Abstract
Objective
In this study, we evaluated the effectiveness and safety of thalidomide by clinically observing 48 individuals with β-thalassemia who have been administered thalidomide in small and medium doses over a period of two years.
Methods
Thalidomide’s efficacy was gauged by tracking hemoglobin (Hb) level alterations post its administration. Liver and kidney function impact was measured through tests for alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, and creatinine. Hemolysis effects were assessed via total bilirubin and indirect bilirubin level measurements. Thrombosis detection was performed using ultrasound examinations of deep vein vessels in the upper and lower extremities. Any adverse effects of thalidomide were recorded during the observation period. Lower dose thalidomide effectiveness was evaluated by monitoring Hb level changes following dosage reduction.
Results
The overall response rate (ORR) among the 48 participants was 91.7% (44 out of 48), with the main reaction (MaR) reaching 72.9% (35 out of 48). Hepatorenal toxicity was not monitored during the 2-year observation period, and there was no improvement in hemolysis. Most adverse effects were mild, with no instances of venous thrombosis and no cases of grade 2 or higher neurotoxicity. When the observation group was divided into three age categories (12–14 years old, 14–18 years old, and over 18 years old), there were no statistically significant differences in the occurrence of adverse reactions among the three groups. As there were some adverse reactions in ten cases, the treatment dose was reduced for them. The maintenance efficacy rate at one year of observation was 90% (9 out of 10).
Conclusion
This study confirmed that thalidomide in small doses over a 2-year observation period is effective, and has no instances of grade 2 or higher neurotoxicity. Long-term maintenance with small doses is recommended for enhanced safety.
Keywords: β-thalassemia, clinical efficacy, safety, thalidomide
Introduction
Thalassemia is an inherited hemolytic disorder. β-thalassemia is particularly prevalent in southern China. According to a 2021 report by the Thalassaemia International Federation, approximately 21 million individuals carry the β-thalassemia gene within China’s southern population of approximately 670 million.1 Epidemiological studies show that β-thalassemia has a higher prevalence in the south than in the north of China. In regions such as Guangdong, Guangxi, and Hainan, the incidence rate is estimated between 2.77% and 6.64%.2 A major congenital defect survey conducted from January 1, 2017, to May 31, 2019, in Guangxi by Dong et al3 identified β-thalassemia major as the fourth most common major birth defect, with an incidence rate of 5.32 per 100,000 births. In the Guilin region alone, there are approximately 300 cases of β-thalassemia major and intermediate beta-thalassemia, where patients typically rely on blood transfusions every 20 to 40 days. Iron chelation therapy becomes necessary once serum ferritin levels exceed 1000 ng/mL, often around the age of two.
Although hematopoietic stem cell transplantation remains the only curative approach, finding a matched human histocompatibility antigen (HLA) donor can be challenging and costly. Therefore, alternative treatments to alleviate anemia have been explored, including hydroxyurea (HU), erythropoietin (EPO), decitabine, butyrate derivatives, and thalidomide. While HU is effective in treating sickle cell disease, it shows limited efficacy for most cases of β-thalassemia. EPO promotes the proliferation, differentiation, and maturation of erythrocytes but has drawbacks, such as bone marrow expansion and the potential for nerve compression. Butyrate derivatives have a short plasma half-life and adverse effects on red blood cell growth, limiting their clinical utility. Decitabine’s primary side effect is thrombocytosis, which has hindered its clinical adoption. Due to the limited efficacy, toxicity, short half-life, inconvenient administration, and high cost associated with these treatments, they are not widely used for β-thalassemia. Currently, research is shifting focus to thalidomide and its derivative, pomalidomide, as potential therapeutic options.
In recent years, thalidomide has gained attention for treating β-thalassemia due to its ability to stimulate γ-protein gene expression, increasing fetal hemoglobin (HbF) synthesis, and thereby enhancing hemoglobin (Hb) levels.4,5 This improvement in efficacy may lead to reduced reliance on blood transfusions, alleviating the financial burden on patients and their families, as well as saving time and conserving blood supplies. While most studies have focused on medium to large doses (50–100 mg/day) of thalidomide, larger doses are associated with a higher risk of neurotoxicity. Although adverse reactions to thalidomide are generally mild, there is limited information on managing these reactions, particularly after reducing the dosage. Additionally, there is a lack of research on thalidomide safety in younger individuals, furthering the need to explore better and accumulate more experience in thalidomide treatment for β-thalassemia.
This study included 52 β-thalassemia cases for a 2-year single-cohort observation. Thalidomide efficacy was assessed based on changes in Hb levels, while effects on liver and kidney function and hemolysis were monitored. Oral aspirin was administered to prevent venous thrombosis. We investigated the efficacy and safety of small to medium doses (25–50 mg/day) of thalidomide and reevaluated efficacy and adverse reaction regression post-adjustment. The main objective of this study was to establish stable and safe efficacy using relatively low doses over an extended period.
Data and Methods
Medical Record Data
Criteria and Typing
Patients with β-thalassemia who were treated with thalidomide at Guilin People’s Hospital from August 2019 to August 2023 were enrolled in this study. Following the criteria outlined in the “Diagnostic and Therapeutic Guidelines for β-Thalassemia Major” and the “Expert Consensus on the Diagnosis and Management of Non-Transfusion-Dependent Thalassemia in Children”, β-thalassemia was classified into transfusion-dependent thalassemia (TDT) and non-transfusion-dependent thalassemia (NTDT).6,7 Prior to treatment, all patients were provided with comprehensive information regarding thalidomide’s potential side effects and benefits. Informed consent was obtained from each patient, and the thalidomide treatment regimen was approved by the Medical Ethics Committee of Guilin People’s Hospital.
Inclusion Criteria
① The patients were diagnosed with β-thalassemia based on both clinical symptoms and genetic testing, confirming either pure or compound heterozygosity.
② The participants, regardless of gender, were aged between 12 and 41 years old and had an Eastern Cooperative Oncology Group (ECOG) physical strength score ranging from 0 to 2.
③ The participants had given their consent in writing before the trial commenced.
Exclusion Criteria
-
%1
Pregnant or lactating women, as well as subjects of childbearing age unwilling to use contraception; ② individuals with severe cardiopulmonary diseases, liver function abnormalities, cerebrovascular or cardiovascular diseases, hepatic or renal conditions, tumors, or other significant primary diseases; ③ those who were allergic to any components of this drug; ④ participants who had taken other medications (ie Hydroxyurea and Luspatercept) a month prior to the clinical trial; ⑤ those with a history of venous or arterial thrombosis; ⑥ prerogative of the investigator who may find someone unsuitable for participation in the study.
Basic Information of Enrolled Cases
In this study, we included 52 cases that met the criteria, consisting of 30 males and 22 females. The age of the individuals ranged from 12 to 41 years, with a median age of 16 years. Among them, 11 cases (22.9%) were between 12 and 14 years old, 20 cases were between 14 and 18 years old, and 21 cases were over 18 years old. Of the 52 cases, 38 had TDT, and 14 had non-transfusion-dependent thalassemia (NTDT). Additionally, 20 out of the 52 cases had undergone splenectomy.
Therapeutic Program
Thalidomide Dosage
Thalidomide dosing was customized as follows: a large dose was defined as more than 50 mg/day, a medium dose as exactly 50 mg/day, and a small dose as less than 50 mg/day. For individuals weighing 25 kg or less, or aged 14 years or younger, the prescribed dose was 37.5 mg/day. For those weighing over 25 kg or older than 14 years, the dose was set at 50 mg/day. Throughout the 6-month observation period within the study group, there were no reported adverse reactions. Patients could request to stop thalidomide, and if they did not, the treatment was continued unless the clinician identified a reason to discontinue. In case of an adverse reaction, the dosage was reduced incrementally by 12.5 mg/day from the current dose as needed.
Splenectomized Patients
For platelet counts exceeding 500×109/L, prophylactic aspirin at a dose of 50–100 mg/day was recommended, with coagulation time monitored every three months. Should platelet counts fall below 500×109/L, aspirin was discontinued.
Blood Transfusion Treatment
If the patient’s Hb level dropped below 90g/L during the medication period, transfusion therapy was initiated. Pre-transfusion test values for the observation indexes were duly recorded.
Detection Methods
Blood Routine
The Sysmex XE-5000 automated blood cell analyzer was utilized for whole blood cell detection.
Blood Biochemistry
The Cobas 701, an automated biochemical analyzer manufactured by Roche, was employed to examine the functionality of the liver and kidneys.
Thalassemia Gene
Various equipment was used to identify different types of α-thalassemia and β-thalassemia mutations. The Bio-Rad T-100 PCR amplifier, Yaneng YN-H16 medical nucleic acid molecular rapid hybridizer, Liuyi-YY-8G electrophoresis system, and AB3500 sequencer were deployed for detecting four deletion types of α-thalassemia (-ɑ3.7/), (-ɑ4.2/), (--SEA/), (--Thai/) using multiplex Gap-PCR. Additionally, PCR probes were employed to detect three non-deletion types of α-thalassemia (ɑCS, ɑQS, ɑWS), and 18 mutation sites commonly associated with β-thalassemia in the Chinese population.
Vascular Ultrasound of Upper and Lower Limb Deep Vein
The ALOKA ARIETTA ultrasound device (Hitachi, Japan) was employed to scan the deep vein vessels in both the upper and lower limbs. Monitoring sessions were scheduled prior to treatment and at intervals of 6 months, 12 months, and 24 months following the treatment.
Observation Indicators
Blood Transfusion
During the treatment period, patients with Hb <90g/L could receive transfusions. The total transfusion volume (U) from the two years prior to enrollment was recorded, and the total transfusion volume (U) from enrollment to the end of the observation period was also documented.
Therapeutic Efficacy Assessment
Total Efficacy
During the 6-month observation period, the reaction to thalidomide was categorized into four levels: A rise in Hb of at least 2.0 g/L or the absence of a requirement for blood transfusion categorized as a Major Response (MaR); An increase in Hb between 1.0–2.0 g/L or a reduction of 50% or more in the total transfusion volume classified as a Minor Response (MiR); A Hb increase of less than 1.0 g/L or a decrease in the total transfusion volume ranging from 25% to 50% considered a Slow Response (SlR); An increase in Hb of less than 1.0 g/L or a decrease in total blood transfusion of less than 25% was deemed a non-response (NR) or largely ineffective. The overall response rate (ORR) was calculated by dividing the sum of MaR, MiR, and SlR cases by the total number of observed cases.
Efficacy After Dose Reduction
Comparing the changes in Hb levels at 3 months and 12 months after reducing thalidomide dosage with those before the reduction, a decrease in Hb greater than 10g/L at the 3-month mark indicated that the effectiveness might not be maintained temporarily, suggesting a potential need to revert to the original dosage. The final effectiveness was assessed at the 12-month mark after thalidomide dosage reduction: a decrease in Hb of less than or equal to 10g/L, or an increase compared to pre-reduction levels, was considered indicative of sustained effectiveness. However, a decrease in Hb greater than 10g/L suggested that the effectiveness could not be maintained.
Safety Assessment
The impact on liver and kidney function was evaluated by monitoring alterations in hematological parameters including enzyme levels, bilirubin, and creatinine levels. Ultrasound imaging of the deep vein vessels in both upper and lower limbs was used to assess the presence or absence of thrombosis, while any adverse reactions related to thalidomide toxicity were observed to determine its harmful effects.
Parameter Collection
Parameters including hemoglobin (Hb), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), indirect bilirubin (IBIL), blood urea nitrogen (BUN), and creatinine (Cr) were measured initially during the baseline period. Subsequently, measurements were taken at 3, 6, 12, and 24 months after the group’s enrollment, and the results of each test were documented.
Observation of Adverse Reactions
Common adverse effects may include drowsiness, skin rash, and gastrointestinal symptoms such as constipation, abdominal pain, and diarrhea. Additionally, potential side effects may involve facial swelling and peripheral neurotoxicity symptoms such as muscle pain, tingling sensations, sensory abnormalities, and limb numbness. The grading system for peripheral neurotoxicity adhered to the criteria outlined in the peripheral motor/sensory nerve disorders section of the 2017 US Department of Health and Human Services’ Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, which comprises five grades.8
Grade 1: Without symptoms or only detected through clinical or diagnostic means.
Grade 2: Moderate symptoms impacting daily instrumental activities.
Grade 3: Intense symptoms that restrict personal independence.
Grade 4: Life-threatening, necessitating immediate treatment or intervention.
Grade 5: Death.
Statistical Methods
Statistical analysis was conducted using SPSS 26.0 software. Descriptive statistics for normally distributed measurement data were presented as mean ± standard deviation. An independent samples t-test was employed to compare data between the two groups undergoing dose changes at each time point. Comparisons across various time points and groups, along with comprehensive analysis of repeated-measurement data, were conducted using repeated-measurement analysis of variance (ANOVA). The significance level was set at α = 0.05, with P < 0.05 indicating statistical significance.
Results
Out of 52 cases, 48 successfully completed the 2-year observation period, including 34 cases of TDT and 14 cases of NTDT. Four cases of TDT were withdrawn from the study for the following reasons: two had developed severe rash within two months of initiating the medication, leading to a decision to halt treatment; one experienced self-perceived menstrual irregularities after five months of drug intake, choosing to discontinue participation; and one opted for hematopoietic stem cell transplantation three months after starting the treatment, bringing an end to the observation as per clinical protocol.
Therapeutic Efficacy and Detachment from Blood Transfusion in the Observation Group of 48 Cases
Among the 48 cases, 14 were of NTDT, which did not require re-transfusion during the 6-month observation period while on medication, achieving a Major Response (MaR) efficacy rating. None of these cases required re-transfusion during the subsequent 2-year observation period. Of the 34 TDT cases, 21 (61.8%) stopped transfusions. Sixteen cases stopped transfusions within one month of treatment, and the remaining 5 within 3 months, all achieving a MaR efficacy rating. None of these 21 cases required re-transfusion during the 2-year observation period. MaR accounted for 72.9% (35/48) of all cases. The remaining 13 TDT cases did not respond to stopping of transfusion. Nevertheless, total blood transfusion decreased by 31.3% compared to baseline, with only 4 cases showing no response (NR). This resulted in an overall response rate (ORR) of 91.7% (44 out of 48) among the total observed cases.
Effects of Thalidomide on Liver and Kidney Functions
There were no statistically significant differences in ALT levels after 6 months, 12 months, and 24 months of treatment compared to the baseline period at 0 months in the 48 cases of the observation group (P > 0.05, Table 1). Similarly, after 6 months and 12 months of treatment, no statistically significant differences (P > 0.05) were observed in the 48 cases of the observation group compared to the baseline period at 0 months. However, after 24 months, a statistically significant difference (P < 0.05) was observed compared to the baseline period at 0 months, with AST levels decreasing after 24 months compared to the previous period (Table 1). Additionally, in the 48 cases, levels of BUN and Cr in the observation group after 6 months, 12 months, and 24 months of treatment were compared with the baseline period (0 months). The results indicated no statistically significant differences (P > 0.05, Table 1).
Table 1.
Changes in Liver and Kidney Function Indicators in 48 Patients After Treatment
| Parameter | (I) Time Point | (J) Time Point | Difference Between Mean Values (I-J) | Standard Error | Significance P |
|---|---|---|---|---|---|
| ALT | 6M | 0M | 1.875 | 3.041 | 0.54 |
| 12M | 0M | 4.833 | 6.614 | 0.469 | |
| 24M | 0M | −4.063 | 4.245 | 0.343 | |
| AST | 6M | 0M | 0.504 | 2.476 | 0.84 |
| 12M | 0M | 1.313 | 3.958 | 0.742 | |
| 24M | 0M | −5.500* | 2.652 | 0.044 | |
| Bun | 6M | 0M | 0.224 | 0.23 | 0.335 |
| 12M | 0M | 0.252 | 0.264 | 0.343 | |
| 24M | 0M | 0.314 | 0.212 | 0.144 | |
| Cr | 6M | 0M | −5.16 | 8.847 | 0.562 |
| 12M | 0M | −5.681 | 8.562 | 0.51 | |
| 24M | 0M | −2.458 | 8.874 | 0.783 |
Note: * P<0.05.
Effect of Thalidomide on Hemolytic Function
To avoid the effects of blood transfusions on hemolytic indexes, the study examined and compared TBIL and IBIL levels in 35 individuals (21 with TDT and 14 with NTDT) out of the total 48 in the observation group who did not undergo transfusions, as shown in Table 2. The data in Table 2 indicate that there were no statistically significant differences in the changes in TBIL and IBIL levels at three time points before and after treatment in these 35 cases (P > 0.05).
Table 2.
Comparison of TBIL and IBIL Values Over Time Following Treatment in 35 Cases
| Parameter | (I) Time Point | (J) Time Point | Difference Between Mean Values (I-J) | Standard Error | Significance P |
|---|---|---|---|---|---|
| TBIL | 0M | 12M | 19.182 | 16.974 | 0.605 |
| 24M | 17.057 | 17.254 | 0.699 | ||
| 12M | 0M | −19.182 | 16.974 | 0.605 | |
| 24M | −2.125 | 2.315 | 0.744 | ||
| 24M | 0M | −17.057 | 17.254 | 0.699 | |
| 12M | 2.125 | 2.315 | 0.744 | ||
| IBIL | 0M | 12M | 3.185 | 3.111 | 0.676 |
| 24M | −0.029 | 4.09 | 1 | ||
| 12M | 0M | −3.185 | 3.111 | 0.676 | |
| 24M | −3.214 | 2.784 | 0.589 | ||
| 24M | 0M | 0.029 | 4.09 | 1 | |
| 12M | 3.214 | 2.784 | 0.589 |
Observation of Adverse Reactions of Thalidomide
The majority of adverse reactions were mild in nature. Out of the 52 cases observed, the most common were mild eyelid/facial swelling in 9 cases, followed by rash in 4 cases (2 of which were severe enough to discontinue treatment), mild joint and muscle pain in the lower limbs in 4 cases (temporary discomfort without mobility issues), skin patterns in 3 cases, drowsiness in 3 cases, constipation in 2 cases, menstrual irregularities in 1 case (self-reported disruption of menstruation), and no instances of venous thrombosis. The 52 individuals were divided into three age groups, and the occurrence of adverse reactions was compared across these groups. The analysis revealed a p-value of 0.052, indicating no statistically significant difference as it was greater than 0.05 (Table 3). The 52 participants were divided into three different age groups to compare adverse reaction rates, and results showed no statistically significant difference (P > 0.05).
Table 3.
Comparison of the Incidence of Adverse Reactions Across Three Distinct Age Categories
| Occurrence of Adverse Reactions | Total | Chi-Square Value | P value | ||
|---|---|---|---|---|---|
| Number of One or More Adverse Reactions | No Adverse Reactions | ||||
| 12–14 years old | 8 | 3 | 11 | 5.901 | 0.052 |
| 14–18 years old | 6 | 14 | 20 | ||
| 18 and above | 12 | 9 | 21 | ||
| Total | 26 | 26 | 52 | ||
Hemoglobin Changes After Thalidomide Reduction
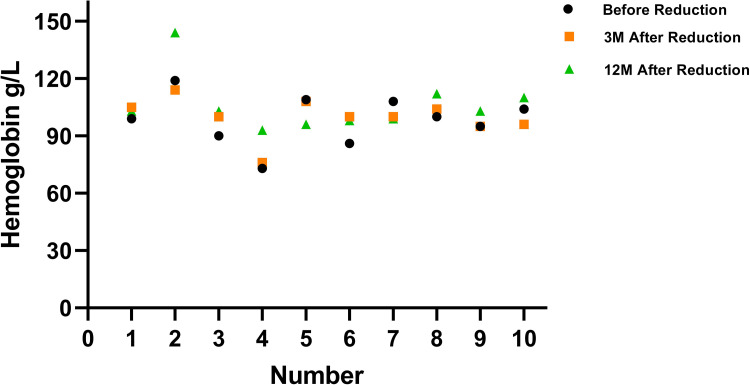
In the observation group of 48 cases, the starting dosages were 50 mg/day for 31 patients, 37.5 mg/day for 16 patients, and 25 mg/day for 1 patient. Due to adverse effects like muscle pain in the lower limbs, skin patterns, drowsiness, and constipation, some patients reduced their dosage. All reductions occurred within the first 6 months of treatment. Specifically, patients numbered 1–6 decreased their dosage from 50 mg/day to 37.5 mg/day, and patients numbered 7–10 reduced their dosage from 37.5 mg/day to 25 mg/day. There were no reductions resulting in a decrease in hemoglobin (Hb) levels of more than 10 g/L at the 3-month mark after the dosage change. However, participant number 5 experienced a decrease in Hb of 13 g/L at the 12-month mark, as shown in Table 4 and Figure 1. By the conclusion of the 2-year study period, the dosages were 50 mg/day for 17 participants, 37.5 mg/day for 25 participants, and 25 mg/day for 6 participants.
Table 4.
Comparison of Hb Levels (g/L) Before and After Thalidomide Dosage Reduction in 10 Cases
| No. | Dose Change | Before Dose Reduction | 3 Months After Dose Reduction | 12 Months After Dose Reduction |
|---|---|---|---|---|
| 1 | 50mg reduced to 37.5mg | 99 | 105 | 103 |
| 2 | 50mg reduced to 37.5mg | 119 | 114 | 144 |
| 3 | 50mg reduced to 37.5mg | 90 | 100 | 103 |
| 4 | 50mg reduced to 37.5mg | 73 | 76 | 93 |
| 5 | 50mg reduced to 37.5mg | 109 | 108 | 96 |
| 6 | 50mg reduced to 37.5mg | 86 | 100 | 98 |
| 7 | 37.5mg reduced to 25mg | 108 | 100 | 99 |
| 8 | 37.5mg reduced to 25mg | 100 | 104 | 112 |
| 9 | 37.5mg reduced to 25mg | 95 | 95 | 103 |
| 10 | 37.5mg reduced to 25mg | 104 | 96 | 110 |
| Hb comparison between time points | Fa=2.602 | Pa=0.105 | ||
| Hb comparison between dose change groups | Fb=0.028 | Pb=0.871 |
Notes: a Comparison of different time points in repeated measures ANOVA. b Comparison among groups with varying dose changes in repeated measures ANOVA.
Figure 1.
Alterations in Hb levels following the reduction of thalidomide dosage in 10 instances.
The results of repeated-measures ANOVA indicated that there were no significant changes in Hb values at various time points before and after dosage reduction, both within the 3-month period prior to reduction and the 3-month period post-reduction, as well as at the 12-month mark post-reduction (Fa = 2.602, Pa = 0.105). Additionally, when comparing the differences in Hb values between groups that underwent different dose reductions (ie, from 50 to 37.5 mg and from 37.5 mg to 25 mg), no significant differences were observed in Hb values between these two groups (Fa = 0.028, Pa = 0.871).
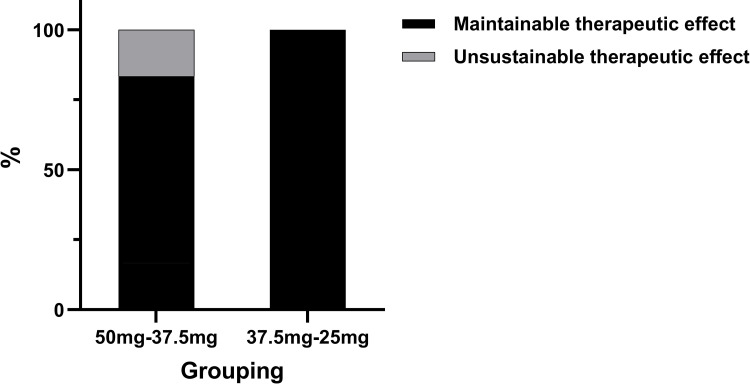
Within the group that reduced their dosage from 50 mg to 37.5 mg, 5 out of 6 individuals maintained efficacy, resulting in an efficacy maintenance rate of 83.3%. Similarly, in the group that reduced their dosage from 37.5 mg to 25 mg, one individual experienced a decrease in Hb from 108 g/L to 99 g/L, which was still regarded as maintaining efficacy, resulting in a 100% efficacy maintenance rate among the 4 individuals in this group (as shown in Figure 2). Overall, among the 10 individuals who underwent dosage reduction, the efficacy maintenance rate was 90% (9 out of 10).
Figure 2.
Effectiveness levels post reduction of thalidomide dosage in each of the groups (totaling 10 cases).
Discussion
Guangxi is known for its high prevalence of thalassemia, particularly in Guilin city where over 200 patients require medical intervention. It faces several economic challenges compared to neighboring provinces such as Hunan and Guangdong. While classical treatments such as blood transfusion and iron removal therapy remain common, hematopoietic stem cell transplantation offers a potential cure for thalassemia. However, due to factors like the lack of suitable HLA-matching donors or financial constraints, many patients and families opt for thalidomide as a safer, more cost-effective, and convenient alternative. This article explores the effectiveness of thalidomide in small and medium doses, examines the impact of dosage reduction on its effectiveness, and assesses its safety over a 2-year period.
Overall Efficacy Assessment of Thalidomide
In this study, 48 cases of β-thalassemia (comprising 34 cases of TDT and 14 cases of NTDT) were observed for a period of 2 years. The ORR was 91.7% (44 out of 48), a figure closely resembling the ORR of 93.5% reported by Yang et al.9 This rate exceeded the ORR of 76.7% reported by Ali et al, which could potentially be attributed to variations in sample size and observation durations.10 Among the 34 TDT cases, 21 stopped transfusion (equating to 61.8%) with an efficacy rating termed MaR, mirroring the MaR efficacy rate of 63.6% observed in 22 TDT cases reported by Xiao Jian et al.11
Safety Assessment of Thalidomide
Effects of Thalidomide on Liver and Kidney Functions
During the 2-year observation period involving 48 cases, thalidomide showed no significant impact on the elevation of ALT and AST, nor on the elevation of BUN and Cr. This indicates a favorable safety profile regarding hepatic and renal function, aligning with findings reported by various researchers.10,12,13 Begum et al noted that ALT elevation occurred in 16% (8 out of 51) of patients post-treatment.14 A meta-analysis of 10 studies involving 338 patients suggested that ALT elevation was a relatively common mild adverse reaction, underscoring the importance of continued monitoring of ALT and other markers during medication.15
Effect of Thalidomide on Hemolytic Function
To mitigate the impact of blood transfusion on hemolytic indexes, the study analyzed TBIL and IBIL levels before and after treatment in 35 out of the 48 cases within the observation group who did not undergo blood transfusions. β-thalassemia, characterized by chronic hemolytic anemia, results from ineffective erythropoiesis in the bone marrow, leading to elevated bilirubin levels, particularly IBIL. Bilirubin serves as a reliable indicator of extravascular hemolysis.16 Prior to treatment, all patients exhibited elevated bilirubin levels compared to normal. However, no statistically significant differences were noted when comparing TBIL and IBIL values at baseline, 12 months, and 24 months post-treatment. These findings are consistent with those reported by Li Li.13 Similarly, this study did not observe any significant effect of thalidomide on improving hemolysis in β-thalassemia, mirroring previous findings.
Assessment of Adverse Reactions to Thalidomide
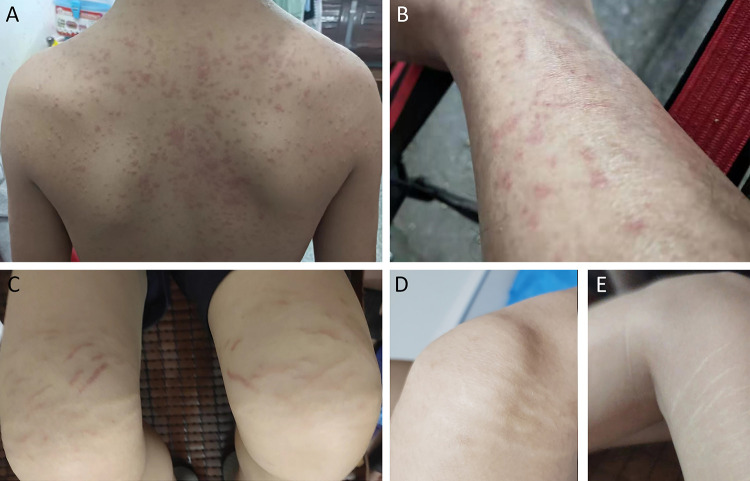
The majority of adverse reactions observed in this dataset were mild, aligning with findings from various studies.11,13,17 Over the two-year period, no significant gastrointestinal discomfort was noted, and side effects like mild drowsiness and constipation were generally well tolerated by patients while on medication. Among the four cases reporting rashes, two were severe and included itching. For instance, Case A displayed a large maculopapular rash on the trunk (Figure 3A), and Case B had papules primarily on the lower limbs (Figure 3B). Discontinuation of the medication led to the disappearance of the rash, which reappeared upon resuming treatment, prompting patients A and B to permanently stop using the drug.
Figure 3.
(A) Case (A) Extensive maculopapular rash present on the back. (B) Case (B) Extensive maculopapular rash on the lower extremities. (C) Pink stripes on the legs of Case C following two months of medication use. (D) Transparent lines appeared on the right leg of Case C several months after reducing the dosage. (E) Transparent lines appeared on the left leg of Case C several months after decreasing the dosage.
The other two patients experienced milder rashes, which eventually faded, allowing them to continue the medication. Additionally, in three cases, a skin pattern manifested as pink, stripe-like markings on the lower limbs, which did not protrude from the skin or cause pain, edema, or itching, with the underlying cause remaining unclear (illustrated in Figure 3C for Case C). In these cases, the stripes faded and turned colorless over a few months after a dosage reduction (depicted in Figure 3D and E for Case C). Mild facial and eyelid edema occurred without worsening during treatment, and patients opted to continue medication. Muscle pain in the lower limbs was reported in four cases, involving transient calf, knee, and ankle joint pain, which resolved with reduced dosages and did not hinder walking. One 16-year-old reported a self-perceived menstrual disorder with a history of irregular menstruation, but venous thrombosis or significant peripheral neuropathy, like limb numbness or sensory issues, was not detected. An age-based analysis of adverse reactions split into groups of 12–14 years, 14–18 years, and over 18 years showed no significant differences in adverse reaction rates across these categories, with the youngest group not experiencing higher incidence rates compared to older groups. The limited number of cases prevented further individual analysis of adverse reaction groups.
Effect of Dose Reduction on Thalidomide Efficacy
Thalidomide was initially prescribed in higher doses for thalassemia, with an awareness that increased dosages could heighten the risk of peripheral neurotoxicity. Yang et al documented a case where a patient experienced numbness in both lower limbs after 18 months on a 100 mg/day dose, ruling out any other potential causes for the neurotoxicity.6 A meta-analysis of 451 patients showed thalidomide dosages ranging from 50 mg/day to 200 mg/day, revealing no significant differences between the lowest and highest doses in terms of Hb level improvement or symptom relief in patients with β-thalassemia.12 The dosage for the 48 participants in this study varied from 25–50 mg/day, with no increases during the observation period. Ten patients reduced their dosage, six from 50 mg/day to 37.5 mg/day and four from 37.5 mg/day to 25 mg/day. The study observed Hb levels before and after dosage reductions at 3 months and 12 months, finding no significant changes in Hb according to repeated measures ANOVA (P > 0.05). At the 12-month mark, 9 of the 10 who reduced their dose maintained treatment efficacy (90%, Figure 2). Jain et al found that a moderate dose of 50 mg/day was effective in maintaining good treatment results.18 Ricchi et al reported similarly on a 44-year-old NTDT patient who managed well on a continuous 50 mg/day regimen for two years without adverse effects, including peripheral neuropathy.19 Recently, Wu Yi studied 23 NTDT cases starting at 50 mg/day using a self-controlled design over two phases.20 In the first phase, the dose was reduced to 37.5 mg/day for three months, and in the second phase, it was further reduced to 12.5 mg/day for another three months. The results showed that mean Hb levels at the end of each phase were significantly higher than baseline levels by 3.0 ± 1.5 g/dL and 3.1 ± 1.4 g/dL, respectively (P <0.001). There were no significant differences in mean Hb values between the phases compared to the situation before each dosage reduction. The study concluded that the dose reductions were safe, well-tolerated, maintained efficacy, delayed the onset of cumulative dose-related peripheral neuropathy, and decreased the incidence of peripheral neuropathy linked to daily dose increases.
Conclusion
We observed 48 cases of β-thalassemia treated with thalidomide at initial doses ranging from 37.5 to 50 mg/day. There were no cases of venous thrombosis. Patients aged 12–14 years did not exhibit a higher incidence of adverse effects compared to the older groups. Ten participants were able to maintain or even improve their Hb levels on reduced doses of 25–37.5 mg/day, achieving a 90% efficacy rate. Given the limited number of cases observed in this study, there is a need to further expand the sample size and conduct longer-term monitoring of adverse reactions to thalidomide. Extending the observation period would also allow a more thorough assessment of the efficacy of low-dose thalidomide, enabling a more accurate evaluation of its effectiveness.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding Statement
This work was supported by Guilin Science and Technology Bureau (No.20210227-10-6).
Abbreviations
HbF, Fetal hemoglobin; TDT, Transfusion-dependent thalassemia; NTDT, Non-transfusion-dependent thalassemia; ECOG, Eastern Cooperative Oncology Group; ORR, Overall response rate; MaR, Main reaction; MiR, Minor response; SlR, Slow response; NR, No response; PCR, Polymerase chain reaction; Hb, Hemoglobin; ALT, Serum alanine aminotransferase; AST, Serum aspartate aminotransferase; TBIL, Total bilirubin; IBIL, Indirect bilirubin; Bun, Urea nitrogen; Cr, Creatinine; CTCAE, Common Terminology Criteria for Adverse Events; HLA, Human leukocyte antigen.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Guilin People’s Hospital (No.2021-035KY). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants, parents or legal guardians gave informed consent for participants under the age of 18.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Eleftheriou A, Angastiniotis M. Global thalassaemia review 2021. Nicosia: Thalassaemia International Federation, 2021. [Google Scholar]
- 2.Beijing Angel Mom Charity Foundation & Beijing Normal University, China Philanthropy Research Institute. China thalassemia blue book: report on the prevention and treatment of thalassemia in China (2020). Beijing: China Social Publishing House, 2021. [Google Scholar]
- 3.Baiqing D, Biyan C, Qiuyu L, et al. Distribution characteristics of 1.69 million cases of total fetal birth defects and major birth defects in the Guangxi zhuang autonomous region. Chin J Epidemiol. 2019;40(12):1554–1559. doi: 10.3760/cma.j.issn.0254-6450.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 4.Jalali FMA, Dehghani FA, Hajizamani S, et al. Thalidomide is more efficient than sodium butyrate in enhancing GATA-1 and EKLF gene expression in erythroid progenitors derived from HSCs with β-globin gene mutation. Int J Hematol Oncol Stem Cell Res. 2016;10(1):37–41. [PMC free article] [PubMed] [Google Scholar]
- 5.Aerbajinai W, Zhu J, Gao Z, et al. Thalidomide induces gamma-globin gene expression through increased reactive oxygen species-mediated p38 MAPK signaling and histone H4 acetylation in adult erythropoiesis. Blood. 2007;110(8):2864–2871. doi: 10.1182/blood-2007-01-065201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Hematology Group of the Pediatric Branch of the Chinese Medical Association, Editorial Committee of Chinese Journal of Pediatrics. Diagnosis and treatment guidelines of β-thalassemia major. Chin J Pediatr. 2018;56(10):724–729. doi: 10.3760/cma.j.issn.0578-1310.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 7.Guangdong Association for the Prevention and Treatment of β-thalassemia, Editorial Committee of Chinese Journal of Practical Pediatrics. Expert consensus on the diagnosis, treatment, and management of non transfusion dependent thalassemia in children. Chinese J Practical Pediatr. 2018;33(12):929–934. [Google Scholar]
- 8.Common Terminology Criteria for Adverse Events (CTCAE)version 5.0: Published November 27, 2017.U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES. [Google Scholar]
- 9.Yang K, Wu Y, Zhou Y, et al. Thalidomide for patients with beta-thalassemia: a multicenter experience.. Mediterr J Hematol Infect Dis. 2020;12(1):e2020021. doi: 10.4084/mjhid.2020.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali Z, Ismail M, Rehman IU, et al. Long-term clinical efficacy and safety of thalidomide in patients with transfusion-dependent beta-thalassemia: results from Thal-Thalido study. Sci Rep. 2023;13(1):13592. doi: 10.1038/s41598-023-40849-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jian X, Liu XD, Peng W, et al. Long-term efficacy and safety of thalidomide treatment in children with β-thalassemia major. Pediatr Blood Cancer. 2023;70(9):e30391. doi: 10.1002/pbc.30391 [DOI] [PubMed] [Google Scholar]
- 12.Ren Q, Zhou YL, Wang L, et al. Clinical trial on the effects of thalidomide on hemoglobin synthesis in patients with moderate thalassemia intermedia. Ann Hematol. 2018;97(10):1933–1939. doi: 10.1007/s00277-018-3395-5 [DOI] [PubMed] [Google Scholar]
- 13.Pinto VM, Romano N, Balocco M, et al. Reduction of extramedullary erythropoiesis and amelioration of anemia in a β-thalassemia patient treated with thalidomide. Am J Hematol. 2024;99(3):463–464. doi: 10.1002/ajh.27189 [DOI] [PubMed] [Google Scholar]
- 14.Zhu W, He Y, Huang M, et al. Long-term follow-up of patients undergoing thalidomide therapy for transfusion-dependent beta-thalassaemia: a single-center experience. Int J Gen Med. 2024;17:1729–1738. doi: 10.2147/IJGM.S462991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y, Wei Z, Yang G, et al. Investigating the efficacy and safety of thalidomide for treating patients with ß-thalassemia: A meta-analysis. Front Pharmacol. 2022;11(12):814302. doi: 10.3389/fphar.2021.814302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barcellini W, Fattizzo B. Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis Markers. 2015;2015:635670. doi: 10.1155/2015/635670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra J, Parakh N, Sidharth, et al. Efficacy and safety of thalidomide in patients with transfusion-dependent thalassemia. Indian Pediatr. 2021;58(7):611–616. doi: 10.1007/s13312-021-2254-y [DOI] [PubMed] [Google Scholar]
- 18.Jain M, Chakrabarti P, Dolai TK, et al. Comparison of efficacy and safety of thalidomide vs hydroxyurea in patients with Hb E-beta thalassemia - a pilot study from a tertiary care Centre of India. Blood Cells Mol Dis. 2021;88:102544. doi: 10.1016/j.bcmd.2021.102544 [DOI] [PubMed] [Google Scholar]
- 19.Ricchi P, Costantini S, Spasiano A, et al. The long-term and extensive efficacy of low dose thalidomide in a case of an untransfusable patient with non-transfusion-dependent thalassemia. Blood Cells Mol Dis. 2016;57:97–99. doi: 10.1016/j.bcmd.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 20.Wu Y Clinical observation of reduced maintenance therapy with thalidomide for non transfusion dependent thalassemia. Guilin Medical College.2021.5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.