risk of COVID-19 infection and sequelae in patients with SCZ
3.1. Impact of sociodemographic factors on COVID-19 risk in individuals with SCZ
Sociodemographic characteristics (e.g., living in group housing, being homeless) and mental disorders (e.g., psychosis and cognitive dysfunction) can affect pandemic-related social distancing measures in individuals with SCZ, thus increasing the risk of contracting COVID-19 (Kozloff, Mulsant et al. 2020). In terms of disease prognosis, physical comorbidities related to the illness and treatment as well as low socioeconomic status may increase the risk of adverse health outcomes for patients with SCZ who also have COVID-19 compared to the general population of patients with SCZ (Kozloff, Mulsant et al. 2020). In addition, high-risk infection factors also include failures to properly recognize self-protection and adherence to preventive behaviors (Yao, Chen et al. 2020), difficulties in evaluating health information, limitations in access to healthcare (Shinn and Viron, 2020), and being easily influenced by ongoing media coverage of the epidemic (Holmes, O'Connor et al. 2020).
3.2. Effect of reduced access to psychiatric healthcare on risk of COVID-19 for individuals with SCZ
The COVID-19 pandemic reduced access to healthcare services, increased rates of early psychiatric discharge, and disrupted face-to-face psychiatric care for people with pre-existing mental illness, which potentially increased the risk of relapse and suicide (Moreno, Wykes et al. 2020). An SCZ spectrum diagnosis was significantly associated with mortality in the 45 days after a positive COVID-19 diagnosis in a U.S. cohort study (Fond et al., 2021, Nemani et al., 2021, Wang et al., 2021). Data from a French national hospital database showed higher in-hospital mortality and lower intensive care unit admission rates for individuals with SCZ than for matched controls. Differences were statistically significant in the age group of ≥65 to <80 years (Fond, Pauly et al. 2021). The COVID-19 pandemic has significantly changed the access to and provision of physical and mental healthcare (Moreno et al., 2020, Mansfield et al., 2021). For example, the number of general telehealth visits increased by 50 % during the first quarter of 2020 compared with the same period in 2019 (Koonin, Hoots et al. 2020). Furthermore, psychiatric emergency admissions decreased at the start of the COVID-19 pandemic lockdown, but the percentage of individuals hospitalized for acute psychiatric care increased significantly (Gómez-Ramiro, Fico et al. 2021).
3.3. Relationship of structural barriers to vaccination with COVID-19 risk for individuals with SCZ
Based on past global experiences with infectious disease control, researchers have identified two types of barriers for vaccination: structural and attitudinal (Zhang and Fisk, 2021). The proportion of individuals with SCZ who were under-vaccinated was higher than unaffected populations despite having similar general attitudes towards vaccination and higher willingness to be vaccinated against COVID-19. Thus, vaccine hesitancy does not appear to be a major barrier for COVID-19 vaccine uptake amongst people with SCZ and disparities in COVID-19 vaccination rates for patients with SCZ appear to be related to structural rather than attitudinal barriers. Structural barriers correspond to systemic issues that may limit the ability of individuals to access vaccine services (Zhang and Fisk, 2021). Several structural barriers related to SCZ have been identified including cost, limited access to online health information, awareness of services, or absence of medical recommendation (Miles et al., 2020, Warren et al., 2021). The development of targeted vaccination programs for this population is urgently needed (De Hert et al., 2021, Mazereel et al., 2021). It is also important to prioritize COVID-19 vaccination in individuals with serious mental illnesses like SCZ (Warren, Kisely et al. 2021).
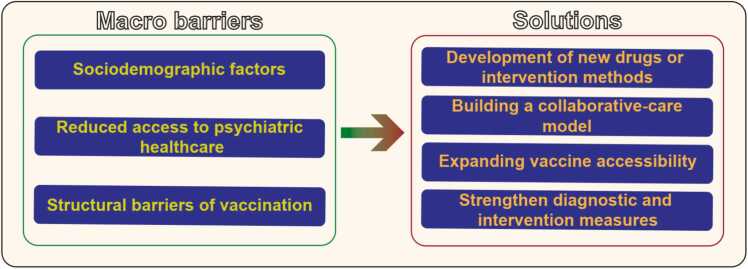
Overall, several micro-factors contribute to the high infection and mortality rates of COVID-19 in patients with SCZ, including sociodemographic factors, limited access to psychiatric health services, structural obstacles hindering COVID-19 immunization (Fig. 3).
Fig. 3.
Micro-factors that could increase the risk of COVID-19 infection and sequelae in patients with SCZ and solutions. COVID-19, coronavirus disease 2019; SCZ, schizophrenia.
4. Solutions
4.1. Development of new drugs or intervention methods
One of the most effective ways to address high infection and mortality rates for an infectious disease like COVID-19 is to develop new drugs or intervention methods that target important signaling or metabolic pathways based on confirmed pathogenic mechanisms. Repurposing of previously developed drugs is also an important approach to obtaining effective treatments.
To date, treatments for COVID-19 include several small-molecule antiviral drugs like nirmatrelvir–ritonavir (Paxlovid), remdesivir and molnupiravir, as well as immunomodulatory drugs, including glucocorticoids like dexamethasone. Cytokine antagonists (e.g., tocilizumab) and Janus kinase inhibitors (e.g., baricitinib) and 11 monoclonal antibodies treatments have also been used (Li, Hilgenfeld et al. 2023). All of these drugs require a prescription. Other drugs including different types of antiviral drugs, antibody therapies, and immune modulators are currently undergoing clinical trials, but have not yet received approval (Li, Hilgenfeld et al. 2023). For the latest information on the progress of clinical trials for COVID-19 therapeutics, websites like clinicaltrials.gov can be consulted.
4.2. Building a collaborative-care model
Due to the increased risk of COVID-19 infection and associated morbidity and mortality, as well as COVID-19 infection control measures that reduce access to out-patient, in-hospital, and group-based psychiatric care for individuals with severe mental illness, a fully integrated, collaborative-care model is the most important aspect of care for individuals with SCZ during and after the COVID-19 pandemic. COVID-19 pandemic-related healthcare service changes include expanding telehealth services and at-home treatment options (Moreno, Wykes et al. 2020). We recognize the increasing importance of family members and caregivers as integral parts of the support network for people with severe mental illness during and after the COVID-19 pandemic and the potential of their inclusion in collaborative-care models. Other aspects of effective collaborative-care models include individual education and disease awareness. The protective effect of psychiatric care and of antipsychotic medication use underscore the importance of continuing care throughout the COVID-19 pandemic (Teixeira, Krause et al. 2021).
4.3. Expanding vaccine accessibility
Overcoming structural obstacles for COVID-19 vaccination of patients with SCZ and improving vaccination rates for these individuals are currently the most direct and effective ways to reduce the infection and mortality rates. Governments and charitable organizations can implement the following measures to overcome the existing barriers and obstacles.
4.3.1. Information dissemination
In addressing the issue that vaccination among individuals with SCZ may be hindered by negative beliefs and misconceptions regarding vaccine safety, healthcare institutions, research organizations, and government health departments should provide easily understandable information about vaccines to patients and their families. This information should include details about the safety and efficacy of vaccines, helping to alleviate their concerns and enhance their willingness to get vaccinated. Furthermore, this initiative will foster a sense of social acceptance for the government’s control measures in response to future epidemic outbreaks.
4.3.2. Priority vaccination policy
The development of targeted vaccination programs for this population is urgently needed (De Hert et al., 2021, Mazereel et al., 2021). It is also essential to prioritize COVID-19 vaccination for individuals with serious mental illnesses, such as schizophrenia (Warren, Kisely et al. 2021), due to their higher risk of infection and greater need for medical resources. China has procured COVID-19 vaccines for the entire population, ensuring that every citizen can benefit from vaccination. Additionally, individuals with moderate to severe mental illnesses—such as schizophrenia, major depressive disorder, bipolar disorder, obsessive-compulsive disorder, intellectual disabilities accompanied by mental disorders, epilepsy with associated mental disorders, and paranoid psychosis—are included in the scope of major illness insurance coverage, alleviating the financial burden on families, including those with schizophrenia. Moreover, patients with severe mental illness should be recognized as a vulnerable group and granted priority access to vaccination and other related services.
4.3.3. Provide vaccination convenience
Setting up dedicated vaccination points in mental health facilities, hospitals, or community centers allows individuals with mental illnesses to receive vaccines in a familiar and comfortable environment. Governments and charitable organizations can offer free transportation services to assist individuals with mental illnesses who are unable to reach vaccination sites on their own. Ensuring that patients can receive vaccinations at home through mobile vaccination units or home visit services can help alleviate their anxiety and discomfort.
4.3.4. Provide healthcare services
Offering mental health support at vaccination sites helps patients cope with the stress and anxiety that may accompany the vaccination process. Training vaccination staff to understand the specific needs of individuals with mental illnesses will enable them to provide more attentive and compassionate care. Encouraging family members to participate in the vaccination process can provide the necessary support and companionship for patients.
By implementing these measures, governments and charitable organizations can significantly improve the accessibility and uptake of COVID-19 vaccinations among individuals with SCZ, ensuring that every patient can benefit from the vaccine.
4.4. Strengthen diagnostic and intervention measures
Diagnostic factors, in terms of preventing disease and identifying populations that have increased susceptibility to COVID-19, especially those that are prone to severe COVID-19, are critical for optimizing preventive measures. Young or obese people with SCZ and those people with SCZ or schizoaffective disorders who have attempted suicide have higher rates of COVID-19 diagnosis. These patients are more prone to long-term neuropsychiatric sequelae associated with COVID-19 and thus should be monitored closely (Okusaga, Kember et al. 2021).
In summary, several strategies have been proposed to address the micro-factors contributing to the high infection and mortality rates of COVID-19 among patients with SCZ. These include developing novel drugs or interventions, establishing collaborative care models, improving vaccine accessibility, and enhancing diagnostic and intervention measures (Fig. 3).
5. Discussion
Although the COVID-19 pandemic has passed, the pathogenic mechanisms of SARS-CoV-2 remain largely unclear. Our literature review reveals that patients with schizophrenia exhibit higher rates of COVID-19 infection and mortality, primarily due to both micro and macro factors. On the micro level, both schizophrenia and COVID-19 are characterized by energy metabolism dysregulation, immune system disruption, and abnormalities in the central nervous system (CNS). Dysregulation of energy metabolism can lead to immune system disturbances and CNS abnormalities. Additionally, immune system dysfunction may further exacerbate CNS abnormalities in both schizophrenia and COVID-19. Of course, there are many metabolic pathways and signaling pathways shared by schizophrenia and COVID-19, but due to space constraints, we will not elaborate on them here. On the macro level, the higher rates of COVID-19 infection and mortality among patients with schizophrenia can be attributed to several factors, including sociodemographic influences, decreased access to psychiatric healthcare, and structural barriers to COVID-19 vaccination.
In the post-pandemic era, the mortality rate among individuals with schizophrenia has significantly decreased, likely due to improved access to healthcare resources and a reduction in the virulence and sporadic transmission of the coronavirus. The current consensus is that the most effective and economical way to lower the high rates of COVID-19 infection and mortality among patients with schizophrenia is to enhance the accessibility of COVID-19 vaccinations, enabling a larger number of these patients to benefit from vaccination. However, numerous barriers and obstacles to vaccine accessibility still exist worldwide. Addressing these barriers will provide critical life-saving support for individuals with schizophrenia in the face of future epidemic diseases.
Therefore, it is essential to examine and consider several recommendations regarding the obstacles to vaccinating people with schizophrenia during epidemic outbreaks. Awareness and Education: There is a need for increased awareness and education regarding the importance of vaccinations among individuals with SCZ, their caregivers, and healthcare providers. Mental Health Stigma: Stigma surrounding mental health issues can deter individuals with SCZ from seeking vaccinations, highlighting the importance of public awareness campaigns to combat these misconceptions. Access to Healthcare Services: Accessibility to healthcare facilities, including transportation challenges and geographical barriers, can impede vaccination efforts. Solutions may include mobile vaccination units and home visit programs. Psychological Barriers: Anxiety and fear related to medical procedures may be heightened in individuals with SCZ. Comprehensive mental health support during the vaccination process is crucial. Healthcare Provider Training: It is essential to train healthcare providers to address the specific needs of individuals with SCZ, ensuring they receive compassionate and informed care. Support Systems: Family involvement and support play a critical role in encouraging vaccination among individuals with SCZ, ensuring they have the necessary encouragement and companionship. Policy and Funding: Governments and organizations should create policies and allocate funding to enhance vaccination accessibility for vulnerable populations, including individuals with mental illnesses. Trust in Medical Systems: Building trust in medical systems among individuals with SCZ is essential for encouraging their participation in vaccination programs. Transparency about vaccine safety and efficacy can contribute to this trust. These reflections emphasize the importance of a multifaceted approach to overcoming the barriers faced by individuals with SCZ in accessing vaccinations during epidemic outbreaks.
6. Conclusion
This article reviews the factors associated with the high COVID-19 infection and mortality rates among patients with SCZ. The micro-factors include energy metabolism dysregulation, immune system disruption, and abnormalities in the CNS. Dysregulation in these pathways is present in both COVID-19 and SCZ. Specifically, disturbances in energy metabolism can lead to immune system disruptions and CNS abnormalities. Moreover, immune system disturbances may also exacerbate CNS abnormalities in both SCZ and COVID-19. Meanwhile, macro factors that can contribute to high rates of COVID-19 infection and mortality of patients with SCZ include sociodemographic factors, reduced access to psychiatric healthcare, and structural barriers for COVID-19 vaccination, which should all be addressed to protect patients with SCZ from SARS-CoV-2 infection. Improving accessibility to vaccines and other medical resources may be the most effective way to reduce COVID-19 infection and mortality rates among patients with SCZ. The COVID-19 pandemic highlighted the importance of a fully integrated collaborative-care model to ensure regular, routine healthcare contact as well as access to prescribed treatments and services for individuals who have SCZ or other severe mental disorders. The present findings highlight the need for improved surveillance and further exploration of possible interventions that can reduce the risk of COVID-19, particularly severe COVID-19, among members of this vulnerable group.
Author contributions
L.-F.L. and R.-J.F. originated the idea for this article and created the manuscript. Z.-Y.L., Y.-Q.L. and J.-R.Z. worked on writing the original draft. L.-F.L. and R.-J.F. performed the review and editing of the manuscript. Additionally, J.W., K.-Z.L., P.W., C.-M.G., H.W., Y.-J.Z., Y.C., Y.G., H.-B.Z. and H.L. assisted with the literature search, and all authors made significant intellectual contributions to the article. Furthermore, all authors critically evaluated the manuscript and approved its publication.
Funding
This work was supported by Key Research and Development Program of Hainan Province (No. ZDYF2022SHFZ310), Hainan Provincial Natural Science Foundation of China (No. 821MS0775, 823RC493), Research Startup Fund for Introduced Talents of Hainan Medical University (No. XRC202033, XRC202118, 821QN261), National Undergraduate Training Program for Innovation and Entrepreneurship of Hainan Medical University (No. 202211810010, X202211810140), Innovative Scientific Research Project for Graduate Students in Ordinary Higher Education Institutions in Hainan Province (No. HYYB2022A16).
Ethical statement
This is a review manuscript, not ethical.
CRediT authorship contribution statement
Yu-Jing Zhang: Software. Han Wang: Visualization. Chun-Mei Gong: Visualization. Kun-Ze Liu: Resources. Li-Fang Lu: Writing – review & editing. Yu-Qian Li: Writing – original draft. Han-Bo Zhang: Software. Zhen-Ying Li: Writing – original draft. Yue Gu: Investigation. Yu Cao: Software. Jie Wang: Resources. Ren-Jun Feng: Writing – review & editing, Conceptualization. Peng Wang: Resources. Hui Lu: Software. Jing-Ru Zhou: Writing – original draft.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
We would like to thank Dr. Rajiv Tandon and Dr. William T Carpenter for his help, comments, and suggestions.
Contributor Information
Li-Fang Lu, Email: lulifang@muhn.edu.cn.
Ren-Jun Feng, Email: fengrenjun@muhn.edu.cn.
References
- Antunes A., Reis-de-Oliveira G., Martins-de-Souza D. Molecular overlaps of neurological manifestations of COVID-19 and schizophrenia from a proteomic perspective. Eur. Arch. Psychiatry Clin. Neurosci. 2024 doi: 10.1007/s00406-024-01842-8. [DOI] [PubMed] [Google Scholar]
- Baranova A., Cao H., Zhang F. "Severe COVID-19 increases the risk of schizophrenia.". Psychiatry Res. 2022;317 doi: 10.1016/j.psychres.2022.114809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielanin J.P., Sun D. Significance of microglial energy metabolism in maintaining brain homeostasis. Transl. Stroke Res. 2023;14(4):435–437. doi: 10.1007/s12975-022-01069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buki A., Kekesi G., Horvath G., Vecsei L. A potential interface between the kynurenine pathway and autonomic imbalance in schizophrenia. Int J. Mol. Sci. 2021;22(18) doi: 10.3390/ijms221810016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-García A., Bolaños J.P., Marsicano G. Metabolic messengers: endocannabinoids. Nat. Metab. 2022;4(7):848–855. doi: 10.1038/s42255-022-00600-1. [DOI] [PubMed] [Google Scholar]
- Chen W., Zhao H., Li Y. "Mitochondrial dynamics in health and disease: mechanisms and potential targets.". Signal Transduct. Target Ther. 2023;8(1):333. doi: 10.1038/s41392-023-01547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer A.L., Carrier M., Tremblay M.E., Cruz-Martin A. "The inflamed brain in schizophrenia: the convergence of genetic and environmental risk factors that lead to uncontrolled neuroinflammation.". Front Cell Neurosci. 2020;14:274. doi: 10.3389/fncel.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario C., Micale V., Di Bartolomeo M., Stark T., Pucci M., Sulcova A., Palazzo M., Babinska Z., Cremaschi L., Drago F., Carlo Altamura A., Maccarrone M., Dell'Osso B. "A preliminary study of endocannabinoid system regulation in psychosis: Distinct alterations of CNR1 promoter DNA methylation in patients with schizophrenia.". Schizophr. Res. 2017;188:132–140. doi: 10.1016/j.schres.2017.01.022. [DOI] [PubMed] [Google Scholar]
- De Hert M., Mazereel V., Detraux J., Van Assche K. Prioritizing COVID-19 vaccination for people with severe mental illness. World Psychiatry. 2021;20(1):54–55. doi: 10.1002/wps.20826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressing A., Bormann T., Blazhenets G., Schroeter N., Walter L.I., Thurow J., August D., Hilger H., Stete K., Gerstacker K., Arndt S., Rau A., Urbach H., Rieg S., Wagner D., Weiller C., Meyer P.T., Hosp J.A. Neuropsychologic profiles and cerebral glucose metabolism in neurocognitive long covid syndrome. J. Nucl. Med. 2022;63(7):1058–1063. doi: 10.2967/jnumed.121.262677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria-Pereira A., Morais V.A. Synapses: the brain's energy-demanding sites. Int J. Mol. Sci. 2022;23(7) doi: 10.3390/ijms23073627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G., Nemani K., Etchecopar-Etchart D., Loundou A., Goff D.C., Lee S.W., Lancon C., Auquier P., Baumstarck K., Llorca P.M., Yon D.K., Boyer L. Association between mental health disorders and mortality among patients with COVID-19 in 7 countries: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(11):1208–1217. doi: 10.1001/jamapsychiatry.2021.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G., Pauly V., Leone M., Llorca P.M., Orleans V., Loundou A., Lancon C., Auquier P., Baumstarck K., Boyer L. Disparities in intensive care unit admission and mortality among patients with schizophrenia and COVID-19: a national cohort study. Schizophr. Bull. 2021;47(3):624–634. doi: 10.1093/schbul/sbaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Ramiro M., Fico G., Anmella G., Vázquez M., Sagué-Vilavella M., Hidalgo-Mazzei D., Pacchiarotti I., Garriga M., Murru A., Parellada E., Vieta E. "Changing trends in psychiatric emergency service admissions during the COVID-19 outbreak: report from a worldwide epicentre.". J. Affect Disord. 2021;282:26–32. doi: 10.1016/j.jad.2020.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E.A., O'Connor R.C., Perry V.H., Tracey I., Wessely S., Arseneault L., Ballard C., Christensen H., Cohen Silver R., Everall I., Ford T., John A., Kabir T., King K., Madan I., Michie S., Przybylski A.K., Shafran R., Sweeney A., Worthman C.M., Yardley L., Cowan K., Cope C., Hotopf M., Bullmore E. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7(6):547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin L.M., Hoots B., Tsang C.A., Leroy Z., Farris K., Jolly T., Antall P., McCabe B., Zelis C.B.R., Tong I., Harris A.M. Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(43):1595–1599. doi: 10.15585/mmwr.mm6943a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff N., Mulsant B.H., Stergiopoulos V., Voineskos A.N. The COVID-19 global pandemic: implications for people with schizophrenia and related disorders. Schizophr. Bull. 2020;46(4):752–757. doi: 10.1093/schbul/sbaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Hilgenfeld R., Whitley R., De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat. Rev. Drug Discov. 2023;22(6):449–475. doi: 10.1038/s41573-023-00672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.F., Wang J., Liu K.Z., Yi X.N., Ma Z.J., Li Y.Q., Feng R.J. "Double-strand breaks induced by learning-like activity may increase risk of de novo mutations in schizophrenia.". Asian J. Psychiatr. 2022;78 doi: 10.1016/j.ajp.2022.103292. [DOI] [PubMed] [Google Scholar]
- Lüscher C., Malenka R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb. Perspect. Biol. 2012;4(6) doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C., Giacopuzzi E., La Via L., Bonini D., Ravasio V., Elhussiny M.E.A., Orizio F., Gangemi F., Valsecchi P., Bresciani R., Barbon A., Vita A., Gennarelli M. "A novel homozygous mutation in GAD1 gene described in a schizophrenic patient impairs activity and dimerization of GAD67 enzyme.". Sci. Rep. 2018;8(1):15470. doi: 10.1038/s41598-018-33924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield K.E., Mathur R., Tazare J., Henderson A.D., Mulick A.R., Carreira H., Matthews A.A., Bidulka P., Gayle A., Forbes H., Cook S., Wong A.Y.S., Strongman H., Wing K., Warren-Gash C., Cadogan S.L., Smeeth L., Hayes J.F., Quint J.K., McKee M., Langan S.M. "Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: a population-based study. Lancet Digit Health. 2021;3(4):e217–e230. doi: 10.1016/S2589-7500(21)00017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazereel V., Vanbrabant T., Desplenter F., De Hert M. COVID-19 vaccine uptake in patients with psychiatric disorders admitted to or residing in a university psychiatric hospital. Lancet Psychiatry. 2021;8(10):860–861. doi: 10.1016/S2215-0366(21)00301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Zelzer S., Schnedl W.J., Baranyi A., Meinitzer A., Enko D. "Assessment of tryptophan and kynurenine as prognostic markers in patients with SARS-CoV-2.". Clin. Chim. Acta. 2022;525:29–33. doi: 10.1016/j.cca.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles L.W., Williams N., Luthy K.E., Eden L. Adult Vaccination Rates in the Mentally Ill Population: An Outpatient Improvement Project. J. Am. Psychiatr. Nurses Assoc. 2020;26(2):172–180. doi: 10.1177/1078390319831763. [DOI] [PubMed] [Google Scholar]
- Mohan M., Perry B.I., Saravanan P., Singh S.P. COVID-19 in people with schizophrenia: potential mechanisms linking schizophrenia to poor prognosis. Front. Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.666067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Wykes T., Galderisi S., Nordentoft M., Crossley N., Jones N., Cannon M., Correll C.U., Byrne L., Carr S., Chen E.Y.H., Gorwood P., Johnson S., Kärkkäinen H., Krystal J.H., Lee J., Lieberman J., López-Jaramillo C., Männikkö M., Phillips M.R., Uchida H., Vieta E., Vita A., Arango C. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813–824. doi: 10.1016/S2215-0366(20)30307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani K., Li C., Olfson M., Blessing E.M., Razavian N., Chen J., Petkova E., Goff D.C. Association of psychiatric disorders with mortality among patients With COVID-19. JAMA Psychiatry. 2021;78(4):380–386. doi: 10.1001/jamapsychiatry.2020.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusaga O.O., Kember R.L., Peloso G.M., Peterson R.E., Vujkovic M., Mitchell B.G., Bernard J., Walder A., Bigdeli T.B. "History of suicide attempts and COVID-19 Infection in Veterans with Schizophrenia or Schizoaffective Disorder: Moderating Effects of Age and Body Mass Index.". Complex Psychiatry. 2021;392:1789–1795. doi: 10.1159/000521230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M., Gerhard T., Huang C., Crystal S., Stroup T.S. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172–1181. doi: 10.1001/jamapsychiatry.2015.1737. [DOI] [PubMed] [Google Scholar]
- Papadopoulos K.I., Sutheesophon W., Aw T.C. "Genetic polymorphisms affecting nitric oxide and beta-cytokine pathways may contribute to increased COVID-19 mortality in schizophrenia.". Asian J. Psychiatr. 2022;69 doi: 10.1016/j.ajp.2021.102981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi M.A., Nassireslami E., Yousefi Zoshk M., Hosseini Y., Abbasian K., Chamanara M. Phosphodiesterase inhibitors in psychiatric disorders. Psychopharmacol. (Berl. ) 2023;240(6):1201–1219. doi: 10.1007/s00213-023-06361-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Rico M., Limosin F., Hoertel N. Is a diagnosis of schizophrenia spectrum disorder associated with increased mortality in patients with COVID-19? Am. J. Psychiatry. 2022;179(1):71–73. doi: 10.1176/appi.ajp.2021.21020196. [DOI] [PubMed] [Google Scholar]
- Scheyer A., Yasmin F., Naskar S., Patel S. Endocannabinoids at the synapse and beyond: implications for neuropsychiatric disease pathophysiology and treatment. Neuropsychopharmacology. 2023;48(1):37–53. doi: 10.1038/s41386-022-01438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieler L., Larsson M.K., Skogh E., Kegel M.E., Orhan F., Abdelmoaty S., Finn A., Bhat M., Samuelsson M., Lundberg K., Dahl M.L., Sellgren C., Schuppe-Koistinen I., Svensson C., Erhardt S., Engberg G. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia--significance for activation of the kynurenine pathway. J. Psychiatry Neurosci. 2015;40(2):126–133. doi: 10.1503/jpn.140126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S., Shuman M., Duncan E. An emerging role of cGMP in the treatment of schizophrenia: a review. Schizophr. Res. 2016;170(1):226–231. doi: 10.1016/j.schres.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Shinn A.K., Viron M. Perspectives on the COVID-19 pandemic and individuals with serious mental illness. J. Clin. Psychiatry. 2020;81(3) doi: 10.4088/JCP.20com13412. [DOI] [PubMed] [Google Scholar]
- Singh T., Poterba T., Curtis D., Akil H., Al Eissa M., Barchas J.D., Bass N., Bigdeli T.B., Breen G., Bromet E.J., Buckley P.F., Bunney W.E., Bybjerg-Grauholm J., Byerley W.F., Chapman S.B., Chen W.J., Churchhouse C., Craddock N., Cusick C.M., DeLisi L., Dodge S., Escamilla M.A., Eskelinen S., Fanous A.H., Faraone S.V., Fiorentino A., Francioli L., Gabriel S.B., Gage D., Gagliano Taliun S.A., Ganna A., Genovese G., Glahn D.C., Grove J., et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604(7906):509–516. doi: 10.1038/s41586-022-04556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub R.H. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat. Rev. Rheuma. 2017;13(12):743–751. doi: 10.1038/nrrheum.2017.172. [DOI] [PubMed] [Google Scholar]
- Taoro-González L., Cabrera-Pastor A., Sancho-Alonso M., Felipo V. "Intracellular and extracelluar cyclic GMP in the brain and the hippocampus.". Vitam. Horm. 2022;118:247–288. doi: 10.1016/bs.vh.2021.11.006. [DOI] [PubMed] [Google Scholar]
- Teixeira A.L., Krause T.M., Ghosh L., Shahani L., Machado-Vieira R., Lane S.D., Boerwinkle E., Soares J.C. "Analysis of COVID-19 infection and mortality among patients with psychiatric disorders, 2020.". JAMA Netw. Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.34969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengholm A., Gylfe E. "cAMP signalling in insulin and glucagon secretion.". Diabetes Obes. Metab. 2017;19(1):42–53. doi: 10.1111/dom.12993. [DOI] [PubMed] [Google Scholar]
- Thomas T., Stefanoni D., Reisz J.A., Nemkov T., Bertolone L., Francis R.O., Hudson K.E., Zimring J.C., Hansen K.C., Hod E.A., Spitalnik S.L., D'Alessandro A. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5(14) doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Xu R., Volkow N.D. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2021;20(1):124–130. doi: 10.1002/wps.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren N., Kisely S., Siskind D. Maximizing the uptake of a COVID-19 vaccine in people with severe mental illness: a public health priority. JAMA Psychiatry. 2021;78(6):589–590. doi: 10.1001/jamapsychiatry.2020.4396. [DOI] [PubMed] [Google Scholar]
- Yao H., Chen J.H., Xu Y.F. "Patients with mental health disorders in the COVID-19 epidemic." Lancet. Psychiatry. 2020;7(4) doi: 10.1016/S2215-0366(20)30090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Liao X., Zhong Y., Wu Y., Lai X., Jiao H., Yan M., Zhang Y., Ma C., Wang S. The candidate schizophrenia risk gene Tmem108 regulates glucose metabolism homeostasis. Front Endocrinol. (Lausanne) 2021;12 doi: 10.3389/fendo.2021.770145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Fisk R.J. Barriers to vaccination for coronavirus disease 2019 (COVID-19) control: experience from the United States. Glob. Health J. 2021;5(1):51–55. doi: 10.1016/j.glohj.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.-P., He Z.-C., Yao X.-H., Tang R., Ma J., Luo T., Zhu C., Li T.-R., Liu X., Zhang D., Zhang S., Ping Y.-F., Leng L., Bian X.-W. COVID-19-associated monocytic encephalitis (CAME): histological and proteomic evidence from autopsy. Signal Transduct. Target. Ther. 2023;8(1) doi: 10.1038/s41392-022-01291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]