Abstract
Background
Numerous systematic reviews and meta-analyses have been published that evaluate the association between periodontal disease and systemic diseases, many of which address similar topics. Moreover, their quality requires assessment. Therefore, we performed a cross-sectional analysis to examine the evidence on the relationship between periodontal disease and systemic diseases.
Methods
The PubMed, Embase, Web of Science, and the Cochrane Library databases were systematically searched to identify relevant systematic reviews and meta-analyses. Only studies that considered periodontal disease as the exposure factor and various systemic diseases as the outcome were included. The basic characteristics and pertinent data from the selected studies were extracted. The modified version of A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR 2) was employed for quality assessment, while R software was used for statistical analysis.
Results
Among the 212 relevant systematic reviews and meta-analyses, 57 were finally included in our analysis. These studies involved 75 diseases and 81 disease-related outcomes, with cancer (19/81) being the most frequently addressed topic. Of the 81 outcomes, 67 demonstrated a significant association. Notably, the highest risk estimate was found for head and neck cancer [odds ratio (OR) = 3.17, 95% confidence interval (CI) 1.78 − 5.64], while the lowest was observed for premature rupture of the amniotic sac [relative risk (RR) = 1.10, 95% CI 1.08 − 1.12]. The methodological quality ratings indicated that approximately 71.93% of included studies were classified as “Critically low”, with another 17.54% rated as “Low”, and only about 10.53% categorized as “Moderate”.
Conclusions
Periodontal disease significantly elevates the risks associated with 15 cancer-related, 8 cardiovascular-related, 8 metabolic-related, and 5 neurological-related outcomes. However, the overall methodological quality of existing systematic reviews and meta-analyses is generally suboptimal and requires enhancement to generate higher-quality evidence in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40779-024-00583-y.
Keywords: Periodontal disease, Periodontitis, Cancer, Cardiovascular diseases, Metabolic disorders, Neurological conditions, Evidence analysis
Background
Periodontal disease is a prevalent oral disease characterized by gingival inflammation and the destruction of periodontal tissues. It encompasses a spectrum of disorders, ranging from gingivitis, which is confined to gum inflammation, to periodontitis, which involves deeper tissue destruction and can be classified into mild to moderate and moderate to advanced stages. These conditions may ultimately lead to both tooth loss and an increased burden of disease [1, 2]. An increasing number of studies have shown that periodontal disease is associated with multiple systemic diseases, including cardiovascular disease, diabetes mellitus, hypertension, respiratory disorders, preterm birth, and low birth weight [3–7].
Although periodontal disease primarily affects the oral cavity, substantial evidence suggests that it may impact systemic health through mechanisms involving innate and adaptive immune responses [8, 9]. In the early stages of gingivitis, oral microbial dysbiosis can promote persistent inflammation. Local inflammatory responses facilitate the entry of pathogens, such as Porphyromonas gingivalis, into the bloodstream, potentially affecting adjacent tissues. As periodontal disease progresses, the host’s immune response intensifies, influencing systemic immune pathways and potentially leading to autoimmune disorders and other systemic diseases. Currently, the mechanisms by which periodontal disease influences systemic conditions remain unclear. The two prevailing theories propose that periodontal disease induces a systemic inflammatory response or that periodontal pathogens or their metabolites spread through the bloodstream to various parts of the body directly [8, 9].
Numerous systematic reviews have explored the association between periodontal disease and various other diseases. Despite addressing similar topics, the conclusions remain controversial due to differences in study design, population characteristics, and diagnostic criteria for diseases [10, 11]. Additionally, the methodological quality of these systematic reviews is often unclear and requires thorough evaluation. To enhance the utilization of evidence, it is necessary to synthesize these findings. This study aims to analyze the existing systematic reviews and meta-analyses regarding the relationship between periodontal disease and systemic diseases, explore the impact of periodontal disease on systemic diseases, comprehensively assess their quality, and evaluate as well as summarize the strength of this correlation.
Methods
Inclusion and exclusion criteria
Inclusion criteria comprising: 1) the research subjects must be human participants; 2) the exposure factor is periodontal disease; 3) the outcome must be a specific systemic disease, such as cancer, coronary heart disease, diabetes mellitus, or stroke; 4) the study design of interest involves non-interventional systematic review or meta-analysis. Only studies published in Chinese or English with accessible full text were included.
Studies presented solely in abstract form, encompassing meeting reports or protocols were excluded. For studies addressing the same topic, both the number of original studies included and the publication date were comprehensively considered during screening. Generally, preference was given to the most recent meta-analysis that contained the largest number of original studies.
Search strategy
This study conducted a comprehensive evaluation of systematic reviews and meta-analyses regarding the association between periodontal disease and systemic diseases. The PubMed, Embase, Web of Science, and the Cochrane Library databases were independently searched until May 31, 2024. The search strategy incorporated Medical Subject Headings (MeSH) as well as free text terms (title/abstract) related to: 1) periodontal disease, such as “periodontal disease”, “periodontitis”, “gingivitis”, “periodontal attachment loss”, “alveolar bone loss”, “clinical attachment loss”; and 2) study design, including “systematic review” and “meta-analysis”. No language restrictions were initially imposed. A complementary screening of the references from the analyzed studies was also performed to include any additional relevant studies.
Data extraction and quality assessment
Three researchers collaboratively retrieved the literature based on predefined inclusion and exclusion criteria. The following information was extracted: first author, year of publication, journal name, study population, number of included studies for each systematic review, ascertainment of exposure and outcomes, whether a meta-analysis was conducted or not, and relevant data on outcomes. Disagreements were resolved through consultation with a third party or discussion among the researchers.
The methodological quality of included systematic reviews and meta-analyses was assessed using a modified version of A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR 2, Additional file 1: Table S1), which comprises 16 evaluation items (Q1 to Q16) that assess the risk of bias and heterogeneity in the included studies [12]. The overall quality of analyzed studies was recorded from high to low as “High”, “Moderate”, “Low”, and “Critically low”. Two researchers cooperatively conducted these quality assessments. Disagreements were addressed through discussion, and if consensus could not be reached, a third-party expert was consulted for a final decision.
Statistical analysis
Each study was reviewed in detail, focusing on the design, methods, and results to analyze the association between periodontal disease and various systemic diseases. A Microsoft Excel spreadsheet was utilized to compile the basic information of included studies. Based on this data, we performed a comprehensive analysis that included examining the publication year and source journal of the studies, counting the number of original studies included in the individual systematic review, describing the ascertainment of exposure and outcomes, as well as assessing the use of meta-analysis. Categorical items were presented as frequencies and percentages. A bar chart was generated to illustrate the results of methodological quality evaluation. All systemic diseases were classified according to the International Classification of Diseases 11th Revision (ICD-11). Tableau was employed for visualizing disease classification, while R software (version 4.3.2) along with the “forestploter” package (version 1.1.2) was used for evidence analysis and generating forest plots.
Results
Basic characteristics
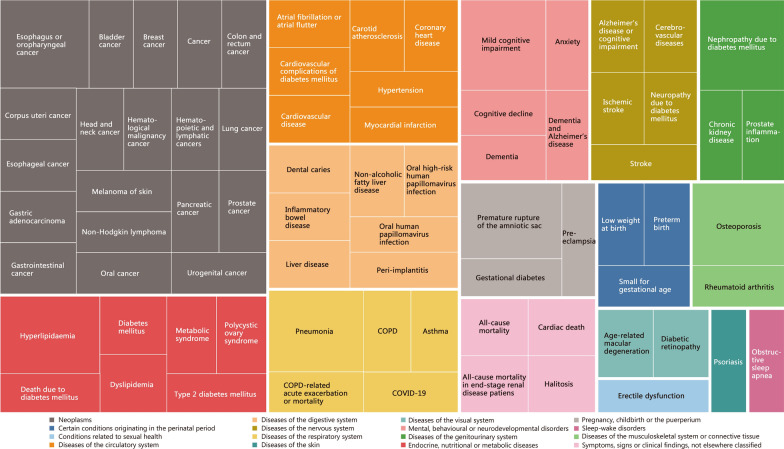
Based on the predefined inclusion and exclusion criteria, a total of 57 systematic reviews and meta-analyses examining the relationship between periodontal disease and systemic diseases were finally included [13–69] (Fig. 1). The basic characteristics of these studies were presented in Additional file 1: Table S2. Among the 57 systematic reviews, the majority (87.72%) were published after 2020, with 40 (70.18%) including more than 10 original studies, 51 (89.47%) reporting the ascertainment of exposure and outcomes, and 54 (94.74%) conducting a meta-analysis for data synthesis. These studies appeared in a total of 48 journals, primarily within the fields of oral medicine and general medicine (Table 1). A cumulative total of 75 diseases were reported and classified into 16 categories, with neoplasms being the most common category (n = 19), followed by circulatory system diseases (n = 7), digestive system diseases (n = 7), and endocrine, nutritional or metabolic diseases (n = 7) (Fig. 2).
Fig. 1.
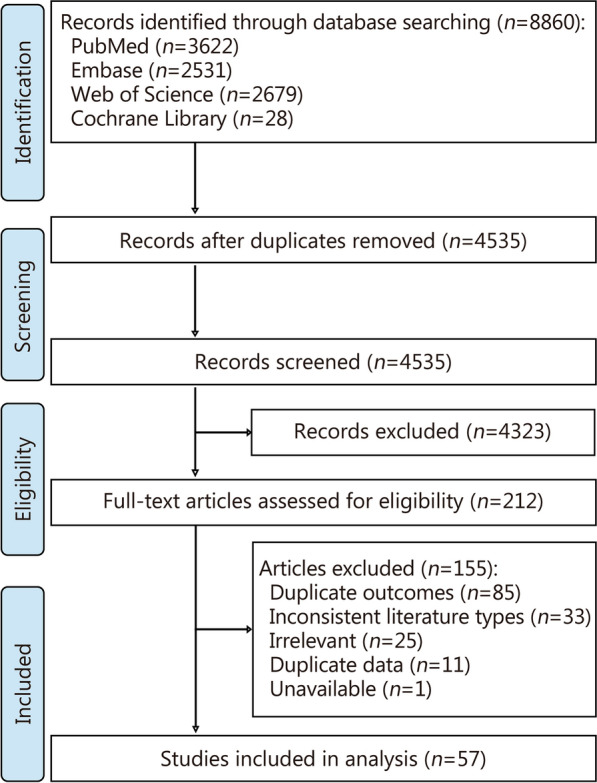
Flow diagram of study selection
Table 1.
Basic characteristics of included systematic reviews (n = 57)
| Item | n (%) |
|---|---|
| Publication year of included systematic reviews | |
| 2024 | 8 (14.04) |
| 2023 | 10 (17.54) |
| 2022 | 7 (12.28) |
| 2021 | 9 (15.79) |
| 2020 | 16 (28.07) |
| 2016 − 2019 | 7 (12.28) |
| Journals where included systematic reviews were published* | |
| Oral Dis | 3 (5.26) |
| Acta Odontol Scand | 2 (3.51) |
| BMC Oral Health | 2 (3.51) |
| Clin Oral Investig | 2 (3.51) |
| J Clin Med | 2 (3.51) |
| J Clin Periodontol | 2 (3.51) |
| Med Oral Patol Oral Cir Bucal | 2 (3.51) |
| PLoS One | 2 (3.51) |
| Number of included studies for individual systematic review | |
| > 50 | 6 (10.53) |
| 40 − 50 | 1 (1.75) |
| 30 − 39 | 5 (8.77) |
| 20 − 29 | 6 (10.53) |
| 10 − 19 | 22 (38.60) |
| ≤ 9 | 17 (29.82) |
| Ascertainment of exposure and outcome | |
| Yes | 51 (89.47) |
| No | 6 (10.53) |
| Use of meta-analysis for individual systematic review | |
| Yes | 54 (94.74) |
| No | 3 (5.26) |
*Only journals that published more than 2 systematic reviews were shown in the table. The full list of journals can be seen in Additional file 1: Table S2
Fig. 2.
Treemap of disease distribution. COPD chronic obstructive pulmonary disease, COVID-19 coronavirus disease 2019
Methodological quality
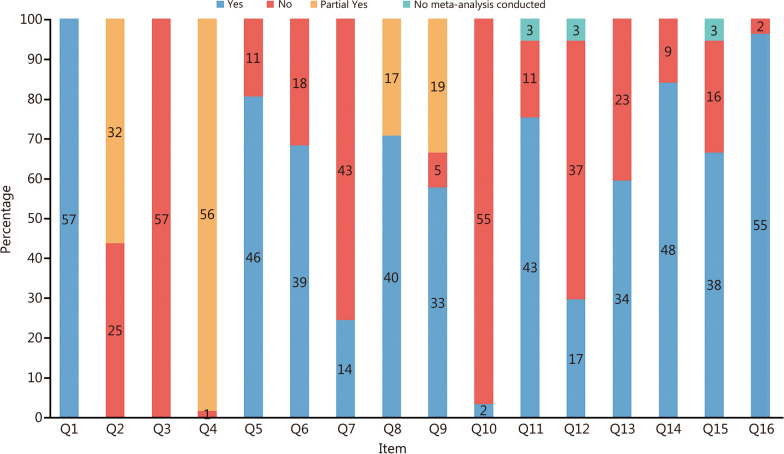
This study utilized a modified version of the AMSTAR 2 tool to evaluate the methodological quality of the included studies. The distribution of results across the 16 evaluation items is shown in Fig. 3. The findings revealed that all studies exhibited varying degrees of methodological flaws (Additional file 1: Table S3). Overall, the methodological quality was rated as “Critically low” (71.93%), “Low” (17.54%), and “Moderate” (10.53%). Notably, nearly all studies demonstrated deficiencies in critical areas, including “explain their selection of the study designs for inclusion” (Q3) and “report on the sources of funding for the studies included” (Q10). The majority only partially satisfied or failed to meet the criteria, particularly in key areas such as “provide a list of excluded studies and justify the exclusions” (Q7) (only 24.56% provided a list of excluded studies with justifications) and “assess the potential impact of risk of bias in individual studies on the results of the meta-analysis or other evidence synthesis” (Q12) (only 31.48% of the 54 studies that underwent meta-analysis assessed potential bias impacts). These findings suggest that the methodological quality of current systematic reviews on periodontal disease and various systemic diseases is generally inadequate. Therefore, future research should address these deficiencies to enhance evidence reliability.
Fig. 3.
Distribution of the results across the 16 evaluation items. Q1 − Q16 16 evaluation items of the modified version of A Measurement Tool to Assess Systematic Reviews 2
Evidence analysis
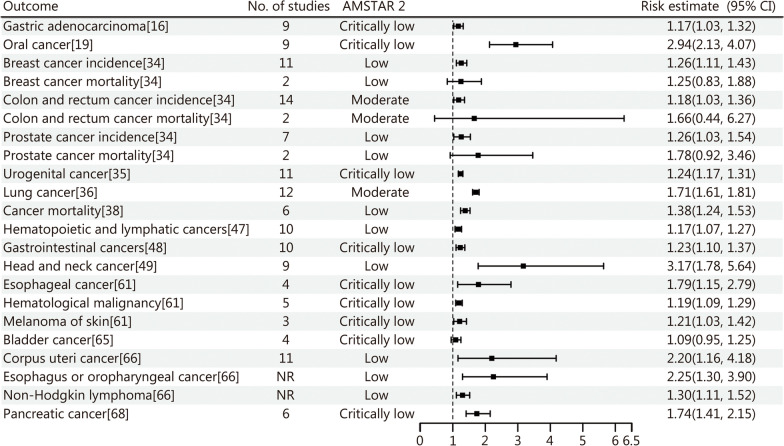
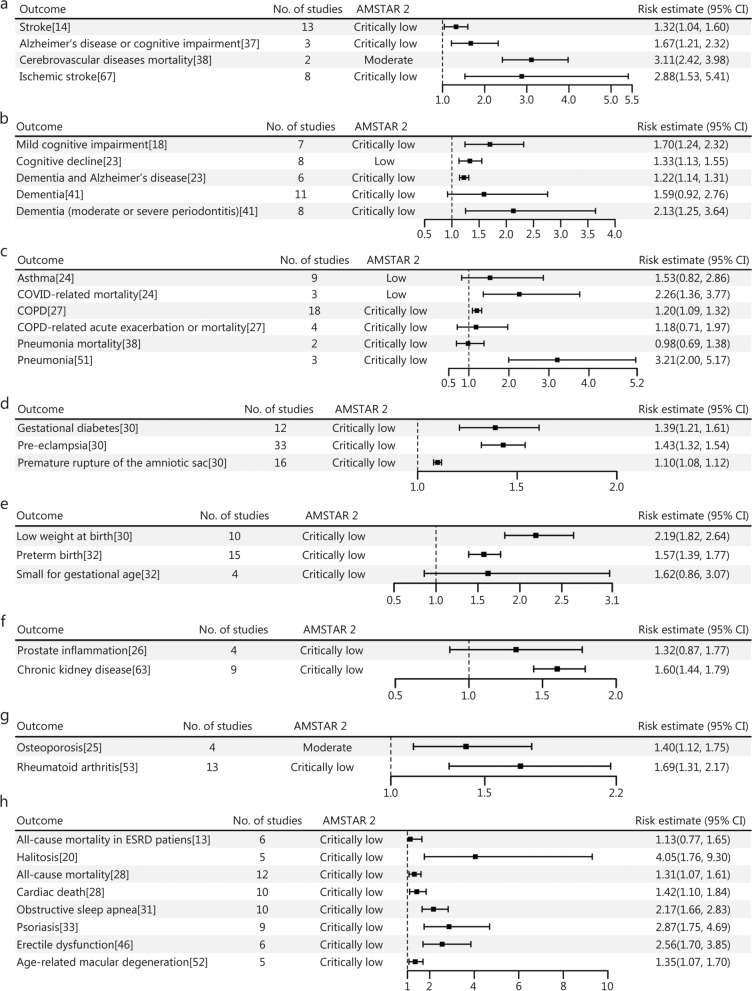
The findings of this study regarding the association between periodontal disease and systemic diseases indicate that periodontal disease significantly elevates the risk of various conditions (Additional file 1: Table S2). Specifically, periodontal disease is notably linked to an increased risk of several cancers, including head and neck cancer [odds ratio (OR) = 3.17, 95% CI 1.78 − 5.64], oral cancer (OR = 2.94, 95% CI 2.13 − 4.07), and esophagus or oropharyngeal cancer [hazard ratio (HR) = 2.25, 95% CI 1.30 − 3.90]. Although the association between periodontal disease and mortality from certain cancers (breast, prostate, colon and rectum) was not statistically significant due to high levels of heterogeneity, the overall results still suggest a link between periodontal disease and cancer risk (Fig. 4).
Fig. 4.
Forest plot of the association between periodontal disease and cancer. No. number, NR no report, AMSTAR 2 A Measurement Tool to Assess Systematic Reviews 2, CI confidence interval
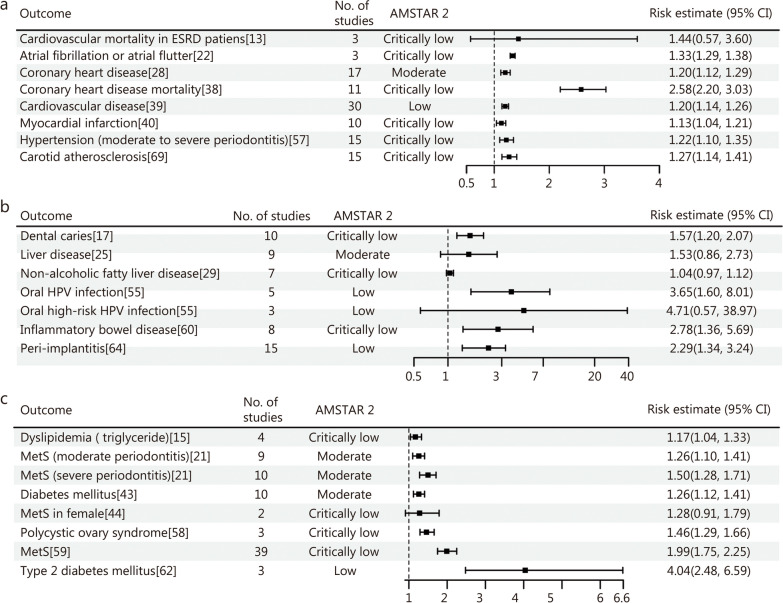
Periodontal disease may increase the risk of cardiovascular disease, including coronary heart disease [relative risk (RR) = 1.20, 95% CI 1.12 − 1.29], myocardial infarction (RR = 1.13, 95% CI 1.04 − 1.21), atrial fibrillation or atrial flutter (RR = 1.33, 95% CI 1.29 − 1.38), carotid atherosclerosis (OR = 1.27, 95% CI 1.14 − 1.41), and hypertension (OR = 1.22, 95% CI 1.10 − 1.35). However, limited evidence indicates a nonsignificant association between periodontal disease and cardiovascular mortality in patients with end-stage renal disease (ESRD; HR = 1.44, 95% CI 0.57 − 3.60) (Fig. 5a). Regarding digestive system diseases, periodontal disease significantly elevates the risk of inflammatory bowel disease (RR = 2.78, 95% CI 1.36 − 5.69), dental caries (OR = 1.57, 95% CI 1.20 − 2.07) and peri-implantitis (OR = 2.29, 95% CI 1.34 − 3.24). However, no relationship was observed between periodontal disease and liver disease. Current evidence demonstrates that periodontal disease increases the risk of oral human papillomavirus (HPV) infection by 3.65 times, but the link between periodontal disease and high-risk oral HPV infections remains inconclusive (Fig. 5b). In terms of metabolic diseases, periodontal disease notably raises the risk of metabolic syndrome, particularly in patients with moderate to severe periodontitis. Nevertheless, in certain female populations, the association between periodontal disease and metabolic syndrome did not achieve statistical significance (Fig. 5c).
Fig. 5.
Forest plot of the association between periodontal disease and circulatory system diseases (a), digestive system diseases (b), and metabolic diseases (c). ESRD end-stage renal disease, HPV human papillomavirus, MetS metabolic syndrome, No. number, AMSTAR 2 A Measurement Tool to Assess Systematic Reviews 2, CI confidence interval
The study also revealed that periodontal disease significantly elevates the risk of mortality from cerebrovascular diseases by 3.11 times, ischemic stroke by 2.88 times, Alzheimer’s disease and cognitive impairment by 1.67 times, and stroke by 1.32 times, highlighting its role as a significant risk factor for neurological disorders (Fig. 6a). No statistical significance was observed in the effect of periodontitis on dementia (OR = 1.59, 95% CI 0.92 − 2.76). However, the analysis indicated that periodontal disease was associated with an increased risk of dementia in patients with moderate or severe periodontitis (OR = 2.13, 95% CI 1.25 − 3.64). Periodontal disease also raises the risk of cognitive disorders (Fig. 6b). Additionally, periodontal disease was linked to a heightened risk of some respiratory diseases, such as pneumonia (OR = 3.21, 95% CI 2.00 − 5.17) and chronic obstructive pulmonary disease (COPD; OR = 1.20, 95% CI 1.09 − 1.32). However, the association did not reach statistical significance for pneumonia mortality, and acute exacerbation or mortality related to COPD (Fig. 6c). The study also found that periodontal disease increases the risk of pre-eclampsia by 1.43 times, premature rupture of the amniotic sac by 1.10 times, and gestational diabetes by 1.39 times (Fig. 6d). And the findings indicate that periodontal disease may contribute to a higher risk of preterm birth (OR = 1.57, 95% CI 1.39 − 1.77) and low weight at birth (RR = 2.19, 95% CI 1.82 − 2.64) among pregnant women (Fig. 6e). Moreover, periodontal disease was significantly correlated with an elevated risk of chronic kidney disease, exhibiting a HR of 1.60 (95% CI 1.44–1.79). In contrast, no significant increase in the risk of prostate inflammation was detected (HR = 1.32, 95% CI 0.87 − 1.77) (Fig. 6f). Furthermore, periodontal disease markedly heightened the risk of osteoporosis by 1.40 times, and rheumatoid arthritis by 1.69 times (Fig. 6g). It also demonstrated a significant association with age-related macular degeneration, increasing the risk by 1.35 times. Nevertheless, while several primary studies unveil an association between periodontal disease and the severity of diabetic retinopathy, the overall quality of evidence remains low, leaving this relationship still unclear. The study further revealed that periodontal disease substantially increased the risk of erectile dysfunction by 2.56 times and psoriasis by 2.87 times, as well as showing a notable correlation with obstructive sleep apnea, the risk increased by 2.17 times; however, this association was predominantly observed in cases involving mild to moderate periodontitis, without any significant link found in severe periodontitis cases. Besides, periodontal disease significantly elevated the risk of halitosis (OR = 4.05, 95% CI 1.76 − 9.30), exhibiting a robust association in both organoleptic testing and volatile sulfur compound reading. In addition, periodontal disease was notably linked to an increased risk of cardiac death (RR = 1.42, 95% CI 1.10 − 1.84) and all-cause mortality (RR = 1.31, 95% CI 1.07 − 1.61) (Fig. 6h).
Fig. 6.
Forest plot of the association between periodontal disease and multiple diseases. a nervous system diseases. b mental, behavioral or neurodevelopmental disorders. c respiratory system diseases. d pregnancy, childbirth, or the puerperium diseases. e perinatal period diseases. f genitourinary system diseases. g musculoskeletal system diseases. h other diseases. COVID coronavirus disease, COPD chronic obstructive pulmonary disease, ESRD end-stage renal disease, No. number, AMSTAR 2 A Measurement Tool to Assess Systematic Reviews 2, CI confidence interval
Discussion
This study encompassed 57 systematic reviews and meta-analyses aimed at investigating the relationship between periodontal disease and multiple systemic diseases. The results revealed a significant association between periodontal disease and systemic conditions, with an increased risk of head and neck cancer as well as circulatory system disorders such as coronary heart disease. Notably, approximately 71.93% of the studies were rated as having “Critically low” methodological quality. In conclusion, this study provides compelling evidence supporting the association between periodontal disease and multiple systemic diseases, underscoring the critical importance of effective management of periodontal health.
Cancer
In recent years, the potential association between periodontal disease and cancer has gained significant attention [70]. Periodontal disease is a complex condition characterized by dynamic interactions among various pathogenic factors and host immune responses. The host immune response is modulated by an interplay of genetic and epigenetic influences, lifestyle choices, comorbidities, and dental health factors. Throughout the progression of different stages of periodontal disease, key mediators such as inflammatory cytokines and immune response play critical roles [71]. The chronic inflammatory state induced by periodontal disease, as an immune-related response, is considered a pivotal factor in this association. Prolonged inflammation may lead to systemic inflammation, continuous activation of the immune system, increased risk of DNA damage, and promotion of cancer development [72]. Studies have shown that individuals with periodontal disease exhibit a significantly elevated risk for cancers affecting the oral cavity, pancreas, esophagus, and colorectum [73–77]. This phenomenon may be attributed to the dissemination of periodontal pathogens and their metabolites to distant tissues via the bloodstream, resulting in localized inflammation [78, 79]. Among the 13 systematic reviews and meta-analyses incorporated in this study, the association between periodontal disease and 19 distinct types of cancer, including gastric adenocarcinoma, oral cancer, colorectal cancer, urogenital cancer, lung cancer, and pancreatic cancer, has been validated to varying extents. Although the study conducted by Xie et al. [65] did not find a significant association between periodontal disease and bladder cancer, the overall findings of the meta-analysis still support a positive correlation between periodontal disease and the risk of most cancer types, particularly head and neck cancer. It is closely linked to chronic inflammation and pathogen infection resulting from periodontal disease. Common pathogens such as Porphyromonas gingivalis may promote the development of oral cancers through multiple mechanisms [9], including dysregulation of the inflammatory microenvironment, inhibition of apoptosis, increased cellular proliferation, enhanced angiogenesis, promotion of epithelial-mesenchymal transition, and production of carcinogenic metabolites [80]. Additionally, the risk of pancreatic cancer is significantly increased in patients with periodontal disease, potentially related to systemic inflammation and immune system dysregulation [61, 68]. Periodontal pathogens and their toxins may be transmitted to the pancreas via the bloodstream, thereby altering the local tissue microenvironment and facilitating carcinogenesis. Wang et al. [34] indicated that while there is a moderate association between periodontal disease and the incidence of breast cancer, prostate cancer, and colorectal cancer, the relationship between the mortality rates of these cancers and periodontal disease remains nonsignificant. This discrepancy may stem from heterogeneity and statistical uncertainty present in the studies.
Circulatory system diseases
The association between periodontal disease and circulatory system disorders has been widely investigated. The 9 included systematic reviews primarily focused on cardiovascular disease, including coronary heart disease and myocardial infarction, among other conditions. Most findings suggest a significant correlation between periodontal disease and these conditions, which is largely attributed to the systemic inflammatory response and vascular endothelial dysfunction induced by periodontal disease [81, 82]. As highlighted in the studies, the severity of periodontitis is associated with carotid intima-media thickness in young adults, and severe periodontitis and elevated leukocyte counts are independent risk factors for increased thickness, potentially linked to vascular endothelial injury [83, 84]. Additionally, periodontal pathogens and their metabolites may disseminate through the bloodstream, invading vascular endothelial cells, triggering localized vascular inflammatory responses, and promoting the development of arteriosclerosis and cardiovascular disease [85]. Furthermore, microorganisms can proliferate on atherosclerotic coronary plaques and worsen cardiovascular disease [86, 87]. However, the study by Chen et al. [13] involving patients with ESRD found no significant association between periodontitis and the risk of cardiovascular death in this population, suggesting that other factors may play a more critical role in determining cardiovascular death in this cohort.
Digestive system diseases
The 6 studies included in this analysis explored the association between periodontal disease and various digestive system disorders, including liver disease, oral HPV infection, and inflammatory bowel disease. The findings indicated a correlation between periodontal disease and these digestive conditions, suggesting that periodontal disease may influence digestive system health through multiple mechanisms [88]. For instance, Larvin et al. [25] noted that while evidence is limited, periodontitis may increase the risk of liver disease, potentially due to the systemic inflammation induced by periodontal disease. Chronic inflammation can lead to abnormal immune responses in the liver, thereby promoting the development of liver disease [89]. Conversely, the study by Ali et al. [55] identified a significant association between periodontal disease and oral HPV infection; however, the relationship with high-risk oral HPV infection remains inconclusive. This may indicate that periodontal disease plays a complex and multifaceted role in oral and digestive tract infections [90, 91]. Given the intricacy of potential mechanisms linking periodontal disease to digestive system diseases [11], future research should employ more detailed molecular biology techniques and clinical studies to elucidate how periodontal disease affects digestive health through pathways such as inflammation and immune response, particularly focusing on its specific effects on various liver and gastrointestinal diseases.
Endocrine, nutritional, or metabolic diseases
The association between periodontal disease and endocrine and metabolic disorders has garnered increasing attention. In this study, 7 systematic reviews and meta-analyses examined the relationship between periodontal disease and various metabolic conditions, such as diabetes, metabolic syndrome, and hyperlipidemia. Most findings indicate a significant correlation between periodontal disease and these metabolic disorders, particularly with diabetes and metabolic syndrome [92, 93]. Gobin et al. [59] provided reliable evidence for the association between periodontitis and metabolic syndrome, while Rosário-Dos-Santos et al. [21] further demonstrated a dose-response relationship showing that as the severity of the periodontal disease increases, so does the risk of developing metabolic syndrome. Periodontal disease may exacerbate metabolic syndrome by inducing systemic inflammation and insulin resistance [94, 95]. However, the study by Sayeed et al. [44] involving female populations found no significant association between periodontal disease and metabolic syndrome. Some studies have also suggested that gender may influence the prevalence and risk estimates of periodontal disease about other diseases, indicating that gender could play a moderating role in this relationship [23]. Overall, while numerous studies support the association between periodontal disease and endocrine and metabolic disorders, there exists some heterogeneity in findings across different investigations, potentially attributable to factors such as study population characteristics, research design methodologies, and criteria used for defining periodontal disease.
Others
In addition to its association with cancer, circulatory, digestive, and metabolic diseases, periodontal disease is potentially linked to a variety of other systemic conditions. 5 studies have reported an association between periodontal disease and neurological disorders, including stroke, Alzheimer’s disease, and related outcomes [14, 37, 38, 50, 67]. In the context of mental health as well as behavioral and neurodevelopmental disorders, periodontal disease has been linked to mild cognitive impairment, cognitive decline, dementia, and anxiety [18, 23, 41, 45]. Although some studies have not identified a significant association between periodontal disease and dementia, the increased risk of dementia associated with moderate to severe periodontal disease suggests that periodontal disease may impact brain function through chronic inflammation and immune responses [23, 41]. Meta-analyses suggest that periodontal disease may increase the risk of COPD, pneumonia, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [coronavirus disease 2019 (COVID-19)]. For instance, one study found that periodontal disease is associated with an elevated risk of pneumonia, while another indicated that it may heighten the risk of mortality related to COVID-19 [24, 51]. These findings imply that periodontal disease could exacerbate respiratory diseases by altering the respiratory microbiome and local immune response. Additionally, periodontal disease is linked to pregnancy-related complications, such as pre-eclampsia, premature rupture of the amniotic sac, and gestational diabetes, potentially due to the systemic inflammatory response and release of bioactive mediators triggered by periodontal inflammation [30]. Regarding urogenital diseases, periodontal disease has been related to chronic kidney disease and diabetic nephropathy; however, its relationship with prostate inflammation remains unclear [26, 50, 63]. There is also evidence connecting periodontal disease with osteoporosis, rheumatoid arthritis, and visual system disorders such as age-related macular degeneration [25, 52, 53]. Associations between periodontal disease and conditions related to sexual health such as erectile dysfunction, and skin diseases like psoriasis, have also been reported [33, 46]. Studies suggest that periodontal disease may increase the risk of these conditions by affecting systemic vascular function and immune responses [96, 97]. While these associations are supported by epidemiological studies, establishing causality necessitates further investigation through mechanistic research and clinical trials.
Limitation and perspective
Despite analyzing a substantial number of systematic reviews and meta-analyses to explore the association between periodontal disease and systemic diseases, this study has several limitations. First, most primary studies included in systematic reviews and meta-analyses were cross-sectional or observational, making it challenging to establish causality. Clinical data were also not directly analyzed in this study. Second, this study did not specifically classify periodontal disease nor investigate the effects of various evolutionary stages such as gingivitis, mild to moderate periodontitis, and moderate to advanced periodontitis on systemic diseases. Third, most studies have inadequately controlled for confounding factors such as smoking habits, dietary patterns, and socioeconomic status, potentially compromising the accuracy of their findings. Additionally, heterogeneity among study populations and inconsistent diagnostic criteria for periodontal disease complicate the interpretation of results. Future research should prioritize: 1) investigating the causal relationship between periodontal disease and systemic diseases through well-designed longitudinal studies and randomized controlled trials; 2) evaluating the effects of different periodontal therapies on both prevention and treatment outcomes of systemic conditions; 3) exploring molecular and genetic mechanisms underlying the association between periodontal disease and systemic disorders to identify new diagnostic and therapeutic targets; and 4) enhancing public health efforts to prevent and control periodontal disease, particularly through early intervention in high-risk populations, thereby mitigating its potential adverse effects on overall health.
Conclusions
Periodontal disease has been linked to a range of systemic conditions, including cancer, cardiovascular diseases, digestive disorders, endocrine and metabolic diseases, as well as neurological disorders. This condition not only shows a significant correlation with the onset of these diseases but may also influence their prognosis. However, the methodological quality of existing systematic reviews and meta-analyses is generally suboptimal, highlighting the need for improvement to generate high-quality evidence. Furthermore, establishing causality requires additional mechanistic research and high-quality randomized controlled trials.
Supplementary Information
Additional file 1: Table S1 Modified version of A Measurement Tool to Assess Systematic Reviews 2. Table S2 Summary of basic information of included studies. Table S3 Detailed evaluation of the methodological quality with modified version of A Measurement Tool to Assess Systematic Reviews 2
Acknowledgements
Not applicable.
Abbreviations
- AMSTAR 2
A Measurement Tool to Assess Systematic Reviews 2
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- COVID-19
Coronavirus disease 2019
- CVD
Cardiovascular disease
- ESRD
End-stage renal disease
- HPV
Human papillomavirus
- HR
Hazard ratio
- MeSH
Medical Subject Headings
- MetS
Metabolic syndrome
- OR
Odds ratio
- OSA
Obstructive sleep apnea
- RR
Relative risk
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- T2DM
Type 2 diabetes mellitus
Authors’ contributions
BM and XZ conceived and designed the study. DH, YYW, and BHL performed the literature search. DH and BHL collected the data. YYW and LW evaluated the quality of the included studies. DH, YYW, and BHL analyzed the data and drafted the figures. DH and BHL drafted the manuscript. BM, XZ, and WZX revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Lanzhou Science and Technology Project (2022-3-8), the Natural Science Foundation of Chongqing (CSTB2024NSCQ-MSX0043 and cstc2020jcyj-msxmX0079), and the Key Scientific Research Project of Education Department of Henan Province (22A320038).
Availability of data and materials
The datasets generated during the current study will be available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Xia Zhou, Email: zhoux20020549@163.com.
Bin Ma, Email: mab@lzu.edu.cn.
References
- 1.Salvi GE, Roccuzzo A, Imber JC, Stähli A, Klinge B, Lang NP. Clinical periodontal diagnosis. Periodontol 2000 2023. 10.1111/prd.12487. [DOI] [PubMed] [Google Scholar]
- 2.Luo LS, Luan HH, Wu L, Shi YJ, Wang YB, Huang Q, et al. Secular trends in severe periodontitis incidence, prevalence and disability-adjusted life years in five Asian countries: a comparative study from 1990 to 2017. J Clin Periodontol. 2021;48(5):627–37. [DOI] [PubMed] [Google Scholar]
- 3.Herrera D, Sanz M, Shapira L, Brotons C, Chapple I, Frese T, et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: consensus report of the joint workshop by the European Federation of Periodontology (EFP) and the European arm of the World Organization of Family doctors (WONCA Europe). J Clin Periodontol. 2023;50(6):819–41. [DOI] [PubMed] [Google Scholar]
- 4.Leng WD, Zeng XT, Kwong JSW, Hua XP. Periodontal disease and risk of coronary heart disease: an updated meta-analysis of prospective cohort studies. Int J Cardiol. 2015;201:469–72. [DOI] [PubMed] [Google Scholar]
- 5.Zhao MJ, Qiao YX, Wu L, Huang Q, Li BH, Zeng XT. Periodontal disease is associated with increased risk of hypertension: a cross-sectional study. Front Physiol. 2019;10:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Zhu B, An Y, Zhou Z, Xiong P, Li X, et al. Gingipain from Porphyromonas gingivalis causes insulin resistance by degrading insulin receptors through direct proteolytic effects. Int J Oral Sci. 2024;16(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant AT, Gupta RD, Akonde M, Reynolds M, Smith-Warner S, Liu J, et al. Association of chlorhexidine use and scaling and root planing with birth outcomes in pregnant individuals with periodontitis: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(12):e2247632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almarhoumi R, Alvarez C, Harris T, Tognoni CM, Paster BJ, Carreras I, et al. Microglial cell response to experimental periodontal disease. J Neuroinflammation. 2023;20(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang SY, Cai Y, Hu X, Li F, Qian XH, Xia LY, et al. P. gingivalis in oral-prostate axis exacerbates benign prostatic hyperplasia via IL-6/IL-6R pathway. Mil Med Res. 2024;11(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperr M, Kundi M, Tursic V, Bristela M, Moritz A, Andrukhov O, et al. Prevalence of comorbidities in periodontitis patients compared with the general Austrian population. J Periodontol. 2018;89(1):19–27. [DOI] [PubMed] [Google Scholar]
- 11.Williams KM, Challa PK, Lopes EW, Burke KE, Ananthakrishnan AN, Richter JM, et al. Periodontal disease is not associated with risk of inflammatory bowel disease: results from two prospective cohort studies in the US. Aliment Pharmacol Ther. 2023;58(10):1052–61. [DOI] [PubMed] [Google Scholar]
- 12.Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better?. Mil Med Res. 2020;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Li J. Association between periodontitis and its treatment on mortality rates of end-stage renal disease: a systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2024;29(3):e334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewan M, Pandit AK, Goyal L. Association of periodontitis and gingivitis with stroke: a systematic review and meta-analysis. Dent Med Probl. 2024;61(3):407–15. [DOI] [PubMed] [Google Scholar]
- 15.Ma W, Zou Z, Yang L, Lin D, Guo J, Shan Z, et al. Exploring the bi-directional relationship between periodontitis and dyslipidemia: a comprehensive systematic review and meta-analysis. BMC Oral Health. 2024;24(1):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguiar FJN, Menezes FDS, Fagundes MA, Fernandes GA, Alves FA, Filho JG, et al. Gastric adenocarcinoma and periodontal disease: a systematic review and meta-analysis. Clin (Sao Paulo). 2024;79:100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Xiang Y, Ren H, Zhang C, Hu Z, Leng W, et al. Association between periodontitis and dental caries: a systematic review and meta-analysis. Clin Oral Investig. 2024;28(6):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J, Pathak JL, Shen Y, Mashrah MA, Zhong X, Chen J, et al. Association between periodontitis and mild cognitive impairment: a systematic review and meta-analysis. Dement Geriatr Cogn Disord. 2024;53(1):37–46. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y, Tuerxun N, Maimaitili G. Periodontitis and the risk of oral cancer: a meta-analysis of case-control studies. Acta Odontol Scand. 2024;83:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang N, Li C, Zhang J, Wang L, Yang J. The association between halitosis and periodontitis: a systematic review and meta-analysis. Clin Oral Investig. 2024;28(6):341. [DOI] [PubMed] [Google Scholar]
- 21.Rosário-Dos-Santos HL, Miranda SS, Gomes-Filho IS, Cruz SSD, Figueiredo A, Souza ES, et al. Periodontitis severity relationship with metabolic syndrome: a systematic review with meta-analysis. Oral Dis. 2023;29(7):2512–20. [DOI] [PubMed] [Google Scholar]
- 22.Leelaviwat N, Kewcharoen J, Poomprakobsri K, Trongtorsak A, Del Rio-Pertuz G, Abdelnabi M, et al. Periodontal disease and risk of atrial fibrillation or atrial flutter: a systematic review and meta-analysis. J Arrhythm. 2023;39(6):992–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larvin H, Gao C, Kang J, Aggarwal VR, Pavitt S, Wu J. The impact of study factors in the association of periodontal disease and cognitive disorders: systematic review and meta-analysis. Age Ageing. 2023;52(2):afad015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina A, Huck O, Herrera D, Montero E. The association between respiratory diseases and periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2023;50(6):842–87. [DOI] [PubMed] [Google Scholar]
- 25.Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J. Periodontitis and risk of immune-mediated systemic conditions: a systematic review and meta-analysis. Community Dent Oral Epidemiol. 2023;51(5):705–17. [DOI] [PubMed] [Google Scholar]
- 26.de Ortíz P, Zubizarreta-Macho Á, Lobo Galindo AB, Montiel-Company JM, Lorenzo-Gómez MF, Flores Fraile J. Relationship between prostate inflammation and periodontal disease-a systematic review and meta-analysis. J Clin Med. 2023;12(18):6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang M, Peng R, Li X, Peng J, Liu L, Chen L. Association between chronic obstructive pulmonary disease and periodontal disease: a systematic review and meta-analysis. BMJ Open. 2023;13(6):e067432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo X, Li X, Liao C, Feng X, He T. Periodontal disease and subsequent risk of cardiovascular outcome and all-cause mortality: a meta-analysis of prospective studies. PLoS One. 2023;18(9):e0290545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu F, Tang J. Is there an association between periodontitis and non-alcoholic fatty liver disease? A systematic review and meta-analysis. Commun Dent Health. 2023;40(1):47–52. [DOI] [PubMed] [Google Scholar]
- 30.Karimi N, Samiee N, Moradi Y. The association between periodontal disease and risk of adverse maternal or neonatal outcomes: a systematic review and meta-analysis of analytical observational studies. Health Sci Rep. 2023;6(10):e1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khodadadi N, Khodadadi M, Zamani M. Is periodontitis associated with obstructive sleep apnea? A systematic review and meta-analysis. J Clin Exp Dent. 2022;14(4):e359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Feng W, Li J, Cui L, Chen ZJ. Periodontal disease and adverse neonatal outcomes: a systematic review and meta-analysis. Front Pediatr. 2022;10:799740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Gu H, Xie S, Su Y. Periodontitis in patients with psoriasis: a systematic review and meta-analysis. Oral Dis. 2022;28(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K, Zhang Z, Wang Z. Assessment of the association between periodontal disease and total cancer incidence and mortality: a meta-analysis. PeerJ. 2022;10:e14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Wang S, He Y, Zhang Y, Lin S, Cen D, et al. Is periodontal disease a risk indicator for urogenital cancer? A systematic review and meta-analysis of cohort studies. Front Oncol. 2022;12:697399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kesharani P, Kansara P, Kansara T, Kini A, Bhat R, Shetty P, et al. Is periodontitis a risk factor for lung cancer? A meta-analysis and detailed review of mechanisms of association. Contemp Clin Dent. 2022;13(4):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaliamoorthy S, Nagarajan M, Sethuraman V, Jayavel K, Lakshmanan V, Palla S. Association of Alzheimer’s disease and periodontitis - a systematic review and meta-analysis of evidence from observational studies. Med Pharm Rep. 2022;95(2):144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romandini M, Baima G, Antonoglou G, Bueno J, Figuero E, Sanz M. Periodontitis, edentulism, and risk of mortality: a systematic review with meta-analyses. J Dent Res. 2021;100(1):37–49. [DOI] [PubMed] [Google Scholar]
- 39.Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J. Risk of incident cardiovascular disease in people with periodontal disease: a systematic review and meta-analysis. Clin Exp Dent Res. 2021;7(1):109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin X, Zhao Y, Guo Y. Periodontal disease and myocardial infarction risk: a meta-analysis of cohort studies. Am J Emerg Med. 2021;48:103–9. [DOI] [PubMed] [Google Scholar]
- 41.Guo H, Chang S, Pi X, Hua F, Jiang H, Liu C, et al. The effect of periodontitis on dementia and cognitive impairment: a meta-analysis. Int J Environ Res Public Health. 2021;18(13):6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alvarenga MOP, Miranda GHN, Ferreira RO, Saito MT, Fagundes NCF, Maia LC, et al. Association between diabetic retinopathy and periodontitis-a systematic review. Front Public Health. 2021;8:550614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stöhr J, Barbaresko J, Neuenschwander M, Schlesinger S. Bidirectional association between periodontal disease and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sci Rep. 2021;11(1):13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayeed G, Varghese SS. Association between periodontitis and metabolic syndrome in females: a systematic review and meta-analysis. J Int Soc Prev Community Dent. 2021;11(6):609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aragão WAB, Souza-Monteiro D, Frazão DR, Ne YGS, Ferreira RO, Rivera LFS, et al. Is there any association between chronic periodontitis and anxiety in adults? A systematic review. Front Psychiatry. 2021;12:710606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farook F, Al Meshrafi A, Mohamed Nizam N, Al Shammari A. The association between periodontitis and erectile dysfunction: a systematic review and meta-analysis. Am J Mens Health. 2021;15(3):15579883211007277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, Shi X, Li Y, Shi X, Gu Y, Qian Q, et al. Hematopoietic and lymphatic cancers in patients with periodontitis: a systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2020;25(1):e21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Sun C, Song EJ, Liang M, Shi T, Min M, et al. Is periodontitis a risk indicator for gastrointestinal cancers? A meta-analysis of cohort studies. J Clin Periodontol. 2020;47(2):134–47. [DOI] [PubMed] [Google Scholar]
- 49.Gopinath D, Kunnath Menon R, Veettil SK, George Botelho M, Johnson NW. Periodontal diseases as putative risk factors for head and neck cancer: systematic review and meta-analysis. Cancers (Basel). 2020;12(7):1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen ATM, Akhter R, Garde S, Scott C, Twigg SM, Colagiuri S, et al. The association of periodontal disease with the complications of diabetes mellitus. A systematic review. Diabetes Res Clin Pract. 2020;165:108244. [DOI] [PubMed] [Google Scholar]
- 51.Gomes-Filho IS, Cruz SSD, Trindade SC, Passos-Soares JS, Carvalho-Filho PC, Figueiredo A, et al. Periodontitis and respiratory diseases: a systematic review with meta-analysis. Oral Dis. 2020;26(2):439–46. [DOI] [PubMed] [Google Scholar]
- 52.Lv X, Li W, Fang Z, Xue X, Pan C. Periodontal disease and age-related macular degeneration: a meta-analysis of 112,240 participants. Biomed Res Int. 2020;2020:4753645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiao Y, Wang Z, Li Y, Han Y, Zhou Y, Cao X. Rheumatoid arthritis risk in periodontitis patients: a systematic review and meta-analysis. Joint Bone Spine. 2020;87(6):556–64. [DOI] [PubMed] [Google Scholar]
- 54.Hussain SB, Botelho J, Machado V, Zehra SA, Mendes JJ, Ciurtin C, et al. Is there a bidirectional association between rheumatoid arthritis and periodontitis? A systematic review and meta-analysis. Semin Arthritis Rheum. 2020;50(3):414–22. [DOI] [PubMed] [Google Scholar]
- 55.Ali A, Lassi ZS, Kapellas K, Jamieson L, Rumbold AR. A systematic review and meta-analysis of the association between periodontitis and oral high-risk human papillomavirus infection. J Public Health (Oxf). 2020;43(4):e610–9. [DOI] [PubMed] [Google Scholar]
- 56.Xu J, Duan X. Association between periodontitis and hyperlipidaemia: a systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2020;47(11):1861–73. [DOI] [PubMed] [Google Scholar]
- 57.Muñoz Aguilera E, Suvan J, Buti J, Czesnikiewicz-Guzik M, Barbosa Ribeiro A, Orlandi M, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. 2020;116(1):28–39. [DOI] [PubMed] [Google Scholar]
- 58.Machado V, Escalda C, Proença L, Mendes JJ, Botelho J. Is there a bidirectional association between polycystic ovarian syndrome and periodontitis? A systematic review and meta-analysis. J Clin Med. 2020;9(6):1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gobin R, Tian D, Liu Q, Wang J. Periodontal diseases and the risk of metabolic syndrome: an updated systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020;11:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorenzo-Pouso AI, Castelo-Baz P, Rodriguez-Zorrilla S, Pérez-Sayáns M, Vega P. Association between periodontal disease and inflammatory bowel disease: a systematic review and meta-analysis. Acta Odontol Scand. 2020;79(5):344–53. [DOI] [PubMed] [Google Scholar]
- 61.Ma H, Zheng J, Li X. Potential risk of certain cancers among patients with periodontitis: a supplementary meta-analysis of a large-scale population. Int J Med Sci. 2020;17(16):2531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu CZ, Yuan YH, Liu HH, Li SS, Zhang BW, Chen W, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kapellas K, Singh A, Bertotti M, Nascimento GG, Jamieson LM. Perio CKDc. Periodontal and chronic kidney disease association: a systematic review and meta-analysis. Nephrol (Carlton). 2019;24(2):202–12. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira SD, Martins CC, Amaral SA, Vieira TR, Albuquerque BN, Cota LOM, et al. Periodontitis as a risk factor for peri-implantitis: systematic review and meta-analysis of observational studies. J Dent. 2018;79:1–10. [DOI] [PubMed] [Google Scholar]
- 65.Xie WZ, Jin YH, Leng WD, Wang XH, Zeng XT, investigators B. Periodontal disease and risk of bladder cancer: a meta-analysis of 298476 participants. Front Physiol. 2018;9:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corbella S, Veronesi P, Galimberti V, Weinstein R, Del Fabbro M, Francetti L. Is periodontitis a risk indicator for cancer? A meta-analysis. PLoS One. 2018;13(4):e0195683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leira Y, Seoane J, Blanco M, Rodriguez-Yanez M, Takkouche B, Blanco J, et al. Association between periodontitis and ischemic stroke: a systematic review and meta-analysis. Eur J Epidemiol. 2017;32(1):43–53. [DOI] [PubMed] [Google Scholar]
- 68.Maisonneuve P, Amar S, Lowenfels AB. Periodontal disease, edentulism, and pancreatic cancer: a meta-analysis. Ann Oncol. 2017;28(5):985–95. [DOI] [PubMed] [Google Scholar]
- 69.Zeng XT, Leng WD, Lam YY, Yan BP, Wei XM, Weng H, et al. Periodontal disease and carotid atherosclerosis: a meta-analysis of 17,330 participants. Int J Cardiol. 2016;203:1044–51. [DOI] [PubMed] [Google Scholar]
- 70.Nwizu N, Wactawski-Wende J, Genco RJ. Periodontal disease and cancer: epidemiologic studies and possible mechanisms. Periodontol 2000. 2020;83(1):213–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loos BG, Van Dyke TE. The role of inflammation and genetics in periodontal disease. Periodontol 2000. 2020;83(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nigam M, Mishra AP, Deb VK, Dimri DB, Tiwari V, Bungau SG, et al. Evaluation of the association of chronic inflammation and cancer: insights and implications. Biomed Pharmacother. 2023;164:115015. [DOI] [PubMed] [Google Scholar]
- 73.Li R, Hou M, Yu L, Luo W, Liu R, Wang H. Association between periodontal disease and oral squamous cell carcinoma: a systematic review and meta-analysis. Br J Oral Maxillofac Surg. 2023;61(6):394–402. [DOI] [PubMed] [Google Scholar]
- 74.Márquez-Arrico CF, Silvestre FJ, Marquez-Arrico JE, Silvestre-Rangil J. Could periodontitis increase the risk of suffering from pancreatic cancer? A systematic review. Cancers (Basel). 2024;16(7):1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haque T, Bin Nabhan A, Akhter F, Nasser Albagieh H. The analysis of periodontal diseases and squamous cell esophageal cancer: a retrospective study. Saudi Dent J. 2023;35(6):714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kerdreux M, Edin S, Löwenmark T, Bronnec V, Löfgren-Burström A, Zingmark C, et al. Porphyromonas gingivalis in colorectal cancer and its association to patient prognosis. J Cancer. 2023;14(9):1479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baima G, Ribaldone DG, Romano F, Aimetti M, Romandini M. The gum-gut axis: periodontitis and the risk of gastrointestinal cancers. Cancers (Basel). 2023;15(18):4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li TJ, Hao YH, Tang YL, Liang XH. Periodontal pathogens: a crucial link between periodontal diseases and oral cancer. Front Microbiol. 2022;13:919633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sobocki BK, Basset CA, Bruhn-Olszewska B, Olszewski P, Szot O, Kaźmierczak-Siedlecka K, et al. Molecular mechanisms leading from periodontal disease to cancer. Int J Mol Sci. 2022;23(2):970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamont RJ, Fitzsimonds ZR, Wang H, Gao S. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontol 2000. 2022;89(1):154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zardawi F, Gul S, Abdulkareem A, Sha A, Yates J. Association between periodontal disease and atherosclerotic cardiovascular diseases: revisited. Front Cardiovasc Med. 2020;7:625579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Q, Ouyang X, Lin J. The impact of periodontitis on vascular endothelial dysfunction. Front Cell Infect Microbiol. 2022;12:998313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsai KZ, Huang WC, Chang YC, Kwon Y, Sui X, Lavie CJ, et al. Localized periodontitis severity associated with carotid intima-media thickness in young adults: CHIEF atherosclerosis study. Sci Rep. 2023;13(1):10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Higashi Y. Roles of oxidative stress and inflammation in vascular endothelial dysfunction-related disease. Antioxid (Basel). 2022;11(10):1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi H, Dey AK, Priyamvara A, Aksentijevich M, Bandyopadhyay D, Dey D, et al. Role of periodontal infection, inflammation and immunity in atherosclerosis. Curr Probl Cardiol. 2021;46(3):100638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Priyamvara A, Dey AK, Bandyopadhyay D, Katikineni V, Zaghlol R, Basyal B, et al. Periodontal inflammation and the risk of cardiovascular disease. Curr Atheroscler Rep. 2020;22(7):28. [DOI] [PubMed] [Google Scholar]
- 87.Hopkins S, Gajagowni S, Qadeer Y, Wang Z, Virani SS, Meurman JH, et al. More than just teeth: how oral health can affect the heart. Am Heart J Plus. 2024;43:100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lam GA, Albarrak H, McColl CJ, Pizarro A, Sanaka H, Gomez-Nguyen A, et al. The oral-gut axis: periodontal diseases and gastrointestinal disorders. Inflamm Bowel Dis. 2023;29(7):1153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21(7):426–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, Huang W, Wang J, Ma J, Zhang M, Lu X, et al. Multifaceted impacts of periodontal pathogens in disorders of the intestinal barrier. Front Immunol. 2021;12:693479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y, Li R, Xue X, Xu T, Luo Y, Dong Q, et al. Periodontal disease and Helicobacter pylori infection in oral cavity: a meta-analysis of 2727 participants mainly based on Asian studies. Clin Oral Investig. 2020;24(7):2175–88. [DOI] [PubMed] [Google Scholar]
- 92.Su Y, Ye L, Hu C, Zhang Y, Liu J, Shao L. Periodontitis as a promoting factor of T2D: current evidence and mechanisms. Int J Oral Sci. 2023;15(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Velioğlu EM, Aydındoğan S, Hakkı SS. Metabolic syndrome and periodontal disease. Curr Oral Health Rep. 2023;10(2):43–51. [Google Scholar]
- 94.Pirih FQ, Monajemzadeh S, Singh N, Sinacola RS, Shin JM, Chen T, et al. Association between metabolic syndrome and periodontitis: the role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontol 2000. 2021;87(1):50–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu Z, Li Y, Chowdhury N, Yu H, Syn WK, Lopes-Virella M, et al. The presence of periodontitis exacerbates non-alcoholic fatty liver disease via sphingolipid metabolism-associated insulin resistance and hepatic inflammation in mice with metabolic syndrome. Int J Mol Sci. 2023;24(9):8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fang C, Wu L, Zhu C, Xie WZ, Hu H, Zeng XT. A potential therapeutic strategy for prostatic disease by targeting the oral microbiome. Med Res Rev. 2021;41(3):1812–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sharma S, Sridhar S, McIntosh A, Messow CM, Aguilera EM, Del Pinto R, et al. Periodontal therapy and treatment of hypertension-alternative to the pharmacological approach. A systematic review and meta-analysis. Pharmacol Res. 2021;166:105511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 Modified version of A Measurement Tool to Assess Systematic Reviews 2. Table S2 Summary of basic information of included studies. Table S3 Detailed evaluation of the methodological quality with modified version of A Measurement Tool to Assess Systematic Reviews 2
Data Availability Statement
The datasets generated during the current study will be available from the corresponding author upon reasonable request.