Abstract
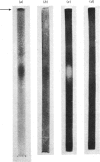
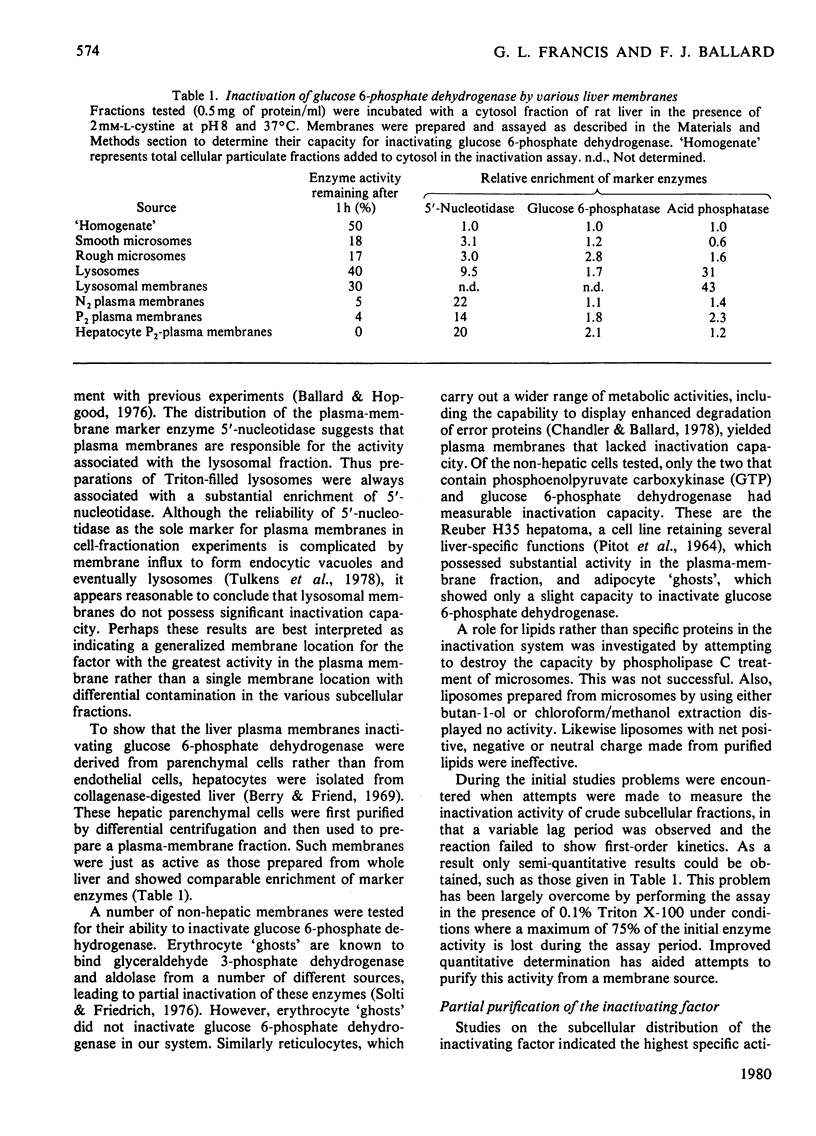
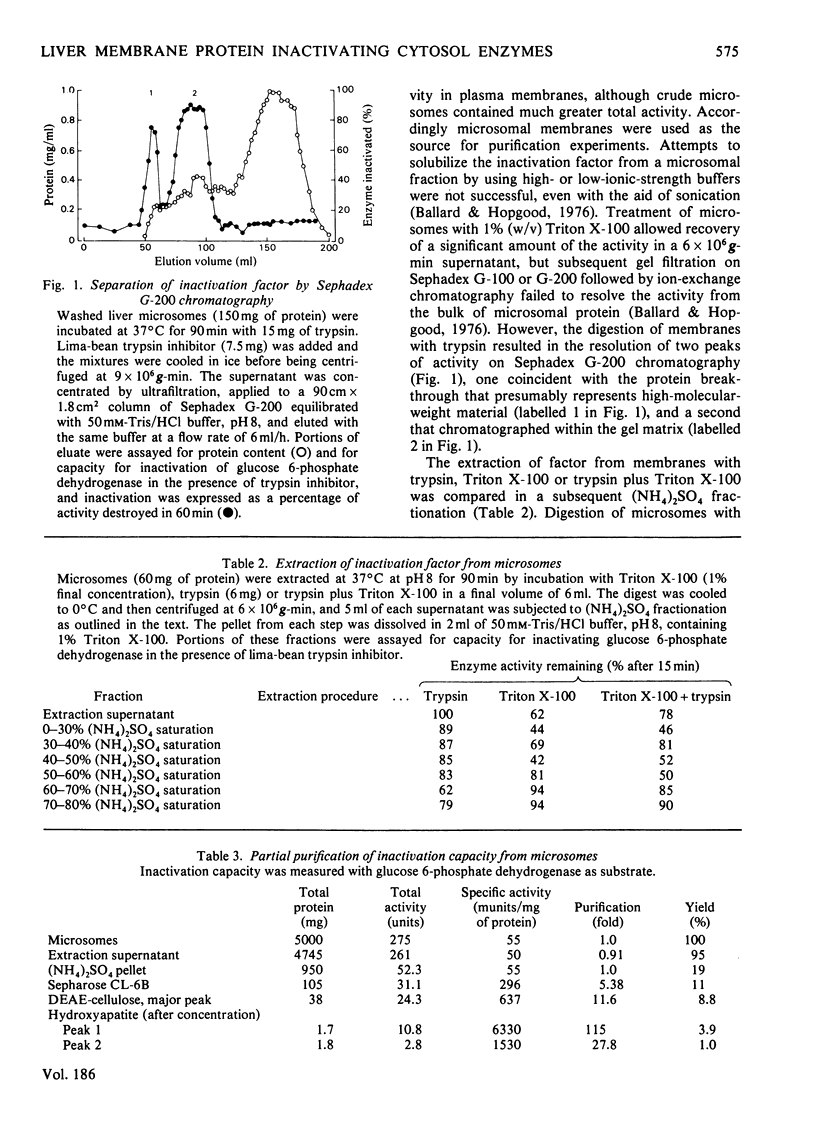
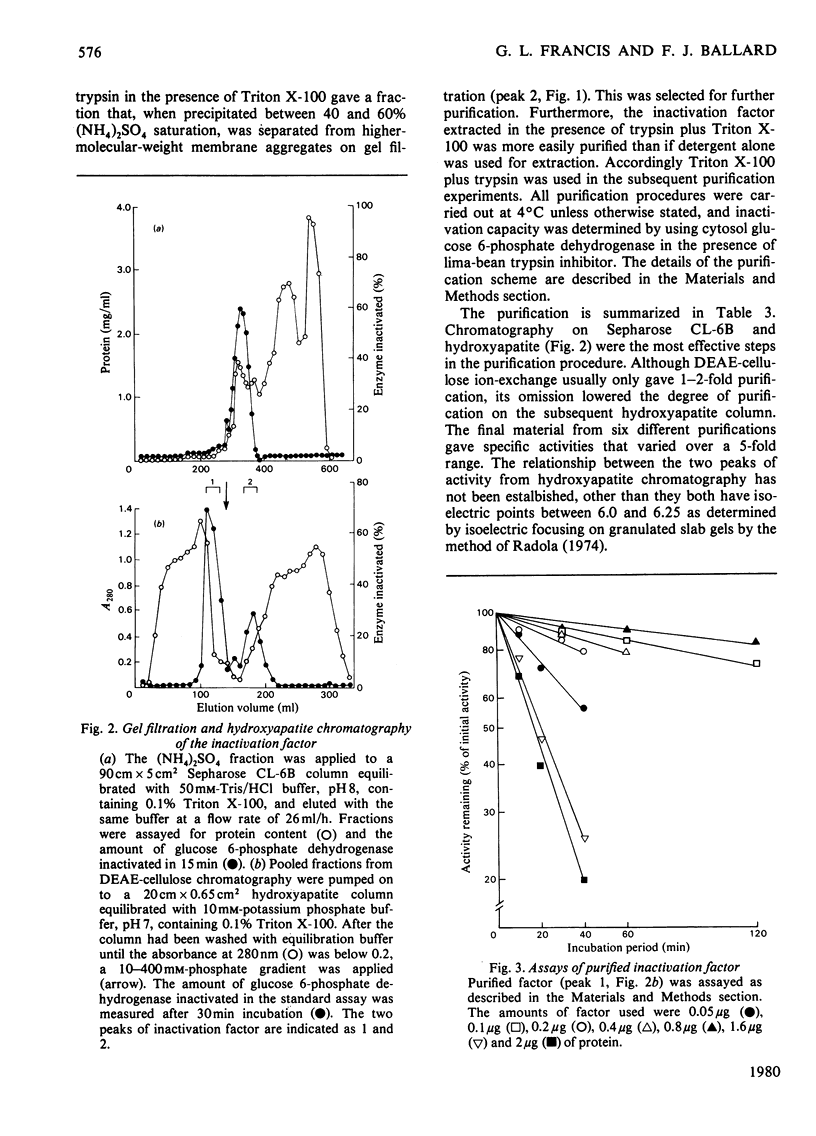
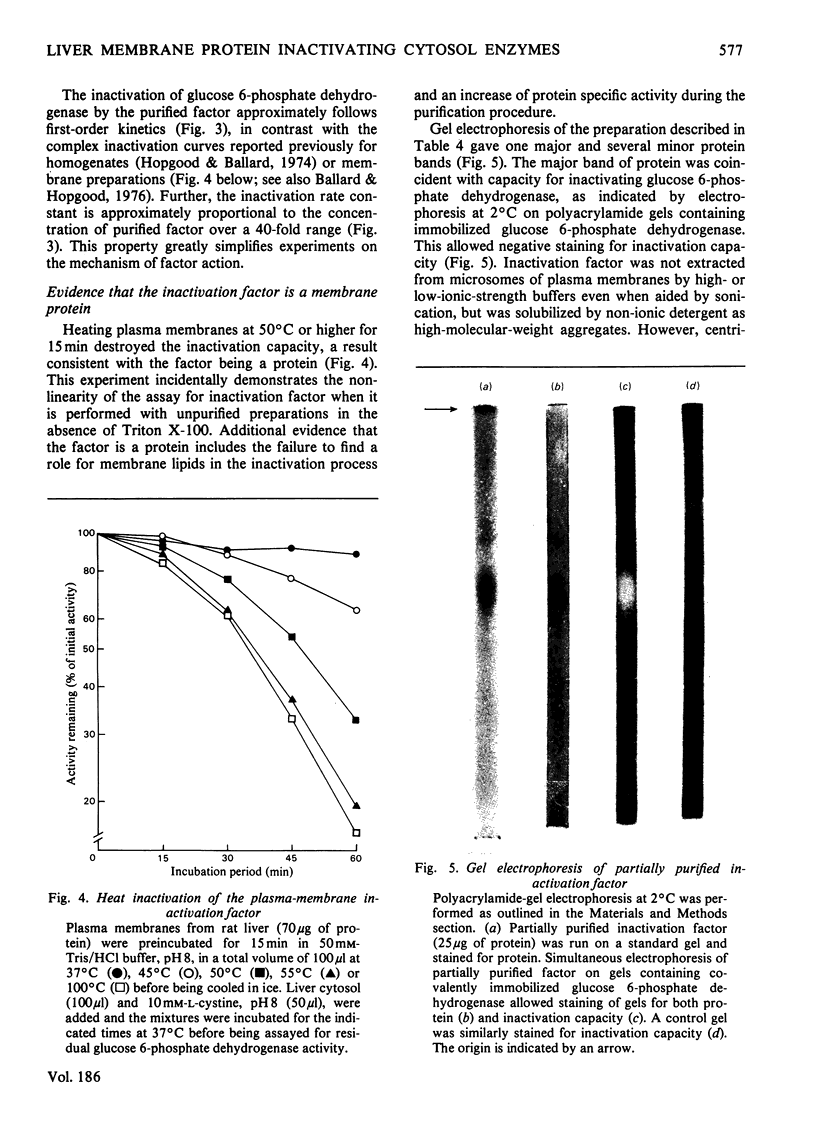
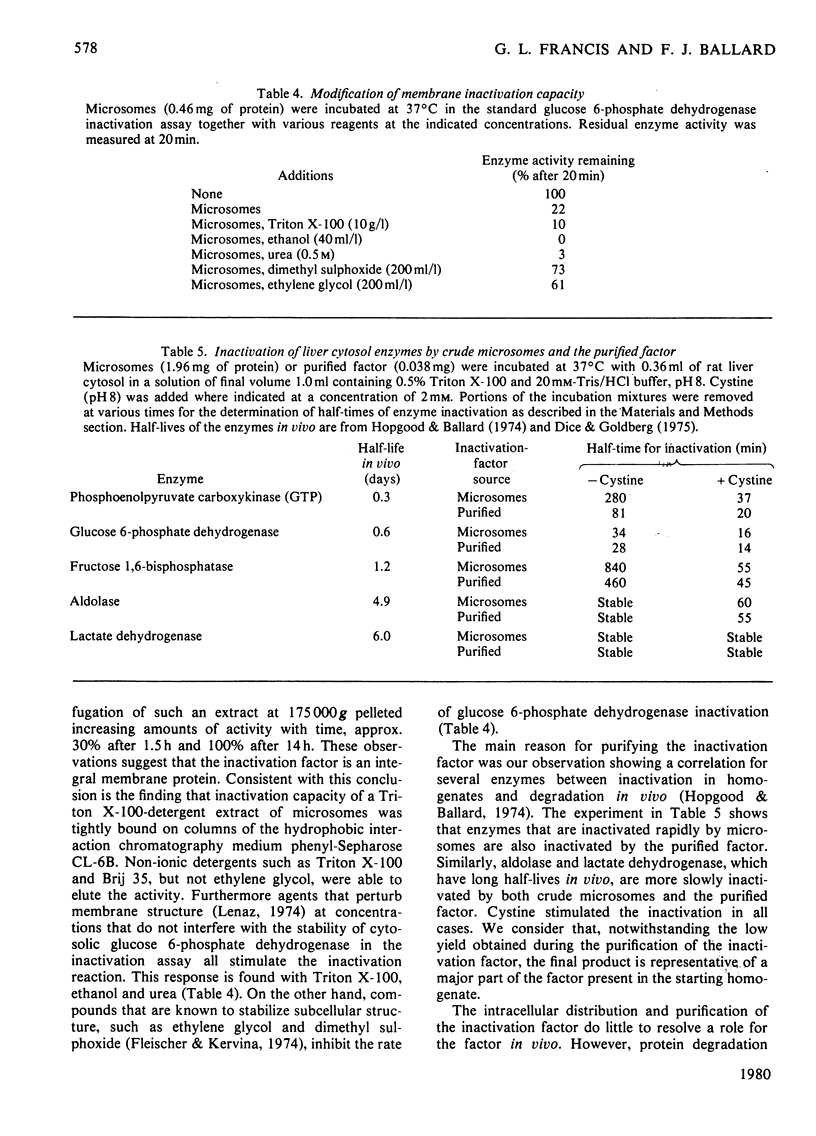
1. The inactivation of cytosol enzymes in liver extracts was carried out by several subcellular fractions, with plasma membranes having the highest specific activity. Rough and smooth microsomal fractions were both active, whereas lysosmal inactivation capacity appeared to be derived entirely from contaminating plasma-membrane fragments. 2. Inactivation capacity in liver fractions was derived from parenchymal cells. Of the non-liver cells tested, plasma membranes from H35 hepatoma cells were able to inactivate glucose 6-phosphate dehydrogenase (EC 1.1.1.49), adipocyte "ghosts" showed slight activity and erythrocyte and reticulocyte "ghosts" were inactive. 3. Liposomes prepared from pure lipids with net negative, positive or neutral charge did not possess inactivation capacity. 4. Liver plasma-membrane inactivation capacity was destroyed by heating at 50 degrees C. 5. Inactivation factor solubilized from membranes by trypsin plus Triton X-100 treatment was partially purified by (NH4)2SO4 fractionation, gel filtration, ion-exchange chromatography and hydroxyapatite chromatography. 6. Partially purified inactivation factor analysed by gel electrophoresis gave a major protein band that co-migrated with capacity for inactivation of glucose 6-phosphate dehydrogenase. 7. It is concluded that inactivation factor is a membrane protein whose intracellular distribution and other properties are consistent with a possible role for this activity in the initial step of protein degradation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Sargus M. J., Baccino F. M. Effect of microtubular or translational inhibitors on general cell protein degradation. Evidence for a dual catabolic pathway. Biochem J. 1977 Nov 15;168(2):223–227. doi: 10.1042/bj1680223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- BLOSTEIN R., RUTTER W. J. COMPARATIVE STUDIES OF LIVER AND MUSCLE ALDOLASE. II. IMMUNOCHEMICAL AND CHROMATOGRAPHIC DIFFERENTIATION. J Biol Chem. 1963 Oct;238:3280–3285. [PubMed] [Google Scholar]
- Baccino F. M., Rita G. A., Zuretti M. F. Studies on the structure-bound sedimentabolity of some rat liver lysosome hydrolases. Biochem J. 1971 Apr;122(3):363–371. doi: 10.1042/bj1220363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Ballard F. J., Hopgood M. F. Inactivation of phosphoenolypyruvate carboxykinase (GTP) by liver extracts. Biochem J. 1976 Mar 15;154(3):717–724. doi: 10.1042/bj1540717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hopgood M. F., Knowles S. E., Francis G. L. Attempts to relate enzyme inactivation to degradation in vivo. Acta Biol Med Ger. 1977;36(11-12):1805–1813. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. S. Acid inactivation of short-lived rat liver enzymes. Biochim Biophys Acta. 1976 Nov 18;451(1):238–249. doi: 10.1016/0304-4165(76)90274-9. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Bloxham D. P., Holland P. C., Lardy H. A. Estimation of the fructose diphosphatase-phosphofructokinase substrate cycle in the flight muscle of Bombus affinis. Biochem J. 1973 Jun;134(2):589–597. doi: 10.1042/bj1340589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G. Isolation of rough and smooth microsomes--general. Methods Enzymol. 1974;31:191–201. [PubMed] [Google Scholar]
- Dean R. T. Concerning a possible mechanism for selective capture of cytoplasmic proteins by lysosomes. Biochem Biophys Res Commun. 1975 Nov 17;67(2):604–609. doi: 10.1016/0006-291x(75)90855-4. [DOI] [PubMed] [Google Scholar]
- Dewald B., Dulaney J. T., Touster O. Solubilization and polyacrylamide gel electrophoresis of membrane enzymes with detergents. Methods Enzymol. 1974;32:82–91. doi: 10.1016/0076-6879(74)32011-3. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Kervina M. Long-term preservation of liver for subcellular fractionation. Methods Enzymol. 1974;31:3–6. doi: 10.1016/0076-6879(74)31004-x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Harrison R. A. The detection of hexokinase, glucosephosphate isomerase and phosphoglucomutase activities in polyacrylamide gels after electrophoresis: a novel method using immobilized glucose 6-phosphate dehydrogenase. Anal Biochem. 1974 Oct;61(2):500–507. doi: 10.1016/0003-2697(74)90417-5. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Ballard F. J. The relative stability of liver cytosol enzymes incubated in vitro. Biochem J. 1974 Nov;144(2):371–376. doi: 10.1042/bj1440371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok C. T., Austin L. Phospholipid composition and metabolism in mouse muscular dystrophy. Biochem J. 1978 Oct 15;176(1):15–22. doi: 10.1042/bj1760015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenaz G. Lipid-protein interactions in the structure of biological membranes. Subcell Biochem. 1974 Sep;3(3):167–248. [PubMed] [Google Scholar]
- Matsuda T., Yugari Y. Glucose-6-phosphate dehydrogenase from rat liver. I. Crystallization and properties. J Biochem. 1967 May;61(5):535–540. doi: 10.1093/oxfordjournals.jbchem.a128583. [DOI] [PubMed] [Google Scholar]
- PITOT H. C., PERAINO C., MORSE P. A., Jr, POTTER V. R. HEPATOMAS IN TISSUE CULTURE COMPARED WITH ADAPTING LIVER IN VIVO. Natl Cancer Inst Monogr. 1964 Apr;13:229–245. [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. II. Preparative isoelectric focusing. Biochim Biophys Acta. 1975 Mar 28;386(1):181–195. doi: 10.1016/0005-2795(75)90258-5. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Krishna G. Preparation of isolated fat cells and fat cell "ghosts"; methods for assaying adenylate cyclase activity and levels of cyclic AMP. Methods Enzymol. 1974;31:103–114. doi: 10.1016/0076-6879(74)31010-5. [DOI] [PubMed] [Google Scholar]
- SHULL K. H., ASHMORE J., MAYER J. Hexokinase, glucose-6-phosphatase and phosphorylase levels in hereditarily obese-hyperglycemic mice. Arch Biochem Biophys. 1956 May;62(1):210–216. doi: 10.1016/0003-9861(56)90104-7. [DOI] [PubMed] [Google Scholar]
- Solti M., Friedrich P. Partial reversible inactivation of enzymes due to binding to the human erythrocyte membrane. Mol Cell Biochem. 1976 Feb 25;10(3):145–152. doi: 10.1007/BF01731685. [DOI] [PubMed] [Google Scholar]
- Trouet A. Isolation of modified liver lysosomes. Methods Enzymol. 1974;31:323–329. doi: 10.1016/0076-6879(74)31034-8. [DOI] [PubMed] [Google Scholar]
- Tulkens P., Schneider Y. J., Trouet A. The fate of the plasma membrane during endocytosis. Biochem Soc Trans. 1977;5(6):1809–1815. doi: 10.1042/bst0051809. [DOI] [PubMed] [Google Scholar]
- Worsfold V., Marshall M. J., Ellis E. B. Enzyme detection using phenazine methosulphate and tetrazolium salts: interference by oxygen. Anal Biochem. 1977 May 1;79(1-2):152–156. doi: 10.1016/0003-2697(77)90389-x. [DOI] [PubMed] [Google Scholar]