Highlights
-
•
EAT-10 total scores were negatively correlated with BMI twelve months later in XDP.
-
•
EAT-10 total scores predicted BMI twelve months later in XDP.
-
•
EAT-10 total scores ≥ 4 predicted malnutrition twelve months later in XDP.
Keywords: X-linked dystonia-parkinsonism, Dysphagia, Malnutrition, BMI, Eating Assessment Tool-10 (EAT-10)
Abstract
Introduction
Malnutrition is a leading cause of death for persons living with X-linked dystonia-parkinsonism (XDP), a degenerative disease endemic to the Philippines. Difficulty swallowing has been linked to malnutrition in other populations; however, knowledge of this relationship is limited in XDP. As such, the purpose of this study was to determine the association between dysphagia and malnutrition in this population.
Method(s)
21 individuals with XDP, 26 controls, and 18 genetic carriers were included in the final data analysis. Spearman’s rank order correlation coefficient was used to determine an association between baseline EAT-10 total scores and 12-month malnutrition status, and multiple linear regression to evaluate the predictive ability of the EAT-10. A baseline EAT-10 score cut-off point predicting 12-month malnutrition status was estimated.
Results
For the XDP group, the baseline EAT-10 total scores had a significant negative correlation (r = -0.68, p < 0.001) with and was a significant predictor (p = 0.001) of 12-month BMI. A baseline EAT-10 total score of ≥ 4 predicted malnutrition twelve months after administration (sensitivity = 0.93; specificity = 1; AUC = 0.95).
Discussion
Dysphagia, as measured using the EAT-10, was associated with BMI in the XDP population. Additionally, an EAT-10 total score ≥ 4 could predict malnutrition in twelve months after test administration. With these findings, healthcare providers could identify patients with XDP at high risk for malnutrition earlier and provide intervention sooner.
1. Introduction
X-linked dystonia-parkinsonism (XDP), a rare degenerative disease endemic to the Philippines, is characterized by signs of either or both dystonia and parkinsonism, including bradykinesia and rigidity [1]. Approximately 94 % of persons with XDP [2] have significant difficulties with swallowing impairments (dysphagia) stemming from dystonic oropharyngeal motor movement [3] or aerodigestive dysfunction [4]. On the resource-limited island of Panay, diagnosing and treating dysphagia in patients with XDP is challenging due to the lack of access to instrumental and clinical swallowing evaluations [5].
Malnutrition, defined as a body mass index (BMI) of less than 18.4 kg/m2, is a leading cause of death in the XDP population [4] and is associated with other poor outcomes including prolonged recovery from illness, increased hospitalization, reduced mobility, depression, and decreased quality of life [6]. Despite the significant impact that malnutrition has on patient health outcomes and quality of life, understanding of malnutrition in XDP is limited and risk factors for the development of malnutrition are unknown.
Dysphagia is a significant risk factor for malnutrition in Parkinson’s disease [7], and has been reported in oromandibular dystonia [8]. However, its impact on nutritional status in XDP is not well understood. In the absence of instrumental and clinical swallowing assessments, a patient-reported outcome measure of dysphagia called the Eating Assessment Tool 10 (EAT-10) could be a sensitive measure of swallowing severity in XDP [5], [9]. Although a patient-reported tool, the EAT-10 has demonstrated high accuracy in identifying individuals with aspiration and other swallowing impairments in degenerative diseases such as amyotrophic lateral sclerosis [10], [11]. The goal of this study was to determine (1) the association between dysphagia and malnutrition in XDP and (2) the predictive ability of the EAT-10 for malnutrition in individuals with confirmed TAF1 SVA repeat expansion mutation causing XDP. Understanding the relationship between dysphagia and malnutrition and identifying those at risk may improve targeted nutritional management for those with XDP.
2. Methods
2.1. Participants
This study was approved by the Institutional Review Boards at Jose Reyes Hospital (Manila, Philippines) and Mass General Brigham Healthcare (Boston, Massachusetts). All participants provided written informed consent to share de-identified data with the Dystonia Partners Research Bank hosted by Mass General Brigham Healthcare.
Initially, a total of 142 potential participants were included in the data set. All of them had undergone a standardized examination by a bilingual neurologist. They did not have a concurrent medical illness, deep brain stimulation, or cognitive dysfunction as determined by the neurologist. Participants were assigned to the XDP, carrier, or control groups based on the presence of the genetic mutation in the X-chromosome and neurologic symptoms (dystonia, parkinsonism, or dystonia-parkinsonism). Thus, individuals in the XDP group had the TAF1 SVA repeat expansion mutation and neurologic symptoms; those in the carrier group had the mutation, but no neurologic symptoms; and the control group had neither the mutation nor neurologic symptoms. To be included in the final data analysis, participants needed to have complete weight, height, and EAT-10 data at both baseline and twelve-month timepoints.
2.2. Assessment
The Eating Assessment Tool-10 (EAT-10) is a well-established dysphagia patient-reported outcome measure that has been translated into various Western (e.g., French, Swedish, and Dutch) and Eastern (e.g., Hindi, Arabic, and Chinese) languages. It is a 10-item patient-reported ordinal scale, with each item ranked from 0 to 4 [12]. For all items, a score of 0 indicated no perceived impairment, while a 4 indicated perceived severe impairment. A total score ranging from 0 to 40 can be derived by adding all of the individual items.
2.3. Data collection
The EAT-10 was adapted into the participants’ native language (Hiligaynon or Aklanon) and administered by trained community advocates. This allowed participants to complete the assessment regardless of their literacy skills. Once completed, the assessment was securely transferred from the Philippines to Boston for analysis.
2.4. Statistical analyses
2.4.1. Aim 1
Spearman’s rank order correlation coefficient was used to evaluate the relationship between the baseline EAT-10 total scores derived from an ordinal scale and twelve-month BMI. Prior to the correlation analysis, we screened for normality using the Shapiro-Wilk test. Spearman’s rank order correlations with a p-value of less than 0.05 were deemed statistically significant.
2.4.2. Aim 2
Multiple regression analysis was used to determine if baseline EAT-10 total scores could predict twelve-month BMI. The time from diagnosis to baseline data collection was used as a covariate in the analysis. We hypothesized that individuals who have had XDP for a longer period of time were more likely to have a lower future BMI given that the disease is progressive. Prior to analysis, regression diagnostics were conducted to assess (1) linearity of the relationships between the dependent variable (BMI) and independent variables (EAT-10 baseline total scores and disease duration); (2) independence of the observations; (3) normality and homoscedasticity of the residuals; (4) outliers; and (5) multicollinearity. Additionally, a baseline EAT-10 total score cut-off point identifying individuals at high risk for developing malnutrition twelve months after evaluation was calculated using a Receiver Operating Characteristic (ROC) curve, which included sensitivity, specificity, and area under the curve (AUC).
3. Results
A total of 65 participants who met the inclusion criteria were included in the final data analysis. Demographic information for the included participants is shown in Table 1.
Table 1.
Clinical data of all participants.
| Participant Distribution | n(%) |
Onset n(%) |
Baseline n(%) |
12 Month n(%) |
|---|---|---|---|---|
| XDP | 21(32) | |||
| Dystonia | 18(86) | 0(0) | 1(5) | |
| Parkinsonism | 3(14) | 0(0) | 0(0) | |
| Dystonia-parkinsonism | 0(0) | 20(95) | 20(95) | |
| Unknown | 0(0) | 1(5) | 0(0) | |
| Carrier | 18 (28) | |||
| Control | 26 (40) | |||
| Demographic Data | XDP | Carrier | Control | |
| Male n(%) | 21(100) | 0(0) | 13(50) | |
|
Baseline Age M(SD; range) |
43.86 (10.45; 25–64) |
41.83 (16.80; 19–70) |
38.77 (13.64; 20–75) |
|
|
12-Month Age M(SD; range) |
44.81 (10.35; 26–64) |
42.83 (16.80; 20–71) |
39.73 (13.64; 21–76) |
|
|
Baseline XDP Duration M(SD; range) |
3.43 (2.01; 0–7) |
Not applicable | Not applicable | |
|
12-Month XDP Duration M(SD; range) |
4.43 (2.01; 1–8) |
Not applicable | Not applicable | |
| Clinical Data | ||||
|
Baseline EAT-10 Total M(SD; range) |
14.14 (13.83; 0–34) |
0.33 (1.19; 0–5) |
0.04 (0.20; 0–1) |
|
|
12-Month EAT-10 Total M(SD; range) |
18.05 (13.81; 0–40) |
0.06 (0.24; 0–1) |
0.19 (0.80; 0–4) |
|
|
Baseline BMI M(SD; range) |
19.90 (3.56; 14.47–27.07) |
24.30 (4.12; 16.75–31.02) |
25.56 (4.68; 17.51–33.68) |
|
|
12-Month BMI M(SD; range) |
18.41 (3.35; 14.09–25.68) |
23.67 (3.66; 16.72–28.93) |
24.77 (4.38; 16.81–32.79) |
|
| EAT-10 and BMI r | −0.68 | −0.03 | −0.31 | |
| Correlation p-value | <0.001** | 0.91 | 0.13 | |
| Malnutrition | XDP | Carrier | Control | |
| Baseline, n(%) | 9(43) | 2(11) | 3(12) | |
| 12-Month, n(%) | 14(67) | 2(11) | 3(12) | |
| Botox | XDP | Carrier | Control | |
| Received injection, n(%) | 11(52) | 0(0) | 0(0) | |
r = 0.1-.3 = weak correlation; 0.4-.6 = moderate correlation; 0.7-.9 = strong correlation
* = p < 0.05.; ** = p < 0.001.
3.1. Aim 1
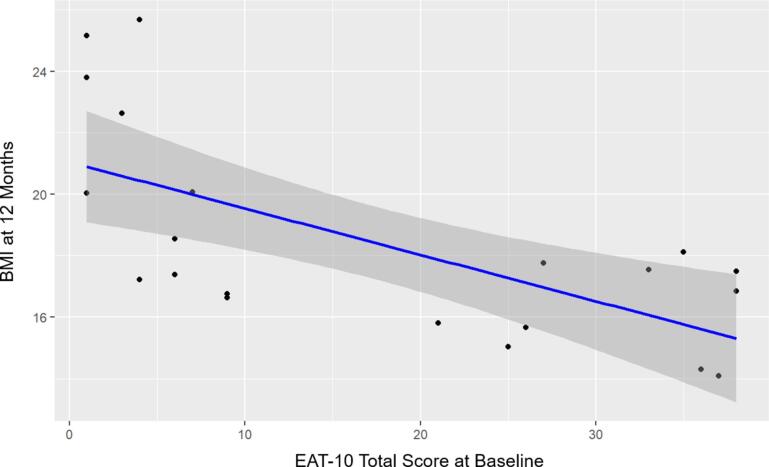
Only the XDP group had a significant, moderately strong inverse relationship between the baseline EAT-10 total scores and BMI at twelve months (p < 0.001, r = -0.68). Neither the carrier (p = 0.91) nor the control (p = 0.13) groups had significant correlations. These results are listed in Table 1 and graphically represented in Fig. 1.
Fig. 1.
Relationship between twelve-month BMI and baseline EAT-10 total scores. Graph displaying the relationship between twelve-month BMI and baseline EAT-10 total score, while accounting for disease duration, in the XDP group.
3.2. Aim 2
In the XDP group, the predictors explained 21.23 % of the variance (R2 = 0.43, F(2,18) = 6.88, p = 0.006). Baseline EAT-10 total scores (β = -0.15, p = 0.005) significantly predicted BMI at 12 months. Disease duration was not statistically significant. When controlling for disease duration, every 1 unit increase in the EAT-10 baseline total scores resulted in a 0.15-unit decrease in 12-month BMI. The resulting regression equation was BMI12 months = 21.24 + (−0.15*EAT-10totalbaseline). When the same multiple regression models were specified for the carrier and control groups, neither disease duration nor baseline EAT-10 total scores were significant predictors of 12-month BMI. Given that neither the carrier nor the control group had disease symptoms, it was not possible to assess disease duration as a predictor for 12-month BMI. The estimated baseline EAT-10 total score cut-off point for determining malnutrition in 12 months was 4, with a sensitivity of 0.93, a specificity of 1, and an AUC of 0.95.
4. Discussion
4.1. Dysphagia is associated with malnutrition in the XDP population
The findings provide evidence of a proportional relationship between dysphagia and malnutrition for people living with XDP: the worse their swallowing impairment (as indexed by EAT-10 scores), the lower their BMI. Over 50 % of individuals with XDP met the criteria for malnutrition (BMI of less than 18.4 kg/m2) over the course of the study.
While the findings link dysphagia and malnutrition in this population, the cause of malnutrition is likely multifactorial and not exclusive to dysphagia [13]. For example, neuropsychiatric symptoms present in XDP, such as depression and anxiety, have been found to contribute to decreased food intake and weight loss in elderly populations. Additionally, symptoms in parkinsonism, such as bradykinesia and rigidity, and oromandibular dystonia, such as involuntary and repetitive muscle contractions of the tongue, lips, jaw, and pharynx, may increase energy expenditure and difficulty self-feeding in individuals with XDP, resulting in increased caloric need and decreased caloric intake. This impact of dystonia severity on weight loss appears to be supported by our study where we found a strong, significant inverse relationship between 12-month BMI and baseline BFMDRS Disability subscale scores.
4.2. The EAT-10 predicts future malnutrition
While malnutrition is likely multifactorial, EAT-10 total scores were found to be predictive of malnutrition while disease duration was not, indicating that dysphagia may be a primary driver of malnutrition in the XDP population. Despite having a variable disease course with mixed symptomology, XDP is progressive, and individuals are likely to experience worsening symptoms over time. XDP phases that are dystonia or parkinsonism dominant occur typically at the time of disease onset, then often transition into more mixed phenotypes that include both dystonia-parkinsonism features over time [1], [3].
Clinically, the results suggest that regardless of the length of their disease, a patient’s nutritional status may be predicted one year after EAT-10 administration. Our findings need to be interpreted cautiously, however, as all of the participants had mixed dystonia-parkinsonism symptoms at the time of data collection and were at least one-year post-symptom onset; it is possible that disease duration could be a significant predictor if the participants had been in the early stages of the disease when only one dominant phenotype was present. Regardless, this work has important clinical implications, as it provides a method of identifying those at risk for malnutrition in this high-risk population in a resource-limited setting.
4.3. Future Directions
Although this study has the potential to assist healthcare providers in identifying and tracking individuals with XDP who are at risk for malnutrition, there are some limitations. First, many physiological, nutritional, and psychological data related to malnutrition could not be collected in our participants who live in the Philippines. This data included instrumental and clinical assessments of swallowing confirming the presence or severity of dysphagia, amount of calorie intake, depressive symptoms, body distribution of fat, and energy expenditure. This was due to the lack of equipment and trained personnel to collect such data in the resource-limited environment of the Philippines. Given such limitations, our goal with this study was to use a patient-reported outcome measure, the EAT-10, which has previously demonstrated high accuracy in detecting aspiration and other swallowing difficulties detectable during instrumental evaluations. Though challenging, it is our hope that future studies can collect this data from individuals with XDP.
Secondly, the data was limited to baseline and 12-month timepoints. Because XDP is a progressive disease, data collected at other time points, such as six or eighteen months, may yield different cut-off points than the 12-month timepoint. Despite the challenges of collecting data in the Philippines on a consistent basis, 6- and 18-month time points should be collected in future studies to better assess changes in swallowing function over time.
Third, the cognitive function of our participants was not assessed at the time of data collection. Because the EAT-10 relies on patient self-report, the presence of cognitive impairment may have skewed the results. Studies have reported the presence of attentional and executive function deficits in the XDP population [14]. Thus, it is possible that the participants in this study also had signs of cognitive impairment.
Finally, we did not account for the potential impact of botulinum toxin injections (Botox) on swallowing in our analysis. Botox injections have been used in the XDP population to alleviate pain from oromandibular dystonia [2]; however, they may negatively impact swallowing function [15]. In our participants with XDP, injection sites included the external nuchal muscles, unilateral sternocleidomastoid muscle, bilateral splenius muscles, and the superior unilateral elevator muscle, depending on the nature of their cervical dystonia. While none of our participants reported changes in their swallowing function following injections, it is possible that botulinum toxin injections may have impacted dysphagia.
5. Conclusion
To our knowledge, this work is the first to explore the relationship between a patient-reported swallowing outcome measure and malnutrition in the XDP population. The analyses have yielded clinically meaningful results that may enable healthcare providers to anticipate future malnutrition and provide timely and appropriate intervention. The findings suggest that the EAT-10 may be a useful assessment that can accurately predict malnutrition in the XDP population. Additionally, it can be administered at any point during the patient’s disease process to predict their nutritional status a year after the evaluation, which is useful for tracking dysphagia and malnutrition risk in a degenerative disease. Future research verifying this work with instrumental or clinical swallowing evaluations, examining the ability of the EAT-10 or other patient-reported outcome measures to predict malnutrition at shorter or more extended timepoints, and extending this work to include more singular XDP phenotypes earlier in the disease process will strengthen the clinical utility of the findings and help improve clinical care for persons living with XDP.
CRediT authorship contribution statement
Tabitha H. Kao: Writing – review & editing, Writing – original draft, Visualization, Formal analysis, Conceptualization. Perman Gochyyev: Writing – review & editing, Formal analysis, Conceptualization. Nutan Sharma: Writing – review & editing. Jan K. de Guzman: Writing – review & editing, Data curation. Melanie Supnet Wells: Writing – review & editing, Data curation. Patrick Acuna: Writing – review & editing. Shasha Li: Writing – review & editing. Hannah P. Rowe: Writing – review & editing. Bridget J. Perry: Writing – review & editing, Writing – original draft, Funding acquisition, Conceptualization.
Funding
This work was supported by the Massachusetts General Hospital Collaborative Center for X-linked Dystonia-Parkinsonism, Boston, MA; the National Institutes of Health (NIH) National Institute on Deafness and Other Communication Disorders [grant number K24DC0016312]; and the NIH National Institute of Neurological Disorders and Stroke [grant number K23NS123369].
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [This work was supported by the Massachusetts General Hospital Collaborative Center for X-linked Dystonia-Parkinsonism, Boston, MA; the National Institutes of Health (NIH) National Institute on Deafness and Other Communication Disorders [grant number K24DC0016312]; and the NIH National Institute of Neurological Disorders and Stroke [grant number K23NS123369]. The funding sources did not participate in the study design; data collection, analysis, and interpretation; report writing; or decision to submit the article for publication.
The authors declare that they have no known competing personal relationships that could have appeared to influence the work reported in this paper].
Acknowledgements
This work was supported by the Massachusetts General Hospital Collaborative Center for X-linked Dystonia-Parkinsonism, Boston, MA; the National Institutes of Health (NIH) National Institute on Deafness and Other Communication Disorders [grant number K24DC0016312]; and the NIH National Institute of Neurological Disorders and Stroke [grant number K23NS123369].
The content of this paper was presented at the 2024 Dysphagia Research Society conference in San Juan, Puerto Rico.
Contributor Information
Tabitha H. Kao, Email: tkao@mghihp.edu.
Perman Gochyyev, Email: pgochyyev@mgb.org.
Nutan Sharma, Email: nsharma@mgb.org.
Jan K. de Guzman, Email: jekneuromd511@gmail.com.
Melanie Supnet Wells, Email: lai.supnet@gmail.com.
Patrick Acuna, Email: pacuna1@mgh.harvard.edu.
Shasha Li, Email: shasha.li@mgh.harvard.edu.
Hannah P. Rowe, Email: h.rowe@northeastern.edu.
Bridget J. Perry, Email: bjperry@mghihp.edu.
References
- 1.Lee L.V., Rivera C., Teleg R.A., Dantes M.B., Pasco P.M.D., Jamora R.D.G., Arancillo J., Villareal-Jordan R.F., Rosales R.L., Demaisip C., Maranon E., Peralta O., Borres R., Tolentino C., Monding M.J., Sarcia S. The unique phenomenology of sex-linked dystonia parkinsonism (XDP, DYT3, “Lubag”) Int. J. Neurosci. 2011;121:3–11. doi: 10.3109/00207454.2010.526728. [DOI] [PubMed] [Google Scholar]
- 2.Song S.A., Go C.L., Acuna P.B., de Guzman J.K.P., Sharma N., Song P.C. Progressive decline in voice and voice-related quality of life in X-linked dystonia parkinsonism. J. Voice. 2020 doi: 10.1016/j.voice.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evidente V.G.H. In: GeneReviews. Adam M.P., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. University of Washington; Washington: 2005. X-linked dystonia-parkinsonism. [Google Scholar]
- 4.Rosales R.L. X-linked dystonia parkinsonism: clinical phenotype, genetics and therapeutics. J. Mov. Disord. 2010;3:32–38. doi: 10.14802/jmd.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaninotto A.L., de Guzman J.K., Stipancic K.L., Perry B.J., Supnet M.L., Go C., Sharma N., Green J.R. Speech and swallowing deficits in X-linked dystonia-parkinsonism. Parkinsonism Relat. Disord. 2021;89:105–110. doi: 10.1016/j.parkreldis.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Neumann S.A., Miller M.D., Daniels L., Crotty M. Nutritional status and clinical outcomes of older patients in rehabilitation. J. Hum. Nutr. Diet. 2005;18:129–136. doi: 10.1111/j.1365-277X.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 7.Sheard J.M., Ash S., Mellick G.D., Silburn P.A., Kerr G.K. Markers of disease severity are associated with malnutrition in Parkinson’s disease. PLoS One. 2013;8:e57986. doi: 10.1371/journal.pone.0057986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papapetropoulos S., Singer C. Eating dysfunction associated with oromandibular dystonia: clinical characteristics and treatment considerations. Head Face Med. 2006;2 doi: 10.1186/1746-160X-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acuna P., Supnet-Wells M.L., Spencer N.A., de Guzman J.K., Russo M., Hunt A., Stephen C., Go C., Carr S., Ganza N.G., Lagarde J.B., Begalan S., Multhaupt-Buell T., Aldykiewicz G., Paul L., Ozelius L., Bragg D.C., Perry B., Green J.R., Miller J.W., Sharma N. Establishing a natural history of X-linked dystonia parkinsonism. Brain Commun. 2023 doi: 10.1093/braincomms/fcad106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plowman E.K., Tabor L.C., Robinson R., Gaziano J., Dion C., Watts S.A., Vu T., Gooch C. Discriminant ability of the Eating Assessment Tool-10 to detect aspiration in individuals with amyotrophic lateral sclerosis. Neurogastroenterol. Motil. 2016;28:85–90. doi: 10.1111/nmo.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donohue C., Tabor Gray L., Anderson A., DiBiase L., Chapin J., Wymer J.P., Plowman E.K. Discriminant ability of the Eating Assessment Tool-10 to detect swallowing safety and efficiency impairments. Laryngoscope. 2022;132:2319–2326. doi: 10.1002/lary.30043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belafsky P.C., Mouadeb D.A., Rees C.J., Pryor J.C., Postma G.N., Allen J., Leonard R.J. Validity and reliability of the Eating Assessment Tool (EAT-10) Ann. Otol. Rhinol. Laryngol. 2008;117:919–924. doi: 10.1177/000348940811701210. [DOI] [PubMed] [Google Scholar]
- 13.Sheard J.M. Malnutrition and neurodegenerative diseases. Curr. Nutr. Rep. 2014;3:102–109. doi: 10.1007/s13668-014-0078-2. [DOI] [Google Scholar]
- 14.Jamora R.D.G., Suratos C.T.R., Bautista J.E.C., Ramiro G.M.I., Westenberger A., Klein C., Ledesma L.K. Neurocognitive profile of patients with X-linked dystonia-parkinsonism. J. Neural Transm. 2021;128:671–678. doi: 10.1007/s00702-021-02317-z. [DOI] [PubMed] [Google Scholar]
- 15.Castelão M., Marques R.E., Duarte G.S., Rodrigues F.B., Ferriera J., Sampaio C., Moore A.P., Costa J. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst. Rev. 2017;12:CD003633. doi: 10.1002/14651858.CD003633.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]