Abstract
Renal impairment and rhabdomyolysis are rare in transplant patients receiving sirolimus. We report the case of a 54-year-old male who underwent liver transplantation and was initially treated with tacrolimus, mycophenolate mofetil, and glucocorticoids for immunosuppression. After the development of renal dysfunction, tacrolimus was replaced with sirolimus. However, one month after taking sirolimus, the patient’s renal function continued to deteriorate, and rhabdomyolysis developed one and a half months later. Serum analysis indicated high sirolimus concentration, whereas renal histopathology revealed acute tubular injury and interstitial arteriopathy. After reducing the dosage of sirolimus, the patient’s creatine kinase levels returned to normal, and renal function improved. Two years after discharge, the patient’s renal function had recovered. This case highlights the importance of monitoring sirolimus blood concentrations in clinical practice, because elevated drug concentrations can lead to renal dysfunction and rhabdomyolysis as adverse reactions. Further investigations into the pathogenic mechanisms of sirolimus-induced renal dysfunction and rhabdomyolysis may contribute to clinical practice.
Keywords: Sirolimus, Liver transplantation, Rhabdomyolysis, Renal dysfunction
Introduction
Sirolimus is a macrolide immunosuppressant that inhibits T-lymphocyte proliferation under cytokine stimulation [2]. Its structure is similar to that of tacrolimus, and its immunosuppressive potency has been reported to be comparable to that of tacrolimus, but with lower renal toxicity [3]. Sirolimus is primarily used to prevent organ rejection after liver, kidney, and other organ transplantations, particularly in patients with concurrent hypertension, renal insufficiency, or tremors. It is a new low-toxicity potent immunosuppressant discovered to date [4]. Previous studies have shown that sirolimus has lower renal toxicity and is less likely to cause renal impairment [5]. When using sirolimus, some adverse reactions were reported, such as dyslipidaemia, bone marrow suppression, delayed wound healing, peripheral oedema, neutropenia, and pneumonitis [6]. However, concurrent creatine kinase (CK) elevation and rhabdomyolysis is rare [11]. Previous studies have reported a few cases of rhabdomyolysis and renal injury in transplant patients taking both sirolimus and statins or fibrate drugs [13–15]. As rhabdomyolysis is a common adverse event associated with lipid-lowering drugs, the effect of sirolimus on rhabdomyolysis in these cases is unclear. Here, we report the case of a liver transplant patient who experienced worsening renal function and rhabdomyolysis after switching from tacrolimus to sirolimus without the use of statin drugs.
Case report
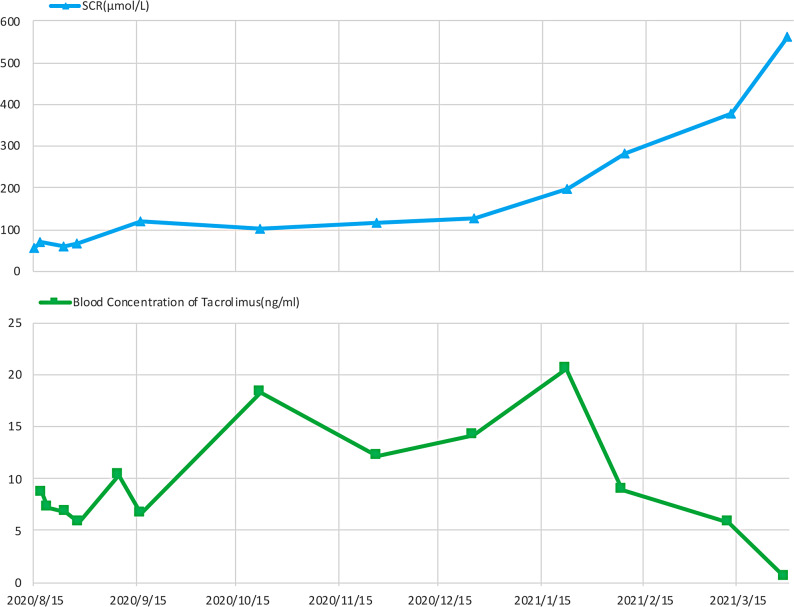
On April 25, 2021, a 54-year-old male (height: 160 cm, weight: 62 kg) was admitted to the Nephrology department of West China Hospital of Sichuan University with elevated serum creatinine for seven months and pitting oedema of both lower extremities for eight days. Eight months prior to admission, the patient underwent an allogeneic liver transplantation for alcoholic liver cirrhosis. The renal function was normal before the surgery. The serum creatinine level was 62 µmol/L after the surgery, and the evaluated glomerular filtration rate (eGFR) was 109.87 mL/min/1.73 m². Following the surgery, the patient was started on regular oral administration of tacrolimus 2 mg twice daily, mycophenolate mofetil 500 mg twice daily, and prednisone 20 mg once daily. During the first two months of tacrolimus treatment, the drug concentration was maintained between 5.0 and 10.2 ng/mL, and serum creatinine gradually increased from 65 µmol/L to 120 µmol/L. The tacrolimus blood concentration fluctuated between 8.97 and 20.6 ng/mL from the third to sixth month, and serum creatinine level rose from 120 µmol/L to 197 µmol/L. In the seventh to eighth months, tacrolimus levels ranged from 5.28 to 8.97 ng/mL, yet renal function continued to deteriorate, with serum creatinine level increasing from 126 µmol/L to 564 µmol/L (Fig. 1). The medication was adjusted to sirolimus 2 mg twice daily (The transplant doctor had informed the patient that sirolimus should be taken once a day. However, for convenience, the patient adjusted the dosage to twice a day in order to take it with other medications), mycophenolate mofetil 500 mg twice daily and prednisone 10 mg once daily. Before the switching to sirolimus, the most recent blood concentration of tacrolimus was 5.78 ng/mL. Additionally, the patient used roxaresta, polysaccharide-iron complex, folic acid tablets for anaemia correction, calcium carbonate, and complex alpha-keto acid tablets for protein supplementation. After multiple verifications, it was confirmed that the patient did not use statins or other related medications. Half a month before admission, his serum creatinine gradually increased to 571 µmol/L. Eight days before admission, the patient developed bilateral lower extremity pitting oedema accompanied by generalised weakness, and his serum creatinine level increased to 677 µmol/L.
Fig. 1.
The clinical course of the present case before admission. The X-axis displays the date before admission. The serum creatinine levels (blue) and tacrolimus blood concentration (green) variations were recorded
Upon admission, the patient had a temperature of 36.8 °C, a respiratory rate of 20 breaths per minute, a heart rate of 91 beats per minute, and a blood pressure of 138/88 mmHg. The 24-h urine output was approximately 1500 mL. Physical examination revealed no significant positive findings in the cardiovascular, respiratory, or abdominal regions; however, bilateral lower limb pitting oedema was observed. Routine examination of the peripheral blood showed a haemoglobin level of 74 g/L, white blood cell count of 20.31 × 109/L, and platelet count of 125 × 109/L. Blood biochemistry analysis showed an albumin level of 31.2 g/L, alanine aminotransferase level of 51 IU/L, aspartate aminotransferase level of 137 IU/L, CK level of 25,468 IU/L, serum creatinine level of 677 µmol/L, uric acid level of 75 µmol/L, calcium ion level of 1.29 mmol/L, potassium ion level of 2.56 mmol/L, anion gap of 20.9 mmol/L, myoglobin level of > 3000 ng/mL, troponin T level of 291.3 ng/L, creatine kinase-MB level of 1.92 ng/mL, pro-B-type natriuretic peptide level of 21,695 ng/L, C-reactive protein level of 20.30 mg/L, procalcitonin level of 0.74 ng/mL, and sirolimus blood concentration of 21.1 ng/mL. Urine analysis revealed the presence of protein 3+, white blood cells of 7/HP, and red blood cells of 8/HP. The protein-to-creatinine ratio in the urine was 0.268 g/mmol creatinine. The 24-hour urine protein excretion was 1.51 g/24 h. Clinical immunological examination revealed no obvious abnormalities. The results for 1,3-β-D-glucan (G) test, aspergillus galactomannan (GM) test, interferon-gamma release assay for tuberculosis infection (TB-IGRA), Epstein-Barr virus DNA real-time fluorescence quantification, BK-JC virus load analysis, cytomegalovirus nucleic acid quantification, mycoplasma pneumoniae antibody, and TORCH panel testing were all negative. Sputum smears, sputum cultures, and blood cultures were negative. Ultrasonography of the urinary system showed slightly enhanced renal parenchymal echogenicity, with the right kidney measuring 12 cm × 5.1 cm × 5.3 cm and the left kidney measuring 11.9 cm × 5.2 cm × 5.6 cm. Single-photon emission computed tomography (SPECT) renal imaging showed reduced blood perfusion and severe impairment of renal function (glomerular filtration rate of 11.3 mL/min in the left kidney and 19.2 mL/min in the right kidney). Chest computed tomography (CT) showed minimal signs of inflammation in both lungs, and a small amount of pleural effusion.
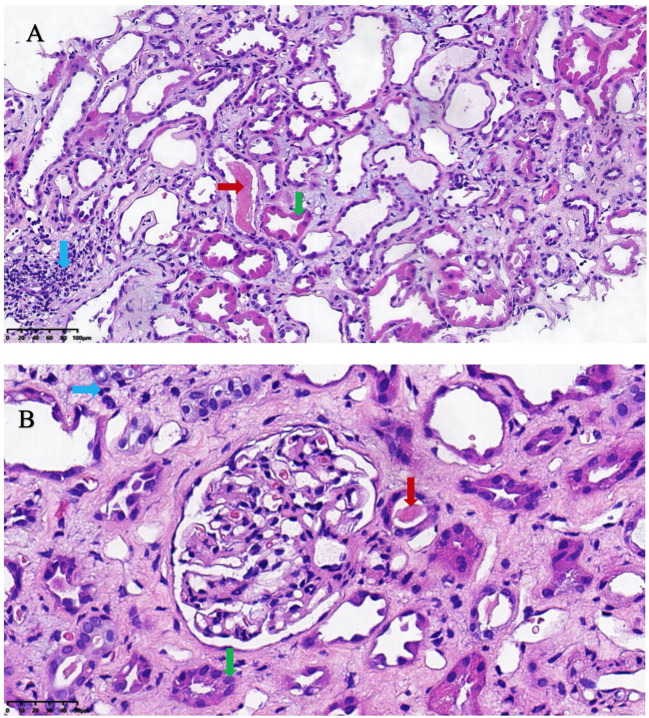
The patient was diagnosed with chronic renal failure with acute exacerbation, rhabdomyolysis, and a pulmonary infection. Upon admission, he received treatment, including citric acid and potassium supplementation to correct electrolyte imbalances, intravenous piperacillin sodium and tazobactam sodium for infection control, water-soluble vitamins for antioxidation, and volume expansion. Due to the elevated blood concentration of sirolimus, the sirolimus dose was reduced to 1 mg once daily. After two weeks of treatment, the patient’s CK level was within the normal range, but the level of serum creatinine was still high (574 µmol/L). On the 16th day after admission, a renal biopsy was performed. Several pathological findings were observed. Optical microscopy findings revealed a total of 22–23 glomeruli, with two exhibiting global sclerosis and one demonstrating fibrous thickening. No significant alterations were observed in the mesangium, basement membrane, or the capillary lumen. Approximately 25% of the tubules displayed atrophy, accompanied by moderate-to-severe degeneration of tubular epithelial cells. Tubular protein casts were observed in some tubular lumens. Approximately 25% of the interstitium exhibited fibrosis accompanied by infiltration of lymphocytes and monocytes. Mild-to-moderate thickening of the small arterial walls was observed, and occasional foam-like changes were noted in the endothelial cells of some arteries (Fig. 2). Immunohistochemistry showed that all four glomeruli were negative for IgG, IgM, IgA, C3, C4, C1q, µ, and λ (Fig. 2). Renal pathology indicated acute tubular injury, which could be explained as a consequence of rhabdomyolysis. This type of lesion is often self-limiting. However, interstitial vascular changes were not typical pathological manifestations of rhabdomyolysis. Based on the patient’s history, vascular changes were highly associated with tacrolimus nephrotoxicity. Therefore, sirolimus was continued instead of tacrolimus and the blood concentration of sirolimus was rechecked. On the 14th day of hospitalisation, the blood concentration of sirolimus was 10.8 ng/mL. As the patient did not exhibit signs of liver dysfunction or rejection, the sirolimus dose was further reduced to 0.5 mg once daily. On the 20th day of hospitalisation, the sirolimus blood concentration was rechecked and a level of 6.6 ng/mL was observed.
Fig. 2.
The histopathological findings of the renal biopsy. (A) Renal interstitial. x200. Hematoxylin and eosin staining. (B) Glomerulus. x400. Hematoxylin and eosin staining. Red arrow: Protein casts in the lumen of renal tubules; Green arrow: Moderate to severe degeneration of renal tubular epithelial cells; Blue arrow: Interstitial fibrosis with infiltration of lymphocytes and monocytes
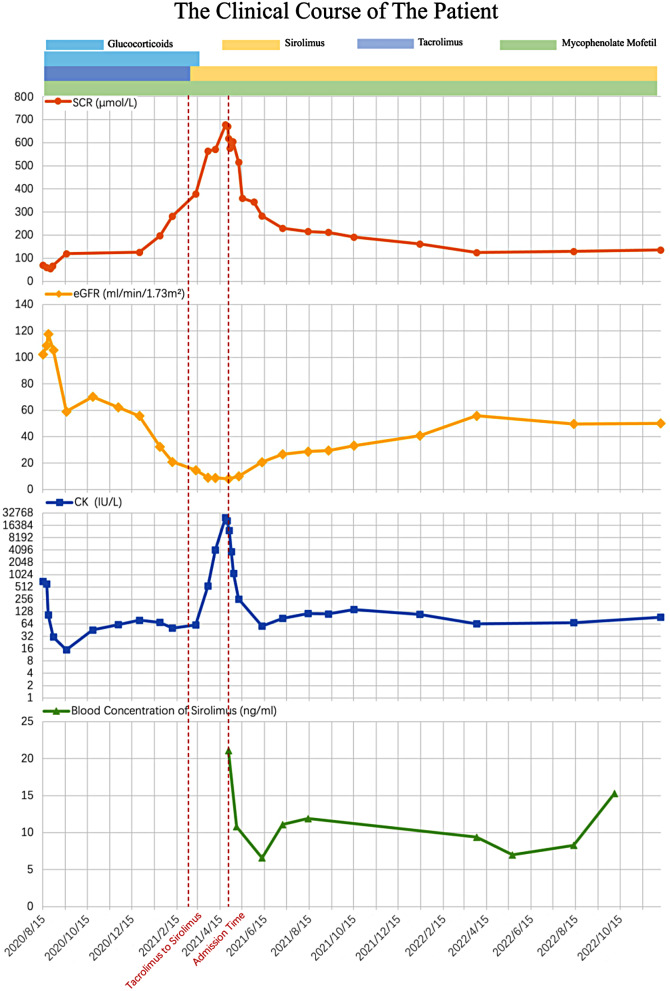
On the 23rd day of hospitalisation, the creatinine decreased to 393 µmol/L. the haemoglobin level had increased to 89 g/L. CK (68IU/L), aspartate aminotransferase, alanine aminotransferase, blood potassium, and calcium levels remained normal. The patient was discharged on the 24th day. After discharge, the patient continued to receive oral sirolimus (0.5 mg) once daily and mycophenolate mofetil (500 mg) twice daily. Blood concentration of sirolimus was regularly monitored, and the concentration fluctuated between 7 and 15 ng/mL. Two months after discharge, the patient’s serum creatinine level decreased to 234 µmol/L. Six months after discharge, the creatinine level was 192 µmol/L. Two years after discharge, the patient’s renal function further improved, with serum creatinine level of 126µmol/L and an eGFR of 54.96 mL/min/1.73 m2.(Fig. 3).
Fig. 3.
The clinical course of the present case. The X-axis shows the date since admission. The serum creatinine levels (Orange), evaluated glomerular filtration rate levels(yellow), creatine kinase (blue), and sirolimus blood drug concentration (green) variations were recorded. Multiple medication were given to the patient. Periods using tacrolimus, mycophenolate mofetil, prednisone and sirolimus consisting of immunosuppressive regimen were marked by purple, green, blue and yellow thick lines, respectively
Discussion
Tacrolimus is a type of calcineurin inhibitor (CNI) [1]. The reliable immunosuppressive effects of CNIs have been widely recognised, but hepatorenal toxicity due to the long-term use of CNIs is common [1]. Sirolimus is a large-ring lactone antibiotic immunosuppressant that inhibits T lymphocyte proliferation upon cytokine stimulation [2]. As a lipophilic 35-membered lactone compound, its structure is similar to that of tacrolimus (a 23-membered lactone compound). However, its immunosuppressive potency has been reported to be higher than that of tacrolimus, while its renal toxicity is lower [3]. Sirolimus is mainly used to prevent organ rejection for organs such as the liver and kidney and is especially suitable for patients with concurrent hypertension, renal insufficiency, and tremors [4]. It is a new potent immunosuppressive agent with low toxicity [4]. In patients with calcineurin inhibitor nephrotoxicity confirmed through biopsy, switching to sirolimus improves renal function. Xu et al. investigated 44 renal transplant recipients with chronic kidney disease (confirmed by transplant kidney biopsy) who had their immunosuppressants switched from tacrolimus to sirolimus. After 1–2 months, the average serum creatinine level of patients decreased, and the average glomerular filtration rate increased. By six months, the difference in serum creatinine levels before and after conversion was statistically significant (P < 0.05), suggesting that sirolimus may have lower renal toxicity than tacrolimus [5].
In this case, the patient was administered tacrolimus 2 mg twice daily for postoperative immunosuppression. However, one month after switching from tacrolimus to sirolimus, the patient’s creatinine level continued to increase, and the glomerular filtration rate continued to decline. Renal pathology revealed an acute tubular injury, tubular epithelial cell shedding, and mild-to-moderate thickening of the interstitial arterial walls accompanied by endometrial fibrosis and individual arterial endarterial cell foam-like changes.
Previous studies have shown that adverse reactions to tacrolimus include acute and chronic renal injury [6]. Acute renal injury is usually associated with afferent arteriolar vasoconstriction and is generally reversible [6]. Chronic nephrotoxicity is often accompanied by characteristic histological changes, including arterial vascular lesions, striped interstitial fibrosis, and focal segmental glomerulosclerosis-like changes, with arterial lesions being the most common and characterised by island-like deposition of hyaline in the small arterial walls [6]. Immunofluorescence of renal biopsies in glomerular lesions often reveals the deposition of immune complexes, such as IgG, IgM, C3, and others [6]. In this case, the patient experienced an increase in creatinine levels after taking tacrolimus for seven months, and pathological biopsy indicated arterial vascular wall lesions, consistent with the pathological features of tacrolimus-induced renal injury. However, after switching from tacrolimus to sirolimus for one month, the patient’s creatinine level continued to rise. Considering high blood sirolimus concentration in the patient (21.1 ng/mL), it is speculated that the elevated sirolimus concentration might have contributed to the deterioration of kidney function. Many transplant physicians believe that the appropriate blood concentration of sirolimus ranges from 5 to 15 µg/L, which can result in higher efficacy while maintaining a lower incidence of adverse reactions. Some studies have indicated that although sirolimus had no impact on renal function at doses sufficient to prevent heart and kidney transplant rejection (0.01–0.08 mg/kg/day intravenously) in a rat model, it did accelerate histological changes, i.e., necrotising vasculopathy and tubular atrophy in the model. At higher doses (0.8 mg/kg), it indeed led to a decline in kidney function [7]. In two multicentre, randomised, double-blind trials, 719 and 576 patients were randomly treated with 2 mg/day sirolimus, 5 mg/day sirolimus, or a placebo. The results showed a negative correlation (P < 0.001) between sirolimus and eGFR [8]. Boratyn et al. reported five cases of renal transplantation in patients who received immunosuppression with cyclosporine and switched to sirolimus after experiencing renal function impairment. The median serum creatinine level, before treatment, was 167.96 µmol/L, and during the treatment, the blood concentration of sirolimus ranged from 9.0 to 18 ng/mL (average 12.5 ng/mL). Three months after using sirolimus, all five patients developed nephrotic syndrome, with a median serum creatinine level increase to 309.4 µmol/L, primarily manifesting as glomerulosclerosis and arteriolar hyalinosis in renal pathology [7]. Dittrich and colleagues also reported two kidney transplant patients who developed proteinuria and elevated serum creatinine levels (increased to 283 µmol/L and 354 µmol/L, respectively) two to nine months after switching from calcineurin inhibitors to sirolimus. Renal biopsy revealed mesangial proliferative glomerulonephritis and IgA nephropathy [9]. Butani et al. observed proteinuria in 12 out of 13 children treated with sirolimus, which resolved upon switching to a different medication. The authors suggested that proteinuria may be due to sirolimus toxicity to the glomeruli, although this has not been supported by experimental models [10]. In this case, the patient’s renal pathology did not indicate glomerular lesions, which is inconsistent with the previously reported sirolimus-induced kidney injury pathology. This suggests that the worsening of renal function after switching to sirolimus in this patient may be related to rhabdomyolysis after switching to sirolimus. It is advisable to monitor sirolimus blood concentrations during use and to remain vigilant for the occurrence of rhabdomyolysis and renal function impairment.
Based on the patient’s clinical presentation, elevated levels of serum CK and creatinine, and the pathological findings of acute renal tubular injury, it is likely that this case involved rhabdomyolysis and acute kidney injury (AKI) following the administration of sirolimus.
Rhabdomyolysis is a syndrome characterised by muscle necrosis and the release of muscle cell contents into the circulation, often presenting with significantly elevated levels of serum CK, and may be accompanied by muscle pain, weakness, and myoglobinuria [11]. Previous studies have reported that rhabdomyolysis is rare in solid organ transplant recipients, particularly in patients undergoing liver transplant [11]. In this case, laboratory tests showed that serum CK levels were 10 times higher than the normal upper limit, confirming the diagnosis of rhabdomyolysis [12]. Based on the patient’s history of sirolimus medication, significantly elevated sirolimus blood concentration upon admission (21.1 ng/mL), and gradual normalisation of CK levels after sirolimus reduction, a relationship between high sirolimus concentrations and rhabdomyolysis was suggested.
Few cases of rhabdomyolysis and renal injury following transplantation with sirolimus have been reported [13–15]. However, the reported transplant patients who developed rhabdomyolysis after using sirolimus often received concomitant treatment with statins. Rhabdomyolysis is a common adverse effect of statin use, and the interaction between sirolimus and statin drugs may increase the risk of rhabdomyolysis [13]. Yu Ah Hong et al. reported a case of a 64-year-old female kidney transplant recipient who had been receiving long-term cyclosporine immunosuppression, concomitantly taking simvastatin for lipid control. After the diagnosis of lung adenocarcinoma, the immunosuppressant was switched from cyclosporine to sirolimus. Rhabdomyolysis developed 20 days after the change in immunosuppression [13]. The authors suggested that both sirolimus and statins are major substrates of the cytochrome P450 3A4 enzyme (CYP 3A4) in the liver. Competition for metabolic processing in the liver may lead to increased levels of statin drugs, enhancing their toxicity and causing rhabdomyolysis [13]. Basic and Dopazo also reported cases of rhabdomyolysis and acute kidney failure in two transplant patients following the concurrent use of statin drugs and sirolimus [14]. However, in this case, the patient received oral tacrolimus, mycophenolate, and corticosteroid immunosuppression after liver transplantation and was switched from tacrolimus to sirolimus after experiencing renal function impairment. The patient developed rhabdomyolysis one month after switching to sirolimus in the absence of statins or other potential causes of rhabdomyolysis. Rhabdomyolysis resolved after reducing the dose of sirolimus. This indicates that renal dysfunction and high-dose sirolimus may lead to rhabdomyolysis. No previous studies have reported cases of rhabdomyolysis with elevated blood sirolimus levels. This case suggests a connection between the two, although the specific pathogenic mechanism remains unclear. This also indicates the importance of monitoring the blood concentrations of sirolimus in patients with liver transplant.
Following a reduction in the oral dose of sirolimus, a reduction in severity of rhabdomyolysis was observed. After 2 years, the patient’s renal function further recovered, with creatinine level decreasing to 126 µmol/L. This suggests that both sirolimus and tacrolimus cause renal damage through various mechanisms. In addition, the gradual improvement in kidney function in this case indicates that tacrolimus-induced renal injury may be partially reversed, although recovery may take several months.
Conclusion
We report the case of a liver transplant patient who developed renal function deterioration and rhabdomyolysis after switching from tacrolimus to sirolimus. This case highlights the importance of monitoring sirolimus blood concentration in patients undergoing liver transplantation.
Author contributions
C.G. wrote the main manuscript text and prepared Figs. 1, 2 and 3. Z.Y.C. participated in data collection. S.J.G. and L.M. presented ideas and planned the writing purpose and methodology. All authors reviewed the manuscript.
Funding
This study is supported by a grant of the Sichuan Provincial Science and Technology Department-Science and Technology Innovation Talent Project, No. 2020JDRC0022. The grant played a role in data collection of the case and follow-up of the patient, as well as the interpretation of data and the manuscript writing.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study received biomedical ethics approval from West China Hospital of Sichuan University and informed consent from the patients (clinical trial number not applicable).
Consent for publication
The patient and his legally authorized guardians have carefully read the contents of this paper and consent to the use of the information and images therein for academic research or publication purposes. The patient and his legally authorized guardians agree to the confidential handling of their personal identity information in this paper and consent to the partial disclosure of relevant treatment information for academic research purposes. Hereby, we confirm that, in compliance with relevant laws and regulations, informed consent has been obtained for the use of patient information and images in this paper.
Donor information
Living.
Relationship of living donor to the recipient
Related.
Data source statement
All data utilized in this study were sourced exclusively from the hospital’s medical record system. This dataset was carefully curated to ensure the complete anonymity and confidentiality of all patients involved. No information or images that could potentially identify any individual patient were included. Furthermore, prior to the commencement of this study, informed consent was meticulously obtained from each and every patient included in the dataset, in accordance with established ethical guidelines and regulations. I hereby declare that the data used comply with the ethical principles of medical research and legal regulations, and strictly adhere to the requirements of personal privacy and data protection. The design and implementation of this study comply with internationally recognized ethical standards and have been approved by the hospital’s research ethics review committee.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chang Gao and Zhi-Yu Chen contributed equally to this work.
References
- 1.De Mattos AM, Olyaei AJ, Bennett WM. Nephrotoxicity of immunosuppressive drugs: long term consequences and challenges for the future. Am J Kidney Dis. 2000;35(2):333–46. [DOI] [PubMed] [Google Scholar]
- 2.Stenton SB, Partovi N, Ensom MH. Sirolimus: the evidence for clinical pharmacokinetic monitoring. Clin Pharmacokinet. 2005;44:769–86. [DOI] [PubMed] [Google Scholar]
- 3.Lin J, Tang YW, Sun W, et al. Clinical study of application of sirolimus with dose-reduced or discontinued cyclosporine A for prevention of acute rejection after kidney transplantatio. Zhonghua Wai Ke Za Zhi. 2009;47(17):1319–21. [PubMed] [Google Scholar]
- 4.Kreis H, Oberbauer R, Camp istol J, et al. Long term benefits with sirolimus based therapy after early cyclosporine with drawal. J Am Soc Nephrol. 2004;15(3):809–17. [DOI] [PubMed] [Google Scholar]
- 5.Xu Zhi-qiang. Wan You-gui, Chen Bo, Conversion treatment using sirolimus for kidney transplant recipients;Journal of Clinical Rehabilitative Tissue Engineering Research.2011;Vol.15, No.5.
- 6.Hatori M, Takahara S, Kyo et al. Clinical and histopathologic examination of renal allografts treated with tacrolimus (FK506) for at least one year.International journal of urology.1998;526 – 33. [DOI] [PubMed]
- 7.Boratynska M, Banasik M, Watorek E, et al. Conversion to sirolimus from cyclosporine may induce nephrotic proteinuria and progressive deterioration of renal function in chronic allograft nephropathy patients. Transpl Proc. 2006;38(1):101–4. [DOI] [PubMed] [Google Scholar]
- 8.Macdomald A, Scarola J, Burke JT et al. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus[J], 2000; 22 (Suppl B): 101–21. [DOI] [PubMed]
- 9.Dittrich E, Schmaldienst S, Soleiman A, et al. Rapamycin-associated post-transplantation glomerulonephritis and its remission after reintroduction of calcineurin-inhibitor therapy. Transpl Int. 200;17:215. [DOI] [PubMed]
- 10.Butani L. Investigation of pediatric renal transplant recipients with heavy proteinuria aftersirolimus rescue. Transplantation. 2004;78:1362. [DOI] [PubMed] [Google Scholar]
- 11.Chavezl O, Leon M, Einav S, et al. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice[J]. Crit Care. 2016;20(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stahlk, Rastellie. Schoserb. Asystematicreviewon the definition of rhabdomyolysis[J]. J Neurol, 2019; 415.
- 13.Kim YAHHD, Jo K, et al. Severe Rhabdomyolysis Associated with Concurrent Use of Simvastatin and Sirolimus after cisplatin-based chemotherapy in a kidney transplant recipient. Exp Clin Transplant. 2014;12(2):152–5. [DOI] [PubMed] [Google Scholar]
- 14.Basic-Jukic N, Kes P, Bubic-Filipi L, Vranjican Z. Rhabdomyolysis and acute kidney injury secondary to concomitant use of fluvastatin and rapamycin in a renal transplant recipient. Nephrol Dial Transpl. 2010;25(6):2036. [DOI] [PubMed] [Google Scholar]
- 15.Dopazo C, Bilbao I, Lázaro JL, et al. Severe rhabdomyolysis and acute renal failure secondary to concomitant use of simvastatin with rapamycin plus tacrolimus in liver transplant patient. Transpl Proc. 2009;41(3):1021–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.