A recent Avian Influenza outbreak has become a significant issue for the dairy business, especially the highly virulent H5N1 strain. The virus, previously limited to wild birds and poultry, has recently been found in dairy cows, raising concerns among scientists and public health officials on possible consequences for animal and human health. This editorial explains the latest information regarding H5N1's effects on dairy cows. It elucidates the threats to public health and consumer safety related to these effects.
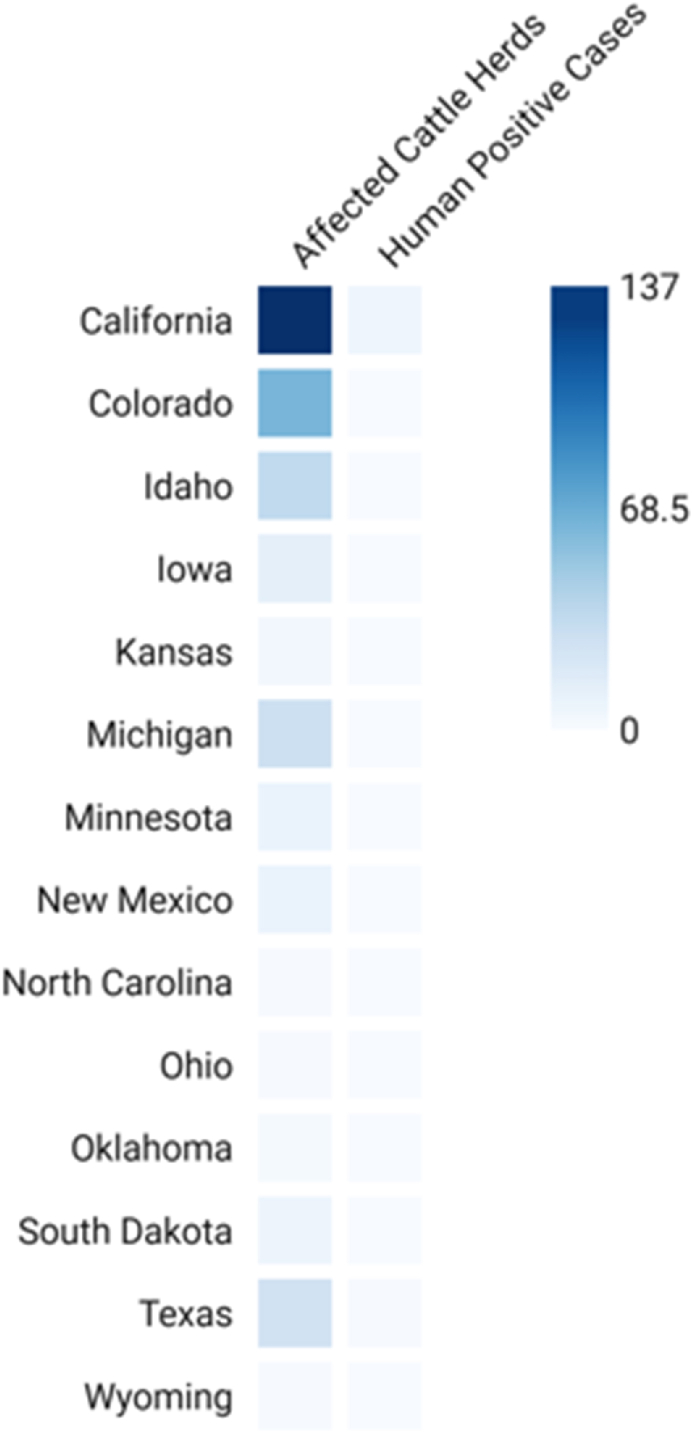
H5N1 outbreak among dairy cows was first detected in the United States on 25th March 2024; some herds in some states such as North Carolina, Ohio, Oklahoma, South Dakota, Texas, Wyoming, Colorado, Idaho, Iowa, Kansas, Michigan, Minnesota, New Mexico, and Colorado (Fig. 1) [1] have tested positive for the virus [2]. Known for its extreme mortality in birds, the H5N1 virus is currently causing alarm since it can spread to infect cattle. This virus has been observed to be present in respiratory secretions from diseased cows and raw (unpasteurized) milk [2]. The disease mainly affects elderly dairy cows and presents as decreased appetite, limited milk output, and overall sickness [3,4].
Fig. 1.
Heat map of H5N1's infected states and positive cattle and human cases.
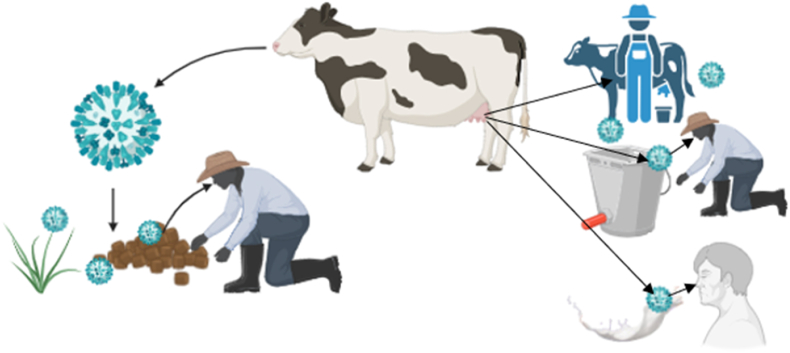
The transmission of this virus to cows is the crucial factor in determining its pathogenicity, clinical identification, treatment, and prevention. It is possible to decide on several pathways by which H5N1 could spread from livestock to people, such as direct contact: Direct contact with infected livestock or contaminated surfaces can result in human infection [5]; Contamination of the environment: The virus can survive in contaminated areas, such as water and feed sources, which raises the possibility of transmission [6]; Animal products: Human infection is directly possible if undercooked or tainted dairy products from infected cattle are consumed [7]. According to current research, the virus may also spread by “fomite transmission,” in which tainted milking equipment helps cows become infected because milk contains a high concentration of the virus (Fig. 2) [2]. This demonstrates the critical necessity for strict biosecurity protocols on dairy farms, such as the need that dairy workers wear personal protective equipment and that milking equipment be regularly disinfected [2,8].
Fig. 2.
Bird flu H5N1 is Potentially transmitted from cow to humans in several ways.
Clinical, diagnostic, and therapeutic considerations have increased significantly since the avian influenza (H5N1) outbreak in dairy cows. Low appetite, decreased milk output, lethargy, and irregular milk appearance—which might include thickening and discolouration—are among the clinical indications seen in affected dairy cows [9]. It has been shown that lactating cows display symptoms more strongly and that less than 10 % of cows in an infected herd show signs of lesser illness [9]. Up to 40 % of dairy cows in certain herds have displayed clinical symptoms. Notably, compared to poultry, the morbidity rate in cattle is much lower because most afflicted animals tend to recover with the proper supportive care and close observation [9]. A few cases of human infection have been reported thus far (Fig. 1), mostly involving workers who came into close contact with diseased cows [2]. Most of these people have only had moderate illness, but because of the naive immune condition of the human population with regard to H5N1, there is still a chance of more severe epidemics [2].
So, it is impossible to ignore the consequences of this outbreak on human health. Because dairy farmers and customers have intimate relationships with animals, there is a higher chance of zoonotic transmission. Several crucial procedures are involved in zoonotic transmission mechanisms, like a). Viral shedding: Feces and respiratory secretions from infected animals spread the virus, contaminating goods and the environment [5], b). Immune response: Cattle that are infected may have changed immune responses, which could allow the virus to continue and possibly change, raising the possibility that it will spread to people [5], c). Interspecies interactions: H5N1's zoonotic potential can be increased by close contact between various animal species, such as cattle and poultry, which can help mix viruses [6]. The probability of H5N1 infecting humans is dependent on a number of factors. Occupational exposure is one of them because they handle sick animals more frequently, farmers and other workers are also more vulnerable to contracting the virus. The necessity of surveillance and monitoring in cattle populations is highlighted by the fact that prior human infection instances suggest possible transmissibility from diseased animals (Fig. 2).
The new host for this virus is becoming an alarming concern for scientists, and more studies are underway to find some insights into this aberrant infection. RT-PCR testing is one diagnostic technique used to identify avian influenza in dairy cows. This test can quickly identify H5N1 in milk samples from critically ill nursing cows [10]. To confirm illness, surveillance programs—which veterinary health officials oversee—involve strategic milk, nasal swab, and tissue sample collection [9]. Subtyping is carried out by additional testing at the National Veterinary Services Laboratories (NVSL) to verify the initial testing results [10]. Moreover, veterinary professionals diagnose and notify municipal or state animal health authorities of incidences of avian influenza in dairy cattle. In-depth case data is gathered, and samples are forwarded to labs for verification. Following confirmation, instances are reported to national and international organizations, including the World Organization for Animal Health (OIE) and the USDA. This information is essential for putting control measures like vaccination and quarantine into place, guaranteeing prompt and efficient disease management, and controlling outbreaks [11].
There is no licensed vaccine for H5N1 avian influenza in dairy cows [12], but there are a number of intriguing areas for future research on avian influenza vaccinations for cattle. Among these are the development of efficient challenge models to evaluate the effectiveness of vaccinations and the quick adaptation of mRNA vaccines to novel strains. In addition, scientists are developing cross-species vaccines to safeguard cattle and poultry, strengthening biosecurity protocols, and advancing surveillance systems with quick diagnostic capabilities. Research institutes, governmental organizations, and the commercial sector must work together to advance these projects [13]. However, biosecurity measures are deemed critical in preventing the virus's spread to and among cattle populations. The USDA recommends that veterinarians and producers maintain robust monitoring practices and isolate any newly received dairy cattle for 30 days upon arrival [5].
The mainstay of treatment for dairy cows with avian influenza is supportive care intended to improve recovery and manage clinical symptoms [9]. Many cases heal quickly—in as little as 10 to 14 days—especially when given enough food and fluids [10]. Careful observation of the patient's condition and the application of biosecurity protocols are essential [2]. In case of human infection, the commercial milk supply is still safe to eat despite these worries as long as the milk is pasteurized. Pasteurization reduces consumer hazards by making the H5N1 virus inactive [2]. It is necessary to launch educational campaigns to warn people about the dangers of raw milk and deter people from buying and consuming unpasteurized goods, which health authorities have already advised against because of the possibility of pathogen contamination [3,8].
The dairy industry is facing severe financial consequences due to the avian influenza (H5N1) outbreak in cows. Reduced milk production is the main financial loss associated with avian influenza in dairy cows; estimates indicate that infected dairy cows lose 10–20 % of their milk production over 7–10 days [14]. According to the American Association of Bovine Practitioners (AABP), H5N1 costs dairy cattle between $100 and $200 per cow [15].
Together, the outbreak of avian influenza in dairy cows is a severe public health concern, and its devastating effects on the industry need to be addressed immediately. As the situation develops, it will be crucial to maintain public awareness, implement biosecurity, and monitor ongoing developments to reduce the hazards to the health of both humans and animals. To safeguard agricultural interests and public health, the dairy industry and health officials must continue to tackle the problems posed by this outbreak diligently.
CRediT authorship contribution statement
Md. Amir Hossain: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Methodology, Conceptualization. Md. Abdul Monem: Writing – review & editing, Writing – original draft, Conceptualization. Asma Akther Rina: Writing – review & editing, Visualization, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no conflicts of interest to report concerning the letter to editor, financial interests, or other affiliations that could be perceived as biasing the content or conclusions presented in this letter.
Contributor Information
Md. Amir Hossain, Email: dr.amir.kau@gmail.com.
Md. Abdul Monem, Email: monemabdul52@gmail.com.
Asma Akther Rina, Email: asma.rina.hossain@gmail.com.
References
- 1.Garg S. Outbreak of highly pathogenic avian influenza A(H5N1) viruses in U.S. Dairy cattle and detection of two human cases — United States, 2024. MMWR Morb Mortal Wkly Rep. 2024;73 doi: 10.15585/mmwr.mm7321e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Highly pathogenic avian influenza (bird flu) outbreak in cattle. https://www.health.ny.gov/diseases/communicable/influenza/avian/status.htm
- 3.Program H.F. FDA; Sep. 2024. Updates on highly pathogenic avian influenza (HPAI)https://www.fda.gov/food/alerts-advisories-safety-information/updates-highly-pathogenic-avian-influenza-hpai [Google Scholar]
- 4.Sah R., et al. Concerns on H5N1 avian influenza given the outbreak in U.S. dairy cattle. Lancet Reg. Health – Am. 2024;35(Jul) doi: 10.1016/j.lana.2024.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butt S.L., Nooruzzaman M., Covaleda L.M., Diel D.G. Hot topic: influenza A H5N1 virus exhibits a broad host range, including dairy cows. JDS Commun. Oct. 2024;5:S13–S19. doi: 10.3168/jdsc.2024-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone H., et al. May 2024. Potential pathways of spread of highly pathogenic avian influenza A/H5N1 clade 2.3.4.4b across dairy farms in the United States. [DOI] [Google Scholar]
- 7.Apostolopoulos V., Chavda V.P., Mehta R., Rodriguez-Morales A.J., Henao-MartÍnez A.F., Sah R. Alert and surveillance on H5N1 influenza virus: risks to agriculture and public health. Ther. Adv. Infect. Dis. Jan. 2024;11 doi: 10.1177/20499361241266521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.What's happening with dairy cows and bird flu | Johns Hopkins. Bloomberg School of Public Health; 2024. https://publichealth.jhu.edu/2024/whats-happening-with-dairy-cows-and-bird-flu [Google Scholar]
- 9.Avian influenza virus type A (H5N1) in U.S. dairy cattle | American Veterinary Medical Association. https://www.avma.org/resources-tools/animal-health-and-welfare/animal-health/avian-influenza/avian-influenza-virus-type-h5n1-us-dairy-cattle
- 10.Bovine Influenza | Iowa State University.” Accessed: September. 21, 2024. https://vetmed.iastate.edu/vdl/resources/client-services/pathogens/bovine-influenza.
- 11.CDC How NNDSS conducts case surveillance | CDC. https://www.cdc.gov/nndss/about/conduct.html
- 12.Avian influenza virus type A (H5N1) in U.S. dairy cattle | American Veterinary Medical Association. https://www.avma.org/resources-tools/animal-health-and-welfare/animal-health/avian-influenza/avian-influenza-virus-type-h5n1-us-dairy-cattle
- 13.Schnirring Lisa. USDA scientists weigh avian flu vaccine for cows; virus may be spreading from cattle to poultry | CIDRAP. https://www.cidrap.umn.edu/avian-influenza-bird-flu/usda-scientists-weigh-avian-flu-vaccine-cows-virus-may-be-spreading-cattle
- 14.Bird Flu, U.S. Cows, and Economic Consequences | Think Global Health,” Council on Foreign Relations. Accessed: September. 21, 2024. https://www.thinkglobalhealth.org/article/bird-flu-us-cows-and-economic-consequences.
- 15.Malinda Larkin, “$200M from federal government aims to stop spread of H5N1 among dairy cows | American Veterinary Medical Association.” Accessed: November. 6, 2024. https://www.avma.org/news/200m-federal-government-aims-stop-spread-h5n1-among-dairy-cows.