Abstract
Background
Patients with severe acute respiratory distress syndrome (ARDS) show a high mortality rate of up to 60 %. In such cases, extracorporeal membrane oxygenation (ECMO) support is often required, which would necessitate anticoagulation therapy, predominantly with heparin. Some bleeding events occurred more frequently in patients during the COVID-19 pandemic who were on venovenous (V-V) ECMO, so it is necessary to investigate whether anticoagulation management should be adjusted.
Methods
We collected data on 90 patients with severe ARDS who underwent ECMO support at the University Hospital Magdeburg between 2014 and 2022. In order to estimate the role of anticoagulation therapy as a cause of bleeding, patients were divided into two groups based on their mean activated partial thromboplastin time (aPTT): one group with a mean aPTT of more than 58 s (45 patients) and another with a mean aPTT of less than 58 s (45 patients). Demographic data, data before, during ECMO support, and bleeding complications were retrospectively recorded. We compared laboratory parameters before ECMO, essential coagulation parameters on days 3, 7, 10 of ECMO support, before the bleeding event occurred, and analyzed hospital survival in both groups.
Results
The incidence of major bleeding complications was significantly higher in the group of patients with higher aPTT (68.9 % vs 33.3 %, p < 0.001), the differences in the occurrence of hemothorax were especially significant (28.9 % vs 2.2 %, p < 0.001). We observed better hospital patients’ survival in the group with lower aPTT (40.0 % vs 68.9 %, p = 0.006). The results of the bivariate analysis indicate that the independent predictors of hospital mortality in adult patients receiving V-V ECMO support due to severe ARDS were COVID-19 (OR: 3.504; 95 % confidence interval [CI]: 1.415–8.681, p = 0.007) acute liver failure (OR: 8.0000; 95 % CI: 1.692–37.822; p = 0.009), high antithrombin level (%) (OR: 1.036; 95 % CI: 1.003–1.071, p = 0.035). A high mean aPTT level increased the risk of major bleeding (OR: 1.080; 95 % CI: 1.016–1.148, p = 0.014) without a significant increase in mortality.
Conclusion
Prolonged aPTT during V-V ECMO support in patients with ARDS significantly impacts the risk of major bleeding, especially hemothorax, without significant increase in hospital mortality.
Keywords: Acute respiratory distress syndrome, Extracorporeal membrane oxygenation, Major bleeding events
1. Introduction
During the Coronavirus Disease 2019 (COVID-19) pandemic, health systems encountered an enormous mass of patients with severe acute respiratory distress syndrome (ARDS) who required extracorporeal membrane oxygenation (ECMO) support. These demanding circumstances in which the issue of bleeding prevention has emerged again should be precisely investigated. According to published data, the incidence of severe bleeding in patients with ARDS due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was 30.9–57.7 % [[1], [2], [3]]. At the same time, many reports have been published regarding the increased risk of thrombosis (11.4–30.1 %) in patients with COVID-19 [4,5]. This conclusion was explained in part due to the finding that patients with severe pneumonia caused by SARS-CoV2 had higher platelet counts as well as markedly elevated D-dimer than patients with non-SARS-CoV2 pneumonia [6]. As a result, a number of medical centers in different countries have confirmed the use of higher than usual doses of anticoagulants in patients on ECMO as a result of ARDS due to COVID-19 [7].
The objective of this study was to compare coagulation parameters not between severe COVID-19 and severe pneumonia caused by other pathogens but based on the level of anticoagulation with heparin/argatroban depending on the level of aPTT by retrospective analysis, which may be useful to choose an adequate level of anticoagulation during ECMO support.
2. Materials and methods
2.1. Study design
We collected data from 154 patients with severe ARDS admitted to the ICU between 2014 and 2022. This study employs a retrospective cohort design, utilizing the database of admissions to multiple intensive care units at the University Hospital Magdeburg. We selected patients who received V-V ECMO because of severe ARDS due to pneumonia, which fulfilled the Berlin Definition Criteria [8]. The study population was limited to patients who did not present with polytrauma (n = 8), recent cardiac surgery or partial lung resection (n = 38), and acute pancreatitis (n = 3).
In a previous study, we observed that despite low aPTT on the monitored days of V-V ECMO support patients could suddenly develop an acute bleeding event on a subsequent day when the aPTT was for some reason dramatically prolonged [3].
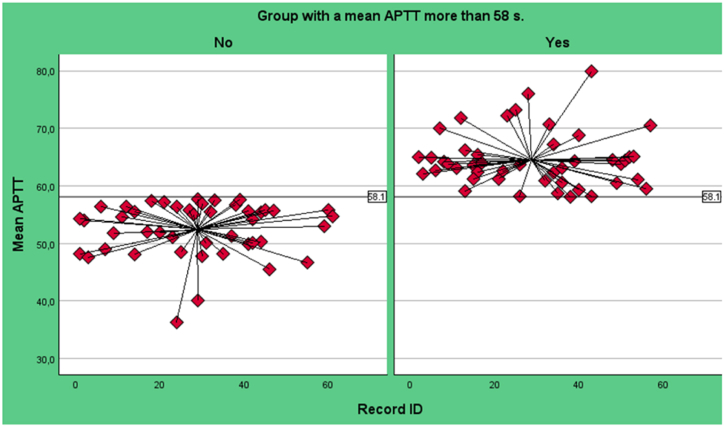
In order to categorise patients into high and low aPTT groups, the median value (58.1 seconds [s]) from the mean aPTT of several determined time points during ECMO support was taken as the dividing point (Fig. 1). Specifically, the values of essential clotting parameters were selected on days 3, 7, and 10 of ECMO support, immediately prior to the onset of major bleeding, and prior to ECMO explantation in the absence of bleeding. This approach enabled the mean aPTT to be monitored throughout the entire ECMO support period, thereby enhancing the objectivity of the analysis.
Fig. 1.
Variation of aPTT values in both groups, grouping by a median APTT 58.1 s.
Following the division of patients into two groups, significant differences in baseline covariates were identified among the 105 patients. In particular, the group with low aPTT included all patients with hematological malignancy and a significantly higher number of immunocompromised patients, and the group with high aPPT had significantly more patients with chronic renal failure. In order to achieve more homogeneity across groups, propensity score matching was performed with the groups aligning on the three aforementioned parameters. Following the matching procedure, the study cohort comprised 90 patients, with 45 patients in each group.
Baseline covariates included demographics (age, sex), comorbidities, duration of pulmonary ventilation before ECMO, and the presence of COVID-19. The time-varying covariates included laboratory parameters obtained prior to and during the course of ECMO support, with a particular focus on the most relevant blood coagulation parameters. Additionally, complications associated with extracorporeal therapy were considered. This observational retrospective study was approved by the Ethics Committee of the Otto-von-Guericke University of Magdeburg (AZ 48/22).
2.2. Anticoagulation therapy
As an anticoagulant, most patients received intravenous heparin therapy (600–2000 U/hour) throughout the entire V-V ECMO support period, the remaining patients received argatroban in case of heparin-induced thrombocytopenia or strong presumption of it. The aPTT was monitored every 8 h. Antithrombin (AT) levels were measured once daily in the morning, and in case of supplementation 12 h after therapy. If AT levels had dropped, patients received 1000 IU of AT III (Beriplex, Behring, Marburg, Germany or Alternativ, Octapharma Biopharmaceuticals, Heidelberg, Germany) as bolus (administered over 30 min) for every missing 10 % AT in the blood to maintain AT activity levels of 70–120 %.
2.3. Outcomes
The primary outcome was a hospital survival rate following the application of ECMO. Secondary outcome included the incidence of complications, major bleeding events (hemothorax, cerebral, gastrointestinal, endobronchial, retroperitoneal, cannulation-side bleeding, epistaxis, neck-bleeding [tracheostoma], chest-wall hematoma), thrombotic events (lung embolism, circuit change because of thrombosis), and duration of ECMO. Secondary outcomes also encompassed laboratory parameters preceding and during V-V ECMO, with a particular focus on the most critical coagulation parameters on days 3, 7, and 10 of ECMO, as well as immediately prior to bleeding or ECMO explantation. These values and frequencies of occurrence were compared in analyses of groups with mean aPTT higher than 58 s and lower than 58 s.
2.4. Statistical analysis
We conducted a comparative analysis of the baseline patient demographics (age, sex, body mass index, comorbidities), duration of ECMO, laboratory values, and complications, as well as blood component transfusions and outcomes between the two groups. Our analysis encompassed a range of complications, including bleeding, thromboembolic events, stroke, acute liver failure, pneumothorax, abdominal compartment syndrome, exploratory laparotomy/intestinal ischemia, thrombocytopenia, lung abscess, pleural empyema, and other complications.
We utilize the SPSS 29 software (IBM, Armonk, New York, USA) for statistical analysis. The graphs were generated using Microsoft Excel and SPSS version 29 (IBM, Armonk, New York, USA). Normality of distribution was proved using Kholmogorov-Smirnov test. The data was subjected to a two-factor independent t-test or Mann-Whitney U test to facilitate a univariate comparison of numerical variables. A chi-square test was employed to analyze categorical variables. The data are presented as either the mean ± standard deviation for numeric variables that follow a normal distribution or the median (interquartile range; IQR) for those that do not. Categorical data are expressed as a number and subsequently transformed into a percentage. The level of significance was defined as α = 0.05. Bivariate logistic regression was employed to ascertain the independent predictors of hospital mortality across the entire population. The most significant complications and laboratory values were subjected to analysis using the logistic regression method [9].
3. Results
3.1. Patients
Between 2014 and 2022, 154 patients required V-V ECMO support due to severe ARDS at the University Hospital of Magdeburg. After applying exclusion criteria and adjustment, 90 patients with severe ARDS receiving V-V ECMO support were included in this study. The etiological cause of ARDS in 54 (60.0 %) patients was COVID-19, 20 (22.3 %) patients had bacterial pneumonia, 12 (13.3 %) patients were identified as suffering from other viral pneumonias (Influenza A and B viruses, respiratory syncytial virus, Cytomegalovirus), and 4 (4.4 %) patients had aspiration without an identified pathogen.
Most patients were men (67/90; 74.4 %), which was the comparable ratio for both groups (Table 1). The mean age of the participants fell within the range of 56.2 ± 13.7 years (56.8 ± 11.1 years in group with aPTT more than 58 s; 55.7 ± 16.0 years in the group with aPTT less than 58 s, p = 0.725). After adjustment, there were essentially no substantial discrepancies in comorbidities between the two groups, except a higher number of COVID-19 in the high-aPTT group. The same applies to laboratory parameters, which did not differ significantly between patients in both groups before the application of V-V ECMO (Table 2). Patients received in most cases (77.7 %) anticoagulation therapy with intravenous heparin (600–2000 IU/hour) during the whole period of V-V ECMO support, the remaining patients received argathra in case of heparin-induced thrombocytopenia or strong presumption of this. Overall, the percentage of patients receiving argatroban was similar in both groups.
Table 1.
Baseline characteristics of the entire patient collective and the subgroups with PTT higher and lower than 58 s.
| Baseline characteristics | All patients, n = 90 | Group with PTT more than 58 s, n = 45 | Group with PTT less than 58 s, n = 45 | p-value |
|---|---|---|---|---|
| Age | 56.2 ± 13.7 | 56.8 ± 11.1 | 55.7 ± 16.0 | 0.725 |
| Male | 67 (74.4 %) | 34 (75.5 %) | 33 (73.3 %) | 0.809 |
| BMI | 29.3 (24.7–32.8) | 29.2 (25.2–33.2) | 29.4 (24.3–32.9) | 0.750 |
| Mechanical ventilation before ECMO (day) | 4.0 (1.0–9.0) | 6.0 (1.0–9.5) | 3.0 (1.0–8.5) | 0.628 |
| Site of cannulation: | ||||
|
20 (22.2 %) | 8 (17.8 %) | 12 (26.7 %) | 0.310 |
|
70 (77.8 %) | 37 (82.2 %) | 33 (73.3 %) | 0.310 |
| Anticoagulation with argatroban | 20 (22.2 %) | 10 (22.2 %) | 10 (22.2 %) | 1.000 |
| Chronic kidney disease | 7 (7.8 %) | 5 (11.1 %) | 2 (4.4 %) | 0.238 |
| Autoimmune disease | 3 (3.3 %) | 2 (4.4 %) | 1 (2.2 %) | 0.557 |
| Hypertension | 54 (60 %) | 27 (60 %) | 27 (60 %) | 1.000 |
| Ischemic heart disease | 13 (14.4 %) | 7 (15.6 %) | 6 (13.3 %) | 0.764 |
| Diabetis | 25 (27.8 %) | 12 (26.7 %) | 13 (28.9 %) | 0.814 |
| Lung disease before | 18 (20 %) | 11 (24.4 %) | 7 (15.6 %) | 0.292 |
| Immunocompromised status | 11 (12.2 %) | 3 (6.7 %) | 8 (17.8 %) | 0.108 |
| Acute liver failure before ECMO | 6 (6.7 %) | 4 (8.9 %) | 2 (4.4 %) | 0.398 |
| COVID-19 | 54 (60.0 %) | 32 (71.1 %) | 22 (48.9 %) | 0.031 |
| Another viral lung infects | 27 (30.0 %) | 10 (22.2 %) | 17 (37.8 %) | 0.107 |
| Influenza A | 2 (2.2 %) | 0 (0 %) | 2 (4.4 %) | 0.153 |
| Influenza B | 1 (1.1 %) | 0 (0 %) | 1 (2.2 %) | 0.315 |
| RSV | 1 (1.1 %) | 0 (0 %) | 1 (2.2 %) | 0.315 |
| Herpes Simplex | 17 (18.9 %) | 8 (17.8 %) | 9 (20.0 %) | 0.788 |
| Cytomegalovirus | 4 (4.4 %) | 1 (2.2 %) | 3 (6.7 %) | 0.306 |
| Epstein Barr Virus | 2 (2.2 %) | 1 (2.2 %) | 1 (2.2 %) | 1.000 |
| Bacterial lung infection | 53 (58.9 %) | 25 (55.6 %) | 28 (62.2 %) | 0.520 |
| Clebsiella pneumoniae | 14 (15.6 %) | 6 (13.3 %) | 8 (17.8 %) | 0.562 |
| Staphylococcus aureus | 8 (8.9 %) | 2 (4.4 %) | 6 (13.3 %) | 0.138 |
| Pseudomonas aeruginosa | 6 (6.7 %) | 2 (4.4 %) | 4 (8.9 %) | 0.398 |
| Stenotrophomonas maltophilia | 3 (3.3 %) | 0 (0 %) | 3 (6.7 %) | 0.078 |
| Serratia marcescens | 4 (4.4 %) | 3 (6.7 %) | 1 (2.2 %) | 0.306 |
| Proteus mirabilis | 5 (5.6 %) | 3 (6.7 %) | 2 (4.4 %) | 0.645 |
| Streptococcus pneumoniea | 6 (6.7 %) | 3 (6.7 %) | 3 (6.7 %) | 1.000 |
| Escherichia coli | 13 (14.4 %) | 5 (11.1 %) | 8 (17.8 %) | 0.368 |
| Enterobacter cloacae | 5 (5.6 %) | 1 (2.2 %) | 4 (8.9 %) | 0.167 |
| Candida species | 2 (2.2 %) | 0 (0 %) | 2 (4.4 %) | 0.153 |
| Pneumocystis jiroveci | 3 (3.3 %) | 2 (4.4 %) | 1 (2.2 %) | 0.557 |
| Acinetobacter baumanii | 2 (2.2 %) | 1 (2.2 %) | 1 (2.2 %) | 1.000 |
| Lung superinfection | 44 (48.9 %) | 22 (48.9 %) | 22 (48.9 %) | 1.000 |
| Aspiration | 4 (4.4 %) | 1 (2.2 %) | 3 (6.7 %) | 0.306 |
Table 2.
Laboratory parameters before ECMO support of the entire patient collective and the subgroups with aPTT higher and lower than 58 s.
| Laboratory parameters before VV ECMO | ||||
|---|---|---|---|---|
| All patients, n = 90 | Group with PTT more than 58 s, n = 45 | Group with PTT less than 58 s, n = 45 | p-value | |
| Horowitz-Index | 76.0 (57.6–113.5) | 81.3 (57.3–120.0) | 76.0 (57.7–108.5) | 1.000 |
| pO2 (mmHg) | 70.1 (57.8–80.3) | 69.0 (57.0–88.0) | 70.5 (58.2–79.0) | 0.843 |
| pCO2 (mmHg) | 72.0 (53.0–98.3) | 67.0 (51.7–90.8) | 75.0 (54.5–101.5) | 0.278 |
| pH | 7.22 ± 0.13 | 7.24 ± 0.14 | 7.20 ± 0.13 | 0.132 |
| Lactate(mmol/l) | 1.6 (1.1–2.6) | 1.5 (1.1–2.6) | 1.8 (1.1–2.7) | 0.566 |
| Hemoglobin (mmol/l) | 6.7 ± 1.2 | 6.8 ± 1.2 | 6.7 ± 1.2 | 0.693 |
| Leucocyte (109/l) | 15.4 (9.5–21.2) | 12.5 (9.1–21.4) | 17.6 (10.2–21.2) | 0.341 |
| Platelet count (109/l) | 274.6 ± 139.2 | 281.4 ± 124.4 | 267.8 ± 153.8 | 0.645 |
| CRP (mg/l) | 244.3 ± 125.3 | 247.5 ± 117.9 | 241.1 ± 133.6 | 0.808 |
| Procalcitonine(μg/l) | 1.0 (0.4–2.4) | 0.9 (0.3–2.1) | 1.0 (0.6–5.7) | 0.136 |
| PTT (s) | 31.7 (28.3–40.0) | 31.7 (28.0–40.7) | 31.7 (28.8–40.0) | 0.717 |
| INR | 1.20 (1.13–1.29) | 1.20 (1.14–1.29) | 1.20 (1.13–1.26) | 0.928 |
| Antithrombin(%) | 69.3 ± 20.0 | 67.4 ± 19.7 | 71.3 ± 20.3 | 0.365 |
| D-Dimer (mg/l) | 6.6 ± 5.8 | 7.5 ± 5.1 | 5.7 ± 5.3 | 0.158 |
| Fibrinogen (g/l) | 6.0 ± 2.3 | 6.2 ± 2.1 | 5.8 ± 2.4 | 0.426 |
| LDH (μmol/ls) | 6.8 (5.4–10.5) | 6.6 (5.4–9.8) | 7.6 (5.4–11.6) | 0.486 |
| ALT (μmol/ls) | 0.6 (0.4–1.6) | 0.6 (0.4–1.0) | 0.8 (0.4–1.8) | 0.282 |
| Creatinine (μmol/l) | 95.0 (62.5–136.5) | 92.5 (64.3–130.0) | 99.0 (60.0–161.5) | 0.684 |
| Total bilirubin (μmol/l) | 10.7 (7.5–22.0) | 10.8 (7.5–20.7) | 10.7 (7.4–24.7) | 0.863 |
3.2. Primary and secondary outcomes
The overall hospital mortality for the total cohort amounted to 45.6 % (n = 49). A lower survival rate was observed in the group with high aPTT: 40.0 % versus 68.9 % (p = 0.006) (Table 3). Duration of the ECMO support was similar in both groups (15.0 [10.5–23.5] days vs. 15.0 [9.0–21.0] days, p = 0.462). No differences were noted in terms of cannulation site or circuit change during V-V ECMO. Furthermore, the incidence of major complications, including renal failure requiring dialysis, abdominal compartment syndrome, pneumothorax, pleural empyema, tracheal/pneumomediastinum rupture, heparin-induced thrombocytopenia, and pulmonary embolism was comparable between the two groups, whereas considerable differences were observed regarding major bleeding events (68.9 % vs. 33.3 %, p < 0.001)) and in specific hemothorax (28.9 % vs. 2.2 %, p < 0.001).
Table 3.
Survival, complications, lengths of stay (LOS) and specificities of the therapy during V-V ECMO of the entire patient collective and the subgroups with PTT higher and lower than 58 s.
| Characteristics | All patients, n = 90 | Group with PTT more than 58 s, n = 45 | Group with PTT less than 58 s, n = 45 | p-value |
|---|---|---|---|---|
| Survived ICU | 49 (54.4 %) | 18 (40.0 %) | 31 (68.9 %) | 0.006 |
| ICU LOS (days), all patients | 26.0 (18.0–40.0) | 24.0 (15.5–41.5) | 31.0 (21.5–39.5) | 0.127 |
| Duration ECMO (days) | 15.0 (10.0–21.5) | 15.0 (10.5–23.5) | 15.0 (9.0–21.0) | 0.462 |
| Site of cannulation: | ||||
|
20 (22.2 %) | 8 (17.8 %) | 12 (26.7 %) | 0.310 |
|
70 (77.8 %) | 37 (82.2 %) | 33 (73.3 %) | 0.310 |
| Major bleeding events | 46 (51.1 %) | 31 (68.9 %) | 15 (33.3 %) | <0.001 |
|
13 (14.4 %) | 8 (17.8 %) | 5 (11.1 %) | 0.368 |
|
10 (11.1 %) | 5 (11.1 %) | 5 (11.1 %) | 1.000 |
|
14 (15.6 %) | 13 (28.9 %) | 1 (2.2 %) | <0.001 |
|
12 (13.3 %) | 8 (17.8 %) | 4 (8.9 %) | 0.215 |
|
5 (5.6 %) | 3 (6.7 %) | 2 (4.4 %) | 0.645 |
|
3 (3.3 %) | 2 (4.4 %) | 1 (2.2 %) | 0.557 |
|
12 (13.3 %) | 8 (17.8 %) | 4 (8.9 %) | 0.215 |
|
6 (6.7 %) | 4 (8.9 %) | 2 (4.4 %) | 0.398 |
| Pneumothorax | 10 (11.1 %) | 7 (15.6 %) | 3 (6.7 %) | 0.180 |
| Pleuraempyem | 5 (5.6 %) | 4 (8.9 %) | 1 (2.2 %) | 0.167 |
| Pleural drainage | 55 (61.1 %) | 31 (68.9 %) | 24 (53.3 %) | 0.130 |
| Renal replacement therapy | 26 (28.9 %) | 14 (31.1 %) | 12 (26.7 %) | 0.642 |
| Tracheal rupture, pneumomediastinum | 4 (4.4 %) | 3 (6.7 %) | 1 (2.2 %) | 0.306 |
| Abdominal compartment syndrome | 9 (10 %) | 3 (6.7 %) | 6 (13.3 %) | 0.292 |
| Peritonitis | 6 (6.7 %) | 1 (2.2 %) | 5 (11.1 %) | 0.091 |
| Lung emboly | 7 (7.8 %) | 4 (8.9 %) | 3 (6.7 %) | 0.694 |
| Circuit change | 22 (24.4 %) | 13 (28.9 %) | 9 (20.0 %) | 0.327 |
| HIT | 9 (10 %) | 6 (13.3 %) | 3 (6.7 %) | 0.292 |
| Acute liver failure | 11 (12.2 %) | 6 (13.3 %) | 5 (11.1 %) | 0.748 |
| Packed red blood cells transfusion (units) | 17.5 (10.0–31.0) | 20.0 (12.0–34.0) | 15.0 (9.5–28.0) | 0.115 |
| Fresh frozen plasma transfusion (units) | 0.0 (0.0–6.0) | 0.0 (0.0–8.0) | 0.0 (0.0–4.0) | 0.585 |
| Platelet transfusion (units) | 2.0 (0.0–9.0) | 1.0 (0.0–7.5) | 2.0 (0.0–10.0) | 0.437 |
| Supplementation of AT/day of ECMO | 222.5 (58.8–465.4) | 270.3 (125.1–531.3) | 130.4 (0.0–362.5) | 0.025 |
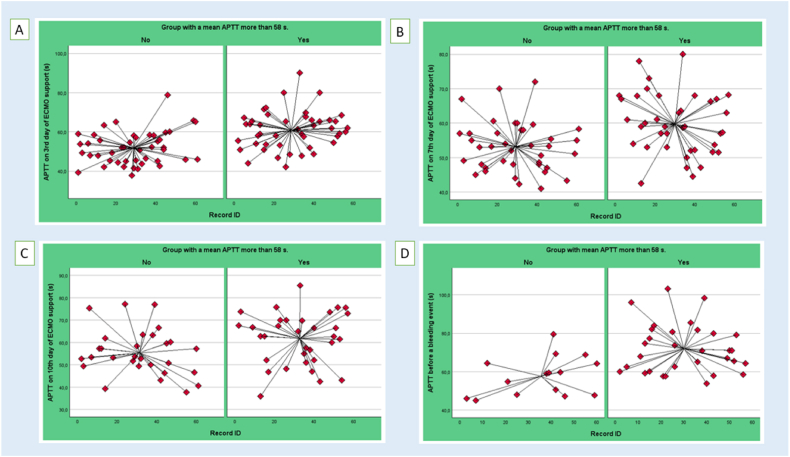
When analyzing the main blood coagulation parameters on day 3, 7, 10 of ECMO application, we observed lower AT levels on day 7 and 10 in patients from the high aPTT group (Table 4). The patient group whith aPTT more than 58 s exhibited also higher levels of platelets (161.0 [112.5–222.5] vs. 122.0 [85.5–184.0], p = 0.040), as well as elevated INR values on day 3 of ECMO therapy (1.21 [1.14–1.38)] vs. 1.15 [1.10–1.25], p = 0.030). However, despite these observations, the INR levels remained below 3, which is considered clinically significant. Consequently, INR levels in the lower range of values between 1.10 and 1.38 had no appreciable effect on the observed bleeding rate [10,11]. Thus, patients differed in blood coagulation parameters mostly by aPTT and AT, working in the same anticoagulation mechanism. It is noteworthy that significant differences were observed at each time point in the mean aPTT. The data set includes all aPTT values for each patient and the mean aPTT scores at various time points, as illustrated in Fig. 2(A–D): the mean aPTT on 3rd day of ECMO support (60.9 ± 9.7 vs. 51.9 ± 8.4 s, p < 0.001) (Fig. 2, A); the mean aPTT on 7th day of ECMO support (59.7 ± 8.7 vs. 53.1 ± 7.2, p < 0.001) (Fig. 2, B); the mean aPTT on 10th day of ECMO support (61.8 ± 11.6 vs. 55.4 ± 10.3, p = 0.024) (Fig. 2C); and the mean aPTT values just before bleeding, which differed most significant (71.9 ± 12.8 vs. 57.6 ± 10.3, p < 0.001) (Fig. 2, D). The latter finding may be indicative of a correlation between peak aPTT and the occurrence of bleeding.
Table 4.
Coagulation parameters on 3rd, 7th and 10th day of the V-V ECMO support of the entire patient collective and the subgroups with PTT higher and lower than 58 s.
| Coagulation parameters on 3rd day of the VV ECMO support | ||||
|---|---|---|---|---|
| Overall, n = 90 | Group with PTT more than 58 s, n = 45 | Group with PTT less than 58 s, n = 45 | p-value | |
| Platelet count (109/l) | 134.0 (94.0–200.3) | 161.0 (112.5–222.5) | 122.0 (85.5–184.0) | 0.040 |
| aPTT (s) | 56.4 ± 10.1 | 60.9 ± 9.7 | 51.9 ± 8.4 | <0.001 |
| INR | 1.17 (1.11–1.30) | 1.21 (1.14–1.38) | 1.15 (1.10–1.25) | 0.030 |
| Antithrombin(%) | 75.7 ± 17.4 | 72.3 ± 18.8 | 78.4 ± 15.6 | 0.137 |
| D-Dimer (mg/l) | 3.9 (2.3–7.9) | 3.8 (2.5–8.2) | 3.9 (2.2–7.7) | 0.564 |
| Fibrinogen (g/l) | 5.5 ± 2.0 | 5.3 ± 2.2 | 5.7 ± 1.8 | 0.271 |
| Coagulation parameters on 7th day of the VV ECMO support | ||||
| Overall, n = 79 | Group with PTT more than 58 s, n = 40 | Group with PTT less than 58 s, n = 39 | p-value | |
| Platelet count (109/l) | 106.0 (84.0–167.0) | 101.5 (80.0–161.5) | 111.0 (87.0–157.0) | 0.806 |
| aPTT (s) | 56.5 ± 8.6 | 59.7 ± 8.7 | 53.1 ± 7.2 | <0.001 |
| INR | 1.21 (1.12–1.40) | 1.24 (1.14–1.50) | 1.20 (1.08–1.37) | 0.121 |
| Antithrombin(%) | 81.1 ± 14.6 | 77.5 ± 12.8 | 84.9 ± 15.4 | 0.022 |
| D-Dimer (mg/l) | 9.6 (4.5–17.4) | 9.5 (5.4–14.6) | 10.3 (3.2–19.8) | 0.915 |
| Fibrinogen (g/l) | 5.0 ± 1.9 | 4.9 ± 2.0 | 5.0 ± 1.7 | 0.735 |
| Coagulation parameters on 10th day of the VV ECMO support | ||||
| Overall, n = 62 | Group with PTT more than 58 s, n = 33 | Group with PTT less than 58 s, n = 29 | p-value | |
| Platelet count (109/l) | 108.0 (78.0–156.0) | 104.5 (75.8–136.8) | 111.0 (85.0–164.0) | 0.521 |
| aPTT (s) | 58.8 ± 11.4 | 61.8 ± 11.6 | 55.4 ± 10.3 | 0.024 |
| INR | 1.24 (1.15–1.41) | 1.24 (1.15–1.47) | 1.23 (1.14–1.43) | 0.841 |
| Antithrombin(%) | 81.6 ± 18.0 | 75.4 ± 17.3 | 89.2 ± 16.0 | 0.002 |
| D-Dimer (mg/l) | 9.5 (4.0–14.0) | 8.4 (4.0–13.9) | 10.5 (4.2–10.0) | 0.308 |
| Fibrinogen (g/l) | 5.4 ± 1.8 | 5.5 ± 2.2 | 5.6 ± 1.9 | 0.774 |
Fig. 2.
Differences in aPTT value at each time point: (A) aPTT on 3rd day of ECMO support, (B) aPTT on 7th day of ECMO support, (C) aPTT on 10th day of ECMO support, (D) aPTT just before a bleeding event.
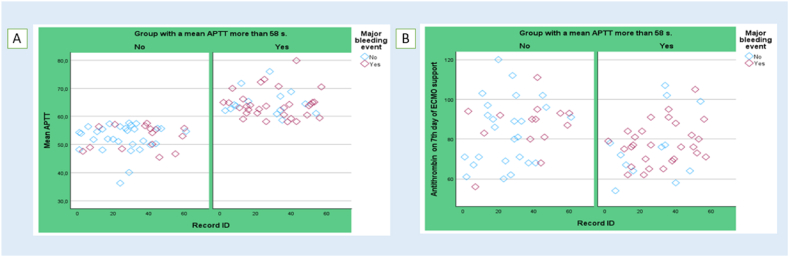
The next finding was that patients with prolonged aPTT had significantly lower levels of AT on the 7th and the 10th day of the V-V ECMO support (Table 4), although its supplementation via AT products was much more intense (270.3 [125.1–531.3] vs.130.4 [0.0–362.5] IU/ECMO-day, p = 0.025) (Table 3). To illustrate, we present the data from Fig. 3 (A, B), which depict the mean aPTT values and AT levels on day 7 of ECMO support. These figures demonstrate a notable trend whereby the majority of bleeding incidents (depicted in purple) occurred in patients with elevated aPTT levels, while AT levels frequently dropped below 80 %.
Fig. 3.
Mean aPTT (A) and AT levels on day 7 of ECMO support (B) and incidence of bleeding (marked in purple). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Prediction model by logistic regression
Based on the results of bivariate analysis, COVID-19 (OR: 3.504; 95 % confidence interval [CI]: 1.415–8.681, p = 0.007), acute liver failure (OR: 8.0000; 95 % CI: 1.692–37.822; p = 0.009), high AT level (%) (OR: 1.036; 95 % CI: 1.003–1.071, p = 0.035) were determined as independent predictors of hospital mortality in adult patients undergoing V-V ECMO due to severe ARDS.
The mean aPTT, which was calculated as the sum of measured aPTT on selected days of ECMO support and before the bleeding event, divided by the number of measurements, demonstrated significance in the occurrence of bleeding events (OR: 1.080; 95 % CI: 1.016–1.148, p = 0.014). The same correlation was also proven for a single measurement point, such as on day 3 of ECMO support (OR: 1.064; 95 % CI: 1.015–1.115, p = 0.010). Concurrently, an increase in mean aPTT, the emergence of major bleeding, and hemothorax individually did not markedly correlate with an elevated risk of mortality, as determined by regression analysis. Interestingly, high antithrombin levels did not lead to an enhanced risk of bleeding [for instance, for antithrombin leven on 7th day of ECMO support (OR: 0.997; 95 % CI: 0.967–1.027, p = 0.823)].
4. Discussion
This retrospective single-center study examined the incidence of major bleeding events among patients with severe ARDS supported by V-V ECMO, with a specific focus on the aPTT subgroup analysis.
The results of our study indicate that a hospital survival rate is significantly higher in patients whose aPTT is more than 58 s, but this increase of mortality was mainly attributed to COVID-19 and acute liver failure. As we examined the incidence of major bleeding, we identified a significant increase in the group with higher aPTT with a prevalence of hemothorax. The relationship between the presence of drains in the pleural cavity and hemothorax was investigated. In two cases, hemothorax was iatrogenically induced because of drains application for other indications, including pneumothorax and pleural effusion. In the majority of cases, pleural drains were placed because of hemothorax. The incidence of thrombotic events did not differ between the two groups.
In previously published studies, the most prevalent sources of bleeding in patients with ARDS on V-V ECMO were intracranial hemorrhage, bleeding on the cannulation side, hemothorax, gastrointestinal, pulmonary, nasal or mucosal bleedings [[12], [13], [14]]. Hemothorax (15.6 %), cerebral bleeding (14.4 %), gastrointestinal bleeding (11.1 %) and bleeding on the cannulation side (13.3 %) were also predominant in our study.
Alternative methods of measuring the anticoagulation effect of unfractionated heparin (UFH) such as activated clotting time, Anti-Xa assay, and ecarin clotting time are currently being investigated, however, the available data remains limited, and aPTT remains the standard measurement [15]. The optimal targets for anticoagulation in patients on ECMO are currently unknown and may vary depending on the patient's underlying disease, comorbidities, and risk of thrombosis or bleeding [16]. Hence, the optimal anticoagulation strategy with intravenous UFH during ECMO support remains a common dilemma and is subject to further debate. Based on several studies in adults, the therapeutic range for aPTT has been set at 1.5 to 2.5 times the patient's baseline aPTT prior to therapy and is 50–80 s [17,18]. Some studies have indicated that low aPTT (45–55 s) has been identified as a significant factor in the prevention of major bleeding events [19]. In a systematic review conducted by Sklar, several studies comparing high and low aPTT, with a cut-off point of 60 s, in patients on ECMO were collected. These data revealed that patients receiving anticoagulation aimed at a low target aPTT had 8 % serious hemorrhage and and 32 % thrombotic complications, whereas among the targeted studies with high aPTT there were 56 % serious haemorrhagic and 7 % thrombotic complications [20]. In our study, patients with high aPTT had bleeding in 68.9 % of cases and thromboembolic complications in 37.8 %, whereas in the group of patients with low aPTT only 33.3 % suffered bleeding and 26.7 % thromboembolic complications. So, despite high aPTT the percentage of thromboembolic complications in the first group was higher.
The utilization of aPTT for the monitoring of UFH is predicated on two fundamental hypotheses: (1) that a patient's initial aPTT is comparable to that of a normal control population and (2) that there exists a linear relationship between UFH dose and aPTT. However, in critically ill patients, the initial aPTT is frequently disparate from that observed in normal controls, thereby limiting the usability of aPTT as an indicator of the impact of UFH [18]. Optimal aPTT during heparin therapy has not yet been confirmed in randomised controlled trials in patients with ECMO support. Aubron et al. reported that aPTT exceeding 70 s on the day preceding the bleeding event was found to be an independently associated factor with an elevated risk of bleeding occurrence [13]. In our study, we have demonstrated that an average aPTT of more than 58 s during the whole period of ECMO support increases the risk of hemorrhage. Furthermore, it has been observed that in this group of patients, there is a notable increase in the aPTT ratio, reaching a peak value of 60–100 s (Abb. 2, D), which has been linked to a significant incidence of bleeding. Consequently, maintaining a longer aPTT will result in the formation of higher peak values.
To date, it's been well researched that the anticoagulant effect of therapeutic heparin is mainly mediated through its interaction with AT [21]. AT is a hepatically synthesized major inhibitor of multiple clotting factors (thrombin [factor IIa], factor Xa, and to a lesser extent factors IXa, XIa, XII, tissue plasminogen activator, plasmin, and kallikrein) that circulates in high concentration in a state that reacts weakly with clotting proteases, and only when interacting with heparin-like glycosaminoglycans does AT become an effective inhibitor [22]. Initially and for the past 50 years, the effect of AT therapy on the outcomes of patients with sepsis and disseminated intravascular coagulation (DIC) has been studied [23]. Patients receiving AT products showed better improvement in the resolution of organ dysfunction and a lower incidence of new organ dysfunction during the follow-up period [24,25]. Current clinical practice guidelines regarding the use of AT in patients with severe sepsis and septic shock are controversial, as there are few clinical studies that have reported that the supplementation of AT does not result in a favorable impact on overall mortality, and the treatment is linked to an elevated risk of bleeding [26]. Hayakawa et al. proposed that patients with sepsis and DIC exhibiting markedly reduced AT activity (less than 43 %) may benefit from AT supplementation, particularly when compared to individuals with AT activity below 70 % [27].
A paucity of studies exists that evaluate the management and outcomes of AT in patients on ECMO. The few reports have argued that dosing strategies may not be adequate to substitute AT in patients receiving ECMO, requiring more frequent and timely measurement of AT levels as well as more detailed studies of the pharmacokinetics of AT products in patients receiving ECMO in order to develop a more accurate dosing scheme [28,29]. There is a suggestion that the balance of pro- and anticoagulant proteins as a result of interaction with the circuit is different in patients on ECMO, which complicates interpretation and decision making related to AT supplementation in patients on ECMO, as it makes the effect of AT administration less predictable [29].
In our study high AT level (%) was identified as independent predictor of hospital mortality (OR: 1.036; 95 % CI: 1.003–1.071, p = 0.035), which is a controversial to previously published data on sepsis. We estimate this finding to mean that nonetheless AT therapy has an unpredictable effect in patients on ECMO. On the other hand, high antithrombin levels did not enhance the risk of bleeding.
Our study has several limitations. These factors include the study's single-center retrospective design and relatively modest sample size. In addition, due to cost and setting limitations, we were unable to measure anti-Xa and perform viscoelastic tests such as rotational thromboelastometry, which are of interest in developing a more effective anticoagulation monitoring strategy.
The study's key strengths include an in-depth analysis of the most crucial clotting parameters, including AT, as well as a comprehensive examination of the impact of AT supplementation on these parameters. Furthermore, we were able to ascertain the relationships between certain clotting parameters and the risk of bleeding.
5. Conclusion
More than half of patients with severe ARDS undergoing V-V ECMO suffered from bleeding complications. A mean aPTT exceeding 58 s during the whole period of ECMO support was identified as a significant prognostic risk factor for the development of bleeding complications, especially hemothorax. Furthermore, elevated AT levels were associated with an increased risk of mortality. Thus, the target aPTT value should be lower than 58 s, and the effect of AT replacement on bleeding complications and survival should be studied further.
CRediT authorship contribution statement
Boris Kuzmin: Writing – original draft, Software, Resources, Project administration, Methodology, Data curation, Conceptualization. Max Wacker: Visualization, Supervision, Formal analysis. Juliana Ponomarenko: Writing – review & editing, Software, Data curation. Arevik Movsisyan: Formal analysis, Data curation. Florian Praetsch: Data curation, Conceptualization. Georg Marsch: Supervision, Methodology. Olaf Keyser: Data curation. Mohammad Fadel: Supervision. Maximilian Scherner: Formal analysis, Methodology, Supervision. Jens Wippermann: Writing – review & editing, Supervision, Project administration, Conceptualization.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used DeepL in order to improve writing and readability. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Antje Wiede for her help throughout this study.
References
- 1.Arachchillage D.J., Rajakaruna I., Scott I., Gaspar M., Odho Z., Banya W., Vlachou A., Isgro G., Cagova L., Wade J., Fleming L., Laffan M., Szydlo R., Ledot S., Jooste R., Vuylsteke A., Yusuff H. Impact of major bleeding and thrombosis on 180‐day survival in patients with severe COVID‐19 supported with veno‐venous extracorporeal membrane oxygenation in the United Kingdom: a multicentre observational study. Br. J. Haematol. 2022;196:566–576. doi: 10.1111/bjh.17870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt M., Hajage D., Lebreton G., Monsel A., Voiriot G., Levy D., Baron E., Beurton A., Chommeloux J., Meng P., Nemlaghi S., Bay P., Leprince P., Demoule A., Guidet B., Constantin J.M., Fartoukh M., Dres M., Combes A., Luyt C.-E., Hekimian G., Brechot N., Pineton De Chambrun M., Desnos C., Arzoine J., Guerin E., Schoell T., Demondion P., Juvin C., Nardonne N., Marin S., D'Alessandro C., Nguyen B.-L., Quemeneur C., James A., Assefi M., Lepere V., Savary G., Gibelin A., Turpin M., Elabbadi A., Berti E., Vezinet C., Bonvallot H., Delmotte P.-R., De Sarcus M., Du Fayet De La Tour C., Abbas S., Maury E., Baudel J.-L., Lavillegrand J.-R., Ait Oufella H., Abdelkrim A., Urbina T., Virolle S., Deleris R., Bonny V., Le Marec J., Mayaux J., Morawiec E. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir. Med. 2020;8:1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuzmin B., Movsisyan A., Praetsch F., Schilling T., Lux A., Fadel M., Azizzadeh F., Crackau J., Keyser O., Awad G., Hachenberg T., Wippermann J., Scherner M. Outcomes of patients with coronavirus disease versus other lung infections requiring venovenous extracorporeal membrane oxygenation. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e17441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M.J.H.A., Van Der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., Van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biancari F., Mariscalco G., Dalén M., Settembre N., Welp H., Perrotti A., Wiebe K., Leo E., Loforte A., Chocron S., Pacini D., Juvonen T., Broman L.M., Perna D.D., Yusuff H., Harvey C., Mongardon N., Maureira J.P., Levy B., Falk L., Ruggieri V.G., Zipfel S., Folliguet T., Fiore A. Six-month survival after extracorporeal membrane oxygenation for severe COVID-19. J. Cardiothorac. Vasc. Anesth. 2021;35:1999–2006. doi: 10.1053/j.jvca.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2021;51:1107–1110. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mang S., Kalenka A., Broman L.M., Supady A., Swol J., Danziger G., Becker A., Hörsch S.I., Mertke T., Kaiser R., Bracht H., Zotzmann V., Seiler F., Bals R., Taccone F.S., Moerer O., Lorusso R., Bělohlávek J., Muellenbach R.M., Lepper P.M., Barrett N., Duerschmied D., Fan E., Fichtner F., Haake H., Langer F., Mutlak H., Kredel M., Müller T., Protti A., Raddatz A., Spangenberg T., Staudacher D., Wehrfritz H., Wengenmayer T., Westheider A., Dang Van S., Daubin C., Gaudard P., Godet T., Guinot P., Le Guennec L., Megarbane B., Mercat A., Sonneville R., Zogheib E., Pham T., Winiszewski H., Schellongowski P., Staudinger T., Wiedemann D., Velik‐Salchner C., Joannidis M., Bodenstein M., Groesdonk H.V., Guth S., Hecker M., Husain‐Syed F., Jung C., Napp L.C., Natanov R., Trummer G., Treskatsch S., Welp H., Avalli L., Ball L., Belliato M., Bonizzoli M., Borrelli E., Cavallaro G., Franci A., Gramaticopolo S., Panigada M., Tritapepe L., Abdulaziz S., Bracco D., Alexandros Y., Joffe A., Nagpal A.D., Sia Y., Auzinger G., Zochios V., Garcia A., Gist K., Lustbader D., Yannopoulos D., Stephens R.S., Tonna J., Paxton L., Hirose H., Kim B., Dalén M., Balik M., Janak D., Castillo L., Bruhn A., Socarras J.L.A., Kim T., Kim H.S., Byun J.H., Mainardi G., Mendes P., Giraud R., Fortuna P., Fukuda T., Maas J., Maciejewski D., Pandit D., Psz Y., Radsel P., Yan G. Extracorporeal life support in COVID‐19‐related acute respiratory distress syndrome: a EuroELSO international survey. Artif. Organs. 2021;45:495–505. doi: 10.1111/aor.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acute Respiratory Distress Syndrome: The Berlin Definition JAMA. 2012;307 doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9.Kirkwood B.R., Sterne J.A.C. 2. ed. Blackwell Science; Oxford: 2009. Essential Medical Statistics. [Nachdr.] [Google Scholar]
- 10.Uetsuka Y., Katsuki T., Aosaki M., Iwade K., Hashimoto A., Koyanagi H., Saito M., Yaginuma Y., Hosoda S. [International normalized ratio (INR) for optimal anticoagulant therapy] Kokyu Junkan. 1993;41:885–890. [PubMed] [Google Scholar]
- 11.Kamthornthanakarn I., Krittayaphong R. Optimal INR level for warfarin therapy after mechanical mitral valve replacement. BMC Cardiovasc. Disord. 2019;19:97. doi: 10.1186/s12872-019-1078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawauchi A., Liu K., Nakamura M., Suzuki H., Fujizuka K., Nakano M. Risk factors for bleeding complications during venovenous extracorporeal membrane oxygenation as a bridge to recovery. Artif. Organs. 2022;46:1901–1911. doi: 10.1111/aor.14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aubron C., DePuydt J., Belon F., Bailey M., Schmidt M., Sheldrake J., Murphy D., Scheinkestel C., Cooper D.J., Capellier G., Pellegrino V., Pilcher D., McQuilten Z. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann. Intensive Care. 2016;6:97. doi: 10.1186/s13613-016-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotz C., Streiber N., Roewer N., Lepper P.M., Muellenbach R.M., Kredel M. Therapeutic interventions and risk factors of bleeding during extracorporeal membrane oxygenation. Am. Soc. Artif. Intern. Organs J. 2017;63:624–630. doi: 10.1097/MAT.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 15.Koster A., Ljajikj E., Faraoni D. Traditional and non-traditional anticoagulation management during extracorporeal membrane oxygenation. Ann. Cardiothorac. Surg. 2019;8:129–136. doi: 10.21037/acs.2018.07.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy J.H., Staudinger T., Steiner M.E. How to manage anticoagulation during extracorporeal membrane oxygenation. Intensive Care Med. 2022;48:1076–1079. doi: 10.1007/s00134-022-06723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu D., Gallus A., Hirsh J., Cade J. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N. Engl. J. Med. 1972;287:324–327. doi: 10.1056/NEJM197208172870703. [DOI] [PubMed] [Google Scholar]
- 18.McMichael A.B.V., Ryerson L.M., Ratano D., Fan E., Faraoni D., Annich G.M. 2021 ELSO adult and pediatric anticoagulation guidelines. Am. Soc. Artif. Intern. Organs J. 2022;68:303–310. doi: 10.1097/MAT.0000000000001652. [DOI] [PubMed] [Google Scholar]
- 19.Shah A., Pasrija C., Kronfli A., Essien E.-O., Zhou Y., Brigante F., Bittle G., Menaker J., Herr D., Mazzeffi M.A., Deatrick K.B., Kon Z.N. A comparison of anticoagulation strategies in veno-venous extracorporeal membrane oxygenation. Am. Soc. Artif. Intern. Organs J. 2022;68:738–743. doi: 10.1097/MAT.0000000000001560. [DOI] [PubMed] [Google Scholar]
- 20.Sklar M.C., Sy E., Lequier L., Fan E., Kanji H.D. Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure. A systematic review. Annals ATS. 2016;13:2242–2250. doi: 10.1513/AnnalsATS.201605-364SR. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg R.D. Heparin, antithrombin, and abnormal clotting. Annu. Rev. Med. 1978;29:367–378. doi: 10.1146/annurev.me.29.020178.002055. [DOI] [PubMed] [Google Scholar]
- 22.Li W., Johnson D.J.D., Esmon C.T., Huntington J.A. Structure of the antithrombin–thrombin–heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat. Struct. Mol. Biol. 2004;11:857–862. doi: 10.1038/nsmb811. [DOI] [PubMed] [Google Scholar]
- 23.Schipper H., Kahlé L., Jenkins C.P., Cate J.W.T. Antithrombin-III transfusion in disseminated intravascular coagulation. Lancet. 1978;311:854–856. doi: 10.1016/s0140-6736(78)90196-4. [DOI] [PubMed] [Google Scholar]
- 24.Baudo F., Caimi T.M., deCataldo F., Ravizza A., Arlati S., Casella G., Carugo D., Palareti G., Legnani C., Ridolfi L., Rossi R., D'Angelo A., Crippa L., Giudici D., Gallioli G., Wolfler A., Calori G. Antithrombin III (ATILL) replacement therapy in patients with sepsis and/or postsurgical complications: a controlled double-blind, randomized, multicenter study. Intensive Care Med. 1998;24:336–342. doi: 10.1007/s001340050576. [DOI] [PubMed] [Google Scholar]
- 25.Eisele B., Heinrichs H., Delvos U., Lamy M., Thijs L.G., Keinecke H.O., Schuster H.P., Matthias F.R., Fourrier F. Antithrombin III in patients with severe sepsis: a randomized, placebo-controlled, double-blind multicenter trial plus a meta-analysis on all randomized, placebo-controlled, double-blind trials with antithrombin III in severe sepsis. Intensive Care Med. 1998;24:663–672. doi: 10.1007/s001340050642. [DOI] [PubMed] [Google Scholar]
- 26.Warren B.L., Eid A., Singer P., Pillay S.S., Carl P., Novak I., Chalupa P., Atherstone A., Pénzes I., Kübler A., Knaub S., Keinecke H.-O., Heinrichs H., Schindel F., Juers M., Bone R.C., Opal S.M. For the KyberSept trial study group, high-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 27.Hayakawa M., Yamakawa K., Kudo D., Ono K. Optimal antithrombin activity threshold for initiating antithrombin supplementation in patients with sepsis-induced disseminated intravascular coagulation: a multicenter retrospective observational study. Clin. Appl. Thromb. Hemost. 2018;24:874–883. doi: 10.1177/1076029618757346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niimi K.S., Fanning J.J. Initial experience with recombinant antithrombin to treat antithrombin deficiency in patients on extracorporeal membrane oxygenation. J. Extra Corpor. Technol. 2014;46:84–90. [PMC free article] [PubMed] [Google Scholar]
- 29.Chlebowski M.M., Baltagi S., Carlson M., Levy J.H., Spinella P.C. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit. Care. 2020;24:19. doi: 10.1186/s13054-020-2726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.