Abstract
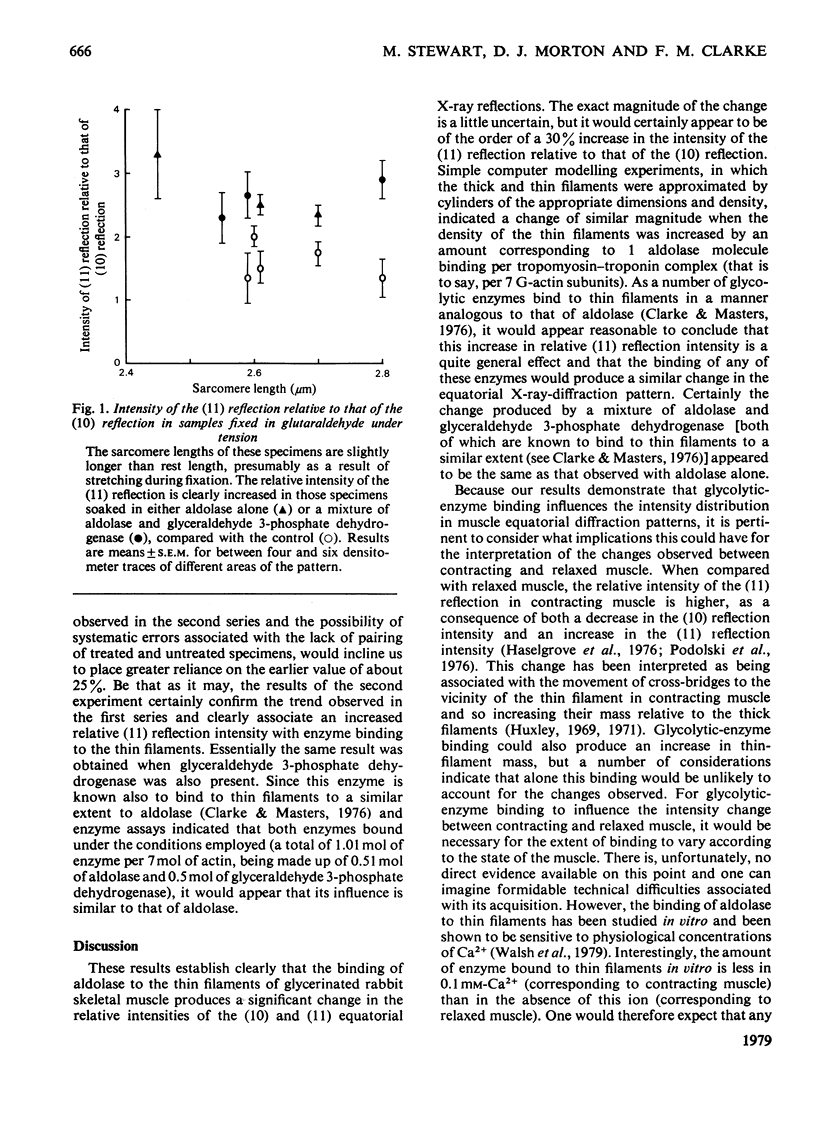
The binding of fructose biphosphate aldolase to the thin filaments of glycerinated rabbit psoas muscle produces a significant change in its low-angle X-ray-diffraction pattern. The intensity of the (11) reflection relative to that of the (10) reflection increases by 26 +/- 3% (mean +/- S.E.M.), which is consistent with the increase in the mass of the thin filaments produced by enzyme binding. A similar effect is found with a mixture of aldolase and glyceraldehyde 3-phosphate dehydrogenase. The significance of the change in intensity is considered with reference to the interpretation of the equatorial patterns obtained from muscles in different physiological states. The magnitude of the increase in the relative intensity of the (11) reflection is lower than that observed between relaxed and contracting muscle and does not bring into question the interpretation linking changes in these patterns to cross-bridge movement. However, the effect due to enzyme binding may be important when making detailed interpretations of these changes. It may also be related to an unusual pattern sometimes observed in cardiac muscle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass A., Brdiczka D., Eyer P., Hofer S., Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969 Sep;10(2):198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Faruqi A. R. High spatial resolution position sensitive counter for use in muscle diffraction. J Phys E. 1975 Aug;8(8):633–635. doi: 10.1088/0022-3735/8/8/006. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954 May 22;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Stewart M., Huxley H. E. Cross-bridge movement during muscle contraction. Nature. 1976 Jun 17;261(5561):606–608. doi: 10.1038/261606a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The structural basis of muscular contraction. Proc R Soc Lond B Biol Sci. 1971 Jun 29;178(1051):131–149. doi: 10.1098/rspb.1971.0057. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Equatorial X-ray reflections and cross arm movement in skeletal muscle. Nature. 1975 Dec 25;258(5537):770–772. doi: 10.1038/258770a0. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Low-angle x-ray diagrams from skeletal muscle: the effect of AMP-PNP, a non-hydrolyzed analogue of ATP. J Mol Biol. 1975 Dec 25;99(4):567–582. doi: 10.1016/s0022-2836(75)80172-0. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Kamiyama A., Suga H. X-ray diffraction study of contracting heart muscle. J Mol Biol. 1977 Apr;111(2):121–128. doi: 10.1016/s0022-2836(77)80118-6. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., St Onge H., Yu L., Lymn R. W. X-ray diffraction of actively shortening muscle. Proc Natl Acad Sci U S A. 1976 Mar;73(3):813–817. doi: 10.1073/pnas.73.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Starlinger H. Uber die Bindung der Muskelaldolase an grobdisperse Partikeln in Homogenaten erregter Muskeln. Hoppe Seylers Z Physiol Chem. 1967 Jul;348(7):864–870. [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]


