Abstract
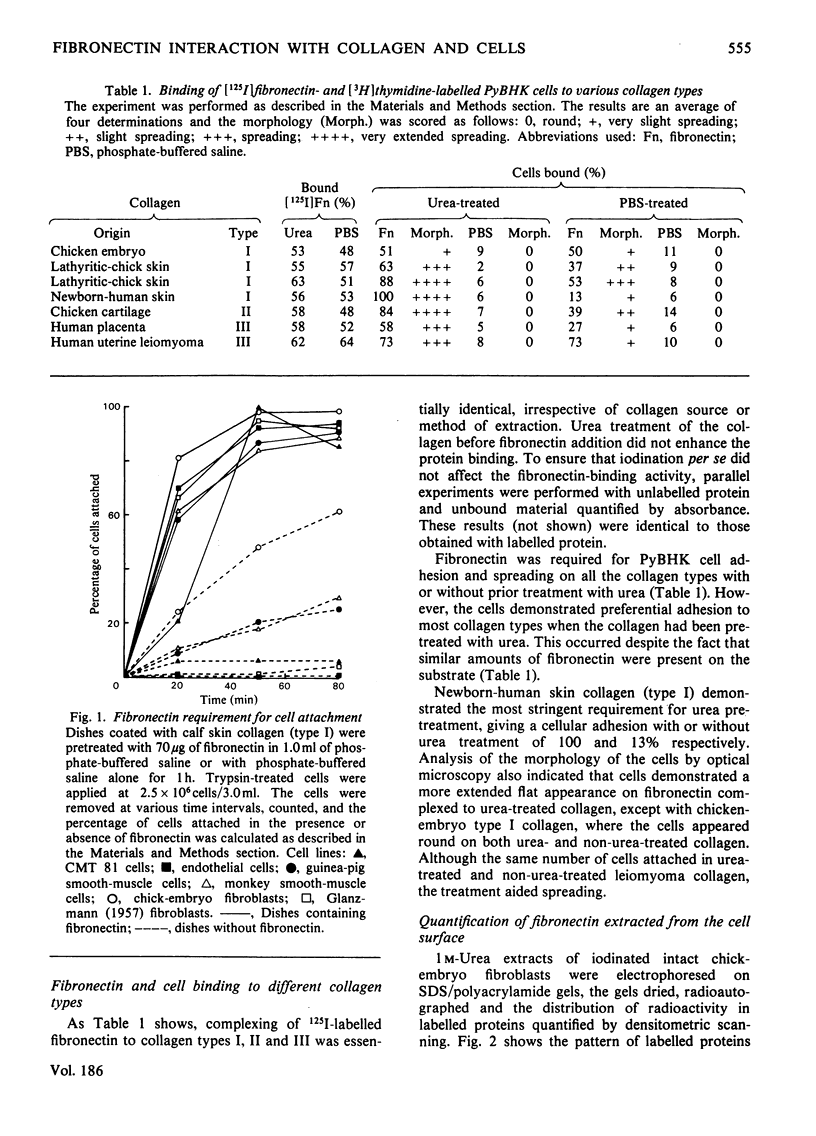
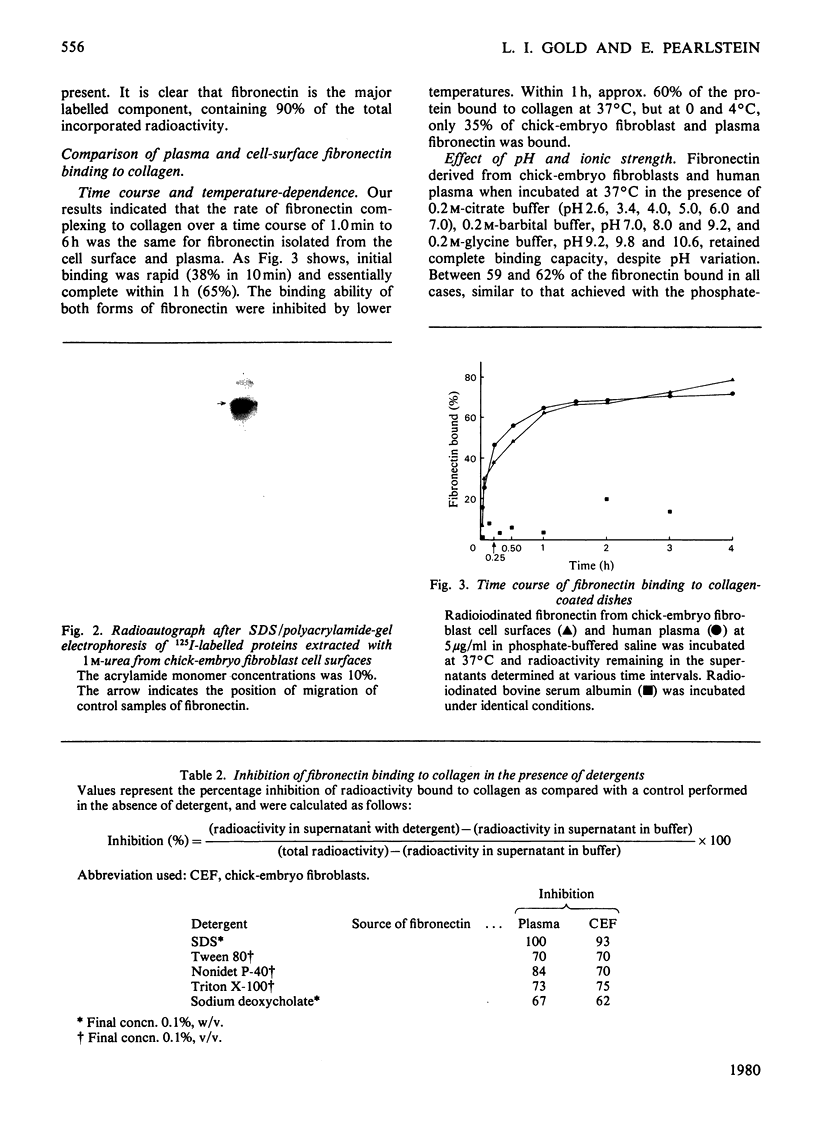
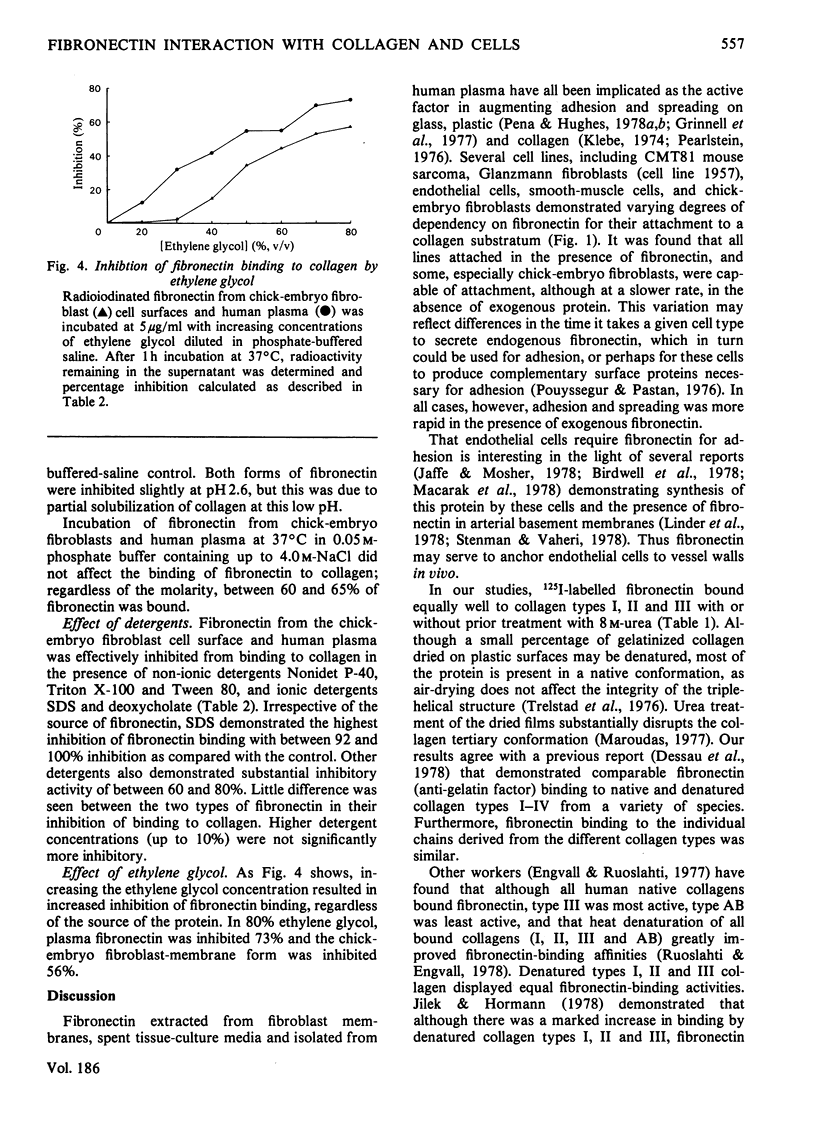
Fibronectin isolated from human plasma and from the extracellular matrices of cell monolayers mediates the attachment in vitro and spreading of trypsin-treated cells on a collagen substratum. Fibronectin-dependent kinetics of cellular attachment to collagen were studied for several adherent cell types. It was shown that trypsin-treated human umbilical-cord cells, mouse sarcoma CMT81 cells, endothelial cells, and human fibroblasts from a patient with Glanzmann's disease were completely dependent on fibronectin for their attachment to collagen, whereas guinea-pig and monkey smooth-muscle cells and chick-embryo secondary fibroblasts displayed varying degrees of dependence on fibronectin for their attachment. Radiolabelled human plasma fibronectin possessed similar affinity for collagen types I, II and III from a variety of sources. The fibronectin bound equally well to the collagens with or without prior urea treatment. However, in the fibronectin-mediated adhesion assay using PyBHK fibroblasts, a greater number of cells adhered and more spreading was observed on urea-treated collagen. Fibronectin extracted from the extracellular matrix of chick-embryo fibroblasts and that purified from human plasma demonstrated very similar kinetics of complexing to collagencoated tissue-culture dishes. Fibronectin from both sources bound to collagen in the presence of 0.05–4.0m-NaCl and over the pH range 2.6–10.6. The binding was inhibited when fibronectin was incubated with 40–80% ethylene glycol, the ionic detergents sodium dodecyl sulphate and deoxycholate, and the non-ionic detergents Nonidet P-40, Tween 80 and Triton X-100, all at a concentration of 0.1%. From these results we proposed that fibronectin–collagen complexing is mainly attributable to hydrophobic interactions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Gospodarowicz D., Nicolson G. L. Identification, localization, and role of fibronectin in cultured bovine endothelial cells. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3273–3277. doi: 10.1073/pnas.75.7.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Ash J. F. Cell surface-associated structural proteins in connective tissue cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2480–2484. doi: 10.1073/pnas.74.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray B. A. Presence of fibronectin in basement membranes and acidic structural glycoproteins from human placenta and lung. Ann N Y Acad Sci. 1978 Jun 20;312:142–150. doi: 10.1111/j.1749-6632.1978.tb16799.x. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Gudor R. C., Sun T. T., Chen A. B., Mosesson M. W. Control of a cell surface major glycoprotein by epidermal growth factor. Science. 1977 Aug 19;197(4305):776–778. doi: 10.1126/science.302030. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Crouch E., Balian G., Holbrook K., Duksin D., Bornstein P. Amniotic fluid fibronectin. Characterization and synthesis by cells in culture. J Cell Biol. 1978 Sep;78(3):701–715. doi: 10.1083/jcb.78.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessau W., Adelmann B. C., Timpl R. Identification of the sites in collagen alpha-chains that bind serum anti-gelatin factor (cold-insoluble globulin). Biochem J. 1978 Jan 1;169(1):55–59. doi: 10.1042/bj1690055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfini E., Azzarone B., Pedulla D., Ottaviano E., De Gaetano G., Donati M. B., Morasca Characterization of human fibroblasts from cancer patients: loss of fibrin clot retractile activity after 'in vitro' spontaneous transformation,. Eur J Cancer. 1976 Oct;12(10):823–825. doi: 10.1016/0014-2964(76)90097-9. [DOI] [PubMed] [Google Scholar]
- Edelhoch H., Osborne J. C., Jr The thermodynamic basis of the stability of proteins, nucleic acids, and membranes. Adv Protein Chem. 1976;30:183–250. doi: 10.1016/s0065-3233(08)60480-5. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furcht L. T., Mosher D. F., Wendelschafer-Crabb G. Immunocytochemical localization of fibronectin (LETS proteins) on the surface of L6 myoblasts: light and electron microscopic studies. Cell. 1978 Feb;13(2):263–271. doi: 10.1016/0092-8674(78)90195-2. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Hays D. G., Minter D. Cell adhesion and spreading factor. Partial purification and properties. Exp Cell Res. 1977 Nov;110(1):175–190. doi: 10.1016/0014-4827(77)90284-1. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Milam M., Srere P. A. Studies on cell adhesion. II. Adhesion of cells to surfaces of diverse chemical composition and inhibition of adhesion by sulfhydryl binding reagents. Arch Biochem Biophys. 1972 Nov;153(1):193–198. doi: 10.1016/0003-9861(72)90436-5. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Shere P. A. Inhibition of cellular adhesiveness by sulfhydryl blocking agents. J Cell Physiol. 1971 Aug;78(1):153–158. doi: 10.1002/jcp.1040780119. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Studies on the mechanism of cell attachment to a substratum with serum in the medium: further evidence supporting a requirement for two biochemically distinct processes. Arch Biochem Biophys. 1974 Dec;165(2):524–530. doi: 10.1016/0003-9861(74)90278-1. [DOI] [PubMed] [Google Scholar]
- Hedman K., Vaheri A., Wartiovaara J. External fibronectin of cultured human fibroblasts is predominantly a matrix protein. J Cell Biol. 1978 Mar;76(3):748–760. doi: 10.1083/jcb.76.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Ali I. U., Destree A. T., Mautner V., Perkins M. E., Senger D. R., Wagner D. D., Smith K. K. A large glycoprotein lost from the surfaces of transformed cells. Ann N Y Acad Sci. 1978 Jun 20;312:317–342. doi: 10.1111/j.1749-6632.1978.tb16811.x. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Mosher D. F. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978 Jun 1;147(6):1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilek F., Hörmann H. Cold-insoluble globulin (fibronectin), IV[1-35 affinity to soluble collagen of various types. Hoppe Seylers Z Physiol Chem. 1978 Feb;359(2):247–250. doi: 10.1515/bchm.1978.359.1.247. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Dimeric character of fibronectin, a major cell surface-associated glycoprotein. Biochem Biophys Res Commun. 1977 Jan 24;74(2):699–706. doi: 10.1016/0006-291x(77)90359-x. [DOI] [PubMed] [Google Scholar]
- Klebe R. J. Isolation of a collagen-dependent cell attachment factor. Nature. 1974 Jul 19;250(463):248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B., Martin G. R., Klebe R. J. Binding of cell attachment protein to collagen: effect of chemical modifications. Ann N Y Acad Sci. 1978 Jun 20;312:436–438. doi: 10.1111/j.1749-6632.1978.tb16829.x. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B., Martin G. R., Klebe R. J., Fietzek P. P., Woolley D. E. Localization of the binding site for cell attachment in the alpha1(I) chain of collagen. J Biol Chem. 1978 Aug 25;253(16):5642–5646. [PubMed] [Google Scholar]
- Kleinman H. K., Murray J. C., McGoodwin E. B., Martin G. R. Connective tissue structure: cell binding to collagen. J Invest Dermatol. 1978 Jul;71(1):9–11. doi: 10.1111/1523-1747.ep12543641. [DOI] [PubMed] [Google Scholar]
- Linder E., Stenman S., Lehto V. P., Vaheri A. Distribution of fibronectin in human tissues and relationship to other connective tissue components. Ann N Y Acad Sci. 1978 Jun 20;312:151–159. doi: 10.1111/j.1749-6632.1978.tb16800.x. [DOI] [PubMed] [Google Scholar]
- Macarak E. J., Kirby E., Kirk T., Kefalides N. A. Synthesis of cold-insoluble globulin by cultured calf endothelial cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2621–2625. doi: 10.1073/pnas.75.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas N. G. Collagen microfibrils and cell attachment proteins. Nature. 1977 May 12;267(5607):183–183. doi: 10.1038/267183b0. [DOI] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Miller E. J. Analysis of changes in collagen biosynthesis that occur when chick chondrocytes are grown in 5-bromo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4511–4515. doi: 10.1073/pnas.72.11.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Miller E. J. Characterization of the collagen chains synthesized by cultured smooth muscle cells derived from rhesus monkey thoracic aorta. Biochemistry. 1978 Feb 7;17(3):446–452. doi: 10.1021/bi00596a011. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Stingl G., Kleinman H. K., Martin G. R., Katz S. I. Epidermal cells adhere preferentially to type IV (basement membrane) collagen. J Cell Biol. 1979 Jan;80(1):197–202. doi: 10.1083/jcb.80.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein E., Gold L. I. High-molecular-weight glycorprotein as a mediator of cellular adhesion. Ann N Y Acad Sci. 1978 Jun 20;312:278–292. doi: 10.1111/j.1749-6632.1978.tb16808.x. [DOI] [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Pearlstein E. Substrate activation of cell adhesion factor as a prerequisite for cell attachment. Int J Cancer. 1978 Jul 15;22(1):32–35. doi: 10.1002/ijc.2910220108. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Waterfield M. D. Metabolic studies on 125I-labeled baby hamster kidney cell plasma membranes. Biochim Biophys Acta. 1974 Aug 7;362(1):1–12. doi: 10.1016/0304-4165(74)90019-1. [DOI] [PubMed] [Google Scholar]
- Pena S. D., Hughes R. C. Fibroblast to substratum contacts mediated by the different forms of fibronectin. Cell Biol Int Rep. 1978 Jul;2(4):339–344. doi: 10.1016/0309-1651(78)90019-x. [DOI] [PubMed] [Google Scholar]
- Pena S. D., Hughes R. C. Fibronectin-plasma membrane interactions in the adhesion and spreading of hamster fibroblasts. Nature. 1978 Nov 2;276(5683):80–83. doi: 10.1038/276080a0. [DOI] [PubMed] [Google Scholar]
- Pouysségur J. M., Pastan I. Mutants of Balb/c 3T3 fibroblasts defective in adhesiveness to substratum: evidence for alteration in cell surface proteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):544–548. doi: 10.1073/pnas.73.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Immunochemical and collagen-binding properties of fibronectin. Ann N Y Acad Sci. 1978 Jun 20;312:178–191. doi: 10.1111/j.1749-6632.1978.tb16802.x. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K., Gross J. Collagen fibrillogenesis: intermediate aggregates and suprafibrillar order. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4027–4031. doi: 10.1073/pnas.73.11.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Kurkinen M., Lehto V. P., Linder E., Timpl R. Codistribution of pericellular matrix proteins in cultured fibroblasts and loss in transformation: fibronectin and procollagen. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4944–4948. doi: 10.1073/pnas.75.10.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E., Westermark B., Ponten J. A common cell-type specific surface antigen in cultured human glial cells and fibroblasts: loss in malignant cells. J Exp Med. 1976 Jan 1;143(1):64–72. doi: 10.1084/jem.143.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Wrann M., Ruoslahti E. Similarity of fibronectins isolated from human plasma and spent fibroblast culture medium. FEBS Lett. 1977 Oct 15;82(2):227–231. doi: 10.1016/0014-5793(77)80590-5. [DOI] [PubMed] [Google Scholar]
- Wartiovaara J., Leivo I., Virtanen I., Vaheri A., Graham C. F. Appearance of fibronectin during differentiation of mouse teratocarcinoma in vitro. Nature. 1978 Mar 23;272(5651):355–356. doi: 10.1038/272355a0. [DOI] [PubMed] [Google Scholar]
- Wartiovaara J., Leivo I., Virtanen I., Vaheri A., Graham C. F. Cell surface and extracellular matrix glycoprotein fibronectin: expression in embryogenesis and in teratocarcinoma differentiation. Ann N Y Acad Sci. 1978 Jun 20;312:132–141. doi: 10.1111/j.1749-6632.1978.tb16798.x. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Fibroblast cellular and plasma fibronectins are similar but not identical. J Cell Biol. 1979 Feb;80(2):492–498. doi: 10.1083/jcb.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Schlesinger D. H., Kennedy D. W., Pastan I. Characterization of a major fibroblast cell surface glycoprotein. Biochemistry. 1977 Dec 13;16(25):5552–5559. doi: 10.1021/bi00644a025. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. The major cell surface glycoprotein of chick embryo fibroblasts is an agglutinin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3158–3162. doi: 10.1073/pnas.72.8.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavada J., Macpherson I. Transformation of hamster cell lines in vitro by a hamster sarcoma virus. Nature. 1970 Jan 3;225(5227):24–26. doi: 10.1038/225024a0. [DOI] [PubMed] [Google Scholar]
- Zetter B. R., Martin G. R. Expression of a high molecular weight cell surface glycoprotein (LETS protein) by preimplantation mouse embryos and teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2324–2328. doi: 10.1073/pnas.75.5.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]



