Abstract
Multisensory decline is common as people age, and aging is the primary risk of Alzheimer’s Disease (AD). Recent studies have begun to shed light on the possibility that age-related sensory decline could accelerate AD pathogenesis, or be a prodromal indicator of AD. Sensory impairments, specifically in taste and smell, often emerge before cognitive symptoms in AD, indicating their potential as early biomarkers. Olfactory dysfunction has been frequently associated with AD and may offer valuable insights into early detection. Hearing impairment is significantly associated with AD, but its causal impact on AD progression remains unclear. The review also discusses visual and tactile deficits in AD, including retinal thinning and changes in tactile perception, highlighting their links to disease progression. Focusing on molecular mechanisms, the review explores the roles of amyloid-β (Aβ) accumulation and tau protein pathology in sensory decline and their bidirectional relationship with AD. In summary, the evidence presented conclusively supports advocating for an integrated approach to understanding AD and sensory decline, to enhance early detection, implementing preventive strategies, and developing therapeutic interventions for AD. This approach underscores the significance of sensory health in addressing neurodegenerative diseases, particularly AD.
Keywords: Alzheimer's Disease (AD), Aging, Sensory impairments, Early biomarkers
Background
The interrelationship between aging, sensory deterioration, and onset of Alzheimer’s Disease (AD) forms a complex nexus within gerontology and neurodegenerative pathology research. Aging is marked by a progressive decline in physiological functions, affecting health, mobility, and quality of life. This decline, resulting from genetic, environmental, and lifestyle factors, leads to changes in various body systems, including the brain, cardiovascular, and musculoskeletal systems. One of the evident impacts of aging is on sensory function. Sensory decline, encompassing the deterioration of vision, hearing, taste, smell, and touch, affects the ability of older adults to interact with their environment. This decline is not just a loss of sensory acuity but also influences daily living, communication, and the ability to maintain social connections.
Age-related changes also lead to alterations in cognitive functions, such as processing speed, attention, memory, language skills, visuospatial abilities, and executive functioning [1]. In the context of the aging landscape, AD emerges as a major health challenge. Defined as a progressive neurodegenerative disorder, AD primarily manifests through cognitive decline and dementia, establishing it as the most prevalent form of dementia among the elderly. The pathogenesis of AD is complex and not entirely understood, involving genetic, environmental, and lifestyle factors. Central to AD are amyloid-β (Aβ) plaques and tau protein tangles in the brain, which interfere with cell communication and trigger inflammatory responses, leading to brain cell death [2, 3]. Notably, AD is marked by a loss of synaptic function and neuronal death, especially in regions critical for memory and cognition [4].
Sensory loss may impact the ability of the cerebral cortex to generate perception in modalities such as vision, hearing, olfaction, and touch, extending beyond the effects of aging. Indeed, sensory deficits, including decreased visual contrast sensitivity [5], hearing loss [6], and olfaction deficits [7], are frequently observed in patients with AD. These observations have prompted extensive research into the association between sensory dysfunction and the pathology of AD. These deficits are intricately linked to alterations of synaptic plasticity and function, potentially precipitating cognitive impairments and suggesting that sensory impairments might not only be consequences of AD but could also contribute to its onset or progression [8] (Fig. 1). Aβ deposition, tau pathology, and neuronal loss within the neocortical regions of both primary and association cortices could potentially be the underlying causes of the sensory perception deficits observed in AD brains. The pathological changes in these areas display similarities across various sensory modalities. They are generally more pronounced in sensory association areas than in primary sensory cortices, particularly in the superficial cortical layers, where changes are most marked [9]. This differential pattern of impairment may explain the variability observed in sensory function: basic sensory functions such as visual acuity and auditory frequency discrimination are typically preserved, while more complex sensory functions, such as visual contrast sensitivity and auditory processing in noisy environments, tend to be compromised at early stages. These deficits, more evident in the sensory association areas and especially in the superficial layers, highlight the areas of the cortex that are vulnerable in AD [10–15].
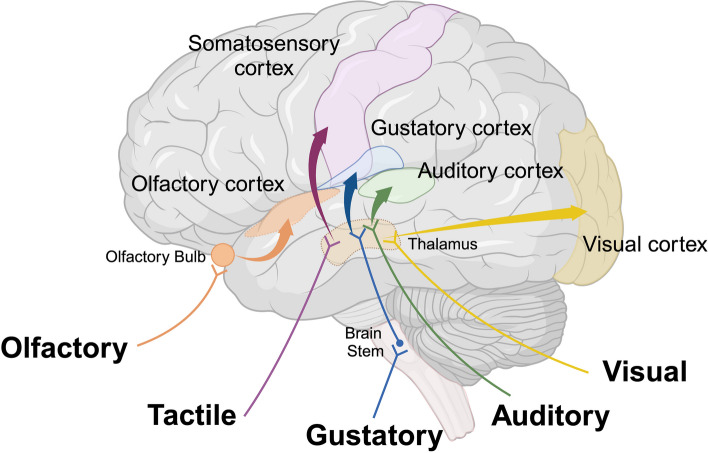
Fig. 1.
Neuroanatomical Regions of Sensory Information in the Cerebral Cortex. Multimodal regions facilitate the processing of information derived from diverse sensory areas within the brain. Image created using Biorender
Research consistently indicates that sensory deficits can exacerbate brain vulnerability to neuropathological changes, including amyloid deposition, tau pathology, and neuronal loss, thereby increasing the risk of developing AD [7, 16–25] (Fig. 2). Understanding this relationship requires a shift in research and therapeutic strategies to address not only the cognitive symptoms of AD but also to consider sensory impairments as potential early indicators and contributing factors in the disease progression. Indeed, sensory stimulation therapies targeting these cortical regions have shown promise in reducing AD symptoms [19, 26–31]. However, understanding the intricate relationship between sensory impairments and AD progression remains an under-researched area. This review examines the intricate relationship between sensory impairments and AD, delving into the multifaceted nature of sensory decline in aging populations and its potential role as a harbinger or accelerator of AD. We aim to provide a nuanced understanding of how sensory dysfunctions, particularly in olfactory, auditory, and visual systems, not only serve as early indicators of AD but may also contribute to its pathogenesis, underscoring the need for an integrated approach in AD research and management.
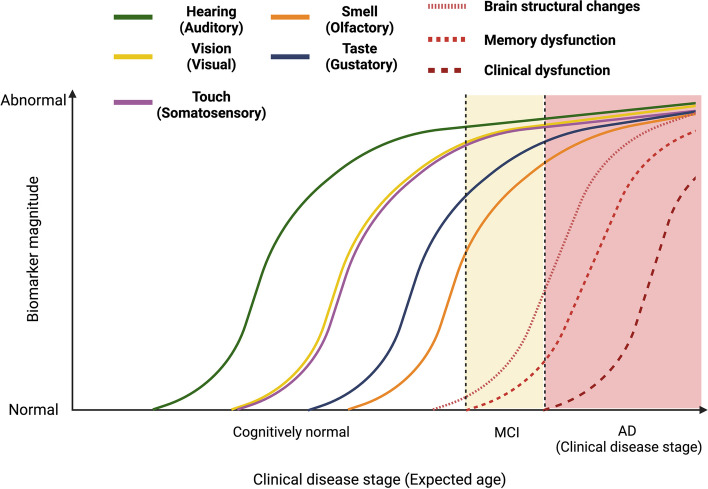
Fig. 2.
Hypothesized Development of Sensory Functional Impairment during the Pathological Progression of AD. This figure depicts the onset of sensory impairment alongside the emergence of other indicators within the context of the AD pathological cascade. MCI, mild cognitive impairment. Image created using Biorender
Main
Auditory impairment
Age-related hearing loss (ARHL), or presbycusis, is a common sensory impairment in older populations. The prevalence of ARHL is often linked with the aging process and is highly prevalent among older adults. The incidence of hearing impairment shows a progressively increasing trend with age, as roughly 25% of individuals aged 60 years and over encounter disabling hearing loss [32]. Clinical hearing loss commonly initiates when bilateral hearing thresholds exceed 25 dB, beginning with higher frequencies and progressively extending to lower ones. Additionally, affected individuals frequently face challenges in hearing comprehension amidst background noise [33].
The auditory system
The auditory system comprises two primary components: the peripheral hearing system and the central auditory system [34, 35]. The peripheral system includes the outer, middle, and inner ears, as well as the cochlear nerve, and is responsible for pivotal auditory functions such as sound detection [35]. Auditory information originating from the cochlea travels through a sequence of neural structures, including the superior olivary complex, lateral lemniscus, inferior colliculus, and medial geniculate nucleus, prior to arriving at the auditory cortex. The auditory cortex is located in the superior temporal gyrus of the temporal lobe and exhibits a precise tonotopic map that corresponds to cochlear frequencies [34]. It is comprised of discrete areas, namely AI (the foremost auditory cortex, Brodmann area 41) and AII (secondary area of auditory processing, spanning Brodmann area 42, anterior, ventral, ventral-posterior, and posterior auditory fields) [36]. AI plays a key role in processing the temporal features of complex auditory signals, including speech and music, sound localization, and identifying sources in auditory scene analysis [37–39]. The hippocampus detects new acoustic stimuli and inhibits duplicate auditory information [40]. Studies on animals show that neurons responsive to modulated tones and noise for frequency and amplitude are found in areas that are anterior and ventral to AI, while the areas posterior to AI contain neurons that have broader frequency tuning, longer tone response latencies, and lower following rates for acoustic frequency and amplitude modulations [41].
Acquired hearing loss mainly occurs due to cochlear damage, whilst AD is associated with cortical degeneration that typically starts in multimodal cortical regions. This raises the fundamental question of how these two ailments are linked. This query has theoretical importance, considering the existence of multiple potential biological and psychological pathways connecting peripheral auditory function with the widespread cortical changes associated with dementia. Moreover, studying the mechanisms underlying the link between hearing loss and cortical degeneration holds practical importance, due to the possibility of effective treatments for hearing loss with cochlear implants or hearing aids, unlike the difficult task of reversing cortical degradation.
Auditory system dysfunction and AD
Several studies have suggested a potential link between AD and ARHL, which is considered a modifiable risk factor for AD dementia [42–44]. Studies also provide evidence that ARHL precedes the clinical onset of dementia by 5 to 10 years [45]. The initial report indicating the possible connection between hearing loss and cognitive impairment suggested that individuals with dementia were more likely to have hearing impairments than cognitively healthy older adults [46]. Since then, numerous investigations have shown an association between ARHL and the risk of developing cognitive decline or dementia. Furthermore, some studies have suggested that the use of hearing aids may delay or even prevent cognitive decline [47–50]. The Lancet International Commission on Dementia, Prevention, Intervention, and Care estimates that eliminating mid-life hearing loss could potentially reduce dementia risk by approximately 7% [23]. Recent research indicates a positive correlation between the severity of peripheral hearing loss, ranging from mild to severe, and the associated risk of dementia, with the risk increasing two to five times higher [51–53].
Studies have shown that hearing impairment is associated with higher levels of tau protein in the cerebrospinal fluid (CSF) and increased tau deposition in the brain. Specifically, poor hearing performance has been linked to elevated tau levels rather than Aβ deposition [54, 55]. This is particularly relevant in the context of hearing loss, as auditory processing deficits may exacerbate cognitive decline through increased neural activity and subsequent tau accumulation [56, 57]. Research indicates that higher tau PET signal correlates with regions of the brain involved in auditory processing and memory, such as the medial temporal lobe (MTL) [58–60]. These findings support the hypothesis that tau pathology in these regions is associated with both cognitive decline and auditory deficits in patients with AD. Also, it has been posited that auditory deficits may lead to modified neuronal activity within the MTL structures, potentially instigating or exacerbating the neuropathological processes of AD [61, 62]. The MTL structures, which are not typically linked to the auditory system, are emphasized here as they play a role in auditory processing, specifically in regard to analyzing sound patterns and memory functions [63, 64]. This may offer a new insight into the complex association between ARHL and the development of AD. Examining and testing the role of MTL in ARHL may considerably enhance our understanding of the fundamental mechanisms that unite these two phenomena.
Potential mechanisms linking hearing sysytem and AD
The precise pathological mechanisms responsible for ARHL are not yet fully elucidated. Nonetheless, it is probable that ARHL results from an amalgamation of acquired pathologies within variegated components of the complex auditory pathway (Fig. 3). Shared pathological processes in the cochlea, auditory pathways, and cortex have been implicated in the connection between hearing loss and dementia. It was suggested that high-frequency hearing loss is common and aligns with age-related cochlear degeneration, as opposed to AD-induced central pathway damage [65]. While current evidence underscores high-frequency hearing loss primarily originating from age-related cochlear changes, the direct attribution of such auditory deficits to central pathway damage by AD pathology is less established. Furthermore, despite the acknowledged role of vascular pathology as a contributory element to auditory function decline, the robust association between hearing loss and dementia endures, even after adjustments for vascular risk factors. This highlights the complexity of their relationship, suggesting that factors beyond vascular pathology may contribute a role in the linkage observed between hearing loss and cognitive decline [66].
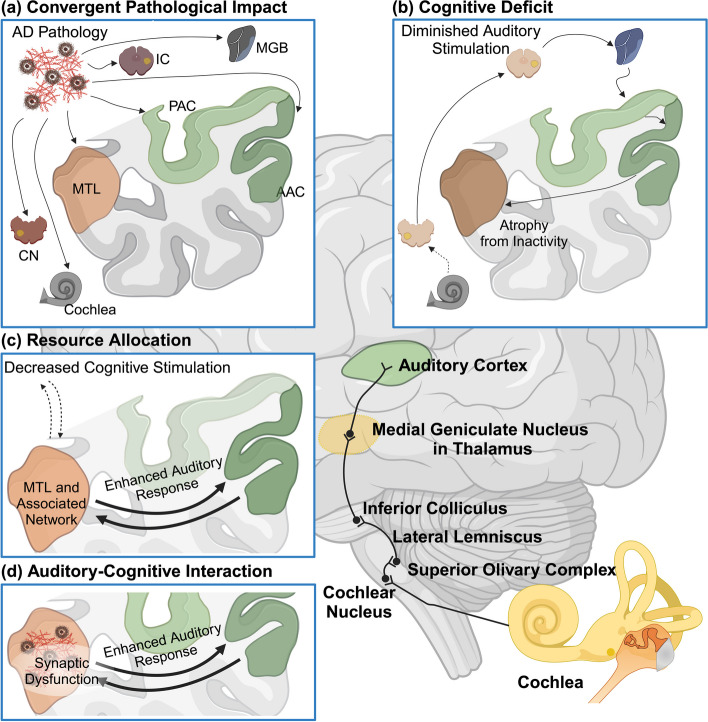
Fig. 3.
Potential Mechanisms Underlying the Association Between Hearing Impairment and AD. (a) Convergent Pathological Impact: Common pathological processes associated with AD or vascular disease impact both the cochlea and the ascending pathway, leading to hearing loss, while simultaneously affecting the MTL and causing dementia. (b) Cognitive Deficit: Hearing loss results in an impoverished cognitive environment, altering brain structure in the auditory cortex and hippocampus. This leads to decreased cognitive reserve and resilience against dementia due to a lack of cognitive stimulation. (c) Resource Allocation: Increased brain activity in the MTL and a broader network during speech-in-noise analysis competes for cognitive resources required for other higher cognitive functions. This mechanism is suggested to better explain cognitive deficits in older individuals with hearing loss rather than dementia itself. (d) Auditory-Cognitive Interaction: Hearing loss leads to increased activity related to pattern analysis in the MTL during challenging listening conditions, interacting with AD pathology. This model incorporates the same activity increase as in resource allocation mechanism but adds the interaction with the molecular bases of AD, specifically synaptic changes associated with the disease. Image created using Biorender
Studies have shown that hearing impairment is associated with higher levels of tau protein in the cerebrospinal fluid (CSF) and increased tau deposition in the brain. Specifically, poor hearing performance has been linked to elevated tau levels rather than Aβ deposition [54, 55]. This is particularly relevant in the context of hearing loss, as auditory processing deficits may exacerbate cognitive decline through increased neural activity and subsequent tau accumulation [56, 57]. Research indicates that higher tau PET signal correlates with regions of the brain involved in auditory processing and memory, such as the medial temporal lobe (MTL) [58–60]. These findings support the hypothesis that tau pathology in these regions is associated with both cognitive decline and auditory deficits in patients with AD. Also, it has been posited that auditory deficits may lead to modified neuronal activity within the MTL structures, potentially instigating or exacerbating the neuropathological processes of AD [61, 62]. The MTL structures, which are not typically linked to the auditory system, are emphasized here as they play a role in auditory processing, specifically in regard to analyzing sound patterns and memory functions [63, 64]. This may offer a new insight into the complex association between ARHL and the development of AD. Examining and testing the role of MTL in ARHL may considerably enhance our understanding of the fundamental mechanisms that unite these two phenomena.
Hearing loss might result in decreased cognitive stimulation and a sensory-deprived environment due to diminished perception of speech and language which could elevate the risk of dementia. Numerous animal studies have demonstrated structural and behavioral changes in response to enriched environments, which may establish the cognitive reserve and serve as a protective factor against dementia [67–69]. Furthermore, impaired hearing can impact brain structure and function due to the reduction of verbal and emotional stimuli, and cognitive decline may result from poor social interactions [70–74]. Moreover, Hearing loss may increase cognitive load during auditory processing, reallocating cognitive resources from other functions such as attention and working memory [75–77]. This cognitive resource reallocation may precede the clinical manifestation of AD, with auditory cognitive networks experiencing diminished stimulation. Specifically, the pathology involves neuroplastic changes and alterations in brain structure arising from decreased sensory stimulation—factors that may precede and subsequently potentiate the risk of AD [78–80]. These neuroplastic changes differ from the alterations in brain activity observed during the progression of AD, which are posited to contribute directly to cognitive deficits. Such impairments may develop independently of the specific molecular and neuronal pathologies characteristic of AD [81], suggesting that the etiology of cognitive decline in the context of hearing loss is multifactorial and extends beyond AD-related neurodegeneration.
Hearing loss and dementia: significance and future directions
Hearing impairment has been strongly correlated with an increased likelihood of mild cognitive impairment (MCI) and accelerated cognitive degeneration. Specifically, those with impaired hearing face a markedly higher risk of developing MCI than individuals with normal hearing levels. Furthermore, evidence suggests that using hearing aids can diminish this risk, implying a protective effect against cognitive decline [82]. The use of hearing aids has been linked to a substantial reduction in the risk of cognitive decline. A study published in The Lancet found that hearing aid use can reduce the risk of cognitive decline by nearly half in older adults at high risk for dementia [83]. Another large-scale study involving over half a million adults indicated that hearing aids might prevent or delay the onset and progression of dementia [84]. Studies have demonstrated that individuals using hearing aids experience a slower rate of cognitive decline compared to those who do not use them. Ongoing research and clinical trials continue to explore the relationship between hearing loss and cognitive decline. For example, randomized controlled trials are investigating whether hearing rehabilitation can improve cognitive function in older adults with hearing impairment and MCI [85]. While auditory impairment alone may not be the most sensitive or specific diagnostic marker for AD, addressing hearing loss through contemporary interventions such as hearing aids holds promising potential in mitigating cognitive decline and delaying the progression of AD. Continued research and early intervention strategies are essential to fully understand and leverage the benefits of hearing rehabilitation in the context of AD.
Olfactory impairment
Olfactory perception is a complex sensory process that results from the integrated function of olfactory receptors, the olfactory nerve, and various central nervous system structures. The confluence of these myriad factors collectively underscores the intricacy of age-related olfactory loss and its profound impact on the aging population. Remarkably, a substantial proportion of affected individuals may persist in a state of unawareness regarding their compromised olfactory faculties [86]. In the elderly population, olfactory dysfunction prevalence surpasses 50% among individuals aged 65 to 80, escalating to 80% in those over the age of 80. This underscores the pervasive nature of olfactory issues within this demographic [87]. Profound olfactory dysfunction substantially detracts from the quality of life, as demonstrated by empirical findings [88]. While some olfactory deficits arise from congenital or idiopathic conditions, it is important to recognize that most olfactory dysfunction in adults is attributable to allergies, nasal polyps, smoking, and prior history of head or facial trauma [89]. These conditions often fall under conductive (transport-related) or sensorineural categories, each affecting olfaction in different ways. Before concluding that changes in olfaction are indicative of neurodegenerative diseases such as AD and Parkinson’s Disease (PD), it is essential to rule out these more common causes. Nevertheless, once these factors are excluded, there remains a notable correlation between olfactory dysfunction and neurodegenerative diseases [90]. The involvement of brain regions integral to olfactory processing, notably the olfactory bulb and entorhinal cortex, in the initial stages of neuropathological processes in AD, underscores the potential of olfactory dysfunction as a promising early biomarker for the disease [91]. Interestingly, olfactory training has been associated with augmented cortical thickness in the hippocampus among patients with MCI, notwithstanding the absence of notable alterations in olfactory bulb volume. These observations posit olfactory training as a potential early intervention capable of mitigating hippocampal atrophy [92]. Consequently, the burgeoning interest in olfactory deficits within the domain of AD has become increasingly evident in both basic scientific and clinical research, underscoring the importance of studying olfactory dysfunction in the context of AD. This focus on both the foundational understanding of the disease and its practical clinical implications may, in turn, offer valuable insights into the early detection and monitoring of this neurodegenerative condition.
The olfactory system
Specialized olfactory nerves, exquisitely adapted to the olfactory sense, are located within the olfactory epithelium. This distinctive lining occupies the upper nasal cavity and assumes a pivotal role in shaping our olfactory perception. In mammals, odor signals processing commences in the olfactory epithelium, wherein olfactory sensory neurons function as the primary neural entities. The olfactory system orchestrates the transmission of chemical signals from the sensory epithelium and bulb to the olfactory cortex through a sequential synaptic interface [93–95]. Functioning as a pivotal convergence site, the olfactory bulb amalgamates the peripheral olfactory system with the central subcortical systems, facilitating the interconnection between the axons of olfactory sensory neurons and the dendrites of mitral cells. Comprising the anterior olfactory nucleus, olfactory tubercle, piriform cortex, entorhinal cortex, and amygdala, including the orbitofrontal regions, the primary olfactory cortex represents a comprehensive neural network. This network further extends to project neural signals towards the secondary olfactory cortex in the orbitofrontal cortex (Fig. 4) [96]. Afferent input originating in the olfactory bulb is transmitted to the primary olfactory cortex via the olfactory tubercles, encompassing axons derived from mitral/tuft cells and GABAergic interneurons [97]. These cells undergo differentiation from progenitor cells migrating from the subventricular zone [97], and the pathway constitutes the inaugural cranial nerve within the central nervous system. Several investigations have consistently reported the existence of neuropathological alterations concomitant with olfactory disorders within the context of neurodegenerative diseases. These changes may impact diverse anatomical components, including the olfactory epithelium, olfactory bulb/tract, primary olfactory pathways, and their secondary targets−specific cerebral regions that receive input from the primary olfactory pathways [98]. Notably, brain regions intricately involved in olfactory processing, such as the olfactory bulb and entorhinal cortex, demonstrate early and pronounced neuropathological changes in AD [99–101]. This observation posits olfactory function as a promising candidate for serving as a potential biomarker for AD.
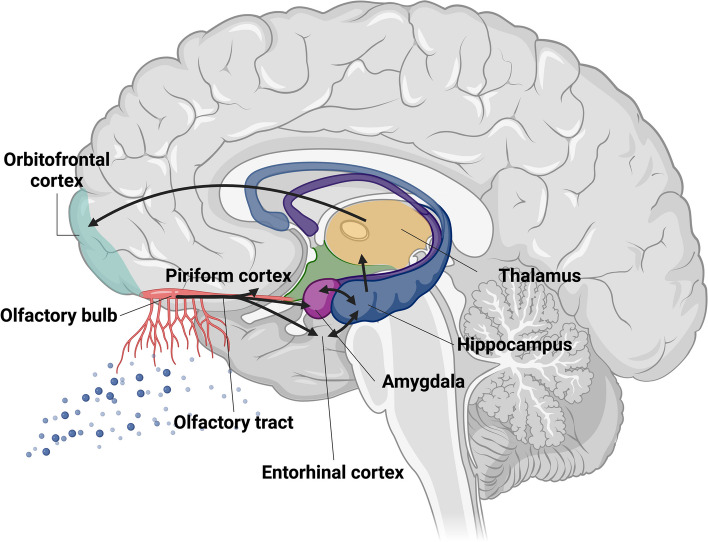
Fig. 4.
Critical Brain Regions Involved in Olfactory Network. Scheme of the olfactory system detailing the process of olfaction. Olfactory sensory neurons transduce odor information through electrical signals, initiating neurotransmitter release in the olfactory bulb. Regions implicated in early olfactory processing encompass the olfactory bulb and tract, the piriform cortex, amygdala, entorhinal cortex, and hippocampus. Image created using Biorender
The olfactory system and AD
The olfactory system in early AD detection has attracted attention due to observations of early neuropathological changes in areas critical to olfaction, such as the olfactory bulb and entorhinal cortex. These early changes suggest that olfactory dysfunction could serve as an early indicator of AD, potentially manifesting before recognizable cognitive deficits become apparent [102–104]. As a result, research into the olfactory system has expanded, with studies examining its implications in AD pathology and its potential utility in early diagnosis [101, 105].
A decline in olfactory capabilities is often observed concurrently with the deterioration of visuospatial cognitive functions, an early symptom of dementia [106]. This concurrent deterioration is likely linked to neurodegeneration in areas such as the parahippocampal gyrus (PHG) and orbitofrontal cortex (OFC), which are responsible for integrating sensory information and are among the first regions to exhibit atrophy in the progression of AD, indicating a potential shared pathway for the decline in both sensory and cognitive domains [107, 108]. Research has shown that lesions in the PHG or its rodent equivalent, the postrhinal cortex (POR), result in significant visuospatial learning and memory deficits, which affect memory retrieval more than encoding [109]. The PHG is involved in contextual processing, which is important for understanding and navigating spatial environments [110]. Additionally, the uncinate fasciculus, a pathway connecting the anterior temporal lobes (including the PHG) to the OFC, plays a role in visuospatial memory, and its disruption has been linked to visuospatial deficits in AD. Therefore, neurodegeneration in these regions likely impairs the circuits responsible for visuospatial processing, contributing to the observed cognitive decline in visuospatial functions in early dementia.
The olfactory test introduced by Richard L. Doty in 1984 has become a mainstay in clinical research and diagnosis [111], with numerous studies confirming severe olfactory loss in patients with AD and Parkinson Disease, especially challenges in odor identification [112–116]. Odor identification tests are not currently used as standard diagnostic markers for AD in clinical practice. However, research has identified them as significant predictive markers for AD progression, with studies showing that individuals with intact olfactory function may have a lower risk of developing dementia [117]. The studies collectively suggest that olfactory dysfunction may serve as an early indicator of AD-related neurodegeneration and is closely associated with tau accumulation in specific brain regions. Lower olfactory identification scores were associated with a higher risk of developing MCI due to AD, and odor identification deficits are predominantly linked to tau accumulation in key olfactory pathway areas [118–120]. Specifically, lower the university of Pennsylvania smell identification test (UPSIT) scores correlate with increased tau deposition in the medial temporal cortex, hippocampus, middle and inferior temporal gyri, inferior parietal cortex, and posterior cingulate [121]. A meta-analysis found that tau PET imaging might be more strongly associated with olfactory impairment than amyloid PET, emphasizing the potential of combining olfactory tests with other biomarkers for better prediction of cognitive decline.
While olfactory dysfunction is often overlooked in clinical practice, it has been linked to an increased likelihood of AD dementia and higher mortality rates [122–125]. Recent findings link olfactory impairments to various neurological conditions, highlighting the broader neurological importance of the olfactory system [126–128].
The underreporting of olfactory dysfunction is notable; while only 6% of patients with AD report a loss of smell, objective measures reveal significant impairment in up to 90% of cases [129]. This discrepancy highlights the critical vulnerability of individuals with undetected olfactory impairments to the progression of AD, particularly when coupled with anosognosia, suggesting the need for heightened clinical attention to olfactory testing in dementia screening.
Potential mechanisms linking olfactory system and AD
Recent research has shed light on potential mechanisms linking the olfactory system to AD, focusing on the accumulation of Aβ peptides and its impact on olfactory dysfunction. Studies using animal models have shown that Aβ peptides accumulate in specific regions of the olfactory system, such as the olfactory epithelium (OE) and olfactory bulb (OB). This accumulation is associated with early olfactory dysfunction in AD, characterized by partial olfactory dysfunction or hyposmia. The olfactory sensory neurons (OSNs) in these regions are particularly affected, leading to disruptions in their turnover and function [130, 131]. It appears that the physiological organization of the olfactory epithelium and bulb, which is conserved across species, is integral to the neurodegenerative processes observed in the olfactory pathways of AD. The degeneration of olfactory pathways, including the OE and OB, contributes significantly to olfactory dysfunction in AD, marking a shift from previous assumptions that higher cortical deficits were the primary cause of olfactory impairments in AD. Emerging insights point to olfactory system neuropathology and neurodegeneration as the primary contributors to olfactory dysfunction in AD [104, 132–134]. Odors serve as potent memory cues, and the connection between the olfactory system and the hippocampus is important for episodic memory, which often declines first in AD. Proficiency in odor identification reflects the depth of semantic information and naming ability, suggesting that identification deficits could indicate wider cognitive dysfunction, including hippocampal degeneration [135, 136].
The pathological processes involved in the amyloidogenic metabolism of amyloid precursor protein (APP) and the resulting neuroinflammatory reactions within the olfactory pathways are key contributors to olfactory dysfunction in AD. These processes lead to the generation and accumulation of toxic Aβ oligomers, which can cause synaptic toxicity and cellular damage [137]. The correlation between cognitive function and oligomerized Aβ proteins in nasal discharge highlights the potential research impact of nasal-fluid biomarkers in understanding AD pathology [138]. This is similar to the use of CSF and plasma biomarkers, providing a non-invasive method to detect AD-related changes early in the disease progression [139]. There is also a hypothesis that external pathogens might exploit the olfactory pathway to initiate neuroinflammation, which could play a role in the pathogenesis of AD [101]. The olfactory system provides a direct anatomical connection to the brain, making it a plausible route for pathogen entry [133]. These insights offer new avenues for early diagnosis and a deeper understanding of AD progression through the lens of olfactory dysfunction.
Olfactory loss and dementia: significance and future directions
Olfactory impairment is increasingly acknowledged as a promising non-invasive biomarker for AD, particularly through the use of odor identification tasks. Despite this, the integration of olfactory measurement with verbal skills and cultural context may confound its application as a diagnostic tool [140]. Olfactory identification is intricately linked to higher cognitive functions and shows a significant decline with age, more so than odor detection thresholds, implicating both sensory and cognitive aspects of the olfaction [141, 142]. Therefore, understanding olfactory tasks, such as discrimination and identification, is pivotal in diagnosing AD. Discrimination involves the sensory ability to differentiate between various odors, a fundamental test of the olfactory system sensitivity. Identification, however, requires the integration of cognitive processes (memory and higher-order thinking) to recognize and name odors. This distinction is pivotal not only in understanding the specificity of olfactory deficits as risk factors for AD but also in their correlation with the progression and severity of the disease [98]. Future research endeavors should strive to characterize these olfactory deficits with more nuanced perceptual and cognitive details, thereby enriching our understanding of the associated pathophysiology. This should encompass in vivo assessments focused on the analysis of Aβ, tau, and alpha-synuclein proteins in individuals exhibiting olfactory dysfunction. Given the prevalence of overlapping neuropathologies in AD and other neurodegenerative diseases, an integrated approach to research, intertwining olfactory impairment with neuropathological markers, is imperative for advancing our understanding of these complex disorders.
The UPSIT is a widely used 40-item smell identification test. Studies have shown that a 10-item subset of the UPSIT can achieve a sensitivity of 88% and a specificity of 71% for identifying AD. For identifying an amnestic disorder, the sensitivity is 74% and specificity is 71% [143]. The test has been validated as a useful screening tool for AD-related amnestic disorders, with sensitivity and specificity comparable to other established biomarkers. The Brief Smell Identification Test (BSIT), a shorter version of the UPSIT, has been proposed for primary care settings due to its quick administration time and cost-effectiveness. It has shown similar effectiveness to the UPSIT in distinguishing and predicting MCI and AD dementia [144, 145]. However, implementing olfactory assessments like the UPSIT in clinical settings presents considerable challenges. These tests are time-consuming, and demand sustained attention and intact cognitive functions, which are frequently compromised in patients with dementia or cognitive impairments. Additional obstacles include physical limitations, environmental distractions, and the influence of cultural or educational differences on odor recognition. Moreover, deploying these tests in practice requires substantial resources such as specialized training, test kits, and dedicated spaces [146, 147]. These factors collectively limit the use of olfactory assessments primarily to research environments rather than routine clinical application. Despite these challenges, the potential of olfactory testing in AD diagnosis and progression monitoring remains meaningful. In clinical trials, odor identification tests have demonstrated similar sensitivity and specificity to CSF biomarkers for detecting progression within the AD spectrum, from amnestic MCI due to AD through to more advanced stages of AD dementia [45, 148]. Cross-sectional and longitudinal population-based studies have further elucidated the association of olfactory identification deficits with impairments in memory and executive functions [149–151]. However, in clinical practice and broad population-level screening, the future of AD diagnosis will likely incorporate blood-based biomarkers. Recent advancements have shown that plasma biomarkers, such as Aβ42 and phosphorylated tau (pTau217), are reliable indicators of AD pathology, offering advantages such as being less invasive, cost-effective, and time-efficient [152–155].
Visual impairment
As the global population ages, the prevalence of visual impairment escalates, presenting considerable challenges to healthcare systems and affects the quality of life for older adults [156]. Epidemiological studies have identified refractive errors, age-related macular degeneration, cataracts, and glaucoma as the leading causes of this condition [157]. Notably, refractive errors, which are readily correctable with glasses or contact lenses, account for a substantial portion of visual impairment cases [158]. This fact underscores the potential for reducing the burden of visual impairment through enhanced access to corrective solutions. The incidence of visual impairment notably increases with age, affecting an estimated 20–22% of individuals aged 70 and above [159]. Projections indicate that the global prevalence of moderate to severe visual impairment is set to rise from 217 million cases in 2015 to approximately 588 million by 2050 [159, 160]. This burgeoning demographic is associated with heightened risks of hospitalization and augmented healthcare costs, underscoring the pressing need for comprehensive care strategies [161, 162].
Pathophysiological changes within the aging eye contribute to visual impairment. The lens becomes denser and less transparent, leading to cataracts, while degenerative changes in the retina, as seen in age-related macular degeneration, directly impair vision [163]. Glaucoma, characterized by increased intraocular pressure, can lead to optic nerve damage and visual field loss, illustrating the complex interplay of age-related changes that compromise visual function [164]. Moreover, these functional limitations are compounded by psychological challenges, as individuals with visual impairment are at an increased risk of experiencing depression and anxiety, exacerbating the overall impact on their well-being [165]. Recent insights from the American Geriatrics Society and the National Institute on Aging have emphasized the frequent co-occurrence of cognitive and sensory impairments, particularly in vision and hearing [166]. In conclusion, addressing visual impairments holds promising potential for enhancing cognitive function.
The visual system
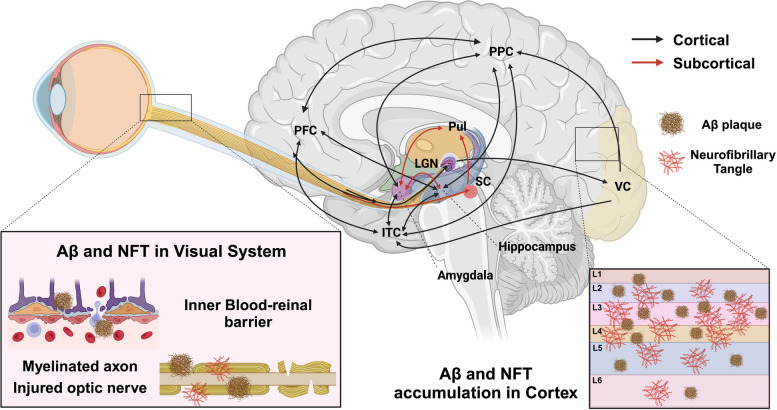
The visual system, a complex network integral to the reception and processing of visual stimuli, operates through afferent and efferent pathways. The afferent pathway begins in the retina, structured with specialized neuronal layers communicating through synapses, functioning as an extension of the central nervous system (CNS). Photoreceptor cells in the outer layer of the retina capture incoming light, initiating a neural signal cascade that culminates at the retinal ganglion cells (RGCs), whose axons form the optic nerve (ON). These axons project to key CNS structures such as the lateral geniculate nucleus (LGN) in the thalamus and the superior colliculus (SC) in the midbrain [167] (Fig. 5). The LGN acts as a vital hub, relaying signals to the primary visual cortex, which, in collaboration with higher-order brain regions, undertakes complex tasks like object recognition and spatial processing [168]. Simultaneously, the efferent visual pathway involves the frontal eye field, parietal eye field, and basal ganglia, orchestrating saccades—rapid, precise movements between fixation points. The signals for these movements are modulated by the superior colliculus and directed by the saccade burst generator in the brainstem, with the dorsolateral prefrontal cortex playing a key role in their voluntary control [169, 170].
Fig. 5.
Impact of Visual System on AD Progression. This visual system illustration delineates discernible alterations observed in individuals affected by AD. Pathological hallmarks of AD, specifically amyloid plaques and tau tangles, manifest prominently in pivotal brain regions associated with visual function, including the visual cortex, pulvinar nucleus, lateral geniculate nucleus, suprachiasmatic nucleus, and superior colliculus. These pathological changes intricately correlate with disruptions in their associated functions. Within the ocular domain, atients with AD exhibit optic nerve degeneration, marked by the loss of axonal projections. Furthermore, AD is concomitant with a reduction in retinal thickness and a decline in retinal vasculature. ITC: inferior temporal cortex; LGN: lateral geniculate nuclei; PFC: prefrontal cortex; PPC: posterior parietal cortex; Pul: pulvinar; SC: superior colliculus; VC: visual cortex. Image created using Biorender
Current research explores the retina as a potential conduit to brain health, investigating how ocular examinations could deepen understanding of CNS disorders. The process of light transduction into neural signals by the retina offers a unique opportunity to observe CNS health [171]. The transmission of these signals from the photoreceptors, through the RGCs, to the visual processing centers in the brain reflects a dynamic interplay between sensory input and neural processing. This pathway facilitates not just our perception of the world but may also hold keys to early detection and monitoring of CNS pathologies, including neurodegenerative diseases. Measurements of retinal nerve fiber layer thickness and macular volume are not only indicators of retinal health but may also signal neurodegenerative conditions. A comprehensive understanding of retinal structure and function is thus essential for advancing ocular assessments in the context of neurological evaluation and could lead to novel diagnostic and therapeutic approaches for neurodegenerative diseases [172–175].
The visual system and AD
In the past few decades, considerable research attention has been directed towards elucidating the ocular manifestations associated with AD [176–179]. A seminal case-control study, focusing on the optic nerves of patients with AD compared to healthy controls, provided robust evidence for RGC atrophy in AD [177]. In many instances, ocular symptoms have been observed to precede cerebral manifestations, suggesting that comprehensive eye examinations could serve as a valuable tool for early diagnosis of underlying neurological conditions [171]. Given the anatomical and functional connection between the eye and the brain, exploring early ocular manifestations in AD is becoming an important research avenue. These studies revealed that, compared to non-AD individuals, patients with AD exhibited constricted retinal veins, reduced retinal blood flow and RGC count, and thinner RNFLs, correlating these anomalies with retinal dysfunction [172, 180–182]. Significantly, a reduction in macular volume among patients with AD was found to correlate with performance on cognitive tests [183]. Prior research suggested that ocular degeneration in AD predominantly occurs in the posterior segments of the optic nerve, near the optic chiasm, indicating that while ocular symptoms are noticeable, the primary site of damage might be intracranial [184]. Furthermore, pathological accumulations of Aβ and p-tau have been identified in the lenses of patients with AD [185], and in the retina and retinal ganglion cells of transgenic AD mouse models [176, 186–190]. Similar to their effects in the brain, Aβ and p-tau in the eye are associated with various forms of ocular damage, including cataract formation, loss of retinal neurons, RNFL thinning, and impaired axonal development [179, 185, 186, 188].
Considering these findings, recent longitudinal cohort studies have illuminated the potential impact of treating visual impairment on future dementia risk. Cataract treatment, in particular, has been associated with a reduced risk of developing dementia including AD dementia, signifying the broader implications of addressing visual impairments. This contrasts with the results for glaucoma surgery, which does not typically lead to improved vision and has not shown a similar reduction in dementia risk [191]. The association between visual impairment and dementia risk is further supported by epidemiological research, which suggests that visual impairment may serve as a marker for short- and medium-term dementia risk [192].
In conclusion, the accumulating body of research underscores the intricate link between visual impairment and the risk of dementia, including AD. The ocular manifestations observed in patients with AD, particularly the changes in retinal structure and function, suggest that the eye could be a window to early detection and intervention in neurodegenerative diseases. As the global population ages, these findings underscore the importance of regular comprehensive eye examinations and the potential benefits of treating visual impairments in mitigating the burden of dementia.
Potential mechanisms linking visual system and AD
The interplay between visual impairment and the risk of dementia, including AD, has gained attention in recent years. The potential mechanisms linking the two conditions, while not fully understood, are believed to involve a variety of indirect pathways. One such pathway is the structural modification of the brain regions associated with vision. Research suggests that ocular impairments leading to reduced visual input can induce changes in and around the primary visual cortex. These changes can sometimes be reversed with appropriate ocular treatments, such as cataract surgery, indicating the plasticity of the brain in response to sensory input [193]. Enhanced sensory input can stimulate neuroplasticity, leading to structural improvements in brain regions affected by visual impairment [194]. This includes increases in grey matter volume and improvements in brain function in areas related to vision and cognition [195, 196]. Recent studies have shown that even functional brain networks far beyond the lesion site can be reorganized, further supporting the potential for visual recovery through neuroplasticity [197]. For instance, a study found that cataract surgery was associated with a slower decline in episodic memory, a key cognitive function, suggesting that improving visual function can positively impact cognitive health in individuals with AD [198]. The Fujiwara-kyo Eye Study found that elderly individuals who underwent cataract surgery had a significantly lower risk of developing MCI, suggesting that improving visual acuity can have broader cognitive benefits [199].
Another intriguing concept is cognitive resilience, which refers to the ability of an individual to maintain cognitive function despite the presence of extensive brain pathology, such as that seen in AD [200]. This concept is important because it helps explain why some individuals can sustain high levels of cognitive function even when their brains exhibit extensive AD-related damage. Consequently, interventions that improve ocular health, such as cataract surgery, might offer the most benefit to those with less innate resilience, potentially reducing their risk of developing dementia. Furthermore, engagement in visual-based leisure activities has been associated with enhanced cognitive resilience and a lower risk of dementia. Cohort studies have provided evidence that active participation in visually stimulating activities can contribute to cognitive health [200]. Better vision reduces the cognitive effort required to process visual information, allowing cognitive resources to be allocated more efficiently. This can lead to improved cognitive function and a lower risk of dementia, as individuals are more likely to engage in cognitively stimulating activities and maintain social interactions [201, 202]. These activities may help maintain cognitive function by promoting neuroplasticity and cognitive reserve.
Visual impairment and dementia: significance and future directions
Visual acuity, while often considered a straightforward measure of ocular health, can be influenced by various factors beyond mere optical clarity [203, 204]. It entails a complex interplay of sensory input and cognitive processing, similar to the way olfactory identification requires memory and higher-order thinking. The decline in visual function, more pronounced with age, implicates both the sensory apparatus and the cognitive domains of the brain. It is this decline that recent research suggests might be an early compensatory response to neural changes in AD, potentially offering a window for early therapeutic intervention [205]. Visual impairment is being increasingly recognized as an early indicator in AD research. Notable findings suggest that changes in visual acuity and related electrophysiological responses may precede the cognitive symptoms traditionally associated with AD, thus presenting an opportunity for early detection [206]. Furthermore, recent studies indicate that visual assessments, particularly those that measure changes in acuity and electrophysiological activity, could be integrated into routine screenings for AD [207]. Eye movement studies, for example, offer promising avenues for early detection and monitoring of AD by analyzing metrics such as saccadic movements, fixation patterns, and smooth pursuit [208–210]. These non-invasive techniques could complement existing diagnostic tools, providing a more comprehensive approach to AD detection and management.
An intriguing observation is the reported increase in visual acuity in some individuals at high risk for AD [211]. This contrasts with earlier research suggesting a decline in visual function in similar cohorts [212–216]. These findings require further investigation to determine whether it indicates an early neural response at the onset of AD or if it is due to hyperactivity in retinal cells caused by Aβ accumulation. Understanding the link between initial ocular signs and the progression of AD is a key focus area. There is a need to investigate the reasons behind altered visual acuity and to ascertain whether these changes are indeed precipitated by the impact of Aβ on the retina or reflect a general increase in neural adaptability.
In clinical settings, evaluating visual function has shown potential as a non-invasive method that may complement CSF biomarkers in detecting the progression from MCI to AD in some individuals. Further studies should investigate the reasons behind altered visual acuity in individuals at high risk for AD. Visual assessments that measure changes in acuity and electrophysiological activity should be integrated into routine screenings for AD to enhance early detection and intervention strategies. Research into the optic nerve and its connections to the brain should be expanded to understand how changes in the visual system might mirror brain health in AD. Integrating these various approaches (visual acuity assessments, electrophysiological measurements, and eye movement studies) can provide a more holistic understanding of how visual impairments relate to AD. By addressing these areas, researchers can develop innovative strategies to mitigate the burden of AD-related dementia and improve the quality of life for older adults.
Gustatory impairment
The phenomenon of diminished taste, or hypogeusia, is a recognized yet understudied condition prevalent among the elderly, often not leading to significant clinical concerns. However, the influence of taste disorders on overall well-being and the ability to perform job-related tasks can be profound, particularly in severe cases where nutritional status and health may be compromised. Gustatory dysfunctions, reported with a prevalence of around 5% in the general population [217], are less frequently documented than olfactory disorders. Nonetheless, the clinical importance of gustatory disturbances warrants attention due to their potential impact on quality of life.
There is a notable lack of detailed research focusing specifically on taste disorders, which may be attributed to the complexity of the relationship between the gustatory system and other sensory modalities, including olfaction, somatosensation, and nociception. The challenge lies in isolating the specific contributions of each sensory system to the overall perception of flavor, a task that complicates research methodologies. The sensory experience of flavor is a result of the intricate integration of gustatory and olfactory stimuli, with concurrent neuronal activations observed in regions such as the insula, amygdala, and orbitofrontal cortex [218]. These areas are critical in the neural circuitry of flavor perception, highlighting the multifaceted nature of how flavor is processed and perceived in the human brain. Understanding these mechanisms is necessary for advancing the diagnosis and treatment of taste disorders, thereby enhancing patient care and quality of life in the aging population.
The gustatory system
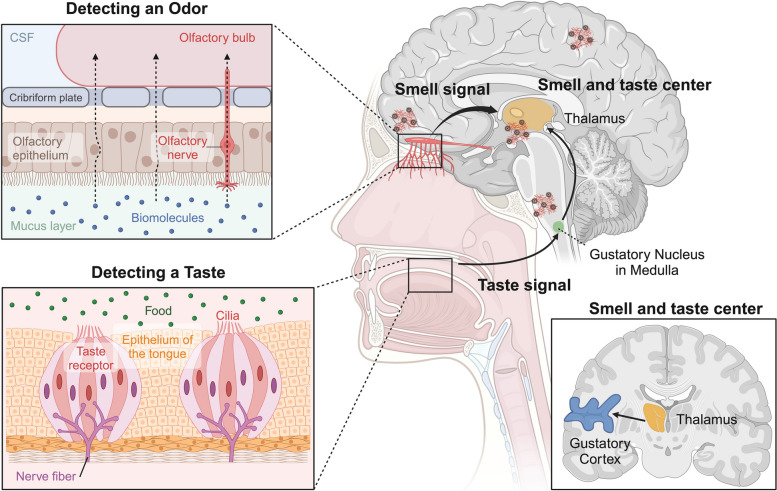
Flavor perception arises from the central integration of various sensory inputs, including taste, smell, texture, temperature, visual, and auditory characteristics associated with food [219]. Psychophysical research and neuroimaging studies in humans, supplemented by electrophysiological data from animal models, are progressively revealing the neural foundations of flavor processing [220, 221]. Neuroimaging studies focused on taste and smell reveal that isolated exposure to tastants or odorants activates specific regions of the insula [222–225]. These insular regions are associated with primary taste perception, the amygdala involvement [222, 226, 227], which is pivotal for emotional processing, and the OFC [222–224, 228, 229], which plays a role in advanced taste and olfactory processing. Additionally, the anterior cingulate cortex is implicated in these complex sensory processes [230]. Figure 6 highlights the distinct yet overlapping neural pathways involved in taste and olfactory processing. Understanding these mechanisms is key to addressing the challenges posed by gustatory disorders, especially in the aging population.
Fig. 6.
Descriptive Anatomy of Odorant Signal Transmission and the Interconnection with the Human Gustatory System in the Context of AD Pathogenesis. The intricate connections revealed in this illustration shed light on the olfactory and gustatory pathways, mapping their course from peripheral nerves to cortical areas. These connections further our comprehension of these sensory pathways within the intricate context of AD pathology. Image created using Biorender
The gustatory system and AD
Recent studies have highlighted a notable pattern of diminished gustatory performance in patient, specifically in those with subjective cognitive decline [231]. This trend is also observed in individuals diagnosed with MCI and mild AD, suggesting a consistent pattern of gustatory dysfunction across these populations [232–234]. Research indicates that individuals with AD often show impaired gustatory function. This impairment is characterized by elevated thresholds for detecting and recognizing various tastes, including umami, sweet, salty, bitter, and sour. For instance, patients with AD commonly experience difficulties in recognizing umami flavors and detecting sweet and salty tastes [232, 235]. In contrast, some studies have found that older subjects with moderate dementia due to AD demonstrate significant reductions in taste recognition, particularly for bitter and salty tastes, compared to cognitively intact individuals [236]. These challenges suggest that there are deficits in taste processing within the neural system of patients with AD, evidenced by elevated thresholds for detecting and recognizing various tastes. This suggests that gustatory assessment could serve as a potential diagnostic tool for distinguishing patients with AD from their healthy individuals [237].
In addition, studies conducted by Kouzuki and colleagues (2018 and 2020) present divergent perspectives on the relationship between gustatory function and neurodegenerative conditions. The 2018 study observed no significant differences in gustatory test scores among AD, MCI, and healthy control groups. This implies that gustatory function may remain relatively intact in the AD and MCI [238]. In contrast, the 2020 study by the same group reported impaired gustatory function in patients with AD. For MCI patients, the results were inconclusive, but a noteworthy number had trouble in recognizing umami. This inconsistency underscores the intricate nature of gustatory changes in AD and emphasizes the imperative for further in-depth investigation [233].
Potential mechanisms linking gustatory system and AD
The gustatory system is closely linked with brain areas affected early in AD, such as the hippocampus, amygdala, and orbitofrontal cortex, which are necessary for taste processing, memory, and emotional regulation [21]. The orbitofrontal cortex, vital for processing taste, shows early neurofibrillary tangle pathology in AD [239]. Damage here can impair taste perception and affect behaviors and decision-making, potentially accelerating cognitive decline. Furthermore, the insula, amygdala, and hippocampus, which are involved in taste processing, are also important in the context of AD [240, 241]. These regions are part of the limbic system, which integrates sensory information, including taste [242]. Disruptions in the gustatory pathways within these areas could influence the progression of AD by affecting memory and emotional processing. Volume changes in these regions, particularly in the MTL structures, might contribute to cognitive decline, suggesting that gustatory pathway dysfunctions could have a cascading effect on AD development. Neuroimaging studies, such as MRI, have revealed significant volume reduction in critical brain areas involved in memory and spatial navigation, including the hippocampus and entorhinal cortex, in individuals with MCI [243]. Furthermore, FDG-PET imaging has highlighted decreased glucose metabolism in the posterior cingulate cortex among MCI group, indicating early signs of neural dysfunction that precede AD [244, 245]. Recent studies using tau-PET imaging have further elucidated the early deposition of tau pathology in the MTL and posterior cingulate cortex of patients with AD [246]. This progression aligns with the known stages of tau pathology in AD, starting from the transentorhinal region and extending to other brain areas.
Changes in neurotransmitter systems, including acetylcholine and serotonin, are associated with both taste perception and cognitive functions [247, 248]. In AD, disruptions in these systems might originate from or be exacerbated by gustatory pathway dysfunctions [249]. The involvement of the cholinergic system in cognitive functions suggests that impairments in taste processing could have downstream effects on cognitive decline. Similarly, changes in serotonin receptors, which are involved in cognitive mechanisms, may be influenced by gustatory dysfunction, potentially accelerating AD progression [250].
The gustatory system, which processes taste sensations, shares similarities with the olfactory pathway in terms of neurological processing. Gustatory fibers might cross paths at the lower midbrain level, a feature reminiscent of the neural architecture of the olfactory system. Importantly, the pathways responsible for conveying taste-related information are closely linked with neural circuits extending to the amygdala and hippocampus (Fig. 6). These brain regions are not only pivotal in emotional responses and memory formation but are also implicated in the integration of taste sensations [251]. This neural overlap suggests that gustatory dysfunction could be related to the broader neural degeneration seen in AD, or potentially arise from various neurodegenerative processes contributing to MCI. In other words, the involvement of the amygdala and hippocampus in taste processing suggests that declines in gustatory function may reflect early neural changes occurring in these key brain regions.
Gustatory impairment and dementia: significance and future directions
The exploration of gustatory impairment as a biomarker for AD and related neurocognitive disorders (NCDs) is becoming increasingly important in the realm of diagnostic advancements. However, the relationship between gustatory thresholds and AD progression remains nuanced, requiring further elucidation through targeted research. A critical analysis integrating clinical diagnoses with biomarker evaluations of AD highlighted that no significant variance in gustatory thresholds exists between patients with amnestic MCI, those with AD dementia, and control groups [238]. This observation underscores the complexity of directly correlating taste performance with AD-specific biomarkers like CSF Aβ42 and phospho-tau levels. The lack of a direct association between these biomarkers and gustatory function underscores the complexities of sensory impairments in AD, calling the need for refined diagnostic criteria that consider the intricate nature of the disease. Given these insights, future directions in research should focus on delineating the specific aspects of gustatory dysfunction that correlate with AD progression. This entails not only the development of standardized gustatory testing protocols that can accurately measure taste impairments but also the examination of how these impairments interact with established clinical biomarkers of AD.
Further investigations employing chemical and electrical testing methods have uncovered gustatory dysfunctions specific to chemical stimuli in patients with AD, as opposed to electrogustometry, highlighting the variability in sensory testing outcomes [252]. Moreover, analyses of National Health and Nutrition Examination Survey data have established a significant link between the inability to identify salty tastes and dementia, further illustrating the specific taste impairments associated with cognitive decline [253]. Likewise, investigating the differential impact of AD on various taste qualities, such as sweet, salty, bitter, and umami, could provide deeper insights into the neurodegenerative processes underlying sensory alterations. Understanding the mechanisms through which AD affects the gustatory system, in conjunction with other sensory modalities, will be important in developing comprehensive diagnostic tools and therapeutic interventions aimed at improving the quality of life for individuals with AD.
Considering these insights, future research should aim to delve deeper into the specific aspects of gustatory dysfunction that align with progression of AD. This includes exploring the differential impact of AD on various taste qualities and how these changes interact with established clinical biomarkers. By adopting a more targeted approach to understanding gustatory impairments in AD, we can enhance diagnostic accuracy and potentially uncover novel pathways for early intervention and management of these complex neurodegenerative diseases.
Tactile impairment
Tactile impairment is prevalent among older adults, with studies indicating that about 70% of individuals over the age of 70 experience some degree of touch impairment [254]. This impairment affects social interactions by reducing the ability to engage in social touch, such as handshakes or hugs, which are important for emotional connections [255]. As tactile sensitivity decreases with age, older adults often face challenges in performing tasks that require fine motor skills, such as buttoning clothes, tying shoelaces, or handling small objects [256]. This decline can lead to a loss of independence in carrying out basic activities of daily living and instrumental activities of daily living, including cooking, cleaning, and maintaining personal hygiene.
Decreased touch sensitivity results from changes in skin elasticity and nervous system function, making it more difficult for the brain to process touch signals [257]. This reduction can lead to decreased sensitivity to temperature, pressure, and pain, increasing the risk of injuries like burns or cuts, as individuals may not perceive these dangers promptly [258, 259]. Diminished pressure sensitivity also heightens the risk of falls and pressure ulcers, further compromising safety and affecting balance and bodily awareness. This can result in social withdrawal and isolation, impacting mental health and increasing the risk of depression. Furthermore, impaired touch can hinder communication, as individuals may struggle with using technology that requires touch inputs, making it difficult to stay connected with family and friends and contributing to social isolation [255].
The tactile system
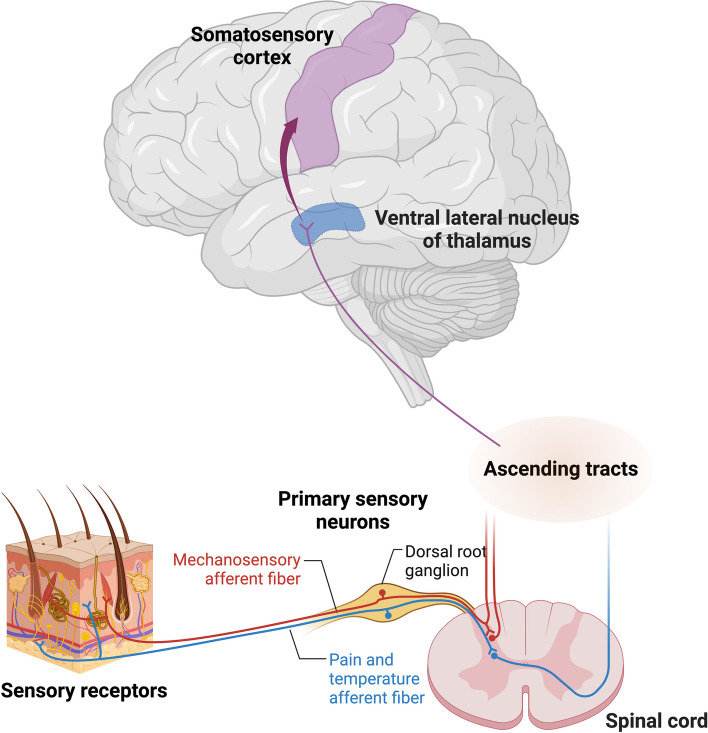
The tactile system, distinct from other sensory systems, exhibits an extensive distribution throughout the body, relying on sensory receptors in the skin for information acquisition. This perceptual modality involves sensing physical contact and pressure on the skin, constituting an integral aspect of the broader somatosensory function [260]. The somatosensory system, responsible for the conscious perception of tactile, thermal, nociceptive, proprioceptive, kinesthetic, and vibratory sensations, receives inputs from various peripheral sources, including musculature, joints, cutaneous structures, and fascial tissues (Fig. 7). The somatosensory system operates through a three-neuron relay mechanism [261] Sensory signals originating from peripheral receptors travel through sensory afferents to the dorsal root ganglia, where the first-order neuron cell bodies reside. These first-order neurons then progress through the spinal cord, intersecting with second-order neurons, whose location varies depending on the specific sensory modality they mediate. The spinal cord houses second-order neurons for processing pain, touch, and temperature signals, while the medulla contains neurons specialized in transmitting tactile, proprioceptive, and vibratory information. Sensory fibers branch into pathways towards the thalamus or the cerebellum, both of which predominantly manage unconscious information processing. The thalamic projections are vital for conveying sensory data to cortical regions, where complex sensory integration and analysis contribute to conscious perception.
Fig. 7.
Schematic representation of major tactile pathway. Fibers conveying fine touch and proprioceptive sensations consolidate in the dorsal column of the spinal cord, ascending to the medulla oblongata. Those originating from the head unite with fibers from the rest of the body in the brainstem region, following the same path to the cerebral cortex. Image created using Biorender
Despite its importance, the body of experimental and clinical evidence concerning age-related changes in the somatosensory system remains equivocal [34, 262]. This ambiguity in findings may be attributed to the intricate interaction of the central and peripheral nervous systems, along with the skin, in processing somatosensory information [263–265]. Understanding the nuanced interplay among these components is essential for elucidating the complexities of age-related alterations in tactile perception and the broader somatosensory system.
It is noteworthy that somatosensory acuity varies significantly among individuals and changes throughout the lifetime [266]. The aging process is inherently linked with a noticeable decline in motor and somatosensory functions [267, 268]. These age-related somatosensory changes in can impact tactile perception. As individuals age, they often experience alterations in cutaneous sensitivity, affecting their ability to perceive and distinguish tactile stimuli [269]. Primarily, aspects of age-related motor decline have been causally linked to reductions in inhibitory function, highlighting a mechanistic aspect. These changes include reductions in brain volume, alteration in patterns of brain functional activity and connectivity, and decreased neurotransmission levels [270–274]. Consequently, it is essential to acknowledge that age-related alterations in sensory perception and motor function might contribute substantially to the heightened vulnerability to AD and other age-related neurodegenerative diseases. Understanding these changes is important for developing comprehensive approaches to managing and treating AD, where sensory and motor functions are integral to the life satisfaction of patients and the effectiveness of therapeutic interventions.
The tactile system and AD
The relationship between the tactile system and AD is an area of growing interest in neuroscience research. Research indicates significant alterations in somatosensory perception and processing among patients with AD, including diminished responsiveness to tactile stimuli. This reduced sensitivity affects their ability to perceive discomfort, pain, and temperature changes. Furthermore, these individuals often struggle to differentiate between various textures and other sensory inputs [275–277]. Studies comparing patients with AD with age-matched controls have found that sensory impairments are more severe in patients diagnosed with AD. For instance, an initial clinical study involving 21 patients with AD, and 15 age-matched controls revealed that patients with AD demonstrated distinct tactile deficits. They made significantly more errors during tactile pattern recognition tasks, with error rates approximately four times higher than those observed in non-affected individuals [278]. Another investigation, involving 40 participants (15 with AD, 10 with MCI, and 15 controls) highlighted similar findings in tactile angle discrimination, showing significant deficits among the AD and MCI groups compared to the controls [276]. Expanding on these observations, a larger study included 120 participants divided into four groups: normal controls, individuals with subjective cognitive decline, those with amnestic MCI, and patients with AD. The study demonstrated that accuracy in tactile angle discrimination progressively worsened from the control group through to the patients with AD, confirming a gradual decline in tactile abilities as part of the AD progression [279].
Tactile stimulation has been found to alleviate AD symptoms in mouse models. Specifically, these models exhibit improvements in cognition, such as enhanced performance in the Morris water maze test. Additionally, tactile stimulation has been shown to improve motor functions, as evidenced by better performance in the rotarod test, and to reduce anxiety-like behaviors, observed through decreased time spent in the open arms of the elevated plus maze. These behavioral improvements are accompanied by a reduction in AD pathology, including decreased Aβ plaque deposition and tau phosphorylation [280]. In humans, a 6-week tactile massage therapy regimen administered to elderly individuals with dementia resulted in decreased aggression and stress levels, suggesting improvements in physiological and psychological functioning [281]. Some studies have found impairments in tactile perception in individuals with AD, which may contribute to cognitive and functional decline. However, the exact nature of this relationship and its implications for disease management require further investigation. To establish an important role for tactile stimulation in AD management and disease progression, larger-scale clinical trials in humans with AD are necessary. These studies should examine the effects of various tactile interventions on cognitive function, behavior, quality of life, and biomarkers of disease progression over extended periods. Additionally, research into the underlying mechanisms by which tactile stimulation might influence AD pathology is needed to support any claims about its potential therapeutic value. This could include investigations into how tactile input affects neural plasticity, inflammation, or other relevant physiological processes in the context of AD.
Potential mechanisms linking tactile function and AD
The mechanisms underlying the relationship between tactile sensory function and AD are still being investigated. Recent studies suggest that tactile stimulation may have positive effects on cerebral and behavioral development, offering insights into novel approaches for managing AD [282–285]. Tactile stimulation has been found to trigger several beneficial neurobiological processes. One key mechanism involves the release of neurotrophic factors. Specifically, tactile stimulation has been shown to induce the epidermal release of fibroblast growth factor-2 (FGF-2) [286], which initiates a cascade of biological processes including neurogenesis, cellular proliferation, cell survival, migration, and neural differentiation [287]. Additionally, increased levels of brain-derived neurotrophic factor (BDNF) have been observed following tactile stimulation, contributing to enhanced synaptic plasticity and neuronal survival [288]. Another important aspect of tactile stimulation is neurotransmitter modulation. Research has associated tactile stimulation with increased levels of acetylcholine [289], a neurotransmitter critical for cognitive function and memory formation. This modulation may play a role in improving cognitive outcomes in patients with AD. Synaptic plasticity, fundamental to learning, memory, and cognitive function, has also been shown to be enhanced by tactile stimulation [290, 291]. This enhancement could potentially contribute to cognitive resilience in individuals at risk for or diagnosed with AD.
Studies in rodent models have demonstrated that tactile stimulation aids in post-brain trauma recovery and reduces Aβ plaque deposition and tau phosphorylation, suggesting a potential neuroprotective effect in AD [280, 285]. This direct impact on AD pathology suggests that tactile stimulation could potentially slow disease progression. This neuroprotective capacity is further supported by observations in preterm infants, where tactile stimulation has been associated with enhanced cognitive and motor skills, indicating a potential for improving brain function through tactile interventions.
While not explicitly demonstrated in current research, it is hypothesized that tactile stimulation may also influence neuroinflammatory processes, which are known to play a role in AD progression [285]. Furthermore, tactile stimulation may enhance overall sensory integration and provide cognitive stimulation, potentially contributing to cognitive reserve and resilience against AD progression. These findings collectively suggest that tactile stimulation could be a promising non-invasive strategy for slowing the onset of dementia in aging individuals. However, it is necessary to note that while these results are encouraging, further research is needed to fully understand the mechanisms involved and to develop effective interventions for patients with AD.
Tactile impairment and dementia: significance and future directions
Current research is intensively exploring tactile impairments and their correlation with cognitive deficits in AD [45, 279, 280, 285, 292–294]. Recent findings suggest that tactile discrimination tasks, which assess the ability to distinguish stimuli through touch, may serve as effective indicators of cognitive decline in individuals with MCI due to AD. These tasks are advantageous because they are quick, easy to compare, and less influenced by educational background, offering a potential method for early diagnosis and intervention [279, 295]. While animal studies have provided insights into abnormal sensory processing in AD models, further human studies are necessary to fully understand how these sensory deficits affect brain function and disease progression in patients with AD [9, 292].
Efforts are also underway to create and validate standardized methods for assessing tactile function in individuals at risk of or exhibiting mild AD [296, 297]. This includes the development of tactile cognitive function tests that leverage both structural and metabolic information to improve early diagnosis [298]. Overall, these research initiatives highlight the importance of sensory processing in AD and underscore the potential of tactile assessments in clinical settings. Concurrently, efforts are underway to create and validate standardized methods and tools for accessing tactile function in individuals at risk of or exhibiting early-stage AD [296, 297].
The involvement of higher-order cortical sensory processing areas, particularly the parietal lobe, in AD has significant implications for sensory dysfunction [301]. The parietal lobe plays a pivotal role in integrating and processing somatosensory information, and its impairment in AD can lead to widespread effects on tactile recognition and spatial perception. Research has shown that patients with AD exhibit significantly higher error rates (approximately four times higher) in tactile pattern recognition tasks compared to healthy controls [278]. This suggests that parietal lobe dysfunction in AD affects not just simple sensory input processing but also complex tactile information interpretation and integration.
Efforts are also underway to create and validate standardized methods for assessing tactile function in individuals at risk of or exhibiting mild AD [296, 297]. This includes the development of tactile cognitive function tests that leverage both structural and metabolic information to improve early diagnosis [298]. Overall, these research initiatives highlight the importance of sensory processing in AD and underscore the potential of tactile assessments in clinical settings. Concurrently, efforts are underway to create and validate standardized methods and tools for accessing tactile function in individuals at risk of or exhibiting early-stage AD [296, 297].
Neurological pathways and brain regions affected by AD that contribute to tactile impairments are being investigated. Advanced neuroimaging techniques like MRI and PET scans have been employed to observe cerebral changes associated with tactile deficits [293, 299]. This includes examining how regions involved in sensory processing are affected in AD, particularly the impact of Aβ plaques and tau tangles on these areas. A deeper understanding of the cellular and molecular mechanisms underlying sensory deficits in AD could illuminate aspects of disease progression and reveal new therapeutic opportunities. Some studies are assessing the potential of tactile stimulation to mitigate cognitive decline in patients with AD. These involve sensory therapies, such as massages or the use of textured materials, designed to enhance cognitive and sensory engagement. The effectiveness of these interventions in improving quality of life, reducing agitation, and potentially slowing disease progression is a key area of research [280, 300].
The involvement of higher-order cortical sensory processing areas, particularly the parietal lobe, in AD has significant implications for sensory dysfunction [301]. The parietal lobe plays a pivotal role in integrating and processing somatosensory information, and its impairment in AD can lead to widespread effects on tactile recognition and spatial perception. Research has shown that patients with AD exhibit significantly higher error rates (approximately four times higher) in tactile pattern recognition tasks compared to healthy controls [278]. This suggests that parietal lobe dysfunction in AD affects not just simple sensory input processing but also complex tactile information interpretation and integration.
The relationship between sensory dysfunction in the elderly and metabolic polyneuropathy presents a complex challenge in establishing correlation and causation. While these two phenomena often co-occur, elucidating the nature of their relationship is not straightforward. The potential for underlying factors such as diabetes, hypertension, dyslipidemia, or nutritional deficiencies to affect both cognitive function and peripheral nerves further complicates this relationship [302]. It is important to consider that the primary contributors to dementia progression may not be sensory deficits themselves, but rather these underlying factors that impact both cognition and peripheral nerves. For instance, diabetes can lead to peripheral neuropathy, causing sensory dysfunction, while simultaneously affecting cognitive function through vascular damage in the brain [303–305]. This suggests that sensory dysfunction and cognitive impairment may be outcomes of shared pathological processes.
The complexity of unraveling the causal relationships between sensory dysfunction, cognitive impairment, and these potential shared risk factors or pathologies presents a considerable challenge. Longitudinal studies and multivariate analyses will be necessary to clarify these relationships. Additionally, neuroimaging techniques and biomarker analyses could provide more precise assessments of how these factors affect brain structure and function.
In conclusion, sensory dysfunction in AD is likely not merely a peripheral nerve issue but a result of higher-order cortical processing deficits, metabolic factors, and complex neurodegenerative processes. Understanding these intricate interactions could provide insights for early diagnosis and development of effective treatment strategies for AD. Future research should focus on disentangling these relationships to better understand the progression of AD and identify potential targets for intervention. As we explore the role of tactile sensation as a biomarker for AD, it becomes imperative to conduct in-depth studies into the relationship between tactile sensory changes and aging. Current hypotheses suggest that the measurement and manipulation of tactile sensation could play a pivotal role in the prevention, diagnosis, and treatment of AD, with future research expected to further solidify this potential.
Multisensory deficits on alzheimer’s disease
Research has increasingly focused on how combinations of sensory impairments, particularly involving hearing, vision, and olfactory functions, impact AD. These studies collectively highlight the significant role that multisensory deficits play in increasing the risk of dementia and accelerating cognitive decline. Research has shown that individuals with dual sensory impairment (DSI) in hearing and vision have a significantly higher risk of developing AD compared to those with a single sensory impairment [306]. While some studies suggest that the risk is more pronounced with hearing loss [307], recent findings indicate a considerably higher hazard of dementia in individuals with combined sensory impairments dementia [308–311]. Further supporting this, the Baltimore Longitudinal Study of Aging explored the impact of multiple sensory impairments, including vision, hearing, olfactory, proprioceptive, and vestibular functions, on brain structure [312]. The study revealed that each additional sensory impairment was associated with lower volumes in critical brain regions like the orbitofrontal gyrus and entorhinal cortex. This indicates that the cumulative effect of multiple sensory deficits can lead to significant brain atrophy and cognitive decline. Similarly, the Health, Aging, and Body Composition Study created a summary measure of multisensory impairment based on vision, hearing, smell, and touch [313]. The findings demonstrated that worsening multisensory function was linked to a higher risk of dementia and faster rates of cognitive decline, emphasizing that even mild levels of multisensory impairment can accelerate cognitive aging. Olfactory impairment has been shown to significantly increase the risk of AD when combined with other sensory deficits such as hearing and vision loss [7, 200]. Olfactory dysfunction is linked to early neuropathological changes in brain areas like the olfactory bulb and entorhinal cortex, which are important for memory processing. When visual impairments are also present, they may compound the cognitive challenges, as both senses are essential for environmental interaction and memory cues. Moreover, another study found that individuals with multiple sensory impairments, including olfactory, hearing, and vision deficits, had a greater association with cognitive decline and dementia than those with a single sensory deficit [314, 315]. This suggests an additive effect where the combination of sensory impairments exacerbates the risk of AD.
In summary, multisensory deficits significantly impact AD by contributing to both the risk and progression of cognitive decline. Each sensory modality plays a role, and their combined effects highlight the importance of comprehensive sensory assessments and interventions in managing AD. Further research is needed to explore the mechanisms underlying these associations and to develop effective interventions targeting sensory impairments in patients with AD.
Conclusions
Approximately 40% of individuals aged 70 to 79 experience dysfunction in at least one sensory system, with over 25% experiencing impairments in multiple senses [316]. These sensory changes often coincide with various comorbidities, specific to the affected sense. Notably, the presence of such sensory deficits is linked with an increased risk of developing AD. The exploration of age-related sensory organ impairments and their impact on AD carries substantial clinical relevance. Early identification and management of sensory impairments in the aging population could serve as a strategic approach to predict or mitigate cognitive decline associated with AD. Intervention targeting sensory deficits may not only improve the quality of life for these individuals but also potentially slow the progression of AD.
This review elucidates the complex interplay between sensory impairments and AD, highlighting the multifaceted nature of sensory decline as both an early indicator and a risk factor for AD. Auditory impairment, particularly age-related hearing loss, is strongly linked to increased cognitive decline and dementia risk, with hearing aids potentially mitigating cognitive deterioration. Olfactory dysfunction, correlating closely with cognitive decline and disease progression, emerges as a promising early biomarker for AD. Similarly, visual impairments, including retinal changes, are associated with an elevated risk of AD, positioning them as potential early indicators of neurodegeneration. Meanwhile, gustatory impairments, though less studied, show promise as markers for AD progression, particularly when occurring alongside other sensory deficits. Tactile impairments suggest broader impacts on somatosensory processing, affecting cognitive function and quality of life. This review further notes that multisensory deficits produce a cumulative effect, significantly increasing the risk of AD and accelerating cognitive decline beyond what is observed with single sensory impairments.
Moving forward, future research should prioritize several key areas to enhance our understanding and response to AD. It is essential to clarify the mechanisms that link sensory impairments to AD pathology, which could reveal new therapeutic targets. Developing standardized protocols for multisensory assessment will ensure consistent and early identification of at-risk individuals. Investigating sensory-based interventions offers potential new strategies for managing AD, possibly slowing or altering its course. This involves investigating the evolution of sensory deficits in the prodromal phase of AD, analyzing their timing in relation to CSF biomarkers, and utilizing measures from structural and functional MRI. Finally, conducting longitudinal studies is crucial for understanding how sensory decline correlates with the onset and progression of AD over time. Such research endeavors are key to improving early detection and intervention strategies for AD, ultimately contributing to more effective management and potentially altering the course of AD.
Acknowledgements
Not applicable.
Authors’ contributions
All authors contributed to the study conception and design. Conceptualization, Investigation and Writing - Original Draft were performed by Suji Hong. Writing - Original Draft, Visualization and Project administration were performed by Seung Hyun Baek.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number) (NRF-2022R1I1A1A01072791, RS-2024-00345742). This work was supported by a Korea Institute of Marine Science & Technology Promotion (KIMST) grant funded by the Ministry of Oceans and Fisheries (RS-2021-KS211513) and the National Health and Medical Research Council of Australia (Grant Identification Number 2019100).
Data availability
Not applicable.
Declarations
Ethics approval and consent to aprticipate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Suji Hong and Seung-Hyun Baek contributed equally to this work.
Contributor Information
Thiruma V. Arumugam, Email: g.arumugam@latrobe.edu.au
Dong-Gyu Jo, Email: jodg@skku.edu.
References
- 1.Peiffer AM, Hugenschmidt CE, Maldjian JA, Casanova R, Srikanth R, Hayasaka S, Burdette JH, Kraft RA, Laurienti PJ. Aging and the interaction of sensory cortical function and structure. Hum Brain Mapp. 2009;30(1):228–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek SH, Park SJ, Jeong JI, Kim SH, Han J, Kyung JW, Baik SH, Choi Y, Choi BY, Park JS, et al. Inhibition of Drp1 ameliorates synaptic depression, Aβ deposition, and cognitive impairment in an Alzheimer’s Disease Model. J Neurosci. 2017;37(20):5099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho Y, Bae HG, Okun E, Arumugam TV, Jo DG. Physiology and pharmacology of amyloid precursor protein. Pharmacol Ther. 2022;235: 108122. [DOI] [PubMed] [Google Scholar]
- 4.Tzioras M, McGeachan RI, Durrant CS, Spires-Jones TL. Synaptic degeneration in Alzheimer disease. Nat Rev Neurol. 2023;19(1):19–38. [DOI] [PubMed] [Google Scholar]
- 5.Risacher SL, Wudunn D, Pepin SM, MaGee TR, McDonald BC, Flashman LA, Wishart HA, Pixley HS, Rabin LA, Paré N, et al. Visual contrast sensitivity in Alzheimer’s disease, mild cognitive impairment, and older adults with cognitive complaints. Neurobiol Aging. 2013;34(4):1133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhlmann RF, Larson EB, Koepsell TD. Hearing impairment and cognitive decline in senile dementia of the Alzheimer’s type. J Am Geriatr Soc. 1986;34(3):207–10. [DOI] [PubMed] [Google Scholar]
- 7.Murphy C. Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol. 2019;15(1):11–24. [DOI] [PubMed] [Google Scholar]
- 8.Desikan RS, Sabuncu MR, Schmansky NJ, Reuter M, Cabral HJ, Hess CP, Weiner MW, Biffi A, Anderson CD, Rosand J, et al. Selective disruption of the cerebral neocortex in Alzheimer’s disease. PLoS ONE. 2010;5(9):e12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang NK, Zhang SK, Zhang LI, Tao HW, Zhang GW. Sensory processing deficits and related cortical pathological changes in Alzheimer’s disease. Front Aging Neurosci. 2023;15: 1213379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graff-Radford J, Yong KXX, Apostolova LG, Bouwman FH, Carrillo M, Dickerson BC, Rabinovici GD, Schott JM, Jones DT, Murray ME. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risacher SL, WuDunn D, Tallman EF, West JD, Gao S, Farlow MR, Brosch JR, Apostolova LG, Saykin AJ. Visual contrast sensitivity is associated with the presence of cerebral amyloid and tau deposition. Brain Commun. 2020;2(1):fcaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol. 2012;11(2):170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, Drake J, Wolf PA. Visual association pathology in preclinical Alzheimer disease. J Neuropathol Exp Neurol. 2006;65(6):621–30. [DOI] [PubMed] [Google Scholar]
- 14.Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer’s disease. Neurology. 1993;43(4):779–85. [DOI] [PubMed] [Google Scholar]
- 15.Aylward A, Auduong P, Anderson JS, Zielinski BA, Wang AY, Weng C, Foster NL, Gurgel RK. Changes in the auditory association cortex in dementing illnesses. Otol Neurotol. 2020;41(10):1327–33. [DOI] [PubMed] [Google Scholar]
- 16.Brewer AA, Barton B. Changes in visual cortex in healthy aging and dementia. In: Update on Dementia. Rijeka: InTech; 2016. p. 273–310.
- 17.den Haan J, Morrema THJ, Verbraak FD, de Boer JF, Scheltens P, Rozemuller AJ, Bergen AAB, Bouwman FH, Hoozemans JJ. Amyloid-beta and phosphorylated tau in post-mortem Alzheimer’s disease retinas. Acta Neuropathol Commun. 2018;6(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusne Y, Wolf AB, Townley K, Conway M, Peyman GA. Visual system manifestations of Alzheimer’s disease. Acta Ophthalmol. 2017;95(8):e668–76. [DOI] [PubMed] [Google Scholar]
- 19.Cheng N, Bai L, Steuer E, Belluscio L. Olfactory functions scale with circuit restoration in a rapidly reversible Alzheimer’s disease model. J Neurosci. 2013;33(30):12208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ubeda-Bañon I, Saiz-Sanchez D, Flores-Cuadrado A, Rioja-Corroto E, Gonzalez-Rodriguez M, Villar-Conde S, Astillero-Lopez V, Cabello-de la Rosa JP, Gallardo-Alcañiz MJ, Vaamonde-Gamo J, et al. The human olfactory system in two proteinopathies: Alzheimer’s and Parkinson’s diseases. Transl Neurodegener. 2020;9(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantovani E, Zanini A, Cecchini MP, Tamburin S. The association between neurocognitive disorders and gustatory dysfunction: a systematic review and meta-analysis. Neuropsychol Rev. 2024;34(1):192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golde TE. Alzheimer’s disease: The journey of a healthy brain into organ failure. Mol Neurodegener. 2022;17(1):18. 10.1186/s13024-022-00512-3. [DOI] [PMC free article] [PubMed]
- 23.Livingston G, Huntley J, Liu KY, Costafreda SG, Selbæk G, Alladi S, Ames D, Banerjee S, Burns A, Brayne C, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet. 2024;404(10452):572–628. [DOI] [PubMed] [Google Scholar]
- 24.Lantero-Rodriguez J, Camporesi E, Montoliu-Gaya L, Gobom J, Piotrowska D, Olsson M, Burmann IM, Becker B, Brinkmalm A, Burmann BM, et al. Tau protein profiling in tauopathies: a human brain study. Mol Neurodegener. 2024;19(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Chen Y, Huo Q, Wang L, Tan S, Misrani A, Jiang J, Chen J, Chen S, Zhang J, et al. Enhancing GABAergic signaling ameliorates aberrant gamma oscillations of olfactory bulb in AD mouse models. Mol Neurodegener. 2021;16(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith BC, D’Amico M. Sensory-based interventions for adults with dementia and Alzheimer’s Disease: a scoping review. Occup Ther Health Care. 2020;34(3):171–201. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Shi X, Wu Y, Zhou Z, Chen S, Han Y, Shan C. Gamma oscillations and application of 40-Hz audiovisual stimulation to improve brain function. Brain Behav. 2022;12(12):e2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murdock MH, Yang CY, Sun N, Pao PC, Blanco-Duque C, Kahn MC, Kim T, Lavoie NS, Victor MB, Islam MR, et al. Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature. 2024;627(8002):149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manippa V, Palmisano A, Filardi M, Vilella D, Nitsche MA, Rivolta D, Logroscino G. An update on the use of gamma (multi)sensory stimulation for Alzheimer’s disease treatment. Front Aging Neurosci. 2022;14: 1095081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Andrea F, Tischler V, Dening T, Churchill A. Olfactory stimulation for people with dementia: a rapid review. Dement (London). 2022;21(5):1800–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang H, Luo Y, Hu Q, Tian X, Wen H. Benefits in Alzheimer’s disease of sensory and multisensory stimulation. J Alzheimers Dis. 2021;82(2):463–84. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO). Deafness and hearing loss: Fact sheet. Geneva: WHO. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss. 2024. Accessed 16 Nov 2024.
- 33.World Health Organization (WHO). Addressing the rising prevalence of hearing loss. Geneva: WHO; 2018. https://www.who.int/publications/i/item/addressing-the-rising-prevalence-of-hearing-loss. Accessed 16 Nov 2024.
- 34.Jayakody DMP, Friedland PL, Martins RN, Sohrabi HR. Impact of aging on the Auditory System and related cognitive functions: a narrative review. Front Neurosci. 2018;12: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayakody DMP, Menegola HK, Yiannos JM, Goodman-Simpson J, Friedland PL, Taddei K, Laws SM, Weinborn M, Martins RN, Sohrabi HR. The peripheral hearing and central auditory processing skills of individuals with subjective memory complaints. Front Neurosci. 2020;14: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Runge C, Friedland D: 127—Neuroanatomy of the auditory system. Cummings otolaryngology head and neck surgery, 7th ed; Flint, PW, Francis, HW, Haughey, BH, Lesperance, MM, Lund, VJ, Robbins, KT, Thomas, JR, Eds 2021:1938–44.
- 37.Eggermont JJ, Wang X. Temporal coding in auditory cortex. In: Winer JA, Schreiner CE, editors. The auditory cortex. New York: Springer; 2010. p. 309–28.
- 38.King AJ, Middlebrooks JC. Cortical representation of auditory space. In: Winer JA, Schreiner CE, editors. The auditory cortex. New York: Springer; 2010. p. 329–41.
- 39.Bregman AS. Auditory scene analysis: The perceptual organization of sound. Cambridge (MA): MIT Press; 1994.
- 40.Kraus KS, Canlon B. Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res. 2012;288(1–2):34–46. [DOI] [PubMed] [Google Scholar]
- 41.Kanwal JS, Ehret G. Communication sounds and their cortical representation. In: Winer JA, Schreiner CE, editors. The auditory cortex. New York: Springer; 2010. p. 343–67.
- 42.Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Capozzo R, Quaranta N, Pilotto A, Logroscino G. Age-related hearing impairment and frailty in Alzheimer’s disease: interconnected associations and mechanisms. Front Aging Neurosci. 2015;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–734. [DOI] [PubMed] [Google Scholar]
- 45.Albers MW, Gilmore GC, Kaye J, Murphy C, Wingfield A, Bennett DA, Boxer AL, Buchman AS, Cruickshanks KJ, Devanand DP, et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 2015;11(1):70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kay DW, Roth M, Beamish P. Old age mental disorders in newcastle upon TYNE. II. A study of possible social and medical causes. Br J Psychiatry. 1964;110:668–82. [DOI] [PubMed] [Google Scholar]
- 47.Dawes P, Emsley R, Cruickshanks KJ, Moore DR, Fortnum H, Edmondson-Jones M, McCormack A, Munro KJ. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS ONE. 2015;10(3): e0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N. Longitudinal relationship between hearing Aid Use and cognitive function in older americans. J Am Geriatr Soc. 2018;66(6):1130–6. [DOI] [PubMed] [Google Scholar]
- 49.Taljaard DS, Olaithe M, Brennan-Jones CG, Eikelboom RH, Bucks RS. The relationship between hearing impairment and cognitive function: a meta-analysis in adults. Clin Otolaryngol. 2016;41(6):718–29. [DOI] [PubMed] [Google Scholar]
- 50.Mamo SK, Nirmalasari O, Nieman CL, McNabney MK, Simpson A, Oh ES, Lin FR. Hearing care intervention for persons with dementia: a pilot study. Am J Geriatr Psychiatry. 2017;25(1):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawes P, Munro KJ. Hearing loss and dementia: where to from Here? Ear Hear. 2024;45(3):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SY, Lim JS, Kong IG, Choi HG. Hearing impairment and the risk of neurodegenerative dementia: a longitudinal follow-up study using a national sample cohort. Sci Rep. 2018;8(1):15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker TD, Hardy C, Keuss S, Coath W, Cash DM, Lu K, Nicholas JM, James SN, Sudre C, Crutch S, et al. Peripheral hearing loss at age 70 predicts brain atrophy and associated cognitive change. J Neurol Neurosurg Psychiatry. 2024;95(9):829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang HF, Zhang W, Rolls ET, Li Y, Wang L, Ma YH, Kang J, Feng J, Yu JT, Cheng W. Hearing impairment is associated with cognitive decline, brain atrophy and tau pathology. EBioMedicine. 2022;86: 104336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng M, Yan J, Hao W, Ren Y, Zhou M, Wang Y, Wang K. Worsening hearing was associated with higher β-amyloid and tau burden in age-related hearing loss. Sci Rep. 2022;12(1):10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Flores R, Das SR, Xie L, Wisse LEM, Lyu X, Shah P, Yushkevich PA, Wolk DA. Medial temporal lobe networks in Alzheimer’s Disease: structural and molecular vulnerabilities. J Neurosci. 2022;42(10):2131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jack CR, Wiste HJ, Botha H, Weigand SD, Therneau TM, Knopman DS, Graff-Radford J, Jones DT, Ferman TJ, Boeve BF, et al. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain. 2019;142(10):3230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolters EE, Ossenkoppele R, Verfaillie SCJ, Coomans EM, Timmers T, Visser D, Tuncel H, Golla SSV, Windhorst AD, Boellaard R, et al. Regional [(18)F]flortaucipir PET is more closely associated with disease severity than CSF p-tau in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2020;47(12):2866–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci. 2014;17(2):304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, et al. PET imaging of tau deposition in the Aging Human Brain. Neuron. 2016;89(5):971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarawneh HY, Jayakody DMP, Sohrabi HR, Martins RN, Mulders W. Understanding the relationship between age-related hearing loss and Alzheimer’s Disease: a narrative review. J Alzheimers Dis Rep. 2022;6(1):539–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griffiths TD, Lad M, Kumar S, Holmes E, McMurray B, Maguire EA, Billig AJ, Sedley W. How can hear loss cause dementia? Neuron. 2020;108(3):401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmed MS, Priestley JB, Castro A, Stefanini F, Solis Canales AS, Balough EM, Lavoie E, Mazzucato L, Fusi S, Losonczy A. Hippocampal network reorganization underlies the formation of a temporal association memory. Neuron. 2020;107(2):283–e291286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav Neurosci. 1986;100(5):729–44. [DOI] [PubMed] [Google Scholar]
- 65.Diao T, Ma X, Zhang J, Duan M, Yu L. The correlation between hearing loss, especially high-frequency hearing loss and cognitive decline among the Elderly. Front Neurosci. 2021;15: 750874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kurata N, Schachern PA, Paparella MM, Cureoglu S. Histopathologic evaluation of vascular findings in the Cochlea in patients with Presbycusis. JAMA Otolaryngol Head Neck Surg. 2016;142(2):173–8. [DOI] [PubMed] [Google Scholar]
- 67.Sale A, Berardi N, Maffei L. Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiol Rev. 2014;94(1):189–234. [DOI] [PubMed] [Google Scholar]
- 68.Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Prog Neurobiol. 2009;89(4):369–82. [DOI] [PubMed] [Google Scholar]
- 69.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Häggström J, Hederstierna C, Rosenhall U, Östberg P, Idrizbegovic E. Prognostic value of a test of central auditory function in conversion from mild cognitive impairment to dementia. Audiol Neurootol. 2020;25(5):276–82. [DOI] [PubMed] [Google Scholar]
- 71.Pronk M, Lissenberg-Witte BI, van der Aa HPA, Comijs HC, Smits C, Lemke U, Zekveld AA, Kramer SE. Longitudinal relationships between decline in speech-in-noise recognition ability and cognitive functioning: the longitudinal aging study Amsterdam. J Speech Lang Hear Res. 2019;62(4s):1167–87. [DOI] [PubMed] [Google Scholar]
- 72.Nixon G, Sarant JZ, Tomlin D, Dowell R. The relationship between peripheral hearing loss and higher order listening function on cognition in older australians. Int J Audiol. 2019;58(12):933–44. [DOI] [PubMed] [Google Scholar]
- 73.Mamo SK, Reed NS, Sharrett AR, Albert MS, Coresh J, Mosley TH, Knopman D, Lin FR, Deal JA. Relationship between domain-specific cognitive function and Speech-in-noise performance in older adults: the atherosclerosis risk in communities hearing pilot study. Am J Audiol. 2019;28(4):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jalaei B, Valadbeigi A, Panahi R, Nahrani MH, Arefi HN, Zia M, Ranjbar N. Central auditory processing tests as diagnostic tools for the early identification of elderly individuals with mild cognitive impairment. J Audiol Otol. 2019;23(2):83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kahneman D. Attention and effort. Englewood Cliffs (NJ): Prentice-Hall; 1973.
- 76.Manohar SG, Zokaei N, Fallon SJ, Vogels TP, Husain M. Neural mechanisms of attending to items in working memory. Neurosci Biobehav Rev. 2019;101:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pichora-Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BW, Humes LE, Lemke U, Lunner T, Matthen M, Mackersie CL, et al. Hearing impairment and cognitive energy: the Framework for understanding Effortful listening (FUEL). Ear Hear. 2016;37(Suppl 1):s5–27. [DOI] [PubMed] [Google Scholar]
- 78.Giroud N, Lemke U, Reich P, Matthes KL, Meyer M. The impact of hearing aids and age-related hearing loss on auditory plasticity across three months - an electrical neuroimaging study. Hear Res. 2017;353:162–75. [DOI] [PubMed] [Google Scholar]
- 79.Rigters SC, Bos D, Metselaar M, Roshchupkin GV, Baatenburg de Jong RJ, Ikram MA, Vernooij MW, Goedegebure A. Hearing impairment is associated with smaller brain volume in aging. Front Aging Neurosci. 2017;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ren F, Ma W, Li M, Sun H, Xin Q, Zong W, Chen W, Wang G, Gao F, Zhao B. Gray matter atrophy is associated with cognitive impairment in patients with Presbycusis: a comprehensive morphometric study. Front Neurosci. 2018;12: 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23:154–66. [DOI] [PubMed] [Google Scholar]
- 82.Bucholc M, Bauermeister S, Kaur D, McClean PL, Todd S. The impact of hearing impairment and hearing aid use on progression to mild cognitive impairment in cognitively healthy adults: an observational cohort study. Alzheimers Dement (N Y). 2022;8(1):e12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin FR, Pike JR, Albert MS, Arnold M, Burgard S, Chisolm T, Couper D, Deal JA, Goman AM, Glynn NW, et al. Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet. 2023;402(10404):786–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cantuaria ML, Pedersen ER, Waldorff FB, Wermuth L, Pedersen KM, Poulsen AH, Raaschou-Nielsen O, Sørensen M, Schmidt JH. Hearing loss, hearing Aid Use, and risk of dementia in older adults. JAMA Otolaryngol Head Neck Surg. 2024;150(2):157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y, Guan L, Chen J, Han K, Yu Q, Zhou J, Wang X, Ma Y, Ji X, Zhao Z, et al. Hearing intervention for decreasing risk of developing dementia in elders with mild cognitive impairment: study protocol of a multicenter randomized controlled trial for Chinese Hearing Solution for Improvement of Cognition in elders (CHOICE). Trials. 2023;24(1):767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–12. [DOI] [PubMed] [Google Scholar]
- 87.Attems J, Walker L, Jellinger KA. Olfaction and aging: a Mini-review. Gerontology. 2015;61(6):485–90. [DOI] [PubMed] [Google Scholar]
- 88.Cha H, Kim S, Son Y. Associations between cognitive function, depression, and olfactory function in elderly people with dementia in Korea. Front Aging Neurosci. 2021;13:799897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schäfer L, Schriever VA, Croy I. Human olfactory dysfunction: causes and consequences. Cell Tissue Res. 2021;383(1):569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ekström IA, Rizzuto D, Grande G, Bellander T, Laukka EJ. Environmental air pollution and olfactory decline in aging. Environ Health Perspect. 2022;130(2):27005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vasavada MM, Martinez B, Wang J, Eslinger PJ, Gill DJ, Sun X, Karunanayaka P, Yang QX. Central olfactory dysfunction in Alzheimer’s disease and mild cognitive impairment: a functional MRI study. J Alzheimers Dis. 2017;59(1):359–68. [DOI] [PubMed] [Google Scholar]
- 92.Haehner A, Chen B, Espin M, Haussmann R, Matthes C, Desser D, Loessner L, Brandt MD, Donix M, Hummel T. Training with odors impacts hippocampal thickness in patients with mild cognitive impairment. J Alzheimers Dis. 2022;88(2):743–55. [DOI] [PubMed] [Google Scholar]
- 93.Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11(9):628–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413(6852):211–8. [DOI] [PubMed] [Google Scholar]
- 95.Nemati NN: A. S. BARWICH, Smellosophy: What the nose tells the mind, Cambridge, Massachusetts: Harvard University Press, 2020. Hist Philos Life Sci 2021;43(2):75.
- 96.Ramachandran VS, editor. Encyclopedia of the human brain. San Diego (CA): Academic Press; 2002.
- 97.Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70(4):582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Attems J, Walker L, Jellinger KA. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 2014;127(4):459–75. [DOI] [PubMed] [Google Scholar]
- 99.Kovács T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. NeuroReport. 2001;12(2):285–8. [DOI] [PubMed] [Google Scholar]
- 100.Arnold SE, Lee EB, Moberg PJ, Stutzbach L, Kazi H, Han LY, Lee VM, Trojanowski JQ. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol. 2010;67(4):462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Son G, Jahanshahi A, Yoo SJ, Boonstra JT, Hopkins DA, Steinbusch HWM, Moon C. Olfactory neuropathology in Alzheimer’s disease: a sign of ongoing neurodegeneration. BMB Rep. 2021;54(6):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64(7):802–8. [DOI] [PubMed] [Google Scholar]
- 103.Park H, Kim H, Kim S, Cha H: The Association between Olfactory Function and Cognitive Impairment in Older Persons with Cognitive Impairments: A Cross-Sectional Study. Healthcare (Basel). 2021;9(4):399. [DOI] [PMC free article] [PubMed]
- 104.Yan Y, Aierken A, Wang C, Song D, Ni J, Wang Z, Quan Z, Qing H. A potential biomarker of preclinical Alzheimer’s disease: the olfactory dysfunction and its pathogenesis-based neural circuitry impairments. Neurosci Biobehav Rev. 2022;132:857–69. [DOI] [PubMed] [Google Scholar]
- 105.Jacobson PT, Vilarello BJ, Tervo JP, Waring NA, Gudis DA, Goldberg TE, Devanand DP, Overdevest JB. Associations between olfactory dysfunction and cognition: a scoping review. J Neurol. 2024;271(3):1170–203. [DOI] [PMC free article] [PubMed]
- 106.Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64(10):871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olofsson JK, Larsson M, Roa C, Wilson DA, Jonsson Laukka E. Interaction between odor identification deficit and APOE4 predicts 6-Year cognitive decline in elderly individuals. Behav Genet. 2020;50(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dintica CS, Marseglia A, Rizzuto D, Wang R, Seubert J, Arfanakis K, Bennett DA, Xu W. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology. 2019;92(7):e700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu L, Wang Z, Du Z, Qi X, Shu H, Liu D, Su F, Ye Q, Liu X, Zhou Z, et al. Impaired parahippocampal gyrus-orbitofrontal cortex circuit associated with visuospatial memory deficit as a potential biomarker and interventional approach for Alzheimer Disease. Neurosci Bull. 2020;36(8):831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn Sci. 2013;17(8):379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489–502. [DOI] [PubMed] [Google Scholar]
- 112.Rey NL, George S, Steiner JA, Madaj Z, Luk KC, Trojanowski JQ, Lee VM, Brundin P. Spread of aggregates after olfactory bulb injection of α-synuclein fibrils is associated with early neuronal loss and is reduced long term. Acta Neuropathol. 2018;135(1):65–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Masaoka Y, Pantelis C, Phillips A, Kawamura M, Mimura M, Minegishi G, Homma I. Markers of brain illness may be hidden in your olfactory ability: a Japanese perspective. Neurosci Lett. 2013;549:182–5. [DOI] [PubMed] [Google Scholar]
- 114.Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol. 1998;55(1):84–90. [DOI] [PubMed] [Google Scholar]
- 115.Rahayel S, Frasnelli J, Joubert S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: a meta-analysis. Behav Brain Res. 2012;231(1):60–74. [DOI] [PubMed] [Google Scholar]
- 116.Doty RL. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 2017;16(6):478–88. [DOI] [PubMed] [Google Scholar]
- 117.Devanand DP, Lee S, Luchsinger JA, Andrews H, Goldberg T, Huey ED, Schupf N, Manly J, Stern Y, Kreisl WC, et al. Intact global cognitive and olfactory ability predicts lack of transition to dementia. Alzheimers Dement. 2020;16(2):326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tu L, Lv X, Fan Z, Zhang M, Wang H, Yu X. Association of odor identification ability with Amyloid-β and tau burden: a systematic review and Meta-analysis. Front Neurosci. 2020;14: 586330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tian Q, Bilgel M, Moghekar AR, Ferrucci L, Resnick SM. Olfaction, cognitive impairment, and PET biomarkers in community-dwelling older adults. J Alzheimers Dis. 2022;86(3):1275–85. [DOI] [PubMed] [Google Scholar]
- 120.Diez I, Ortiz-Terán L, Ng TSC, Albers MW, Marshall G, Orwig W, Kim CM, Bueichekú E, Montal V, Olofsson J, et al. Tau propagation in the brain olfactory circuits is associated with smell perception changes in aging. Nat Commun. 2024;15(1):4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klein J, Yan X, Johnson A, Tomljanovic Z, Zou J, Polly K, Honig LS, Brickman AM, Stern Y, Devanand DP, et al. Olfactory impairment is related to Tau Pathology and Neuroinflammation in Alzheimer’s Disease. J Alzheimers Dis. 2021;80(3):1051–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ekström I, Josefsson M, Larsson M, Rönnlund M, Nordin S, Olofsson JK. Subjective olfactory loss in older adults concurs with long-term odor identification decline. Chem Senses. 2019;44(2):105–12. [DOI] [PubMed] [Google Scholar]
- 123.Stanciu I, Larsson M, Nordin S, Adolfsson R, Nilsson LG, Olofsson JK. Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. J Int Neuropsychol Soc. 2014;20(2):209–17. [DOI] [PubMed] [Google Scholar]
- 124.Welge-Lüssen A. Ageing, neurodegeneration, and olfactory and gustatory loss. B-ENT. 2009;5(Suppl 13):129–32. [PubMed] [Google Scholar]
- 125.Devanand DP, Lee S, Manly J, Andrews H, Schupf N, Masurkar A, Stern Y, Mayeux R, Doty RL. Olfactory identification deficits and increased mortality in the community. Ann Neurol. 2015;78(3):401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaya E, Göker AE. Olfactory dysfunction: its association with subjective cognitive impairment in patients with major depression. J Nerv Ment Dis. 2022;210(3):172–8. [DOI] [PubMed] [Google Scholar]
- 127.Dong J, Zhan X, Sun H, Fang F, Wei Y. Olfactory dysfunction is associated with cognitive impairment in patients with obstructive sleep apnea: a cross-sectional study. Eur Arch Otorhinolaryngol. 2022;279(4):1979–87. [DOI] [PubMed] [Google Scholar]
- 128.Mahali M, Coolidge FL. On the relationship between neurocognitive measures and olfactory dysfunction in COVID-19 patients with and without anosmia. Brain Behav Immun Health. 2023;30:100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry. 2000;157(9):1399–405. [DOI] [PubMed] [Google Scholar]
- 130.Mitrano DA, Houle SE, Pearce P, Quintanilla RM, Lockhart BK, Genovese BC, Schendzielos RA, Croushore EE, Dymond EM, Bogenpohl JW, et al. Olfactory dysfunction in the 3xTg-AD model of Alzheimer’s disease. IBRO Neurosci Rep. 2021;10:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ziegler-Waldkirch S, Friesen M, Loreth D, Sauer JF, Kemna S, Hilse A, Erny D, Helm C, Prinz PDE. Seed-induced Aβ deposition alters neuronal function and impairs olfaction in a mouse model of Alzheimer’s disease. Mol Psychiatry. 2022;27(10):4274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Attems J, Jellinger KA. Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin Neuropathol. 2006;25(6):265–71. [PubMed] [Google Scholar]
- 133.Zhou X, Kumar P, Bhuyan DJ, Jensen SO, Roberts TL, Münch GW. Neuroinflammation in alzheimer’s disease: a potential role of nose-picking in pathogen entry via the olfactory system? Biomolecules 2023;13(11):1568. [DOI] [PMC free article] [PubMed]
- 134.GoodSmith MS, Wroblewski KE, Schumm LP, McClintock MK, Pinto JM. Association of APOE ε4 status with long-term declines in odor sensitivity, odor identification, and cognition in older US adults. Neurology. 2023;101(13):e1341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Frank RA, Rybalsky K, Brearton M, Mannea E. Odor recognition memory as a function of odor-naming performance. Chem Senses. 2011;36(1):29–41. [DOI] [PubMed] [Google Scholar]
- 136.Kjelvik G, Saltvedt I, White LR, Stenumgård P, Sletvold O, Engedal K, Skåtun K, Lyngvær AK, Steffenach HA, Håberg AK. The brain structural and cognitive basis of odor identification deficits in mild cognitive impairment and Alzheimer’s disease. BMC Neurol. 2014;14:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Son G, Yoo SJ, Kang S, Rasheed A, Jung DH, Park H, Cho B, Steinbusch HWM, Chang KA, Suh YH, et al. Region-specific amyloid-β accumulation in the olfactory system influences olfactory sensory neuronal dysfunction in 5xFAD mice. Alzheimers Res Ther. 2021;13(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoo SJ, Son G, Bae J, Kim SY, Yoo YK, Park D, Baek SY, Chang KA, Suh YH, Lee YB, et al. Longitudinal profiling of oligomeric Aβ in human nasal discharge reflecting cognitive decline in probable Alzheimer’s disease. Sci Rep. 2020;10(1):11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu D, Lu J, Wei L, Yao M, Yang H, Lv P, Wang H, Zhu Y, Zhu Z, Zhang X, et al. Olfactory deficit: a potential functional marker across the Alzheimer’s disease continuum. Front Neurosci. 2024;18: 1309482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc. 2008;56(8):1517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441–3. [DOI] [PubMed] [Google Scholar]
- 142.Rawson NE. Olfactory loss in aging. Sci Aging Knowl Environ. 2006;2006(5):pe6. [DOI] [PubMed] [Google Scholar]
- 143.Woodward MR, Amrutkar CV, Shah HC, Benedict RH, Rajakrishnan S, Doody RS, Yan L, Szigeti K. Validation of olfactory deficit as a biomarker of Alzheimer disease. Neurol Clin Pract. 2017;7(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu S, Jiang Z, Zhao J, Li Z, Li R, Qiu Y, Peng H. Disparity of smell tests in Alzheimer’s disease and other neurodegenerative disorders: a systematic review and meta-analysis. Front Aging Neurosci. 2023;15: 1249512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Menon C, Westervelt HJ, Jahn DR, Dressel JA, O’Bryant SE. Normative performance on the brief smell identification test (BSIT) in a multi-ethnic bilingual cohort: a Project FRONTIER study. Clin Neuropsychol. 2013;27(6):946–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Doty RL. Olfactory dysfunction and its measurement in the clinic. World J Otorhinolaryngol Head Neck Surg. 2015;1(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim SM, Kim HR, Min HJ, Kim KS, Jin JC, Han DH. A novel olfactory threshold test for screening cognitive decline among elderly people. PLoS ONE. 2021;16(7): e0254357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Finkel D, Pedersen NL, Larsson M. Olfactory functioning and cognitive abilities: a twin study. J Gerontol B Psychol Sci Soc Sci. 2001;56(4):P226–233. [DOI] [PubMed] [Google Scholar]
- 149.Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 2010;32(10):1062–7. [DOI] [PubMed] [Google Scholar]
- 150.Palmquist E, Larsson M, Olofsson JK, Seubert J, Bäckman L, Laukka EJ. A prospective study on risk factors for olfactory dysfunction in aging. J Gerontol Biol Sci Med Sci. 2020;75(3):603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Challakere Ramaswamy VM, Schofield PW. Olfaction and executive cognitive performance: a systematic review. Front Psychol. 2022;13: 871391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pais MV, Forlenza OV, Diniz BS. Plasma biomarkers of Alzheimer’s disease: a review of available assays, recent developments, and implications for clinical practice. J Alzheimers Dis Rep. 2023;7(1):355–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hampel H, Hu Y, Cummings J, Mattke S, Iwatsubo T, Nakamura A, Vellas B, O’Bryant S, Shaw LM, Cho M, et al. Blood-based biomarkers for Alzheimer’s disease: current state and future use in a transformed global healthcare landscape. Neuron. 2023;111(18):2781–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Palmqvist S, Tideman P, Mattsson-Carlgren N, Schindler SE, Smith R, Ossenkoppele R, Calling S, West T, Monane M, Verghese PB, et al. Blood biomarkers to detect Alzheimer Disease in primary care and secondary care. JAMA. 2024;332(15):1245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo Q, Ping L, Dammer EB, Duong DM, Yin L, Xu K, Shantaraman A, Fox EJ, Golde TE, Johnson ECB, et al. Heparin-enriched plasma proteome is significantly altered in Alzheimer’s disease. Mol Neurodegener. 2024;19(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Verbeek E, Drewes YM, Gussekloo J. Visual impairment as a predictor for deterioration in functioning: the Leiden 85-plus study. BMC Geriatr. 2022;22(1):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Al-Namaeh M. Common causes of visual impairment in the elderly. Med Hypothesis Discov Innov Ophthalmol. 2021;10(4):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Congdon N, O’Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–85. [DOI] [PubMed] [Google Scholar]
- 159.Bourne RRA, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(9):e888–97. [DOI] [PubMed] [Google Scholar]
- 160.Zheng C, Zeng R, Wu G, Hu Y, Yu H: Beyond Vision: A View from Eye to Alzheimer’s Disease and Dementia. J Prev Alzheimers Dis. 2024;11(2):469–83. [DOI] [PubMed]
- 161.Deardorff WJ, Liu PL, Sloane R, Van Houtven C, Pieper CF, Hastings SN, Cohen HJ, Whitson HE. Association of sensory and cognitive impairment with Healthcare utilization and cost in older adults. J Am Geriatr Soc. 2019;67(8):1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, Klein R, Tielsch JM, Vijan S, Saaddine J. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124(12):1754–60. [DOI] [PubMed] [Google Scholar]
- 163.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration–emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Davis BM, Crawley L, Pahlitzsch M, Javaid F, Cordeiro MF. Glaucoma: the retina and beyond. Acta Neuropathol. 2016;132(6):807–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marks R. Falls among the elderly and vision: A Narrative Review. Open Med J. 2014;1(1):54–65.
- 166.Whitson HE, Cronin-Golomb A, Cruickshanks KJ, Gilmore GC, Owsley C, Peelle JE, Recanzone G, Sharma A, Swenor B, Yaffe K, et al. American Geriatrics Society and National Institute on Aging Bench-to-Bedside Conference: sensory impairment and cognitive decline in older adults. J Am Geriatr Soc. 2018;66(11):2052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu SZ, Masurkar AV, Balcer LJ. Afferent and efferent visual markers of Alzheimer’s Disease: a review and update in early stage disease. Front Aging Neurosci. 2020;12: 572337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Prasad S, Galetta SL. Anatomy and physiology of the afferent visual system. Handb Clin Neurol. 2011;102:3–19. [DOI] [PubMed] [Google Scholar]
- 169.Vermersch A. Cortical control of saccades. Annals Neuroloa. 1995;37:557–67. [DOI] [PubMed] [Google Scholar]
- 170.Girard B, Berthoz A. From brainstem to cortex: computational models of saccade generation circuitry. Prog Neurobiol. 2005;77(4):215–51. [DOI] [PubMed] [Google Scholar]
- 171.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9(1):44–53. [DOI] [PubMed] [Google Scholar]
- 172.Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48(5):2285–9. [DOI] [PubMed] [Google Scholar]
- 173.Kesler A, Vakhapova V, Korczyn AD, Naftaliev E, Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg. 2011;113(7):523–6. [DOI] [PubMed] [Google Scholar]
- 174.Paquet C, Boissonnot M, Roger F, Dighiero P, Gil R, Hugon J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2007;420(2):97–9. [DOI] [PubMed] [Google Scholar]
- 175.Gao L, Liu Y, Li X, Bai Q, Liu P. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch Gerontol Geriatr. 2015;60(1):162–7. [DOI] [PubMed] [Google Scholar]
- 176.Chiu K, Chan TF, Wu A, Leung IY, So KF, Chang RC. Neurodegeneration of the retina in mouse models of Alzheimer’s disease: what can we learn from the retina? Age (Dordr). 2012;34(3):633–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guo L, Duggan J, Cordeiro MF. Alzheimer’s disease and retinal neurodegeneration. Curr Alzheimer Res. 2010;7(1):3–14. [DOI] [PubMed] [Google Scholar]
- 178.Snyder PJ, Alber J, Alt C, Bain LJ, Bouma BE, Bouwman FH, DeBuc DC, Campbell MCW, Carrillo MC, Chew EY, et al. Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimers Dement. 2021;17(1):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mirzaei N, Shi H, Oviatt M, Doustar J, Rentsendorj A, Fuchs D-T, Sheyn J, Black KL, Koronyo Y, Koronyo-Hamaoui M. Alzheimer’s retinopathy: seeing disease in the eyes. Front NeuroSci. 2020;14: 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315(8):485–7. [DOI] [PubMed] [Google Scholar]
- 181.Blanks JC, Hinton DR, Sadun AA, Miller CA. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res. 1989;501(2):364–72. [DOI] [PubMed] [Google Scholar]
- 182.Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol. 2001;112(10):1860–7. [DOI] [PubMed] [Google Scholar]
- 183.Iseri PK, Altinaş O, Tokay T, Yüksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26(1):18–24. [DOI] [PubMed] [Google Scholar]
- 184.Sadun AA, Bassi CJ. Optic nerve damage in Alzheimer’s disease. Ophthalmology. 1990;97(1):9–17. [DOI] [PubMed] [Google Scholar]
- 185.Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X, Mavros C, Coccia JA, Faget KY, Fitch KA, et al. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet. 2003;361(9365):1258–65. [DOI] [PubMed] [Google Scholar]
- 186.Ning A, Cui J, To E, Ashe KH, Matsubara J. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci. 2008;49(11):5136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu B, Rasool S, Yang Z, Glabe CG, Schreiber SS, Ge J, Tan Z. Amyloid-peptide vaccinations reduce {beta}-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer’s transgenic mice. Am J Pathol. 2009;175(5):2099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gasparini L, Crowther RA, Martin KR, Berg N, Coleman M, Goedert M, Spillantini MG. Tau inclusions in retinal ganglion cells of human P301S tau transgenic mice: effects on axonal viability. Neurobiol Aging. 2011;32(3):419–33. [DOI] [PubMed] [Google Scholar]
- 189.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, Schwartz M, Farkas DL. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. NeuroImage. 2011;54(Suppl 1):S204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schultz N, Byman E, Wennström M. Levels of retinal amyloid-β correlate with levels of retinal IAPP and hippocampal amyloid-β in neuropathologically evaluated individuals. J Alzheimers Dis. 2020;73(3):1201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee CS, Gibbons LE, Lee AY, Yanagihara RT, Blazes MS, Lee ML, McCurry SM, Bowen JD, McCormick WC, Crane PK, et al. Association between cataract extraction and development of Dementia. JAMA Intern Med. 2022;182(2):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Naël V, Pérès K, Dartigues JF, Letenneur L, Amieva H, Arleo A, Scherlen AC, Tzourio C, Berr C, Carrière I, et al. Vision loss and 12-year risk of dementia in older adults: the 3 C cohort study. Eur J Epidemiol. 2019;34(2):141–52. [DOI] [PubMed] [Google Scholar]
- 193.Hanson RLW, Gale RP, Gouws AD, Airody A, Scott MTW, Akthar F, Waterson S, Wells MT, Wright AJ, Bell K, et al. Following the status of visual cortex over time in patients with macular degeneration reveals atrophy of visually deprived brain regions. Invest Ophthalmol Vis Sci. 2019;60(15):5045–51. [DOI] [PubMed] [Google Scholar]
- 194.Ferguson EL, Thoma M, Buto PT, Wang J, Glymour MM, Hoffmann TJ, Choquet H, Andrews SJ, Yaffe K, Casaletto K, et al. Visual impairment, eye conditions, and diagnoses of neurodegeneration and dementia. JAMA Netw Open. 2024;7(7):e2424539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lin H, Zhang L, Lin D, Chen W, Zhu Y, Chen C, Chan KC, Liu Y, Chen W. Visual restoration after cataract surgery promotes functional and structural brain recovery. EBioMedicine. 2018;30:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pedersini CA, Miller NP, Gandhi TK, Gilad-Gutnick S, Mahajan V, Sinha P, Rokers B. White matter plasticity following cataract surgery in congenitally blind patients. Proc Natl Acad Sci U S A. 2023;120(19): e2207025120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sabel BA, Gao Y, Antal A. Reversibility of visual field defects through induction of brain plasticity: vision restoration, recovery and rehabilitation using alternating current stimulation. Neural Regen Res. 2020;15(10):1799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N. Cataract surgery and age-related cognitive decline: a 13-year follow-up of the English Longitudinal Study of Ageing. PLoS ONE. 2018;13(10): e0204833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Miyata K, Yoshikawa T, Morikawa M, Mine M, Okamoto N, Kurumatani N, Ogata N. Effect of cataract surgery on cognitive function in elderly: results of Fujiwara-Kyo Eye Study. PLoS ONE. 2018;13(2): e0192677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lad M, Sedley W, Griffiths TD. Sensory loss and risk of dementia. Neuroscientist. 2024;30(2):247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yoshida Y, Ono K, Sekimoto S, Umeya R, Hiratsuka Y. Impact of cataract surgery on cognitive impairment in older people. Acta Ophthalmol. 2024;102(4):e602–11. [DOI] [PubMed] [Google Scholar]
- 202.Hecht I, Kanclerz P, Tuuminen R. Secondary outcomes of lens and cataract surgery: more than just best-corrected visual acuity. Prog Retin Eye Res. 2023;95:101150. [DOI] [PubMed] [Google Scholar]
- 203.Hart NJ, Koronyo Y, Black KL, Koronyo-Hamaoui M. Ocular indicators of Alzheimer’s: exploring disease in the retina. Acta Neuropathol. 2016;132:767–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cabrera DeBuc D, Gaca-Wysocka M, Grzybowski A, Kanclerz P. Identification of retinal biomarkers in alzheimer’s disease using optical coherence tomography: recent insights, challenges, and opportunities. J Clin Med. 2019;8(7):996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2012;109(22):8740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cerquera-Jaramillo MA, Nava-Mesa MO, González-Reyes RE, Tellez-Conti C. de-la-Torre A: visual features in alzheimer’s disease: from basic mechanisms to clinical overview. Neural Plast. 2018;2018:2941783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sartucci F, Porciatti V. Psychophysiology and electrophysiology of the visual system. In: Psychophysiology methods. edn. Edited by Valeriani M, de Tommaso M. New York, NY: Springer US; 2024:115–156.
- 208.Molitor RJ, Ko PC, Ally BA. Eye movements in Alzheimer’s disease. J Alzheimers Dis. 2015;44(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wilcockson TDW, Mardanbegi D, Xia B, Taylor S, Sawyer P, Gellersen HW, Leroi I, Killick R, Crawford TJ. Abnormalities of saccadic eye movements in dementia due to Alzheimer’s disease and mild cognitive impairment. Aging. 2019;11(15):5389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wolf A, Tripanpitak K, Umeda S, Otake-Matsuura M. Eye-tracking paradigms for the assessment of mild cognitive impairment: a systematic review. Front Psychol. 2023;14: 1197567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.López-Cuenca I, Nebreda A, García-Colomo A, Salobrar-García E, de Frutos-Lucas J, Bruña R, Ramírez AI, Ramirez-Toraño F, Salazar JJ, Barabash A, et al. Early visual alterations in individuals at-risk of Alzheimer’s disease: a multidisciplinary approach. Alzheimers Res Ther. 2023;15(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sadun AA, Borchert M, DeVita E, Hinton DR, Bassi CJ. Assessment of visual impairment in patients with Alzheimer’s disease. Am J Ophthalmol. 1987;104(2):113–20. [DOI] [PubMed] [Google Scholar]
- 213.Bernardin F, Schwan R, Lalanne L, Ligier F, Angioi-Duprez K, Schwitzer T, Laprevote V. The role of the retina in visual hallucinations: a review of the literature and implications for psychosis. Neuropsychologia. 2017;99:128–38. [DOI] [PubMed] [Google Scholar]
- 214.Cronin-Golomb A. Vision in Alzheimer’s disease. Gerontologist. 1995;35(3):370–6. [DOI] [PubMed] [Google Scholar]
- 215.Leroi I, Voulgari A, Breitner JC, Lyketsos CG. The epidemiology of psychosis in dementia. Am J Geriatr Psychiatry. 2003;11(1):83–91. [PubMed] [Google Scholar]
- 216.Liu D, Zhang L, Li Z, Zhang X, Wu Y, Yang H, Min B, Zhang X, Ma D, Lu Y. Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol. 2015;15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Welge-Lüssen A, Dörig P, Wolfensberger M, Krone F, Hummel T. A study about the frequency of taste disorders. J Neurol. 2011;258(3):386–92. [DOI] [PubMed] [Google Scholar]
- 218.Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol. 2004;92(3):1892–903. [DOI] [PubMed] [Google Scholar]
- 219.Bartoshuk LM, Beauchamp GK. Chemical senses. Annu Rev Psychol. 1994;45:419–49. [DOI] [PubMed] [Google Scholar]
- 220.Ai Y, Han P. Neurocognitive mechanisms of odor-induced taste enhancement: a systematic review. Int J Gastronomy Food Sci. 2022;28:100535. [Google Scholar]
- 221.Rolls ET. Taste, olfactory, and food reward value processing in the brain. Prog Neurobiol. 2015;127:64–90. [DOI] [PubMed] [Google Scholar]
- 222.Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22(24):10819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Poellinger A, Thomas R, Lio P, Lee A, Makris N, Rosen BR, Kwong KK. Activation and habituation in olfaction—an fMRI study. NeuroImage. 2001;13(4):547–60. [DOI] [PubMed] [Google Scholar]
- 224.Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26(3):735–45. [DOI] [PubMed] [Google Scholar]
- 225.Cerf-Ducastel B, Van de Moortele PF, MacLeod P, Le Bihan D, Faurion A. Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic resonance imaging study. Chem Senses. 2001;26(4):371–83. [DOI] [PubMed] [Google Scholar]
- 226.Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci. 1997;94(8):4119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6(2):196–202. [DOI] [PubMed] [Google Scholar]
- 228.O’doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. NeuroReport. 2000;11(4):893–7. [DOI] [PubMed] [Google Scholar]
- 229.Francis S, Rolls ET, Bowtell R, McGlone F, O’Doherty J, Browning A, Clare S, Smith E. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. NeuroReport. 1999;10(3):453–9. [DOI] [PubMed] [Google Scholar]
- 230.Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39(4):701–11. [DOI] [PubMed] [Google Scholar]
- 231.Schmicker M, Frühling I, Menze I, Glanz W, Müller P, Noesselt T, Müller NG. The potential role of gustatory function as an early diagnostic marker for the risk of Alzheimer’s Disease in subjective cognitive decline. J Alzheimers Dis Rep. 2023;7(1):249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sakai M, Ikeda M, Kazui H, Shigenobu K, Nishikawa T. Decline of gustatory sensitivity with the progression of Alzheimer’s disease. Int Psychogeriatr. 2016;28(3):511–7. [DOI] [PubMed] [Google Scholar]
- 233.Kouzuki M, Ichikawa J, Shirasagi D, Katsube F, Kobashi Y, Matsumoto H, Chao H, Yoshida S, Urakami K. Detection and recognition thresholds for five basic tastes in patients with mild cognitive impairment and Alzheimer’s disease dementia. BMC Neurol. 2020;20(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sakai M, Kazui H, Shigenobu K, Komori K, Ikeda M, Nishikawa T. Gustatory dysfunction as an early Symptom of Semantic Dementia. Dement Geriatr Cogn Dis Extra. 2017;7(3):395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Martín ISM, Barato VP, Oliva SL, Rodríguez M, Yurrita LC, Cabañas MJC, Rojo SS, de la Calle L, Díaz E, Santos YQ, et al. Body composition, dietary, and gustatory function assessment in people with Alzheimer’s Disease. Am J Alzheimers Dis Other Demen. 2018;33(8):508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Contri-Degiovanni PV, Degiovanni GC, Ferriolli E, da Costa Lima NK, Moriguti JC. Impact of the severity of dementia due to Alzheimer’s disease on the gustatory sensitivity of older persons. Aging Clin Exp Res. 2020;32(11):2303–9. [DOI] [PubMed] [Google Scholar]
- 237.Naudin M, Mondon K, El-Hage W, Perriot E, Boudjarane M, Desmidt T, Lorette A, Belzung C, Hommet C, Atanasova B. Taste identification used as a potential discriminative test among depression and Alzheimer׳s disease in elderly: a pilot study. Psychiatry Res. 2015;228(2):228–32. [DOI] [PubMed] [Google Scholar]
- 238.Kouzuki M, Suzuki T, Nagano M, Nakamura S, Katsumata Y, Takamura A, Urakami K. Comparison of olfactory and gustatory disorders in Alzheimer’s disease. Neurol Sci. 2018;39(2):321–8. [DOI] [PubMed] [Google Scholar]
- 239.Van Hoesen GW, Parvizi J, Chu CC. Orbitofrontal cortex pathology in Alzheimer’s disease. Cereb Cortex. 2000;10(3):243–51. [DOI] [PubMed] [Google Scholar]
- 240.Naidich TP, Kang E, Fatterpekar GM, Delman BN, Gultekin SH, Wolfe D, Ortiz O, Yousry I, Weismann M, Yousry TA. The insula: anatomic study and MR imaging display at 1.5 T. AJNR Am J Neuroradiol. 2004;25(2):222–32. [PMC free article] [PubMed] [Google Scholar]
- 241.Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC. Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Res. 2011;194(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rolls ET. Limbic systems for emotion and for memory, but no single limbic system. Cortex. 2015;62:119–57. [DOI] [PubMed] [Google Scholar]
- 243.De Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, De Santi S, McRae T, Ferris SH, Reisberg B, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiol Aging. 1997;18(1):1–11. [DOI] [PubMed] [Google Scholar]
- 244.Chételat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60(8):1374–7. [DOI] [PubMed] [Google Scholar]
- 245.Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2003;54(3):343–51. [DOI] [PubMed] [Google Scholar]
- 246.Ayubcha C, Rigney G, Borja AJ, Werner T, Alavi A. Tau-PET imaging as a molecular modality for Alzheimer’s disease. Am J Nucl Med Mol Imaging. 2021;11(5):374–86. [PMC free article] [PubMed] [Google Scholar]
- 247.Heath TP, Melichar JK, Nutt DJ, Donaldson LF. Human taste thresholds are modulated by serotonin and noradrenaline. J Neurosci. 2006;26(49):12664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Smith SA, Trotter PD, McGlone FP, Walker SC. Effects of acute tryptophan depletion on human taste perception. Chem Senses. 2021;46:bjaa078. [DOI] [PubMed] [Google Scholar]
- 249.Garcia-Alloza M, Gil-Bea FJ, Diez-Ariza M, Chen CP, Francis PT, Lasheras B, Ramirez MJ. Cholinergic-serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia. 2005;43(3):442–9. [DOI] [PubMed] [Google Scholar]
- 250.Eremin DV, Kondaurova EM, Rodnyy AY, Molobekova CA, Kudlay DA, Naumenko VS. Serotonin receptors as a potential target in the treatment of Alzheimer’s Disease. Biochem (Mosc). 2023;88(12):2023–42. [DOI] [PubMed] [Google Scholar]
- 251.Sewards TV. Dual separate pathways for sensory and hedonic aspects of taste. Brain Res Bull. 2004;62(4):271–83. [DOI] [PubMed] [Google Scholar]
- 252.Ogawa T, Irikawa N, Yanagisawa D, Shiino A, Tooyama I, Shimizu T. Taste detection and recognition thresholds in Japanese patients with Alzheimer-type dementia. Auris Nasus Larynx. 2017;44(2):168–73. [DOI] [PubMed] [Google Scholar]
- 253.Churnin I, Qazi J, Fermin CR, Wilson JH, Payne SC, Mattos JL. Association between olfactory and gustatory dysfunction and cognition in older adults. Am J Rhinol Allergy. 2019;33(2):170–7. [DOI] [PubMed] [Google Scholar]
- 254.Correia C, Lopez KJ, Wroblewski KE, Huisingh-Scheetz M, Kern DW, Chen RC, Schumm LP, Dale W, McClintock MK, Pinto JM. Global sensory impairment in older adults in the United States. J Am Geriatr Soc. 2016;64(2):306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kuang L, Hu H, Dai H, Ma H, Jia Y, Sheng Y. Interventions to improve social network in older people with sensory impairment: a systematic review. Aging Clin Exp Res. 2024;36(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Elboim-Gabyzon M, Weiss PL, Danial-Saad A. Effect of age on the touchscreen manipulation ability of community-dwelling adults. Int J Environ Res Public Health. 2021;18(4):2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wickremaratchi MM, Llewelyn JG. Effects of ageing on touch. Postgrad Med J. 2006;82(967):301–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lipsitz LA, Manor B, Habtemariam D, Iloputaife I, Zhou J, Travison TG. The pace and prognosis of peripheral sensory loss in advanced age: association with gait speed and falls. BMC Geriatr. 2018;18(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Elli C, Novella A, Nobili A, Ianes A, Pasina L. Factors associated with a high-risk profile for developing pressure injuries in long-term residents of nursing homes. Med Princ Pract. 2022;31(5):433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055–68. [DOI] [PubMed] [Google Scholar]
- 261.Gebhart GF, Schmidt RF: Dorsal Horn. In: Encyclopedia of pain. edn. Edited by Gebhart GF, Schmidt RF. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013:1049–1050.
- 262.Andersen GJ. Aging and vision: changes in function and performance from optics to perception. Wiley Interdisciplinary Reviews: Cogn Sci. 2012;3(3):403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Decorps J, Saumet JL, Sommer P, Sigaudo-Roussel D, Fromy B. Effect of ageing on tactile transduction processes. Ageing Res Rev. 2014;13:90–9. [DOI] [PubMed] [Google Scholar]
- 264.Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87(2):193–207. [DOI] [PubMed] [Google Scholar]
- 265.Tseng MT, Chiang MC, Yazhuo K, Chao CC, Tseng WI, Hsieh ST. Effect of aging on the cerebral processing of thermal pain in the human brain. Pain. 2013;154(10):2120–9. [DOI] [PubMed] [Google Scholar]
- 266.Grunwald M, Busse F, Hensel A, Riedel-Heller S, Kruggel F, Arendt T, Wolf H, Gertz HJ. Theta-power differences in patients with mild cognitive impairment under rest condition and during haptic tasks. Alzheimer Dis Assoc Disord. 2002;16(1):40–8. [DOI] [PubMed] [Google Scholar]
- 267.Skedung L, El Rawadi C, Arvidsson M, Farcet C, Luengo GS, Breton L, Rutland MW. Mechanisms of tactile sensory deterioration amongst the elderly. Sci Rep. 2018;8(1):5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.García-Piqueras J, García-Mesa Y, Cárcaba L, Feito J, Torres-Parejo I, Martín-Biedma B, Cobo J, García-Suárez O, Vega JA. Ageing of the somatosensory system at the periphery: age-related changes in cutaneous mechanoreceptors. J Anat. 2019;234(6):839–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cavazzana A, Röhrborn A, Garthus-Niegel S, Larsson M, Hummel T, Croy I. Sensory-specific impairment among older people. An investigation using both sensory thresholds and subjective measures across the five senses. PLoS ONE. 2018;13(8): e0202969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehavioral Reviews. 2010;34(5):721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sala-Llonch R, Bartrés-Faz D, Junqué C. Reorganization of brain networks in aging: a review of functional connectivity studies. Front Psychol. 2015;6:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Heft M, Robinson M. Somatosensory function in old age. J Rehabil. 2017;44(4):327–32. [DOI] [PubMed] [Google Scholar]
- 273.Levin O, Fujiyama H, Boisgontier MP, Swinnen SP, Summers JJ. Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neurosci Biobehav Rev. 2014;43:100–17. [DOI] [PubMed] [Google Scholar]
- 274.Maes C, Gooijers J, Orban de Xivry JJ, Swinnen SP, Boisgontier MP. Two hands, one brain, and aging. Neurosci Biobehav Rev. 2017;75:234–56. [DOI] [PubMed] [Google Scholar]
- 275.Oishi Y, Imamura T, Shimomura T, Suzuki K. Visual texture agnosia in dementia with Lewy bodies and Alzheimer’s disease. Cortex. 2018;103:277–90. [DOI] [PubMed] [Google Scholar]
- 276.Yang J, Ogasa T, Ohta Y, Abe K, Wu J. Decline of human tactile angle discrimination in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2010;22(1):225–34. [DOI] [PubMed] [Google Scholar]
- 277.Kunz M, Lautenbacher S. [The impact of Alzheimer’s disease on the pain processing]. Fortschr Neurol Psychiatr. 2004;72(7):375–82. [DOI] [PubMed] [Google Scholar]
- 278.Müller G, Richter RA, Weisbrod S, Klingberg F. Impaired tactile pattern recognition in the early stage of primary degenerative dementia compared with normal aging. Arch Gerontol Geriatr. 1992;14(3):215–25. [DOI] [PubMed] [Google Scholar]
- 279.Zhang Z, Chen G, Zhang J, Yan T, Go R, Fukuyama H, Wu J, Han Y, Li C. Tactile angle discrimination decreases due to subjective cognitive decline in Alzheimer’s Disease. Curr Alzheimer Res. 2020;17(2):168–76. [DOI] [PubMed] [Google Scholar]
- 280.Hossain SR, Karem H, Jafari Z, Kolb BE, Mohajerani MH. Tactile stimulation improves cognition, motor, and anxiety-like behaviors and attenuates the Alzheimer’s disease pathology in adult APP(NL-G-F/NL-G-F) mice. Synapse. 2023;77(2):e22257. [DOI] [PubMed] [Google Scholar]
- 281.Suzuki M, Tatsumi A, Otsuka T, Kikuchi K, Mizuta A, Makino K, Kimoto A, Fujiwara K, Abe T, Nakagomi T, et al. Physical and psychological effects of 6-week tactile massage on elderly patients with severe dementia. Am J Alzheimers Dis Other Demen. 2010;25(8):680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Li Q, Zhao W, Kendrick KM. Affective touch in the context of development, oxytocin signaling, and autism. Front Psychol. 2022;13: 967791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Richards S, Mychasiuk R, Kolb B, Gibb R. Tactile stimulation during development alters behaviour and neuroanatomical organization of normal rats. Behav Brain Res. 2012;231(1):86–91. [DOI] [PubMed] [Google Scholar]
- 284.Cerritelli F, Chiacchiaretta P, Gambi F, Ferretti A. Effect of continuous touch on brain functional connectivity is modified by the Operator’s tactile attention. Front Hum Neurosci. 2017;11: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Hossain SR, Karem H, Jafari Z, Kolb BE, Mohajerani MH. Early tactile stimulation influences the development of Alzheimer’s disease in gestationally stressed APP (NL-G-F) adult offspring (NL-G-F/NL-G-F) mice. Exp Neurol. 2023;368:114498. [DOI] [PubMed] [Google Scholar]
- 286.Comeau WL, Hastings E, Kolb B. Pre- and postnatal FGF-2 both facilitate recovery and alter cortical morphology following early medial prefrontal cortical injury. Behav Brain Res. 2007;180(1):18–27. [DOI] [PubMed] [Google Scholar]
- 287.Gibb RL, Gonzalez CL, Wegenast W, Kolb BE. Tactile stimulation promotes motor recovery following cortical injury in adult rats. Behav Brain Res. 2010;214(1):102–7. [DOI] [PubMed] [Google Scholar]
- 288.Antoniazzi CT, Metz VG, Roversi K, Freitas DL, Vey LT, Dias VT, Segat HJ, Duarte MM, Burger ME. Tactile stimulation during different developmental periods modifies hippocampal BDNF and GR, affecting memory and behavior in adult rats. Hippocampus. 2017;27(2):210–20. [DOI] [PubMed] [Google Scholar]
- 289.Dudar J, Whishaw I, Szerb J. Release of acetylcholine from the hippocampus of freely moving rats during sensory stimulation and running. Neuropharmacology. 1979;18(8–9):673–8. [DOI] [PubMed] [Google Scholar]
- 290.Kolb B, Gibb R. Tactile stimulation after frontal or parietal cortical injury in infant rats facilitates functional recovery and produces synaptic changes in adjacent cortex. Behav Brain Res. 2010;214(1):115–20. [DOI] [PubMed] [Google Scholar]
- 291.Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry. 2011;20(4):265–76. [PMC free article] [PubMed] [Google Scholar]
- 292.Löffler A, Beier F, Bekrater-Bodmann R, Hausner L, Desch S, Silvoni S, Kleinböhl D, Löffler M, Nees F, Frölich L, et al. Reduced tactile sensitivity is associated with mild cognitive impairment. EBioMedicine. 2023;99:104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wiesman AI, Mundorf VM, Casagrande CC, Wolfson SL, Johnson CM, May PE, Murman DL, Wilson TW. Somatosensory dysfunction is masked by variable cognitive deficits across patients on the Alzheimer’s disease spectrum. EBioMedicine. 2021;73: 103638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bessi V, Giacomucci G. Hidden functional derangement of somatosensory cortices in Alzheimer’s Disease. EBioMedicine. 2021;74:103708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Xu J, Sun Y, Zhu X, Pan S, Tong Z, Jiang K. Tactile discrimination as a diagnostic indicator of cognitive decline in patients with mild cognitive impairment: a narrative review. Heliyon. 2024;10(10): e31256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Silverberg NB, Ryan LM, Carrillo MC, Sperling R, Petersen RC, Posner HB, Snyder PJ, Hilsabeck R, Gallagher M, Raber J, et al. Assessment of cognition in early dementia. Alzheimers Dement. 2011;7(3):e60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Muller K, Frohlich S, Germano AMC, Kondragunta J, Agoitia Hurtado M, Rudisch J, Schmidt D, Hirtz G, Stollmann P, Voelcker-Rehage C. Sensor-based systems for early detection of dementia (SENDA): a study protocol for a prospective cohort sequential study. BMC Neurol. 2020;20(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Yang J, Syafiq UM, Yu Y, Takahashi S, Zhang Z, Wu J. Development and evaluation of a tactile cognitive function test device for Alzheimer’s Disease Early Detection. Neurosci Biomedical Eng (Discontinued). 2015;3(2):58–65. [Google Scholar]
- 299.Sanganahalli BG, Herman P, Behar KL, Blumenfeld H, Rothman DL, Hyder F. Functional MRI and neural responses in a rat model of Alzheimer’s disease. NeuroImage. 2013;79:404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Saarinen A, Harjunen V, Jasinskaja-Lahti I, Jääskeläinen IP, Ravaja N. Social touch experience in different contexts: a review. Neurosci Biobehav Rev. 2021;131:360–72. [DOI] [PubMed] [Google Scholar]
- 301.Jacobs HI, Van Boxtel MP, Jolles J, Verhey FR, Uylings HB. Parietal cortex matters in Alzheimer’s disease: an overview of structural, functional and metabolic findings. Neurosci Biobehav Rev. 2012;36(1):297–309. [DOI] [PubMed] [Google Scholar]
- 302.Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lin YJ, Kao TW, Chen WL. Relationship between peripheral neuropathy and cognitive performance in the elderly population. Med (Baltim). 2021;100(20):e26071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Taouis M, Torres-Aleman I. Editorial: insulin and the brain. Front Endocrinol (Lausanne). 2019;10:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Jun JH, Kim JS, Palomera LF, Jo DG. Dysregulation of histone deacetylases in ocular diseases. Arch Pharm Res. 2024;47(1):20–39. [DOI] [PubMed] [Google Scholar]
- 306.Hwang PH, Longstreth WT Jr, Brenowitz WD, Thielke SM, Lopez OL, Francis CE, DeKosky ST, Fitzpatrick AL. Dual sensory impairment in older adults and risk of dementia from the GEM study. Alzheimers Dement (Amst). 2020;12(1):e12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Pabst A, Bär J, Röhr S, Löbner M, Kleineidam L, Heser K, Hajek A, van der Leeden C, Wiese B, Maier W, et al. Do self-reported hearing and visual impairments predict longitudinal dementia in older adults? J Am Geriatr Soc. 2021;69(6):1519–28. [DOI] [PubMed] [Google Scholar]
- 308.Lee ATC, Richards M, Chan WC, Chiu HFK, Lee RSY, Lam LCW. Higher dementia incidence in older adults with poor visual acuity. J Gerontol Biol Sci Med Sci. 2020;75(11):2162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Tran EM, Stefanick ML, Henderson VW, Rapp SR, Chen JC, Armstrong NM, Espeland MA, Gower EW, Shadyab AH, Li W, et al. Association of visual impairment with risk of incident dementia in a women’s Health Initiative Population. JAMA Ophthalmol. 2020;138(6):624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Zhu Z, Shi D, Liao H, Ha J, Shang X, Huang Y, Zhang X, Jiang Y, Li L, Yu H, et al. Visual impairment and risk of dementia: the UK Biobank Study. Am J Ophthalmol. 2022;235:7–14. [DOI] [PubMed] [Google Scholar]
- 311.Littlejohns TJ, Hayat S, Luben R, Brayne C, Conroy M, Foster PJ, Khawaja AP, Kuźma E. Visual impairment and risk of dementia in 2 Population-based prospective cohorts: UK Biobank and EPIC-Norfolk. J Gerontol Biol Sci Med Sci. 2022;77(4):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Brenowitz WD, Kaup AR, Lin FR, Yaffe K. Multiple sensory impairment is associated with increased risk of dementia among black and white older adults. J Gerontol Biol Sci Med Sci. 2019;74(6):890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Brenowitz WD, Kaup AR, Yaffe K. Incident dementia and faster rates of cognitive decline are associated with worse multisensory function summary scores. Alzheimers Dement. 2020;16(10):1384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Hwang PH, Longstreth WT Jr, Thielke SM, Francis CE, Carone M, Kuller LH, Fitzpatrick AL. Longitudinal changes in hearing and visual impairments and risk of dementia in older adults in the United States. JAMA Netw Open. 2022;5(5):e2210734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Dintica CS, Calderón-Larrañaga A, Vetrano DL, Xu W. Association between sensory impairment and dementia: the roles of social network and leisure activity. J Alzheimers Dis. 2023;94(2):585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Völter C, Thomas JP, Maetzler W, Guthoff R, Grunwald M, Hummel T. Sensory dysfunction in Old Age. Dtsch Arztebl Int. 2021;118(29–30):512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.