Abstract
BACKGROUND
“Dangling choroid” is a prenatal sonographic marker of ventriculomegaly that measures the angle of choroid plexus (ChP) displacement in the lateral ventricle. To the authors’ knowledge, postnatal sequelae related to this pathology, besides hydrocephalus, have never been reported.
OBSERVATIONS
A female fetus was diagnosed with bilateral ventriculomegaly. Postnatally, the patient was diagnosed with hydrocephalus and macrocephaly secondary to aqueductal stenosis and underwent endoscopic third ventriculostomy with ChP cauterization. Intraoperatively, the septum pellucidum was incomplete, and the right-sided ChP glomus was contralaterally displaced and entangled with the left, with evidence of ischemic torsion and hemorrhage.
LESSONS
In this case of an ischemic ChP secondary to transventricular displacement of the glomus, at least two biomechanical events are relevant. First, the choroid fissure and velum interpositum can be thinned in the setting of ventriculomegaly. Second, stretching and perforation of the septum pellucidum can occur. Both changes can increase the mobility of a dangling choroid, occasionally leading to entanglement of the vascular pedicles. Preoperative recognition of this complication can help optimize surgical planning, e.g., using flexible endoscopy to facilitate complete ChP cauterization and changing the surgical approach if the ChP has been displaced.
Keywords: dangling choroid plexus, glomus, septum pellucidum, intraventricular hemorrhage, IVH, ventriculomegaly, hydrocephalus, pediatric neurosurgery, case report
ABBREVIATIONS: ChP = choroid plexus, CPC = ChP cauterization, ETV = endoscopic third ventriculostomy, IVH = intraventricular hemorrhage, MRI = magnetic resonance imaging.
The choroid plexus (ChP) is a highly vascularized intraventricular brain structure that is known for its roles in regulating the composition, volume, and general homeostasis of cerebrospinal fluid. It also plays an important role in brain development, neurogenesis, immune responses, and circadian rhythms.1–5 Anatomically, the ChP can be identified by its ventricular location. Within the atrium (trigone) of the lateral ventricle lies the glomus choroidea, or “glomus”: a swelling of the ChP that is identifiable in large mammals.6–8 Here, we present a rare case of a “dangling” ChP that led to contralateral, transventricular displacement of the right glomus, entanglement with the left glomus, and the creation of a glomus torsion complex, ultimately resulting in ChP ischemia and mild intraventricular hemorrhage (IVH) in a term infant.
Illustrative Case
Presentation
The patient was initially diagnosed with bilateral ventriculomegaly on routine prenatal ultrasound at 18 weeks’ gestational age. Amniocentesis demonstrated no abnormalities on the chromosomal microarray, and later postnatal whole exome sequencing was unremarkable. At 22 weeks’ gestational age, the patient’s mother underwent fetal magnetic resonance imaging (MRI), which showed severe ventriculomegaly with thinning of the corpus callosum and septum pellucidum. The patient’s mother was a 38-year-old gravida 2 para 1 who had conceived spontaneously and had had an unremarkable pregnancy thus far, with no reported exposure to alcohol, tobacco, illicit substances, teratogenic medications, or infections. Term delivery occurred via cesarean section, with no subsequent complications during a 4-day stay in the neonatal intensive care unit. The patient’s examination was notable for mild nystagmus, no upgaze limitation, a full fontanelle, and a 42-cm (99th percentile) head circumference. She was discharged to home with a plan for outpatient MRI and neurosurgical clinic follow-up within 3 weeks.
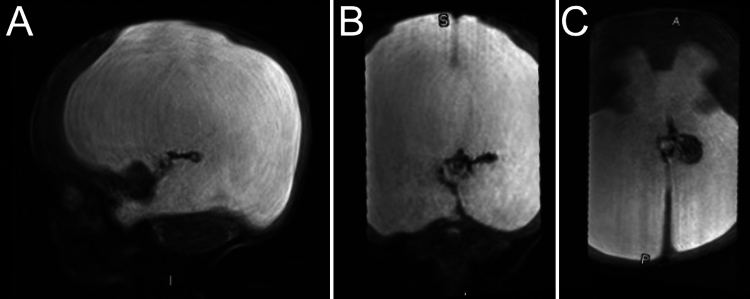
The patient’s interval evaluation in the clinic was notable for an increase in head circumference (48.5 cm, 100th percentile), irregular eye movements with newly impaired upgaze, splayed sutures with prominent scalp veins, and a full fontanelle. MRI revealed cerebral aqueductal stenosis as the likely etiology of her severe triventricular ventriculomegaly, and the glomus of the ChP was asymmetric, visible on the left side only (Fig. 1). Imaging was further notable for an incomplete falx, diffuse thinning of the supratentorial parenchyma, and blood products within or near the ChP of the left lateral ventricle.
FIG. 1.
Preoperative imaging. T2-weighted sequences centered on the ChP glomera deviated to the left ventricle. A: Sagittal view. B: Coronal view. C: Axial view. A = anterior; I = inferior; P = posterior; S = superior.
Intraoperative Findings
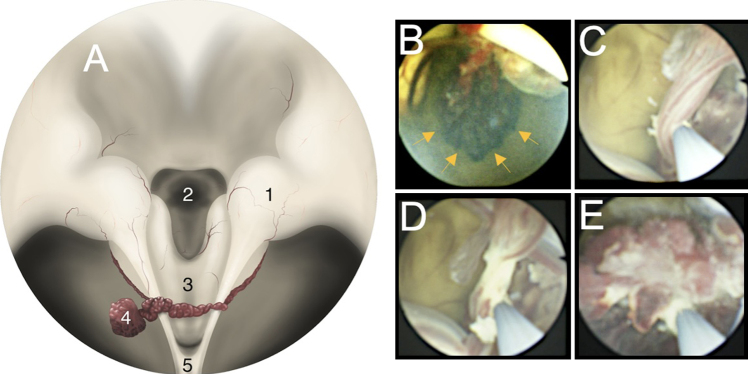
Shortly thereafter, at 28 days of age, the patient underwent endoscopic third ventriculostomy (ETV) and bilateral endoscopic ChP cauterization (CPC). Upon endoscopic entry into the right lateral ventricle, we observed that the glomus of the right ChP was contralaterally displaced and was entangled with the vascular pedicle of its counterpart on the left, causing thrombosed veins of both glomera with associated discoloration, suggestive of ischemia (see Fig. 2A for artistic rendering, Fig. 2B–E for photographs from the flexible, fiberoptic neuroendoscope). The third ventricle was further notable for compression of its floor into the sella turcica, absence of its roof, and anterior bowing of the lamina terminalis. Other intraoperative findings included abnormal brain architecture with knobby separated thalami, an absent septum pellucidum, and an atretic cerebral aqueduct. The procedure concluded with complete cauterization of the bilateral ChP within the lateral ventricles, including the entangled glomera.
FIG. 2.
Intraoperative, intraventricular visualization of glomus entanglement and torsion, using a flexible endoscope. A: Artistic rendering of the endoscopic survey of the ventricular system, showing contralateral draping of glomus torsion in this patient. 1, thalamus; 2, tuber cinereum; 3, brainstem; 4, entangled glomera (ChP); 5, tentorium. B: Endoscopic view of the left lateral ventricle with the red-colored vascular pedicle draped over the white-colored tentorial incisura; the dark/ischemic glomus is connected to its entangled vascular pedicle (yellow arrows). A portion of the other glomus is partially visible beneath the incisura. C: Endoscopic view of the vascular pedicle leading to the two glomera, partially displaced by the cauterization wire protruding from the endoscope. D: Endoscopic view of the blanching of the pedicle in response to cauterization. E: View of the glomus deep to the incisura, within the left lateral ventricle.
Postoperative Course
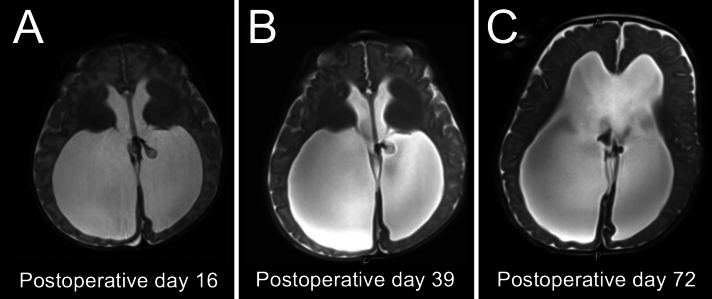
The patient had an uncomplicated postoperative course. At the first postoperative visit, the patient’s parents reported no concerning symptoms such as vomiting, abnormal eye movements, gaze restrictions, or lethargy. Interval MRI demonstrated an overall decrease in ventricle size and gradual atrophy of the cauterized ChP over 2 months (Fig. 3). Head circumference began to parallel an appropriate growth trajectory by the 2nd postoperative month. At 16 months of age, the patient was meeting appropriate developmental milestones, except for gross and fine motor skills, for which she was receiving support from regular physical and speech therapy. At the long-term follow-up (3.5 years of age), physical examination was remarkable for continued gross and fine motor skill delay, accompanied by proximal muscle weakness, appendicular hypotonia, and macrocephaly (with a stable fronto-occipital horn ratio of 0.64).
FIG. 3.
Postoperative imaging. A: MRI at postoperative day 16. B: MRI at postoperative day 39. C: MRI at postoperative day 72, showing complete atrophy of the left-sided ChP glomera following ETV/CPC.
Other Imaging Examples
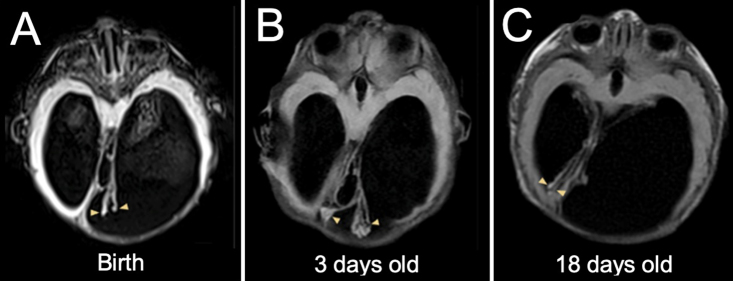
We observed ChP displacement and entanglement on review of imaging in two other patients with severe ventriculomegaly, as demonstrated in Figs. 4 and 5. These findings occurred in the setting of a septum pellucidum defect and bilateral ventriculomegaly, as in our illustrative case. Figure 4 represents a full-term patient born with myelomeningocele and hydrocephalus, which were treated with closure of the myelomeningocele and ventriculoperitoneal shunt placement within 24 hours of life. Prior to shunting, both glomera were seen in the right lateral ventricle due to a perforated septum pellucidum and highly mobile glomera. After shunting and on interval imaging, the right glomus descended into the third ventricle through the foramen of Monro, while the left glomus returned to its normal position in the left lateral ventricle. Figure 5 shows a third patient, born at 34 weeks’ gestational age with severe aqueductal stenosis, who also underwent shunt placement within 48 hours of birth. In this case, the glomera were entangled from birth and showed mobility as a unit, in contrast to the prior case. Imaging revealed bilateral ChP entanglement within the left lateral ventricle (Fig. 5A), mobility as demonstrated by the 90° rotation of both flat glomera (Fig. 5B), and later contralateral displacement of the same entangled pair of glomera (Fig. 5C).
FIG. 4.
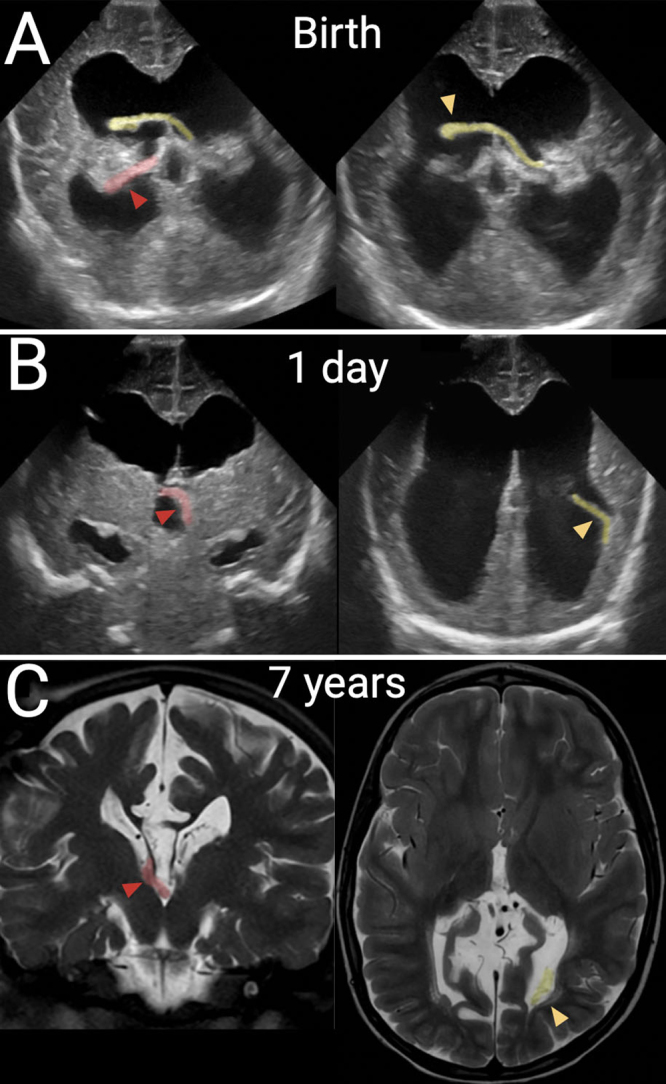
Second case of dangling choroid. A full-term patient was born with myelomeningocele that was repaired and treated with a shunt on the 1st day of life. In all panels, the right-sided glomus is shaded in red with a red arrowhead; the left-sided glomus is shaded in yellow with a yellow arrowhead. A: Prior to shunting, coronal transfontanelle ultrasound images show displacement of a mobile left glomus across the perforated septum and a right glomus draped over the posterior thalamus. B: After shunting, coronal ultrasound images show a dangling left glomus in the left ventricle and a newly displaced right glomus in the third ventricle. C: T2-weighted MRI at 7 years of age in the same patient, showing stable locations of both ChPs as compared to the postshunt ultrasound image.
FIG. 5.
Third case of dangling choroid. The patient was born at 34 weeks’ gestational age with severe aqueductal stenosis that was treated within 48 hours. Fluid-attenuated inversion recovery MRI showing entangled ChP (yellow arrowheads) in the left lateral ventricle (A), 90° rotation of both flat glomera in the same ventricle (B), and then contralateral displacement of the same entangled pair of glomera (C).
Informed Consent
The necessary informed consent was obtained in this study.
Discussion
Observations
A dangling choroid is a documented sonographic finding in the obstetrics literature and is occasionally found on prenatal ultrasound in the setting of ventriculomegaly.9, 10 The term describes a condition in which the ChP migrates from its usual position against the lateral ventricle wall to “dangle” within the ventricle due to ventriculomegaly.11 Unique to this illustrative case of a dangling choroid was the resultant ischemic torsion and contralateral displacement of the glomus in the postnatal period. The mobility of the ChP complex was particularly evident during the ETV/CPC: as we applied cautery to the underside and circumferential portions of the glomus, the mobile portions of the ChP along its pedicle required significant manipulation, which was facilitated by the use of a flexible endoscope.
The precise chronology for the entanglement of the two glomera in this case of a dangling choroid remains unknown, but the loss of the septum pellucidum during the progression of ventriculomegaly was essential for contralateral choroidal crossing and rotational mobility. Although the absence of the septum pellucidum can be a congenital defect,12 ,13 its absence in the main illustrative and subsequent cases presented here was secondary to ventriculomegaly, given that 1) the septum pellucidum was initially noted on prenatal imaging, and 2) severe and progressive ventriculomegaly stretched the septum pellucidum over time.
Although the gradual perforation of the septum pellucidum can independently cause a transient hematoma (our observations), the laterality and focal finding of hemorrhage near the entangled glomera in our case argue in favor of a ChP source. This is consistent with the finding that IVH in term infants most commonly originates from the posterior tufts of the glomus, which has a tortuous microvascular architecture,6, 14 in contrast to IVH in preterm infants, which more commonly originates from the germinal matrix.15, 16 Additionally, the sequelae of ChP ischemia and IVH most likely reflect postnatal events, given subacute findings of hemorrhage on postnatal MRI.
There are other examples of the importance of the glomus as a nexus for ChP pathologies, including vascular malformations17 and intraventricular tumors. ChP papillomas are commonly localized to the glomus,14, 18 and a robust mouse model of ChP carcinoma has identified specific localization of this tumor to the posterior domain of the lateral ventricle ChP,19 in the same relative location as the human glomus.20 Intraventricular meningiomas are also most commonly observed in the atrium of the lateral ventricle and are postulated to arise from stromal tissue in the glomus.21–24 Future studies should continue to assess the clinical relevance of the underlying vascular and structural changes associated with the dangling choroid.
Lessons
The increased pedicle length and mobility at the base of the ChP due to ventriculomegaly, combined with the relative size and weight of the glomus, led to increased ChP mobility that resulted in the dangling choroid sign seen on prenatal imaging for this patient. The contralateral displacement, entanglement, and ischemic torsion of a highly mobile ChP following gradual perforation of the septum pellucidum, as described here, can provide an explanation for unexpected findings on postnatal imaging and intraoperatively for patients with severe hydrocephalus. We suggest the use of flexible rather than rigid endoscopy to facilitate complete cauterization of the ChP in these cases, given the mobility and altered anatomy of the ChP. The pre-operative recognition of ChP torsion and displacement may affect surgical planning, both in the use of a flexible endoscope and in the surgical approach to target the new site of the ChP. Whether clinical outcomes are different for individuals with severe ventriculomegaly and these complications of a dangling choroid remains to be seen.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Sadegh, Moyer, Sudhof, Warf. Acquisition of data: Sadegh, Warf. Analysis and interpretation of data: Sadegh, Jones, Sudhof, Soufi, Warf. Drafting the article: Sadegh, Jones, Moyer, Sudhof, Soufi, Mashouf. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Sadegh. Study supervision: Sadegh. Illustration: Moyer.
Correspondence
Cameron Sadegh: UC Davis Medical Center, Sacramento, CA. csadegh@ucdavis.edu.
References
- 1.Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat Rev Neurosci. 2015;16(8):445-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fame RM, Lehtinen MK. Emergence and developmental roles of the cerebrospinal fluid system. Dev Cell. 2020;52(3):261-275. [DOI] [PubMed] [Google Scholar]
- 3.Ghersi-Egea JF, Strazielle N, Catala M, Silva-Vargas V, Doetsch F, Engelhardt B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018;135(3):337-361. [DOI] [PubMed] [Google Scholar]
- 4.Sadegh C, Xu H, Sutin J, et al. Choroid plexus-targeted NKCC1 overexpression to treat post-hemorrhagic hydrocephalus. Neuron. 2023;111(10):1591-1608.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders NR, Dziegielewska KM, Fame RM, Lehtinen MK, Liddelow SA. The choroid plexus: a missing link in our understanding of brain development and function. Physiol Rev. 2023;103(1):919-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millen JW, Woollam DHM. Vascular patterns in the choroid plexus. J Anat. 1953;87(2):114-123. [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson AJ. The development of the vascular pattern of the choroid plexus of the lateral ventricles. J Comp Neurol. 1960;115(2):171-186. [DOI] [PubMed] [Google Scholar]
- 8.Gomez DG, Potts DG. The lateral, third, and fourth ventricle choroid plexus of the dog: a structural and ultrastructural study. Ann Neurol. 1981;10(4):333-340. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi DW, Crombleholme TM, D’Alton ME, Malone FD. Fetology: Diagnosis and Management of the Fetal Patient. 2nded. The McGraw-Hill Companies, Inc.; 2010. [Google Scholar]
- 10.Woodward PJ. Diagnostic Imaging: Obstetrics. 4th ed. Elsevier; 2021. [Google Scholar]
- 11.Cardoza JD, Filly RA, Podrasky AE. The dangling choroid plexus: a sonographic observation of value in excluding ventriculomegaly. AJR Am J Roentgenol. 1988;151(4):767-770. [DOI] [PubMed] [Google Scholar]
- 13.Sarwar M. The septum pellucidum: normal and abnormal. AJNR Am J Neuroradiol. 1989;10(5):989-1005. [PMC free article] [PubMed] [Google Scholar]
- 14.Marinković S, Gibo H, Milisavljević M, Djulejić V, Jovanović VT. Microanatomy of the intrachoroidal vasculature of the lateral ventricle. Oper Neurosurg. 2005;57(1 suppl):22-36. [DOI] [PubMed] [Google Scholar]
- 15.Szpecht D, Frydryszak D, Miszczyk N, Szymankiewicz M, Gadzinowski J. The incidence of severe intraventricular hemorrhage based on retrospective analysis of 35939 full-term newborns—report of two cases and review of literature. Childs Nerv Syst. 2016;32(12):2447-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacey DJ, Terplan K. Intraventricular hemorrhage in full-term neonates. Dev Med Child Neurol. 1982;24(3):332-337. [DOI] [PubMed] [Google Scholar]
- 17.Moftakhar R, Salamat MS, Sahin S, Iskandar BJ. Endoscopically-assisted resection of a choroid plexus vascular malformation traversing the cerebral aqueduct: technical case report. Oper Neurosurg. 2006;59(1 suppl 1):ONS-E161. [DOI] [PubMed] [Google Scholar]
- 18.Raimondi AJ, Gutierrez FA. Diagnosis and surgical treatment of choroid plexus papillomas. Childs Brain. 1975;1(2-3):81-115. [DOI] [PubMed] [Google Scholar]
- 19.Shannon ML, Fame RM, Chau KF, et al. Mice expressing Myc in neural precursors develop choroid plexus and ciliary body tumors. Am J Pathol. 2018;188(6):1334-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortazavi MM, Griessenauer CJ, Adeeb N, et al. The choroid plexus: a comprehensive review of its history, anatomy, function, histology, embryology, and surgical considerations. Childs Nerv Syst. 2014;30(2):205-214. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A, Kanekar S. Intraventricular tumors. Semin Ultrasound CT MR. 2016;37(2):150-158. [DOI] [PubMed] [Google Scholar]
- 22.Grujicic D, Cavallo LM, Somma T, et al. Intraventricular meningiomas: a series of 42 patients at a single institution and literature review. World Neurosurg. 2017;97:178-188. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Roser F, Bundschuh O, Vorkapic P, Samii M. Intraventricular meningiomas: a review of 16 cases with reference to the literature. Surg Neurol. 2003;59(6):491-503. [DOI] [PubMed] [Google Scholar]
- 24.Mayol Del Valle M, De Jesus O. Intraventricular meningioma. In: StatPearls. Internet. StatPearls; Publishing; 2024 Jan–. Accessed October 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK562202/ [PubMed] [Google Scholar]