Abstract
Objectives:
The study aimed to develop evidence-based recommendations for the treatment of rapidly progressive interstitial lung disease (RPILD) associated with the anti-Melanoma Differentiation-Associated Gene 5-positive dermatomyositis (DM) syndrome.
Methods:
The task force comprised an expert panel of specialists in rheumatology, intensive care medicine, pulmonology, immunology, and internal medicine. The study was carried out in two phases: identifying key areas in the management of DM-RPILD syndrome and developing a set of recommendations based on a review of the available scientific evidence. Four specific questions focused on different treatment options were identified. Relevant publications in English, Spanish or French up to April 2018 were searched systematically for each topic using PubMed (MEDLINE), EMBASE, and Cochrane Library (Wiley Online). The experts used evidence obtained from these studies to develop recommendations.
Results:
A total of 134 studies met eligibility criteria and formed the evidentiary basis for the recommendations regarding immunosuppressive therapy and complementary treatments. Overall, there was general agreement on the initial use of combined immunosuppressive therapy. Combination of high-dose glucocorticoids and calcineurin antagonists with or without cyclophosphamide is the first choice. In the case of calcineurin antagonist contraindication or treatment failure, switching or adding other immunosuppressants may be individualized. Plasmapheresis, polymyxin B hemoperfusion and/or intravenous immunoglobulins may be used as rescue options. ECMO should be considered in life-threatening situations while waiting for a clinical response or as a bridge to lung transplant.
Conclusions:
Thirteen recommendations regarding the treatment of the anti-MDA5 positive DM-RPILD were developed using research-based evidence and expert opinion.
Keywords: Intensive care, Dermatomyositis, Rapidly progressive interstitial lung disease, Glucocorticoid, Cyclosporine, Tacrolimus, Cyclophosphamide, Mycophenolate, Rituximab, Basiliximab, Tofacitinib, Intravenous immunoglobulins, Plasmapheresis, Polymixyn B hemoperfusion, Extracorporeal membrane oxygenation, Lung transplant, Review, systematic
Introduction
Idiopathic inflammatory myopathies are a heterogeneous group of systemic autoimmune diseases usually characterized by inflammatory infiltrates in the muscle biopsy. Several phenotypes are included, being dermatomyositis (DM) one of the best recognized [1]. The autoantibody profile allows individualizing the clinical presentations in DM patients being some manifestations linked to specific autoantibodies. This is the case of the clinically amyopathic dermatomyositis (CADM) with anti-melanoma differentiation-associated gene 5 (MDA5) antibodies [2]. Those are patients with the characteristic skin rash of the disease, with Gottron’s papules and heliotrope sign, but without muscle weakness, herein the name of clinically amyopathic DM. The distribution of classic DM and CADM in anti-MDA5 positive patients varies among ethnic groups, being CADM around 82% in Japan and between 42–50% in non-Japanese patients [3–5]. At least three different subsets of DM positive for anti-MDA5 antibodies can be identified [6–9], a cutaneous form without muscle or lung involvement, a chronic form of cutaneous features with interstitial lung disease resembling the antisynthetase syndrome, and lastly the most severe form of cutaneous manifestations with rapidly progressive ILD (RPILD). Patients with DM anti-MDA5 with RPILD usually have a bad prognosis, and, although mortality figures may vary among different ethnicities [10,11], up to 80% of these patients do not survive even after an early diagnosis or intensive immunosuppressive therapy [12]. Therefore, the aim of this study, with the participation of the different areas of knowledge implicated in its treatment (i.e. intensive care unit, rheumatology, pulmonology, immunology and internal medicine) is to provide evidence-based recommendations on the different treatments until now used in these patients in order to define which will be the best treatment to offer, and to define an algorithm of actuation.
Recommendations’ questions
In order to address our objective, the main clinical question formulated was, 1) “Which is the effectiveness, efficacy, and safety of the different treatments administered in anti-MDA5 positive DM-RPILD patients?”
However, given that scarce scientific evidence on the matter was expected, and that some CDM patients with RPILD were reported before the detection of anti-MDA5 antibodies was available, the Expert Panel decided to analyze also other groups of related conditions. Thus, three additional clinical questions were formulated to prepare the scientific evidence search strategy and further facilitate the reaching of our objective:
2. Which is the effectiveness, efficacy, and safety of the different treatments administered in anti-MDA5 positive patients with non-RPILD or other type of ILD such as usual interstitial pneumonia (UIP), non-specific interstitial pneumonia (NSIP), or cryptogenic organizing pneumonia (COP)?
3. Which is the effectiveness, efficacy, and safety of the different treatments administered in patients with inflammatory myopathy and RPILD negative to or with unknown status of anti-MDA5 antibodies?
4. Which is the effectiveness, efficacy, and safety of the different treatments administered in RPILD anti-MDA5 negative antibody patients with systemic autoimmune diseases other than dermatomyositis?
Methods
Study design.
A qualitative synthesis of the scientific evidence currently available was performed. Consensus techniques of methodology were used to collect expert opinion based on the participants’ clinical experience when only no or low-quality scientific evidence was available.
Study stages.
This study has been developed according to the different stages for elaborating Clinical Practice Guidelines (CPG) in the Spanish National Health System [13]. The process was divided into seven different stages.
Recommendations of the working group.
The guidelines working group made up of 7 healthcare professionals from different disciplines in the area of myositis and progressive interstitial lung disease (rheumatology, internal medicine, intensive care medicine, immunology and pulmonology). The expert group has been managed by a clinical and methodological coordination team. The different Scientific Societies involved were contacted agreeing to be represented in the development group.
Identification of key areas.
The expert group defined the main objectives of the recommendations. They identified those clinical questions expected to have the greatest impact on the management of DM-RPILD syndrome in anti-MDA5 positive patients.
Analysis of scientific evidence.
The research question was formulated according to the Population, Intervention, Comparison, Outcome (PICO) format. The question related to lung transplantation was not framed in the PICO format, and was based on a non-systematic review of the studies published on the topic. A systematic literature review was performed in PubMed (MEDLINE), EMBASE (Elsevier), and Cochrane Library (Wiley Online) until April 2018; subsequently the expert group identified some studies which had been published till July 2019 and were included in the evidence corpus. The search strategy was constructed by an experienced medical librarian; included studies published in English, Spanish or French and were limited to studies in humans. The search strategy was developed initially in PubMed using controlled vocabulary and free text terms, and then it was adapted for each of the other databases to find publications about “interstitial lung diseases” and synonyms. Articles were excluded if they were (1) meeting abstracts not subsequently published in peer-reviewed journals; (2) editorials, commentaries and narrative reviews. Additional information about the search strategy can be consulted as on-line supplementary material (available in the Data Supplement).
Analysis and summary of scientific evidence.
Evaluation of the quality of the studies and summary of the evidence for each question was performed using the critical reading tool of the Agency for Healthcare Technology Assessment of the Basque Country (OSTEBA) [14]. Furthermore, the determination of the evidence levels and the recommendations grade was based on SIGN methodology (Scottish Intercollegiate Guidelines Network) [13]. (Appendix 1).
Formulation of recommendations.
Formulation of recommendations was based on the “formal evaluation” or “justified opinion” of SIGN [13]. To determine the strength of each one of the formulated recommendations, the development group has considered not only the level of evidence available but also the equilibrium between desirable and undesirable consequences of carrying out the recommendation. The good clinical practice recommendations have been formulated and agreed by consensus following a transparent methodology with a face-to-face meeting of the development group and a subsequent series of successive consultation rounds with a panel of experts. These recommendations have been divided into four complementary areas: general management, combination therapy, therapy for the refractory patient and other therapeutic options (Table 1).
Table 1.
Recommendations for the treatment of anti-MDA5 positive CADM-RPILD*.
| Set ofRecommendations | RG** | |
|---|---|---|
| General management | ||
| 1 | Patients with DM-associated rapidly progressive interstitial lung disease anti-MDA5 (+) should be treated with combination therapy as a first option. | D |
| Combination therapy | ||
| 2 | A combination therapy which include glucocorticoids plus a calcineurin inhibitor (cyclosporine A or tacrolimus), or triple therapy adding intravenous cyclophosphamide†ฎ to the previous schedule, are both considered good initial alternatives. | D |
| 2a | Both, cyclosporine A and tacrolimus are considered equally good therapeutic options. The choice of any of them will depend on the safety profile and patients’ characteristics. | √ |
| 2b | Monitoring of calcineurin inhibitors blood levels are recommended in order to adjust posology and minimize toxicity. | √ |
| 3 | When calcineurin inhibitors are not feasible, consider combination therapy with glucocorticoids and other immunosuppressive drugs such as cyclophosphamide†ฎ and/or mycophenolate mofetil☨ or adding rituximab☨ to any one of the previous schedules. | D |
| 3a | The choice of one of these drugs will depend on the individual characteristics of the patient and the clinician experience. | √ |
| Therapy for the refractory patient | ||
| 4 | In patients with CADM-associated RPILD anti-MDA5 (+) who do not respond to combination therapy with glucocorticoids plus immunosuppressive drugs, clinicians have to take into account the following alternatives: | |
| - Adding one ofthese immunosuppressive drugs (cyclophosphamide, mycophenolate mofetil, rituximab, basiliximab or tofacitinibʃ) to the current therapy. | D | |
| - Change one immunosuppressant for another | √ | |
| 5 | In patients who do not respond to combined immunosuppressive drugs, the use of the following alternative rescue therapies, either separate or in a sequential manner, might be considered: | |
| - Polymyxin B hemoperfusion | D | |
| - Plasmapheresis | D | |
| - Intravenous immunoglobulins | √ | |
| 6 | Assistance with ECMO should be considered in patients with life threatening severe and refractory respiratory insufficiency in order to maintain the patient alive while waiting for a clinical response to intensive and combined immunosuppressive treatment or as a bridge to lung transplantation. | √ |
| 7 | Lung transplantation should be considered as a therapeutic option in patients with refractory RPILD associated to anti- MDA5. Early referral for transplant eligibility assessment is recommended at the time of ILD diagnosis. | √ |
| Other treatment options | ||
| 8 | Azathioprine, methotrexate and leflunomide are not recommended for the treatment of RPILD associated to anti-MDA5. | √ |
| 9 | Infliximab is not recommended in anti-MDA-5 associated RPILD treatment | √ |
| 10 | Although pirfenidone has been added to conventional immunosuppressant treatment in CADM-associated subacute interstitial pneumonia with data of pulmonary fibrosis, the expert panel may not recommend its use in patients with RPILD associated to anti-MDA5. | √ |
Level of evidence was 3 in all the recommendations.
Avoid its administration in young female or male who are willing to have offspring.
Avoid its administration in women prone to be pregnant due to the risk of fetal embryopathy.
There is not available data on the safety of combined therapy with biologic agents and tofacitinib. Abbreviations: R, Recommendation. RG, Recommendation Grade based on SIGN methodology, see Appendix 1. RPILD, Rapidly Progressive Interstitial Lung Disease. MDA5, Melanoma Differentiation-Associated protein 5. Anti-MDA5, anti-MDA5 antibodies. ECMO, Extracorporeal Membrane Oxygenation.
External review.
External reviewers have participated in the review of the second draft. The purpose of submitting the CPG to external review was to improve the overall quality, to ensure the appropriateness of recommendations, to disseminate the evidence, as well as to assess its applicability and feasibility.
Public Display.
The draft of recommendations was subject to public comment by the Spanish Society of Rheumatology associate members and different interest groups (the pharmaceutical industry, other scientific societies, and patient associations). The objective was to collect scientific input on the methodology and recommendations put forth by the document.
Conflicts of interest
All members of the Expert Panel completed the disclosure form, which requires disclosure of financial and other interests, including relationships with commercial entities that are reasonably likely to experience direct regulatory or commercial impact as a result of promulgation of the guideline. Categories for disclosure include employment; leadership; stock or other ownership; honoraria, consulting or advisory role; speaker’s bureau; research funding; patents, royalties, other intellectual property; expert testimony; travel, accommodations, expenses; and other relationships. In accordance with the Policy, the majority of the members of the expert panel did not disclose any relationships constituting a conflict under the Policy.
Overarching principles
Diagnostic accuracy and rationale of the different questions, methods of anti-MDA5 detection and brief description of the different therapies administered
Not generally accepted diagnostic criteria for patients with the anti-MDA5 syndrome do exist. Therefore, most studies included patients with definite or probably DM, usually clinically amyopathic, and antibodies positive to MDA5 detected using home-made ELISA or blot, protein immunoprecipitation or commercial tests such as EUROIMMUN. Altogether RPILD was considered when worsening of radiologic interstitial changes with progressive dyspnea and hypoxemia within 1 month after the onset of respiratory symptoms appeared. The diagnosis of ILD was established by chest X-ray and/or high-resolution CT scan showing reticular opacities, ground-glass opacity (GGO) or honeycomb appearance [15].
One of the proposed strategies to treat properly these patients includes risk stratification. In this setting, it is important to evaluate those parameters that can act as an activity surrogate. Although a myriad of biomarkers has been described [16], ferritin is the most recognized factor. Hoa et al [17] found in a series of anti-MDA5 (+) RP-ILD associated DM, that levels of ferritin were in the range of 370–13,878 ng/ml (NV < 200 ng/ml). Blood values higher than 1000 ng/ml, seem to be associated with higher mortality in Caucasians and Asian ethnicities [18–21]; moreover, ferritin values run in parallel to the activity of the disease [22]. Beside the ferritin, Krebs von den Lungen-6 (KL-6), a type II pneumocyte glycoprotein has been postulated as a biomarker of ILD in different ethnicities [23,24]. Nevertheless, although in anti-MDA5 (+) patients the value of KL6 is high, it does not correlate with activity, treatment response, or mortality [20,22,25,26]. Values of C reactive protein higher than 1 mg/dL and age older than 60 years seem to be risk factors of bad prognosis [11]. Finally, several articles focused on the level of the anti-MDA5 values measured using ELISA test. Higher values of anti-MDA5 antibodies correlate with a worst outcome [16,20,27,28] and seem to be a good biomarker of relapse [22].
The different therapies that have been administered to these patients are described in Table 2.
Table 2.
Reported therapies in anti-MDA5 positive DM associated RPILD.
| Therapy | Dose, schedule and route ofadministration |
|---|---|
| Prednisone/prednisolone1 | 0.5–1 mg/kg/day p.o. |
| Pulsed methylprednisolone1 | 500 mg-1 gr/day (×3consecutive days) i.v. |
| Cyclosporine A2 | 2–5 mg/kg/day p.o. or i.v. |
| Tacrolimus3 | 0.06–0.075 mg/kg/day p.o. |
| Cyclophosphamide | 0.5–1 gr/m2/2–4 weeks i.v. |
| Azathioprine4 | 2–3 mg/kg/day p.o. |
| Leflunomide5 | 10–20 mg/day p.o. |
| Methotrexate6 | Up to 25 mg/week p.o. or s.c. |
| Mycophenolate mofetil | 1–3 g/day p.o. |
| Basiliximab | 20 mg/week (×2) i.v. |
| Infliximab | 5 mg/kg i.v. at week 0,2,6 and every 8 weeks |
| Rituximab | 350–375 mg/m2/week (×2–4) i.v. or 1 gr/2 week (×2) i.v. |
| Tofacitinib | 5 mg b.i.d. p.o. |
| Pirfenidone | 267 mg t.i.d. p.o. |
| Immunoglobulin | 0.4 g/kg/5 days i.v. |
| Polymyxin B and plasmapheresis | Hemoperfusion with polymyxin B at a flow rate of 100 ml/h for 3 h/day (×2) and plas mapheresis with 3.51 of 5% seroalbumin replacement followed by intravenous immunoglobulin |
Corticosteroids as initial or induction/rescue therapy.
To achieve a blood level of 1000 ng/mL during induction therapy, if possible.
To achieve a blood level of 10–15 ng/mL during induction therapy, if possible.
Depending on thiopurine methyl transferase activity.
Dose not reported.
Not administered in anti-MDA5 associated RPILD. p.o.: per os. i.v.: intravenous. s.c.: subcutaneous; bid: twice in a day. tid: three in a day.
Results
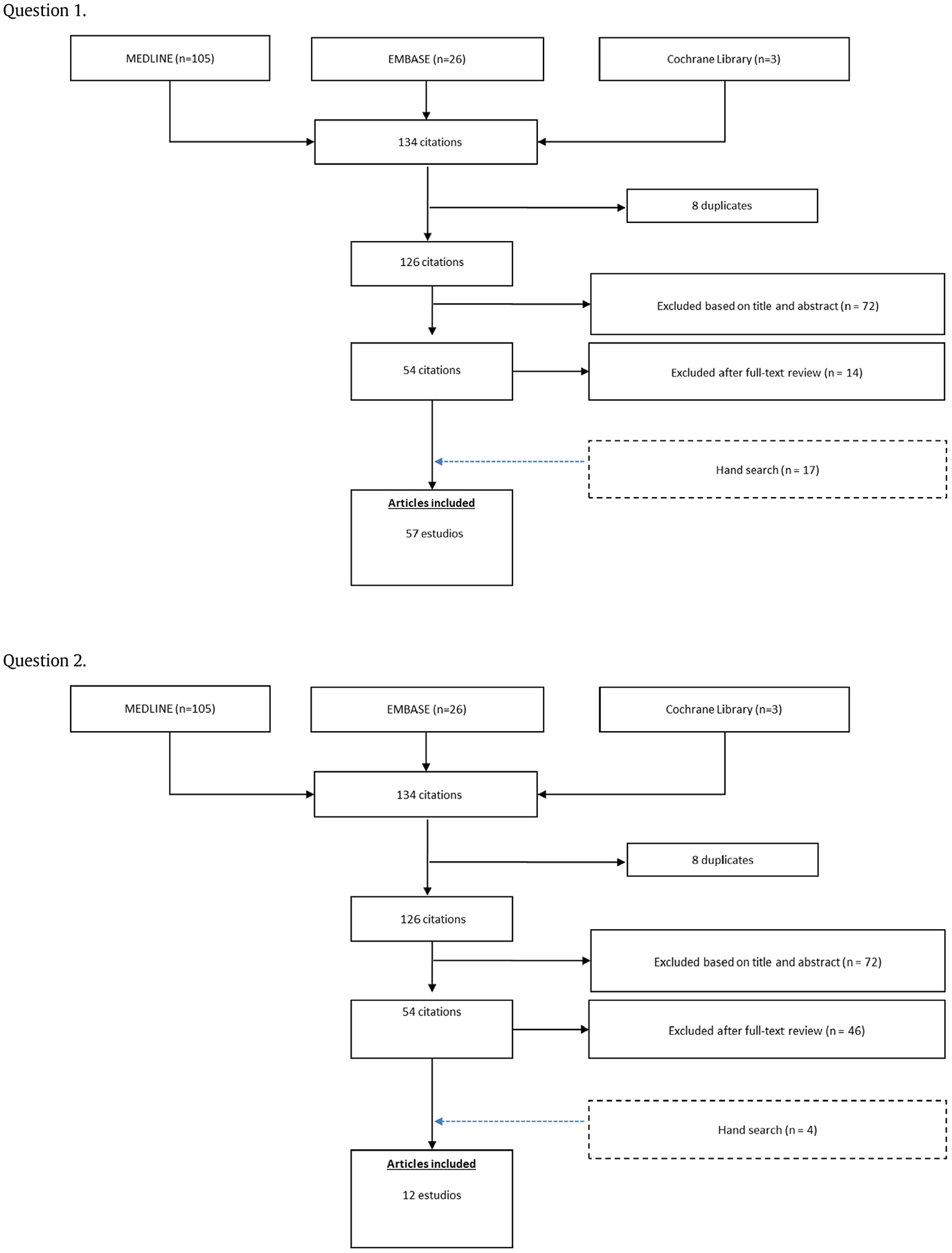
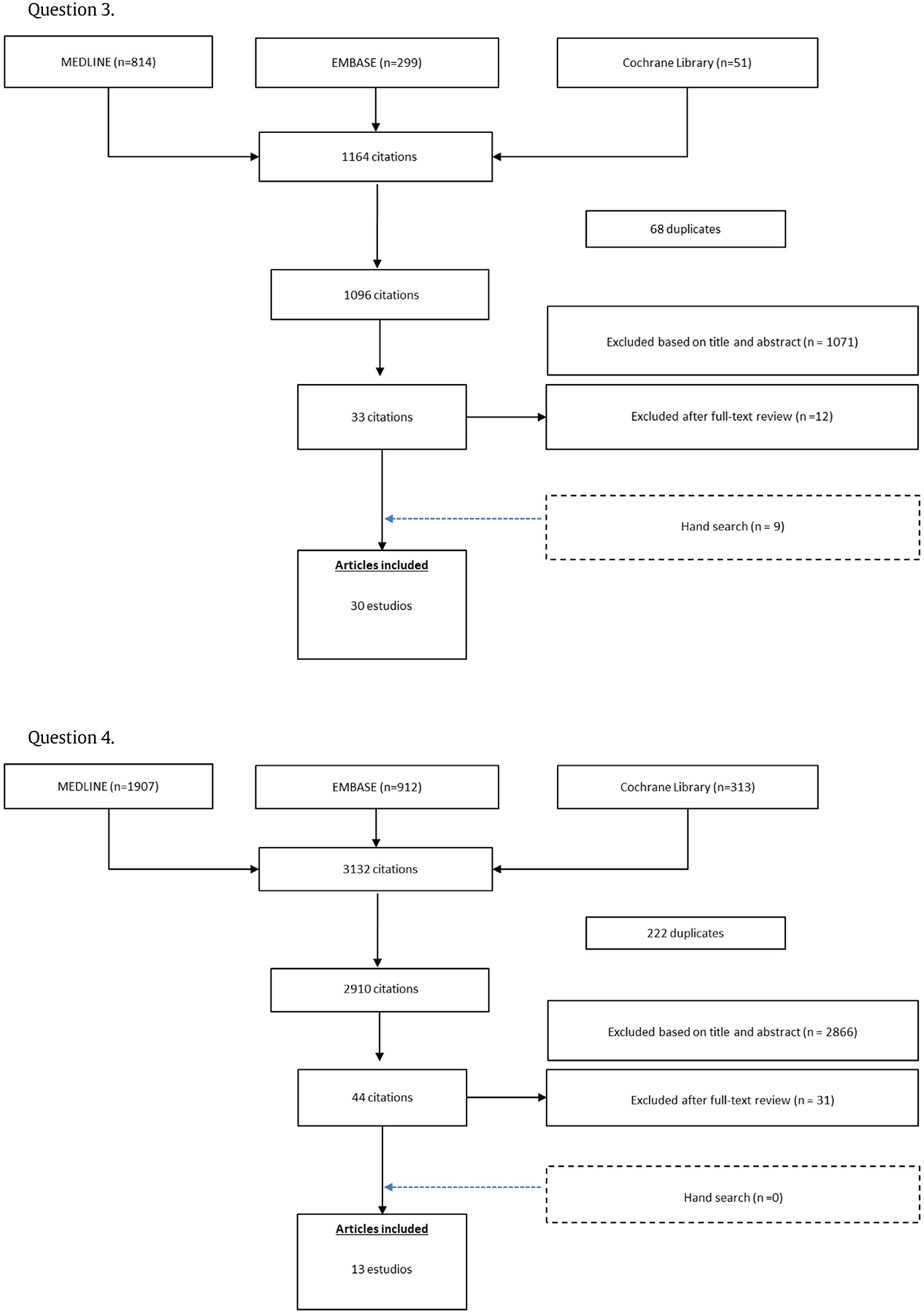
By the search strategy, 134, 134, 1164, and, 3132 references were respectively identified. Of these, 49, 8, 30, 13 full-text papers respectively were included in the systematic review. A detailed flow chart with the results of the literature search is shown in Appendix 2.
General Management
Recommendation 1: Patients with DM-associated rapidly progressive interstitial lung disease anti-MDA5 (+) should be treated with combination therapy as a first option. (Recommendation grade D).
Scientific evidence on efficacy and safety of the drugs used for the treatment of anti-MDA5 (+) associated RPILD comes from observational studies and case reports. All the identified studies include a combined or progressive administration of immunosuppressive drugs with or without support therapies. The usual approach comprises a combined schedule of glucocorticoids (oral prednisone or prednisolone, intravenous methylprednisolone pulse therapy, or both), immunosuppressive drugs (intravenous cyclophosphamide or calcineurin antagonists such as cyclosporine A or tacrolimus), and intravenous immunoglobulin as an adjuvant therapy [10,16–19,22,28–51] (Level of evidence 3).
Obtained data is mainly focused on mortality and prognosis factors that contribute to an interstitial pneumonia favorable outcome. In summary, all the studies gave support to the combination therapy. Accordingly, and considering their clinical expertise, the elaborating group also supports combination therapy as the best available treatment in order to improve the clinical outcome and reduce the mortality in these patients.
Combination therapy
Recommendation 2: A combination therapy including glucocorticoids plus a calcineurin antagonist (cyclosporine A or tacrolimus), or triple therapy adding intravenous cyclophosphamide to the previous schedule, are both considered good initial alternatives. (Recommendation grade D).
Recommendation 2a: Both, cyclosporine A and tacrolimus are considered equally good therapeutic options. The choice of any of them will depend on the safety profile and patients’ characteristics. (Recommendation grade √).
Recommendation 2b: Monitoring of calcineurin antagonists blood levels is recommended to adjust posology and minimize toxicity (Recommendation grade √).
A systematic review of the scientific evidence allowed us to identify several observational studies (case series) focused on the pharmacological combination therapy in patients with DM-associated RPILD and anti-MDA5 positive antibodies.
Three retrospective studies [16,19,37] aimed to analyze the differences in clinical activity and pulmonary function parameters between patients with anti-MDA5 positive antibodies and RPILD who died or survived, and to determine the main prognostic factors.
The first study [16], included 20 RPILD anti-MDA5 (+) patients, 12 of them received treatment with a combination of prednisolone and cyclophosphamide plus calcineurin antagonists (triple therapy). Seven out of 12 (58%) died and the other 5 (42%) developed a favorable outcome and survived. Eight patients received treatment with a combination of prednisolone and either cyclophosphamide or a calcineurin antagonist (2 died and 6 survived). The number of patients treated with the combination including a calcineurin antagonist is not specified.
At the second study [19] the authors identify 17 anti-MDA5 positive patients who develop RPILD among a series of 95 DM patients. In this study only one (16%) out of 6 patients who received triple therapy (prednisolone, cyclophosphamide and calcineurin antagonists) died. Among the other 11 who were treated with a combination therapy including prednisolone plus either cyclophosphamide or calcineurin antagonists, 3 (27%) died.
Finally, the third of the 3 retrospective studies previously mentioned [37] included 12 patients diagnosed with DM anti-MDA5 positive who develop a RPILD. Eight of these patients received combination therapy with prednisolone and cyclosporine, and only 3 (25%) died. The other 4 patients received triple therapy (prednisolone, cyclophosphamide and cyclosporine), being the mortality of 75% (3 patients) (Level of evidence 3).
Other study [22] analyzed 11 patients positive to anti-MDA5 with RPILD, who were also treated with triple therapy, being tacrolimus the calcineurin inhibitor used. A good clinical response was noticed and none of the patients died, although a non-significant trend to clinical relapse was observed in those patients who received a reduced number of intravenous cyclophosphamide cycles (Level of evidence 3).
Hozumi et al [40] reported 15 patients diagnosed with DM anti-MDA5 positive and ILD, 13 of them with anti-MDA5 positive and RPILD. Ten were treated with combination therapy that included prednisolone plus a calcineurin antagonist (cyclosporine in 8 patients and tacrolimus in 2), and 5 received a triple therapy scheme (prednisolone, cyclophosphamide and cyclosporine). Six out of 15 patients died, 5 of them due to respiratory failure and the other one of unknown cause (Level of evidence 3).
Other 4 retrospective studies adding indirect evidence were identified. Patients reported in these studies were mostly but not all anti-MDA5 positive, and there was no specific information for this subgroup. Tanizawa et al. [39] included 12 anti-MDA5 positive patients, five of whom developed RPILD. Seven out of the 12 patients died, five of them with RPILD, being six of them treated with triple therapy (glucocorticoid, cyclophosphamide and cyclosporine) and the other one with the combination of glucocorticoids and cyclosporine. Ikeda et al. [34] reported 10 patients positive to anti-MDA5 who developed ILD, 6 (60%) of them died, all with the RPILD phenotype, even though they received triple therapy. Ma X et al [35], reported 7 anti-MDA5 positive patients with RPILD, being treated with triple therapy including mycophenolate, leflunomide, intravenous immunoglobulin, and some naturist therapies (i.e. Chinese herbs). Six out of 7 (85%) died. A study published by Nakashima, et al [10], compare a cohort of 14 anti-MDA5 patients who develop RPILD and were treated with triple therapy (prednisolone, cyclophosphamide and cyclosporine) with a historical cohort who received standard therapy (not described). Mortality in the group treated with triple therapy was 25% in comparison with 71.4% of the historical cohort (Level of evidence 3).
Overall, published data are scarce and the level of evidence of the studies is weak. Hence, case reports were also included in the analysis, with a total of 53 anti-MDA5 positive DM patients with RPILD. The outcome of the reported cases that were treated with combination therapy (glucocorticoids, plus either cyclophosphamide or cyclosporine, or a combination of both immunosuppressive drugs) [28–32,36,38] was good, and only 2 cases died [30,36]. Other reported cases that used tacrolimus instead of cyclosporine [33,41–43,45,46], also had a good prognosis, except for two cases [45,46] and one out of the three reported cases in the Koguchi-Yoshioka H et al study [42] (Level of evidence 3).
In summary, from the analysis of the reported cases, 21 patients (40%) died, and 32 (60%) improved after immunosuppressive therapy. Most cases received combination therapy with glucocorticoids (either oral prednisone or prednisolone or pulsed methylprednisolone), cyclophosphamide and/or a calcineurin antagonist (cyclosporine or tacrolimus). Outcome data, in terms of mortality, on this combination therapies from retrospective studies and case reports are summarized in Table 3.
Table 3.
Research supporting the role of combined therapy, with glucocorticoids plus either cyclophosphamide or calcineurin inhibitors or triple therapy with the three of them, for the treatment of anti-MDA5 positive rapidly progressive interstitial lung disease (RPILD).
| Reference | Type of study | Patients (n) RPILD | Exitus |
|---|---|---|---|
| 16 | Retrospective | n=20 | 9/20 (45%) |
| 12 (GC+CYC+CNI) | 7/12 (58%) | ||
| 8 (GC+CYC or CNI) * | 2/8 (25%) | ||
| 40 | Retrospective | n=15†ฎ | 6/15 (40%) |
| 5 (GC+CYC+CNI) | Not specified | ||
| 10 (GC+CNI) | Not specified | ||
| 34 | Retrospective | n=10 | 6/10 (60%) |
| 10 (GC+CYC+CNI) | |||
| 35 | Retrospective | n=7 | 6/7 (85%) |
| 7 (GC+CYC+CNI) | |||
| 22 | Retrospective | n=11 | 0/11 (0%) |
| 11 (GC+CYC+CNI) | |||
| 19 | Retrospective | n=17 | 1/6 (16%) |
| 6 (GC+CYC+CNI) | 3/11 (27%) | ||
| 11 (GC+CYCor CNI)* | |||
| 10 | Retrospective | n=28 | 25% |
| 14 (GC+CYC+CNI) | 71% | ||
| 14 (Historical cohort) | |||
| 37 | Retrospective | n=12 | 3/4 (75%) |
| 4 (GC+CYC+CNI) | 3/8 (37%) | ||
| 8 (GC+CNI) | |||
| 39 | Retrospective | n=7†ฎ | 6/6 (100%) |
| 6 (GC+CYC+CNI) | 1/1 (100%) | ||
| 1 (GC+CNI) | |||
| 28,29,30,31, | Case reports (sum up) | n=16 | 5/16 (31%) |
| 32,33,36, | 10 (GC+CYC+CNI) | 5/10 (50%) | |
| 38,41,42, | 5 (GC+CNI) | 0/5 (0%) | |
| 43,45,46. | 1 (gc+cyc) | 0/1 (0%) |
GC, glucocorticoids; CYC, cyclophosphamide; CNI, calcineurin inhibitors; RP-ILD, rapidly progressive interstitial lung disease.
Number of patients treated with each combination not specified.
patients with chronic NI.
Two more published cases that included from the onset mycophenolate added to the combination therapy of glucocorticoid and calcineurin antagonists were identified. One is the case number 9 from Hoa et al [17] who presented a good outcome after being treated with mycophenolate, tacrolimus and glucocorticoids, and the other one (case 9) with RPILD reported by Takada et al. [44] developed a progressive course and died in spite of triple therapy with glucocorticoid, mycophenolate and cyclosporine (Level of evidence 3).
The expert group, therefore, considers that there is no enough information for a triple therapy recommendation including mycophenolate plus glucocorticoids and calcineurin antagonists from the disease onset.
Lastly, other studies providing indirect information have been identified. They included patients diagnosed with DM and negative for or with unknown anti-MDA5 antibodies status, who developed a RPILD. Combination therapy (glucocorticoid and calcineurin antagonists from the onset) effectively reduced mortality in comparison with historical controls treated only with glucocorticoids, mainly in those patients with acute ILD (6.7% vs. 28.6%, p=0.043) and (31% vs. 68%, p=0.049) [16,19]. Moreover, those DM patients with acute or subacute ILD who received triple therapy with glucocorticoids, cyclophosphamide and cyclosporine, had a survival of 50% [40,44].
Improvement of pulmonary function parameters, creatine-kinase and manual muscle test (MMT) score and a reduction in glucocorticoid requirement with an increase in disease-free survival (HR: 0.25; CI 95% 0.010–0.66, p=0.005) [34,35] was observed when tacrolimus was added to the standard immunosuppressive therapy (prednisolone and/or cyclophosphamide and/or cyclosporine). (Level of evidence 3).
Considering these results, the expert group stated that the first therapeutic option in anti-MDA5 positive patients with RPILD is a combination therapy including glucocorticoids plus the administration of a calcineurin antagonist, or alternatively a triple therapy with glucocorticoids, calcineurin antagonists and pulses of intravenous cyclophosphamide. If cyclophosphamide is not feasible, the administration of mycophenolate may be a good option.
Otherwise, although studies performed in myositis patients with RPILD, negative for or with unknown anti-MDA5 antibodies, suggest that adding tacrolimus to other immunosuppressive drugs (glucocorticoids and/or cyclophosphamide and/or cyclosporine) may improve the outcome of these patients, the evidence is so scarce that it does not allow to establish a preference for tacrolimus over cyclosporine.
Although cyclosporine A has been the most commonly used calcineurin antagonist in patients with RPILD and positive anti-MDA5 antibodies, and the benefits of adding tacrolimus to other immunosuppressive drugs have not been specifically evaluated, the expert group considered that the choice of tacrolimus or cyclosporine will depend on the safety profile and the patient clinical background.
Recommendation 3: When calcineurin antagonists are not feasible, consider combination therapy with glucocorticoids and other immunosuppressive drugs such as cyclophosphamide and/or mycophenolate mofetil, or adding rituximab to any one of the previous schedules (Recommendation grade 3).
Recommendation 3a: The choice of one of these drugs will depend on the individual characteristics of the patient and the clinician experience (Recommendation grade √).
Double therapy with glucocorticoid and cyclophosphamide was used in several retrospective studies and case reports. Two retrospective studies previously mentioned in recommendation 216, 19 describe 19 cases (8 and 11 patients, respectively) treated with a double therapy combining glucocorticoid and cyclophosphamide or a calcineurin antagonist, 14 patients of whom survived (6 and 8, respectively). The number of patients treated with the combination including cyclophosphamide is not specified. Besides, the case reported by Goussot [32] received this double therapy and also survived (Level of evidence 3).
The evidence about the efficacy and safety of mycophenolate in the treatment of RPILD associated with anti-MDA5 is scarce and indirect, based on 12 patients from case series and reports [47–50,52]. Mycophenolate was combined with other immunosuppressants resulting in three patients who died and nine with clinical improvement. Six out of nine patients who improved did not receive calcineurin antagonists as part of the therapeutic strategy. Two of the three patients who died received sequential treatment with several immunosuppressants, which did not include calcineurin antagonists [48,49] (Level of evidence 3).
In assessing these results, the expert panel considered that when calcineurin antagonists are not feasible, either double therapy with glucocorticoid and cyclophosphamide or mycophenolate or triple therapy with the three of them with or without intravenous immunoglobulins might also be a valid therapeutic option.
Thirteen patients treated with rituximab due to RPILD associated to anti-MDA5 antibodies have been reported. Six of them did not receive calcineurin antagonists as part of the combined therapy with cyclophosphamide with or without mycophenolate [17,47–49,53]. Of these, four patients died [17,48,49] and only two improved [47,53] (Level of evidence 3). According to these data, the expert panel considers that adding rituximab to the combination of glucocorticoid and cyclophosphamide must be taken with caution.
Therapy for the refractory patient
Recommendation 4: In patients with DM-associated rapidly progressive interstitial lung disease anti-MDA5 (+) who do not respond to combination therapy with glucocorticoids plus immunosuppressive drugs, clinicians have to consider the following alternatives:
Adding one of these immunosuppressive drugs (cyclophosphamide, mycophenolate mofetil, rituximab, basiliximab or tofacitinib) to the current therapy (Recommendation grade D)
Change one immunosuppressant for another (Recommendation grade √)
Although definition of a refractory patient can differ from one study to another, it is generally accepted as a lack of response after administration of the classic therapeutic schedule following recommendations 2 and 3. Some studies have defined treatment failure in these patients when they fulfill the following conditions at least 1 week after the institution of triple therapy: deteriorating respiratory symptoms; increasing alveolo-arterial O2 tension difference (A-aDO2); newly-emerging or expanding GGO/consolidation on chest imaging; increasing ferritin levels, and the personal impression of clinical worsening of the patient under triple therapy by the attending physicians [54].
Evidence-based analysis identified several drugs used as rescue therapy in refractory patients with anti-MDA5 positive DM-associated RPILD. Rituximab has been added to the standard immunosuppressive therapy (recommendations 1 and 2) in patients with RPILD impairment [17,48,49,53,55–59]. Eight out of 13 reported patients died, even though rituximab had been added [17,48,49,55,59], and 5 improved [17,53,56,57], although in a single case relapse did not involve the lung [47] (Level of evidence 3).
As previously reported, recommendations 2 and 3 gather the available evidence (case reports) on the use of mycophenolate in combination with other immunosuppressive drugs. Only a single patient refractory to the initial triple therapy that finally improved after adding mycophenolate has been identified [50] (Level of evidence 3).
A single study highlighted the efficacy of basiliximab (an anti-CD25/sIL-2R monoclonal antibody) [60]. It included 4 patients who were refractory to immunosuppressive therapy including prednisone, cyclosporine, and intravenous immunoglobulin. Basiliximab showed efficacy in 3 of the 4 patients [60] (Level of evidence 3)
Another option in the case of failure to the conventional triple therapy is to replace one immunosuppressant for another. Nevertheless, in the case of calcineurin antagonists, Yoshida et al [46] described the case of a patient refractory to triple immunosuppressive therapy who died despite switching cyclosporine by tacrolimus (Level of evidence 3).
Finally, two studies have found a good response adding the Janus kinase inhibitor tofacitinib to conventional triple therapy in six refractory cases. Kurasawa et al. [54] reported a survival rate of 60% in tofacitinib-treated patients (three out of five) compared to none out of six historical controls with similar poor-prognostic factors. However, 80% of tofacitinib-treated patients presented varicella-zoster virus reactivation and 100% developed cytomegalovirus infection. Kato et al. [61] reported a case of refractory ILD with pneumomediastinum responsive to tofacitinib add-on therapy (Level of evidence 3).
Considering these results, the expert group suggests that in refractory cases to standard triple immunosuppressive therapy (recommendations 2 and 3), adding to a new immunosuppressant or switching one for another may be considered valid therapeutic alternatives.
Recommendation 5: In patients who do not respond to combined immunosuppressive drugs, the use of the following alternative rescue therapies, either separate or in a sequential manner, might be considered:
Polymyxin B hemoperfusion (Recommendation grade D)
Plasmapheresis (Recommendation grade D)
Intravenous immunoglobulins (Recommendation grade √)
Use of non-pharmacologic therapies such as polymyxin B, plasmapheresis or intravenous immunoglobulin (IVIg) administration is accepted as rescue therapy in these patients. Adsorption and elimination of inflammatory cytokines, mediators and activated leukocytes, as well as removing anti-MDA5 antibodies could be the rationale of its efficacy.
A retrospective study [62] aimed to evaluate the efficacy of polymyxin B hemoperfusion analyzed 14 clinically amyopathic dermatomyositis associated RPILD patients (10 with anti-MDA5 antibodies). Before polymyxin B use, all the patients have been treated with standard triple therapy including prednisolone, cyclophosphamide and calcineurin antagonists (cyclosporine or tacrolimus). Polymyxin B administration was performed by using a polymyxin B-immobilized fiber column and conventional equipment for hemoperfusion and hemodialysis circuit. Nine out of 10 (90%) of anti-MDA5 positive patients died, and only one case survived (Level of evidence 3).
Takada et al. [44] reported in a retrospective study 2 out of 13 patients diagnosed with CADM and positive anti-MDA5 antibodies refractory to combined immunosuppressive therapy in whom polymyxin hemoperfusion was performed; one of them survived.
Four more patients refractory to conventional immunosuppressive therapy have also been published [63–66] reporting a significant improvement when polymyxin hemoperfusion was added. Ichiyasu et al. [67] described 3 cases of CADM with RPILD who responded to polymyxin B hemoperfusion after previous failure of triple combination immunosuppressive therapy (cyclophosphamide pulses, cyclosporine and glucocorticoids), although the anti-MDA5 status was not reported. The same author reported a study of 77 patients diagnosed with RPILD, 41 being treated with polymyxin B hemoperfusion in comparison with 36 from a historical control group. They found a 90-day reduced mortality in the polymyxin group vs the historical group (41.5% vs 66.7%, p=0.019). Half of the patients studied were diagnosed with connective tissue disease, and 12 with DM with unknown MDA5 status. All received concurrent immunosuppressive therapy [68]. Moreover, Furosawa [66] published a series of 24 patients with an acute exacerbation of interstitial pneumonia, 12 of them were DM, who were negative for anti-MDA5 antibodies. Data reported in this study showed a better outcome of those patients in whom polymyxin hemoperfusion was performed, although it did not reduce the mortality. Nevertheless, only one out of 5 DM patients in whom polymyxin B hemoperfusion was performed died in comparison with 6 out of 7 who did not receive this therapy (p=0.045). Therefore, direct hemoperfusion using a polymyxin B-immobilized fiber column after triple standard immunosuppressive therapy, even in patients negative to anti-MDA5 antibodies, may support the potential use of this technique as a rescue therapy in this clinical setting (Level of evidence 3).
Considering this data, and that a third (5 out of 14, 35%) of RPILD anti-MDA5 positive patients who received polymyxin hemoperfusion as an add-on therapy to the triple immunosuppressive therapy survived, the expert group made a favorable recommendation.
Ten patients treated with plasmapheresis [12,48,55,64,69] have been identified. All the reported cases included this therapy as additional treatment to triple conventional combined/progressive immunosuppressive schedule. Only 2 patients survived, and one of them also received also polymyxin hemoperfusión [64,69] (Level of evidence 3).
Taking into account the above reported data, the expert group suggests that plasmapheresis may be included as a part of the scheduling approach in patients with anti-MDA5 positive and RPILD.
Intravenous immunoglobulin rescue therapy is usually administered as an adjuvant therapy. A total of 22 patients with anti-MDA5 positive RPILD associated DM were retrieved from published case reports, more than half of them (13 out of 22, 59%) were alive at the end of the therapy, which was usually combination of different immunosuppressive drugs and glucocorticoids. Ma et al. [35] in a single study reported 7 out of 11 anti-MDA5 positive patients with pneumomediastinum and RPILD who received treatment with IVIgs. No information on the specific outcome in those 7 patients was reported.
Although there is not enough data to support that IVIgs are useful as a direct therapy for anti-MDA5 positive rapidly progressive ILD associated DM, the expert panel agreed on that IVIgs should be considered as a potential useful adjuvant treatment (Recommendation grade √).
Recommendation 6: Assistance with veno-venous extracorporeal membrane oxygenation (VV-ECMO) should be considered in patients with life-threatening severe and refractory respiratory insufficiency to maintain the patient alive while waiting for a clinical response to intensive and combined immunosuppressive treatment or as a bridge to lung transplantation (Recommendation grade √).
Veno-venous extra-corporeal Membrane Oxygenation (VV-ECMO), a method of life support used to oxygenate the blood is a technique aimed to provide prolonged respiratory support in those patients with respiratory failure. VV-ECMO assistance can maintain lung function during days or weeks. Nevertheless, it is a complex procedure and consumes high human and technical requirements that only should be performed in high specialized centers. It is considered the very last therapeutic option when standard therapy had failed, and always as a bridge to a definitive solution of the original cause of respiratory failure.
The use of VV-ECMO in refractory anti-MDA5 positive DM patients that develop RPILD is exceptional and it has only been described in 6 studies. Among them, a retrospective study [12] reported 6 patients with refractory respiratory failure who received VV-ECMO as organ support. However, despite this procedure, all (100%) of them finally died. Alqatari et al [55] and Gorka et al [70] reported 2 cases that developed a poor outcome and died. In contrast, Broome et al [71] and Leclair et al [72] reported the case of a middleaged man with anti-MDA5-associated RPILD refractory to immunosuppressants who was treated with ECMO for 52 days as a bridge therapy to successful bilateral lung transplant. More recently, Deitchman et al. [73] and Huang et al. [74], reported one and three refractory patients, respectively, who also survived after VV-ECMO bridging to lung transplant (Level of evidence 3).
Taking together all this information, the expert panel considered that the use of ECMO as life support may be effective in anti-MDA5 positive patients who develop RPILD while a complete response to combination immunosuppressive therapy has not yet been achieved or as a bridge therapy to lung transplantation.
Recommendation 7: Lung transplantation should be considered as a therapeutic option in patients with refractory RPILD associated with anti-MDA5. Early referral for transplant eligibility assessment is recommended at the time of ILD diagnosis (Recommendation grade √).
In patients with interstitial lung disease associated with connective tissue disease (CTD), lung transplantation is contraindicated at many centers because of the impact of pre-existing conditions on post-transplant outcomes. Potential contributors to poor outcomes include gastroesophageal reflux (thought to cause bronchiolitis obliterans syndrome), renal disease (as it complicates management of immunosuppressive and antimicrobial agents commonly used after transplantation), and extra-pulmonary disease such as myositis (which complicates management of immunosuppression and rehabilitation after transplantation and the risk of malignancy association). In fact, less than 1% of all lung transplants worldwide between 1995 and 2015 were given to patients with CTD associated with lung disease [75]. However, recent studies suggest that post-transplant outcomes in these patients do not differ significantly from those in patients with non-CTD [76–78] which supports CTD patients to be considered as part of lung transplant candidates [79].
Data on lung transplantation in anti-MDA5 positive DM associated RPILD are scarce and limited to case series and reports. Selva-O’Callaghan et al. [80] reported two cases of unsuccessful lung transplantation in patients with DM-associated RPILD complicated with pneumomediastinum, subcutaneous emphysema and acute alveolar injury. Several years later, stored serum samples of these patients, which were obtained at the beginning of the disease, were analyzed. They turned out to be positive for anti-MDA 5 antibodies (author personal communication). On the other hand, Shoji et al. [81] reported a case of bilateral living-donor lobar lung transplantation with uneventful postoperative course, who also was able to perform daily activities without oxygen seven months after surgery. Besides, a patient reported by Leclair et al. [72], who underwent bilateral lung transplantation after prolonged VV-ECMO, was able to resume his normal life with a survival period to date of twelve years in remission. More recently, a patient reported by Deitchman et al. [73] and three out of eight anti-MDA5 positive RPILD refractory patients reported by Huang et al. [74] survived after lung transplant being previously supported by VV-ECMO. (Level of evidence 3).
Therefore, the expert panel strongly recommends referring soon patients with ILD associated with anti-MDA5 antibodies to centers with experience in the evaluation and management of lung transplantation in CTD.
Other treatment options
Recommendation 8: Azathioprine, methotrexate and leflunomide are not recommended as an induction therapy in RPILD associated with anti-MDA5 antibodies (Recommendation grade √).
The evidence about the efficacy and safety of azathioprine in RPILD associated with anti-MDA5 is scarce with uneven results in the only five reported cases. With respect to this, two patients received azathioprine as part of sequential therapy with non-calcineurin antagonists immunosuppressants (cyclophosphamide, mycophenolate and rituximab) but they did not survive [48,49]. However, case 5 of the Hoa series [17] who developed pleural effusion, improved after adding azathioprine to glucocorticoid and tacrolimus double therapy. Finally, azathioprine monotherapy plus glucocorticoid resulted in ILD improvement in one case [82] and fatal outcome in another [83] (Level of evidence 3).
Information about the use of methotrexate in anti-MDA5-associated ILD has only been retrieved from seven patients with the non-RP form. In all of them, methotrexate was used as part of the combined treatment with other immunosuppressants (mycophenolate, hydroxychloroquine, azathioprine, or rituximab). All patients presented a good clinical course without progression of the pulmonary involvement [8,84]. Both, the scarce number of patients and the association with other immunosuppressants make difficult to evaluate the real effect of methotrexate in the observed outcome (Level of evidence 3).
Leflunomide has only been evaluated in seven patients with anti-MDA5-associated RPILD [35]. It was used in combination with Chinese herbs and other immunosuppressants, including glucocorticoid, cyclophosphamide, calcineurin antagonists, mycophenolate and intravenous immunoglobulins, thus being very difficult to evaluate, in this context, the role of this drug in the fatal outcome of 6 out of the 7 patients (85%) (Level of evidence 3).
Considering the results of all these studies and the scarce clinical experience, the elaborating group considered that azathioprine, methotrexate and leflunomide should not be recommended in the management of RPILD, particularly as an induction therapy.
Recommendation 9: Infliximab is not recommended in anti-MDA-5 associated RPILD treatment (Recommendation grade √).
Regarding the use of infliximab in inflammatory myopathy-associated RPILD, only a retrospective case series of fourteen non-MDA5 treated patients in combination with conventional immunosuppressant therapy has been identified [9]. Ten of them had the amyopathic clinical form. All the fourteen patients were initially treated with methylprednisolone combined with cyclophosphamide in seven, mycophenolate in one, tacrolimus in three, cyclosporine in one, methotrexate in another one and immunoglobulins in five. Also, all of them received infliximab at a dose of 5 mg/kg/i.v. at week 0, 2, 6 and then every eight weeks. The ten patients (71%) treated in the early phase did have a favorable response while the other four (29%) who received infliximab after the respiratory failure, died (Level of evidence 3).
Despite this data, the expert panel has considered the clinical evidence showing that anti-TNF agents may cause serious ILD and, therefore, cannot recommend infliximab use in the therapeutic management of these ILD’s patients.
Recommendation 10: Although pirfenidone has been added to conventional immunosuppressant treatment in DM-associated subacute interstitial pneumonia with pulmonary fibrosis, the expert panel may not recommend its use in patients with RPILD associated to anti-MDA5 antibodies (Recommendation grade √).
Data on the use of antifibroticxagents comes from a prospective study [52] that included 30 patients with CADM-associated RPILD treated with pirfenidone in addition to conventional immunosuppressive treatment (glucocorticoids, cyclosporine and mycophenolate) compared with a historical cohort of 27 patients treated with conventional therapy. Twenty-two of 30 patients from the pirfenidone-treated group were anti-MDA5 positive versus 4 of 27 patients of the control group. Overall, mortality in the pirfenidone-treated group was lower although did not reach statistical significance compared with the control group (36.7% vs. 51.9%, p=0.223). An analysis of the subgroup of patients with acute ILD (<3 month) (n=30) disclosed identical mortality for case and control groups (50% vs. 50%, respectively; p=0.386). However, in patients with subacute ILD (3 to 6 month) (n=19), the mortality in pirfenidone-treated patients was lower than that of the control group (90% vs. 44%, p=0.045). A subgroup analysis describing only anti-MDA-5 patients was not performed. No serious adverse events were described (Level of evidence 3).
Based on all the previous recommendations, the expert panel proposes two flow charts for the diagnosis and treatment (Figs. 1 and 2, respectively) of RPILD in patients with anti MDA5 antibodies.
Fig. 1.
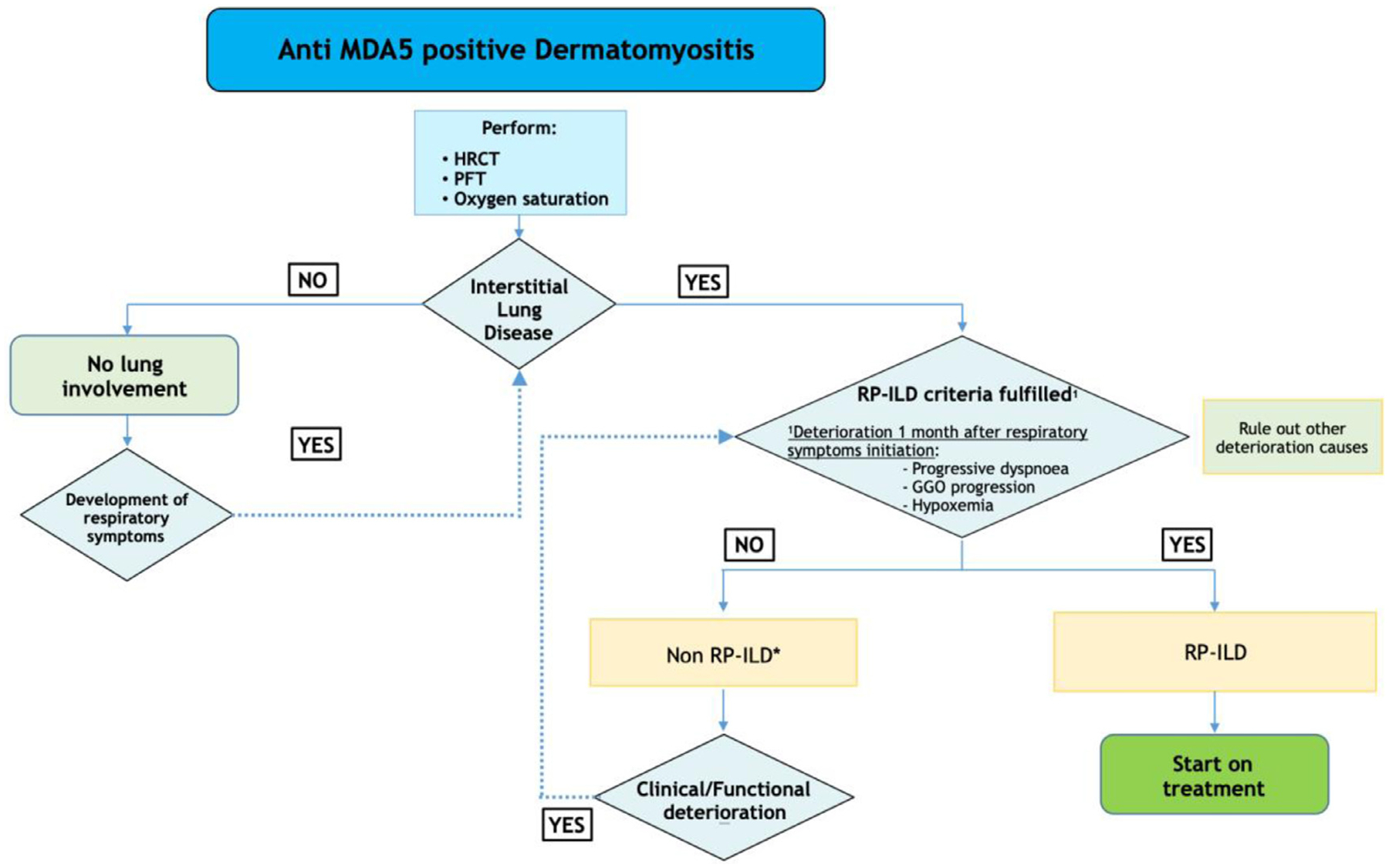
Diagnosis of RPILD in patients with anti MDA5 antibodies. *Combined therapy with glucocorticoids and calcineurin antagonists is recommended specially if some risk factors are present (>60 years old, hyperferritinemia >500 nm/mL, C reactive protein > 1 mg/dl). HRCT, High Resolution CT Scan. PFT, Pulmonary Function Tests. RP-ILD, Rapidly Progressive Interstitial Lung Disease. GGO, Ground Glass Opacity.
Fig. 2.
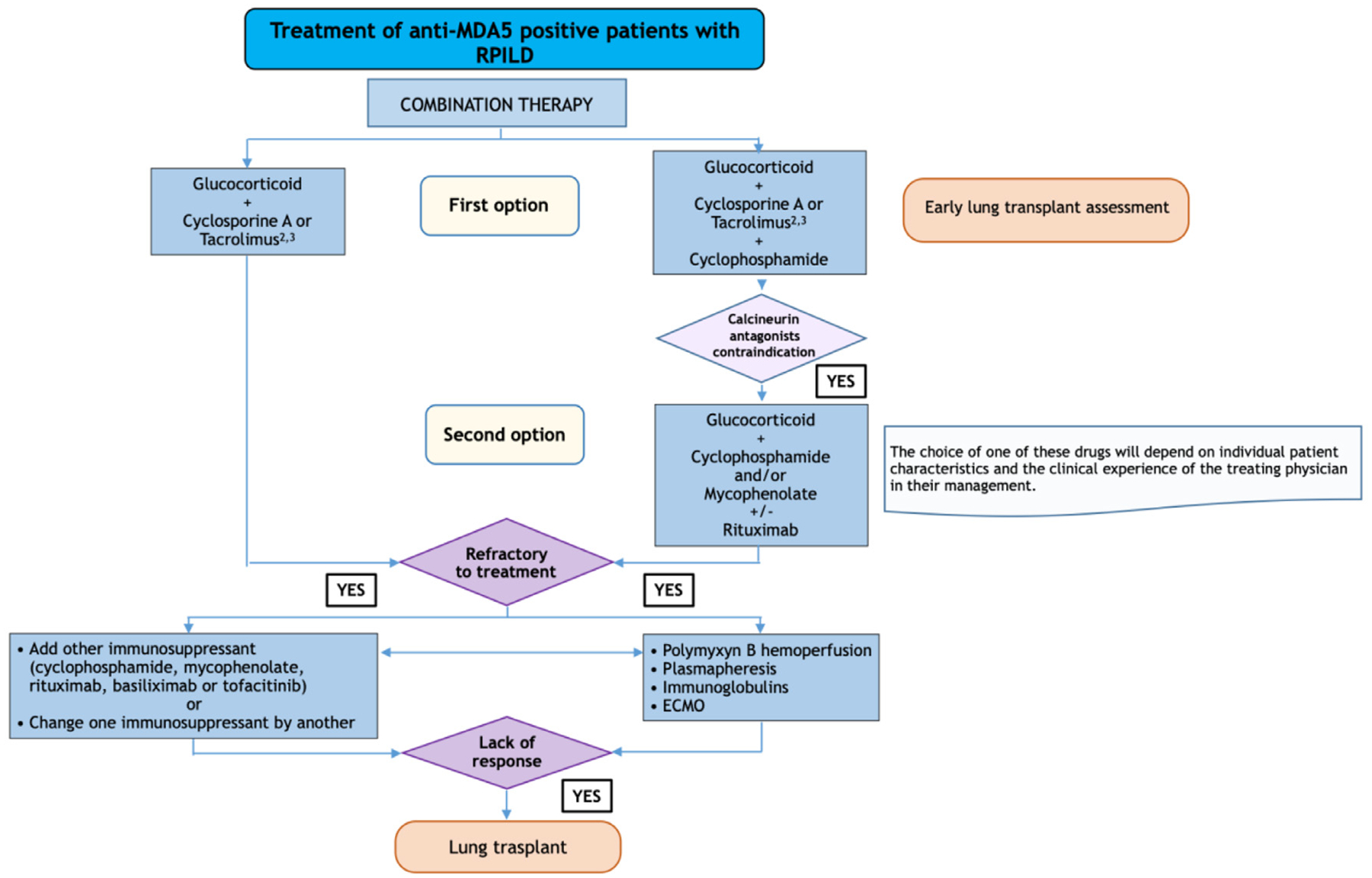
Treatment of RPILD in patients with anti MDA5 antibodies.
Summary and conclusions
Medical literature searching discloses a generally poor prognosis and bad outcome in patients with DM who are positive to anti-MDA5 antibodies and develop a RPILD. The small number of patients with this syndrome precludes performing randomized clinical trials to know which would be the best treatment for this catastrophic situation. These recommendations are based on observational studies, mainly cohort studies and case reports, therefore the level of scientific evidence is not higher than 3. We have completed them summing up the experience of the clinicians from different specialties who participated in the task force (clinical recommendations by the expert panel).
However, this study has a series of limitations. First, the largest number of reports from Japan may have skewed our interpretation of the data towards the treatment modalities and practices favored in this region. Second, none of the studies identified have a randomized clinical trial design to meet the research objective. They correspond to open studies, case series or case reports and include a small number of patients. Third, systematic reviews are susceptible to problems like reporting and selection bias, incomplete outcome data, confounding by indication and local trends in medical practices, among others.
Considering these limitations, there is a consensus to treat these patients from the onset with a combination therapy that, besides glucocorticoids, includes immunosuppressive drugs such as calcineurin antagonists, and following the experience from Asian cohorts, adding cyclophosphamide as a third drug. Nevertheless, this combination therapy approach is not always enough to get good outcome and to date, more than half of the patients follow a fatal course. Then, adding on or switching immunosuppressants could be a plausible option; monoclonal antibodies such as basiliximab, rituximab, or new immunosuppressive drugs such as mycophenolate mofetil or JAK inhibitors (tofacitinib) may be good options. Moreover, tofacitinib combined with glucocorticoids has recently shown to be a promising therapy in the early stage of anti-MDA5 positive CADM-ILD [85] as the six-month survival after ILD onset was significantly higher in tofacitinib-treated patients (18 of 18, 100%) than in the historical controls who met the same criteria and received conventional therapy (25 of 32, 78%) (p=0.04). Further studies are warranted to determine its role in anti MDA5 positive RPILD initial therapy. In addition to the immunosuppressive treatment and given the bad outcome that usually experienced these patients, some rescue therapies such as plasmapheresis, intravenous immunoglobulins or polymyxin B hemoperfusion are also indicated when the patient does not respond adequately in terms of respiratory failure. Lastly, extracorporeal membrane oxygenation, as a strategy to allow time for immunosuppressive therapy to be effective or as a bridge therapy to lung transplantation, is another option to be considered.
Future research agenda
Multicenter prospective studies are mandatory to gather enough number of patients that allow performing randomized clinical trials, tuning up definitions of improvement and outcome, and the proper use of reliable biomarkers to define the risk strategy and the best therapeutic option at any moment will undoubtedly contribute to the better outcome and improvement of this severe syndrome. On the other hand, a consortium that allows going deeper into the knowledge of the intrinsic mechanisms or epidemiological issues will be of paramount importance for the understanding of this syndrome.
Supplementary Material
Acknowledgments
We would like to express our sincere gratitude to investigators of the JAMI cohort for collecting data regarding myositis-associated ILD.
Funding
This project was supported by Spanish Rheumatology Society and Spanish Society of Internal Medicine (GEAS, Study Group on Autoimmune Diseases).
Appendix 1. Levels of evidence and grades of recommendation
SIGN Levels of evidence and grades of recommendation [13]
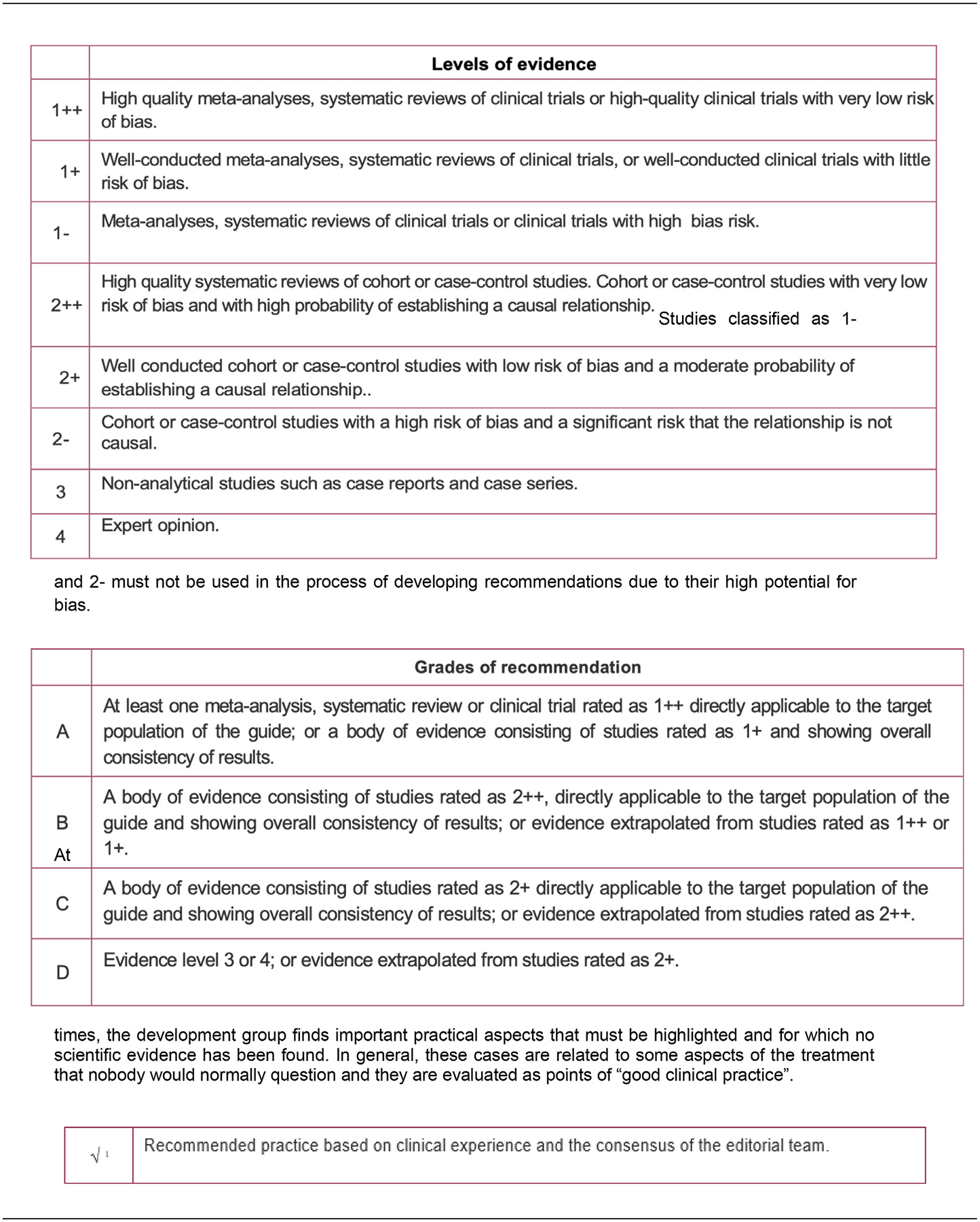
Studies classified as 1- and 2- must not be used in the process of developing recommendations due to their high potential for bias.
At times, the development group finds important practical aspects that must be highlighted and for which no scientific evidence has been found. In general, these cases are related to some aspects of the treatment that nobody would normally question and they are evaluated as points of “good clinical practice”.
Appendix 2. Flow chart with the results of the literature search
Footnotes
Ethics approval
This study did not involve human participants, therefore the ethical approval was not needed.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.semarthrit.2020.03.007.
References
- [1].Selva-O’Callaghan A, Pinal-Fernandez I, Trallero-Araguas E, Milisenda JC, Grau-Junyent JM, Mammen AL. Classification and management of adult inflammatory myopathies. Lancet Neurol 2018;17(9):816–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005;52(5):1571–6. [DOI] [PubMed] [Google Scholar]
- [3].Chen Z, Hu W, Wang Y, Guo Z, Sun L, Kuwana M. Distinct profiles of myositis-specific autoantibodies in Chinese and Japanese patients with polymyositis/dermatomyositis. Clin Rheumatol 2015;34(9):1627–31. [DOI] [PubMed] [Google Scholar]
- [4].Koga T, Fujikawa K, Horai Y, Okada A, Kawashiri SY, Iwamoto N, et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology (Oxford) 2012;51(7):1278–84. [DOI] [PubMed] [Google Scholar]
- [5].Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016;68(5):689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A. Anti-MDA5 antibodies in a large Mediterranean population of adults with dermatomyositis. 2014;2014:290797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical spectrum in north american patients with dermatomyositis. J Rheumatol 2017;44(3):319–25. [DOI] [PubMed] [Google Scholar]
- [8].Hall JC, Casciola-Rosen L, Samedy LA, Werner J, Owoyemi K, Danoff SK, et al. Antimelanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res (Hoboken) 2013;65(8):1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen Z, Cao M, Plana MN, Liang J, Cai H, Kuwana M, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res (Hoboken) 2013;65(8):1316–24. [DOI] [PubMed] [Google Scholar]
- [10].Nakashima R, Hosono Y, Mimori T. Clinical significance and new detection system of autoantibodies in myositis with interstitial lung disease. Lupus 2016;25(8):925–33. [DOI] [PubMed] [Google Scholar]
- [11].Sato S, Masui K, Nishina N, Kawaguchi Y, Kawakami A, Tamura M, et al. Initial predictors of poor survival in myositis-associated interstitial lung disease: a multicentre cohort of 497 patients. Rheumatology (Oxford) 2018;57(7):1212–21. [DOI] [PubMed] [Google Scholar]
- [12].Vuillard C, Pineton de Chambrun M, de Prost N, Guerin C, Schmidt M, Dargent A, et al. Clinical features and outcome of patients with acute respiratory failure revealing anti-synthetase or anti-MDA-5 dermato-pulmonary syndrome: a French multicenter retrospective study. Ann Intensive Care 2018;8(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Grupo de trabajo sobre GPC. Elaboración de Guías de Practica Clínica en el Sistema Nacional de Salud. Manual Metodológico. Madrid: Plan Nacional para el SNS del MSC. Instituto Aragonés de Ciencias de la Salud-I+CS; 2007. Guías de Practica Práctica Clínica en el SNS: I+CS N° 2006/0I. [Google Scholar]
- [14].López de Argumedo MRE, Gutiérrez A, Bayón JC. Actualización del Sistema de Trabajo Compartido para Revisiones Sistemáticas de la Evidencia Científica y Lectura Crítica (Plataforma FLC 3.0). Ministerio de Sanidad, Servicios Sociales e Igualdad. Servicio de Evaluación de Tecnologías Sanitarias del País Vasco; 2017. Informes de Evaluación de Tecnologías Sanitarias: OSTEBA. [Google Scholar]
- [15].Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183(6):788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gono T, Sato S, Kawaguchi Y, Kuwana M, Hanaoka M, Katsumata Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology (Oxford) 2012;51(9):1563–70. [DOI] [PubMed] [Google Scholar]
- [17].Hoa S, Troyanov Y, Fritzler MJ, Targoff IN, Chartrand S, Mansour AM, et al. Describing and expanding the clinical phenotype of anti-MDA5-associated rapidly progressive interstitial lung disease: case series of nine Canadian patients and literature review. Scand J Rheumatol 2017:1–15. [DOI] [PubMed] [Google Scholar]
- [18].Gono T, Kawaguchi Y, Satoh T, Kuwana M, Katsumata Y, Takagi K, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 2010;49(9):1713–9. [DOI] [PubMed] [Google Scholar]
- [19].Muro Y, Sugiura K, Akiyama M. Limitations of a single-point evaluation of anti-MDA5 antibody, ferritin, and IL-18 in predicting the prognosis of interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Clin Rheumatol 2013;32(3):395–8. [DOI] [PubMed] [Google Scholar]
- [20].Sakamoto S, Okamoto M, Kaieda S, Fujimoto K, Nagata S, Tominaga M, et al. Low positive titer of anti-melanoma differentiation-associated gene 5 antibody is not associated with a poor long-term outcome of interstitial lung disease in patients with dermatomyositis. Respir Investig 2018;56(6):464–72. [DOI] [PubMed] [Google Scholar]
- [21].Gono T, Kawaguchi Y, Ozeki E, Ota Y, Satoh T, Kuwana M, et al. Serum ferritin correlates with activity of anti-MDA5 antibody-associated acute interstitial lung disease as a complication of dermatomyositis. Mod Rheumatol 2011;21(2):223–7. [DOI] [PubMed] [Google Scholar]
- [22].Matsushita T, Mizumaki K, Kano M, Yagi N, Tennichi M, Takeuchi A, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol 2017;176(2):395–402. [DOI] [PubMed] [Google Scholar]
- [23].Chen F, Lu X, Shu X, Peng Q, Tian X, Wang G. Predictive value of serum markers for the development of interstitial lung disease in patients with polymyositis and dermatomyositis: a comparative and prospective study. Intern Med J 2015;45(6):641–7. [DOI] [PubMed] [Google Scholar]
- [24].Fathi M, Barbasso Helmers S, Lundberg IE. KL-6: a serological biomarker for interstitial lung disease in patients with polymyositis and dermatomyositis. J Intern Med 2012;271(6):589–97. [DOI] [PubMed] [Google Scholar]
- [25].Nishioka A, Tsunoda S, Abe T, Yoshikawa T, Takata M, Kitano M, et al. Serum neopterin as well as ferritin, soluble interleukin-2 receptor, KL-6 and anti-MDA5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti-MDA5 antibody-positive dermatomyositis. Mod Rheumatol. 2019;29(5):814–20. [DOI] [PubMed] [Google Scholar]
- [26].Osawa T, Morimoto K, Sasaki Y, Matsuda S, Yamana K, Yano R, et al. The serum ferritin level is associated with the treatment responsivity for rapidly progressive interstitial lung disease with amyopathic dermatomyositis, irrespective of the anti-MDA5 antibody level. Internal Medicine 2018;57(3):387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sato S, Kuwana M, Fujita T, Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol 2013;23(3):496–502. [DOI] [PubMed] [Google Scholar]
- [28].Sato S, Kuwana M, Fujita T, Suzuki Y. Amyopathic dermatomyositis developing rapidly progressive interstitial lung disease with elevation of anti-CADM-140/MDA5 autoantibodies. Mod Rheumatol 2012;22(4):625–9. [DOI] [PubMed] [Google Scholar]
- [29].Charbit L, Bursztejn AC, Mohamed S, Kaminsky P, Lerondeau B, Barbaud A, et al. [Extensive digital necrosis during dermatomyositis associated with MDA-5 antibodies]. Ann Dermatol Venereol 2016;143(8–9):537–42. [DOI] [PubMed] [Google Scholar]
- [30].Fujimoto N, Honda S, Wakabayashi M, Hamaguchi Y, Fujimoto M, Tanaka T. Anti-MDA-5 antibody-positive bullous dermatomyositis with palmar papules complicating rapidly progressive interstitial lung disease. Mod Rheumatol 2016;26(4):614–6. [DOI] [PubMed] [Google Scholar]
- [31].González-Moreno J, Raya-Cruz M, Losada-Lopez I, Cacheda AP, Oliver C, Colom B. Rapidly progressive interstitial lung disease due to anti-MDA5 antibodies without skin involvement: a case report and literature review. Rheumatology International 2018:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Goussot R, Theulin A, Goetz J, Sibilia J, Gottenberg JE, Meyer A. An arthro-dermato-pulmonary syndrome associated with anti-MDA5 antibodies. Joint Bone Spine 2014;81(3):266. [DOI] [PubMed] [Google Scholar]
- [33].Horai Y, Isomoto E, Koga T, Okada A, Kawashiri SY, Tamai M, et al. Early diagnosis and treatment for remission of clinically amyopathic dermatomyositis complicated by rapid progress interstitial lung disease: a report of two cases. Mod Rheumatol 2013;23(1):190–4. [DOI] [PubMed] [Google Scholar]
- [34].Ikeda S, Arita M, Morita M, Ikeo S, Ito A, Tokioka F, et al. Interstitial lung disease in clinically amyopathic dermatomyositis with and without anti-MDA-5 antibody: to lump or split? BMC Pulm Med. 2015;15:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ma X, Chen Z, Hu W, Guo Z, Wang Y, Kuwana M, et al. Clinical and serological features of patients with dermatomyositis complicated by spontaneous pneumomediastinum. Clin Rheumatol 2016;35(2):489–93. [DOI] [PubMed] [Google Scholar]
- [36].Morita Y, Kuwagata S, Kato N, Tsujimura Y, Mizutani H, Suehiro M, et al. 18F-FDG PET/CT useful for the early detection of rapidly progressive fatal interstitial lung disease in dermatomyositis. Intern Med 2012;51(12):1613–8. [DOI] [PubMed] [Google Scholar]
- [37].Nara M, Komatsuda A, Omokawa A, Togashi M, Okuyama S, Sawada K, et al. Serum interleukin 6 levels as a useful prognostic predictor of clinically amyopathic dermatomyositis with rapidly progressive interstitial lung disease. Mod Rheumatol 2014;24(4):633–6. [DOI] [PubMed] [Google Scholar]
- [38].Parronchi P, Radice A, Palterer B, Liotta F, Scaletti C. MDA5-positive dermatomyositis: an uncommon entity in Europe with variable clinical presentations. Clin Mol Allergy 2015;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tanizawa K, Handa T, Nakashima R, Kubo T, Hosono Y, Watanabe K, et al. HRCT features of interstitial lung disease in dermatomyositis with anti-CADM-140 antibody. Respir Med 2011;105(9):1380–7. [DOI] [PubMed] [Google Scholar]
- [40].Hozumi H, Fujisawa T, Nakashima R, Johkoh T, Sumikawa H, Murakami A, et al. Comprehensive assessment of myositis-specific autoantibodies in polymyositis/dermatomyositis-associated interstitial lung disease. Respir Med 2016;121:91–9. [DOI] [PubMed] [Google Scholar]
- [41].Koga T, Kaieda S, Okamoto M, Masuda K, Fujimoto K, Sakamoto S, et al. Successful treatment of rapidly progressive unclassifiable idiopathic interstitial pneumonia with anti-melanoma differentiation-associated gene-5 antibody by intensive immunosuppressive therapy. Intern Med 2018;57(7):1039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Koguchi-Yoshioka H, Okiyama N, Iwamoto K, Matsumura Y, Ogawa T, Inoue S, et al. Intravenous immunoglobulin contributes to the control of antimelanoma differentiation-associated protein 5 antibody-associated dermatomyositis with palmar violaceous macules/papules. Br JDermatol 2017;177(5):1442–6. [DOI] [PubMed] [Google Scholar]
- [43].Suzuki A, Kondoh Y, Taniguchi H, Tabata K, Kimura T, Kataoka K, et al. Lung histopathological pattern in a survivor with rapidly progressive interstitial lung disease and anti-melanoma differentiation-associated gene 5 antibody-positive clinically amyopathic dermatomyositis. Respir Med Case Rep 2016;19:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Takada T, Aoki A, Asakawa K, Sakagami T, Moriyama H, Narita I, et al. Serum cytokine profiles of patients with interstitial lung disease associated with anti-CADM-140/MDA5 antibody positive amyopathic dermatomyositis. Respir Med 2015;109(9):1174–80. [DOI] [PubMed] [Google Scholar]
- [45].Yamada K, Asai K, Okamoto A, Watanabe T, Kanazawa H, Ohata M, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. Rheumatol Int 2018;11(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yoshida N, Kaieda S, Tomozoe K, Tajiri M, Wakasugi D, Okamoto M, et al. An autopsy case of anti-melanoma differentiation-associated gene-5 antibody-positive clinical amyopathic dermatomyositis complicated by rapidly progressive interstitial lung disease. Intern Med 2016;55(12):1653–9. [DOI] [PubMed] [Google Scholar]
- [47].Clottu A, Laffitte E, Prins C, Chizzolini C. Response of mucocutaneous lesions to rituximab in a case of melanoma differentiation antigen 5-related dermatomyositis. Dermatology 2012;225(4):376–80. [DOI] [PubMed] [Google Scholar]
- [48].Gil B, Merav L, Pnina L, Chagai G. Diagnosis and treatment of clinically amyopathic dermatomyositis (CADM): a case series and literature review. Clin Rheumatol 2016;35(8):2125–30. [DOI] [PubMed] [Google Scholar]
- [49].Girard C, Vincent T, Bessis D. [Dermatomyositis and acute interstitial lung disease associated with MDA-5 antibodies: an atypical case]. Ann Dermatol Venereol 2013;140(10):628–34. [DOI] [PubMed] [Google Scholar]
- [50].Hayashi M, Aoki A, Asakawa K, Sakagami T, Kikuchi T, Takada T. Cytokine profiles of amyopathic dermatomyositis with interstitial lung diseases treated with mycophenolate. 2017;5(4):e00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li T, Guo L, Chen Z, Gu L, Sun F, Tan X, et al. Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci Rep 2016;6:33226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lee LW, Narang NS, Postolova A, Seminara N, Kantor MA. Anti-MDA5-positive dermatomyositis presenting as fever of unknown origin. J Gen Intern Med 2016;31(12):1530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sharma A, Hudson M, Watters K, Billick R, Fallavolita S, Netchiporouk E. Rapidly progressive melanoma differentiation–associated protein 5–positive amyopathic dermatomyositis in an HIV-positive patient. JAAD Case Reports 2017;3(2):158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kurasawa K, Arai S, Namiki Y, Tanaka A, Takamura Y, Owada T, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology (Oxford) 2018;57(12):2114–9. [DOI] [PubMed] [Google Scholar]
- [55].Alqatari S, Riddell P, Harney S, Henry M, Murphy G. MDA-5 associated rapidly progressive interstitial lung disease with recurrent Pneumothoraces: a case report. BMC Pulm Med 2018;18(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Koichi Y, Aya Y, Megumi U, Shunichi K, Masafumi S, Hiroaki M, et al. A case of anti-MDA5-positive rapidly progressive interstitial lung disease in a patient with clinically amyopathic dermatomyositis ameliorated by rituximab, in addition to standard immunosuppressive treatment. Mod Rheumatol 2017;27(3):536–40. [DOI] [PubMed] [Google Scholar]
- [57].Ogawa Y, Kishida D, Shimojima Y. Effective administration of rituximab in anti-MDA5 antibody-positive dermatomyositis with rapidly progressive interstitial lung disease and refractory cutaneous involvement: a case report and literature review. 2017;2017:5386797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tamai K, Tachikawa R, Otsuka K, Ueda H, Hosono Y, Tomii K. Early pulmonary involvement of anti-CADM-140 autoantibody-positive rapidly progressive interstitial lung disease preceding typical cutaneous symptoms. Intern Med 2014;53(21):2515–9. [DOI] [PubMed] [Google Scholar]
- [59].Tokunaga K, Hagino N. Dermatomyositis with rapidly progressive interstitial lung disease treated with rituximab: a report of 3 cases in Japan. Intern Med 2017;56(11):1399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zou J, Li T, Huang X, Chen S, Guo Q, Bao C. Basiliximab may improve the survival rate of rapidly progressive interstitial pneumonia in patients with clinically amyopathic dermatomyositis with anti-MDA5 antibody. Ann Rheum Dis 2014;73(8):1591–3. [DOI] [PubMed] [Google Scholar]
- [61].Kato M, Ikeda K, Kageyama T, Kasuya T, Kumagai T, Furuya H, et al. Successful treatment for refractory interstitial lung disease and pneumomediastinum with multidisciplinary therapy including tofacitinib in a patient with anti-MDA5 antibody-positive dermatomyositis. J Clin Rheumatol 2019. [DOI] [PubMed] [Google Scholar]
- [62].Okabayashi H, Ichiyasu H, Hirooka S, Akaike K, Kojima K, Jodai T, et al. Clinical effects of direct hemoperfusion using a polymyxin B-immobilized fiber column in clinically amyopathic dermatomyositis-associated rapidly progressive interstitial pneumonias. BMC Pulm Med 2017;17(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sasaki O, Dohi M, Harada H, Harada H, Imamura M, Imamura M , Tsuchida Y, Tsuchida Y , Yamaguchi K, Yamaguchi K , Komai T, et al. A Case of Polymyxin b-Immobilized Fiber Column Treatment for Rapidly Progressive Interstitial Pneumonia Associated with Clinically Amyopathic Dermatomyositis. (1687–9627 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Silveira MG, Selva-O’Callaghan A, Ramos-Terrades N, Arredondo-Agudelo KV, Labrador-Horrillo M, Bravo-Masgoret C. Anti-MDA5 dermatomyositis and progressive interstitial pneumonia. Qjm 2016;109(1):49–50. [DOI] [PubMed] [Google Scholar]
- [65].Teruya A, Kawamura K, Ichikado K, Sato S, Yasuda Y, Yoshioka M. Successful polymyxin B hemoperfusion treatment associated with serial reduction of serum anti-CADM-140/MDA5 antibody levels in rapidly progressive interstitial lung disease with amyopathic dermatomyositis. Chest 2013;144(6):1934–6. [DOI] [PubMed] [Google Scholar]
- [66].Furusawa H, Sugiura M, Mitaka C, Inase N. Direct hemoperfusion with polymyxin B-immobilized fibre treatment for acute exacerbation of interstitial pneumonia. Respirology 2017;22(7):1357–62. [DOI] [PubMed] [Google Scholar]
- [67].Ichiyasu H, Horio Y, Tsumura S, Hirosako S, Sakamoto Y, Sakata S, et al. Favorable outcome with hemoperfusion of polymyxin B-immobilized fiber column for rapidly progressive interstitial pneumonia associated with clinically amyopathic dermatomyositis: report of three cases. Mod Rheumatol 2014;24(2):361–5. [DOI] [PubMed] [Google Scholar]
- [68].Ichiyasu H, Horio Y, Masunaga A, Migiyama Y, Sakamoto Y, Jodai T, et al. Efficacy of direct hemoperfusion using polymyxin B-immobilized fiber column (PMX-DHP) in rapidly progressive interstitial pneumonias: results of a historical control study and a review of previous studies. Ther Adv Respir Dis 2017;11(7):261–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Endo Y, Koga T, Suzuki T, Hara K, Ishida M, Fujita Y, et al. Successful treatment of plasma exchange for rapidly progressive interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis: A case report. Medicine (Baltimore) 2018;97(15):e0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gorka J, Szczeklik W, Wludarczyk A, Loboda P, Chmura L, Musial J. Rapidly progressive interstitial lung fibrosis in a patient with amyopathic dermatomyositis and antiMDA5 antibodies. Pol Arch Med Wewn 2015;125(9):685–6. [DOI] [PubMed] [Google Scholar]
- [71].Broome M, Palmer K, Schersten H, Frenckner B, Nilsson F. Prolonged extracorporeal membrane oxygenation and circulatory support as bridge to lung transplant. Ann Thorac Surg 2008;86(4):1357–60. [DOI] [PubMed] [Google Scholar]
- [72].Leclair V, Labirua-Iturburu A, Lundberg IE. Successful lung transplantation in a case of rapidly progressive interstitial lung disease associated with antimelanoma differentiation-associated gene 5 antibodies. J Rheumatol 2018;45(4):581–3. [DOI] [PubMed] [Google Scholar]
- [73].Deitchman AR, Kalchiem-Dekel O, Todd N, Reed RM. Rapidly progressive interstitial lung disease due to anti-melanoma differentiation associated protein-5 requiring a bilateral lung transplant, and complicated by kennel cough. Respir Med Case Rep 2019;28:100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Huang K, Vinik O, Shojania K, Yeung J, Shupak R, Nimmo M, et al. Clinical spectrum and therapeutics in Canadian patients with anti-melanoma differentiation-associated gene 5 (MDA5)-positive dermatomyositis: a case-based review. Rheumatol Int 2019;39(11):1971–81. [DOI] [PubMed] [Google Scholar]
- [75].Yusen RD, Edwards LB, Dipchand AI, Goldfarb SB, Kucheryavaya AY, Levvey BJ, et al. The registry of the international society for heart and lung transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016;35(10):1170–84. [DOI] [PubMed] [Google Scholar]
- [76].Bernstein EJ, Peterson ER, Sell JL, D’Ovidio F, Arcasoy SM, Bathon JM, et al. Survival of adults with systemic sclerosis following lung transplantation: a nationwide cohort study. Arthritis Rheumatol 2015;67(5):1314–22. [DOI] [PubMed] [Google Scholar]
- [77].Takagishi T, Ostrowski R, Alex C, Rychlik K, Pelletiere K, Tehrani R. Survival and extrapulmonary course of connective tissue disease after lung transplantation. J Clin Rheumatol 2012;18(6):283–9. [DOI] [PubMed] [Google Scholar]
- [78].Courtwright AM, El-Chemaly S, Dellaripa PF, Goldberg HJ. Survival and outcomes after lung transplantation for non-scleroderma connective tissue-related interstitial lung disease. J Heart Lung Transplant 2017;36(7):763–9. [DOI] [PubMed] [Google Scholar]
- [79].Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014–an update from the pulmonary transplantation council of the international society for heart and lung transplantation. J Heart Lung Transplant 2015;34(1):1–15. [DOI] [PubMed] [Google Scholar]
- [80].Selva-O’Callaghan A, Labrador-Horrillo M, Munoz-Gall X, Martinez-Gomez X, Majo-Masferrer J, Solans-Laque R, et al. Polymyositis/dermatomyositis-associated lung disease: analysis of a series of 81 patients. Lupus 2005;14(7):534–42. [DOI] [PubMed] [Google Scholar]
- [81].Shoji T, Bando T, Fujinaga T, Chen F, Sasano H, Yukawa N, et al. Living-donor lobar lung transplantation for rapidly progressive interstitial pneumonia associated with clinically amyopathic dermatomyositis: report of a case. Gen Thorac Cardiovasc Surg 2013;61(1):32–4. [DOI] [PubMed] [Google Scholar]
- [82].Chaisson NF, Paik J, Orbai AM, Casciola-Rosen L, Fiorentino D, Danoff S, et al. A novel dermato-pulmonary syndrome associated with MDA-5 antibodies: report of 2 cases and review of the literature. Medicine (Baltimore) 2012;91(4):220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].El Euch M, Bani W, Mahfoudhi M, Jaziri F, Ben Abdelghani K, Turki S, et al. A severe anti MDA-5 antibodies associated amyopathic dermatomyositis. Tunis Med 2017;95(6):444–5. [PubMed] [Google Scholar]
- [84].Chan CWS, Chung HY, Lau CS, Tsang HHL. Spontaneous pneumomediastinum in a dermatomyositis patient with anti-melanoma differentiation-associated gene-5 antibody and interstitial lung disease despite an initial response to immunosuppressant. Int J Rheum Dis 2017. [DOI] [PubMed] [Google Scholar]
- [85].Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N Engl J Med. 2019;381:291–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


