Abstract
Background
Due to its rarity, it is challenging to predict the survival of patients with synchronous multiple primary esophageal squamous carcinomas (SMPESCs). We aimed to construct nomograms to predict survival outcomes and help to make therapeutic strategy for patients with SMPESCs.
Materials and Methods
The clinical and survival data of 135 patients with SMPESCs were analyzed retrospectively. Univariate and multivariate Cox analyses were used to identify independent prognostic factors. Nomograms were constructed to predict 1-year, 3-year and 5-year disease-free survival (DFS) and overall survival (OS). In addition, we further evaluated the effect of postoperative adjuvant therapy on SMPESCs patients with lymph node metastasis.
Results
In univariate and multivariate analyses of DFS and OS, age, site of the main lesion, lymph node metastasis, total number of lymph nodes dissected, lactate dehydrogenase level and lymphocyte-to-monocyte ratio were identified as independent prognostic factors. These characteristics were further included to establish nomograms. For the internal validation of the nomogram predictions of survival outcomes, the concordance indices were 0.752 and 0.756, respectively. Decision curve analysis also proved the efficacy of the nomograms. Furthermore, adjuvant therapy had a statistically significant benefit for OS but not DFS in patients with lymph node metastasis.
Conclusions
These nomograms could effectively predict the 1-year, 3-year and 5-year survival outcomes of patients with SMPESCs. Furthermore, adjuvant therapy has the potential to improve OS in patients with lymph node metastasis.
Keywords: Multiple esophageal squamous carcinomas, synchronous, survival, nomogram
Introduction
Multiple primary esophageal carcinoma is a relatively unusual tumor, defined as two or more carcinomas in different parts of the esophagus confirmed by pathologic diagnosis simultaneously or successively. The mechanism of multiple primary cancers may be explained by the theory of field cancerization, which argues that exposure of the epithelium of the aerodigestive tract (e.g. head and neck, esophagus and lung) to carcinogens (e.g. tobacco and alcohol) results in the development of multiple primary tumors [1], but the validity and reliability of this theory has been revisited by recently published article [2]. The incidences of patients with multiple primary esophageal carcinomas have ranged from 1% to 31% in previous studies [3–6]. To date, there have been several studies on the prognosis of synchronous multiple primary esophageal squamous carcinomas (SMPESCs) [3,4,7]. However, in these studies, the sample size of patients who underwent surgery was relatively small, and patients who received radical chemoradiotherapy or radiotherapy were also included. In addition, the clinical data of patients are insufficient, such as preoperative blood tests and operation details. The above deficiencies will inevitably affect the accurate assessment of the prognosis of patients with SMPESCs.
This study aimed to construct nomograms to predict the survival outcomes of patients with SMPESCs. In addition, we also explored the role of postoperative adjuvant therapy on survival in patients with lymph node metastasis, which may guide appropriate clinical decision making.
Material and methods
Patient selection
Clinicopathological characteristics and survival data in the present retrospective study were obtained from a prospectively collected database. Recommended by Warren and Gates [8], the inclusion criteria for SMPESCs in this study were as follows: (1) the tumors must be clearly malignant on histologic examination; (2) all the lesions must be separated by normal mucosa from endoscopic inspection and distant metastases must be excluded; (3) no distant organ or supraclavicular lymph node metastasis; (4) R0 resection; and (5) patients did not undergo any treatment before surgery, such as chemotherapy and chemoradiotherapy. Furthermore, cases with the following conditions were excluded: (1) the coexistence of cardiac and hypopharyngeal carcinoma; (2) other pathologic types, including adenocarcinoma, adenosquamous carcinoma and Barrett carcinoma; (3) the existence of the precancerous lesion in two tumor lesions; (4) perioperative death (within 30 days); and (5) a history of cancers or simultaneously accompanying other cancers. Written informed consent for all participants in this study has been obtained. This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (B2022-163-01).
Study population and variables
From January 2001 to July 2019, a total of 3032 consecutive patients with a pathological diagnosis of esophageal squamous carcinoma who underwent surgery (McKeown, Ivor Lewis or Sweet) in the cancer center at which the corresponding author works, were retrospectively screened. Eventually, 135 of these patients met the above inclusion and exclusion criteria. Upper gastroenterography, enhanced computed tomography (CT) and endoscopic ultrasonography were carried out for all patients before surgery. The tumor location and pathological stage were defined according to the 8th edition of the Union for International Cancer Control or American Joint Committee on Cancer staging system [9]. The tumor length was calculated based on the surgically removed specimen. The main lesion was defined as the one with the deepest invasive depth by postoperative pathology, and the second lesion was less invasive than the former [5,6].
The variables, including patient demographics, preoperative hematological parameters, tumor pathological features and surgery-related indicators, were collected retrospectively. The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to monocyte-ratio (LMR), and albumin/globulin ratio (A/G) were calculated by division of the absolute values of the corresponding hematological parameters.
Study endpoints and follow-up
The primary endpoint was disease-free survival (DFS), which was defined as the interval from the date of surgery to the date of disease recurrence or death. The secondary endpoint was overall survival (OS), which was defined as the period from the date of surgery to the date of death from any disease cause or the last follow-up. Patients were followed up in the outpatient clinic every 3 months for the first 2 years after esophagectomy, every 6 months for the next 3 years, and annually thereafter.
Statistical analysis
The cutoff values of age, length of the main lesion, total number of lymph nodes dissected (TNLD) and blood test indicators were calculated by X-tile software (Yale University, New Haven, Connecticut, USA) according to prognosis. The Kaplan–Meier method was used to plot survival curves. To minimize the statistical bias caused by sample size limitations, factors with P values less than 0.20 in univariate analysis were entered into a multivariate Cox regression model (backward stepwise) for multivariate analyses. All statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Nomograms were constructed based on the results of multivariate analyses using the “regplot” package in R version 4.4.0 (available at http://www.r-project.org/). The concordance index (C-index) was used to evaluate the reliability of the nomograms. Calibration curves were used to compare the conformity between the predicted and actual survival. In addition, decision curve analysis (“dcurves” package) was performed to compare the efficacy in different models. A two-tailed P value <0.05 was considered statistically significant.
Results
Patient characteristics
This study recruited 135 of 3032 patients (4.5%), including 113 males and 22 females, who met the inclusion criteria. The median follow-up time for all patients was 54.8 months. As shown in Table 1, the average age of included patients was 60 years (range from 39 to 83).
Table 1.
Baseline characteristics and univariate analysis of the survival of SMPESCs patients.
| Variables | Value, n (%) | Univariate analysis P value |
|
|---|---|---|---|
| DFS | OS | ||
| Age, years | 0.068 | 0.040 | |
| ≤ 67 | 110 (81.5) | ||
| >67 | 25 (18.5) | ||
| Sex | 0.702 | 0.232 | |
| Male | 113 (83.7) | ||
| Female | 22 (16.3) | ||
| BMI (kg/m²) | 0.032 | 0.019 | |
| ≤ 18.5 | 11 (8.1) | ||
| 18.5–22.9 | 86 (63.7) | ||
| ≥ 23.0 | 38 (28.2) | ||
| Smoking and drinking history | 0.960 | 0.904 | |
| No | 47 (34.8) | ||
| Yes | 88 (65.2) | ||
| Preoperative comorbidity | 0.826 | 0.873 | |
| No | 117 (86.7) | ||
| Yes | 18 (13.3) | ||
| Family history | 0.074 | 0.312 | |
| No | 109 (80.7) | ||
| Yes | 26 (19.3) | ||
| Hemoglobin (g/L) | 0.023 | 0.015 | |
| ≤125.5 | 18 (13.3) | ||
| >125.5 | 117 (86.7) | ||
| A/G | 0.041 | 0.280 | |
| ≤1.71 | 103 (76.3) | ||
| >1.71 | 32 (23.7) | ||
| LDH (U/L) | 0.011 | 0.026 | |
| ≤183.7 | 96 (71.1) | ||
| >183.7 | 39 (28.9) | ||
| ALP (U/L) | 0.308 | 0.156 | |
| ≤ 92.0 | 116 (85.9) | ||
| >92.0 | 19 (14.1) | ||
| NLR | 0.225 | 0.557 | |
| ≤ 2.4 | 87 (64.4) | ||
| >2.4 | 48 (35.6) | ||
| PLR | 0.149 | 0.317 | |
| ≤ 144.9 | 94 (69.6) | ||
| >144.9 | 41 (30.4) | ||
| LMR | 0.013 | 0.003 | |
| ≤ 4.00 | 75 (55.6) | ||
| >4.00 | 60 (44.4) | ||
| Surgical procedure | 0.457 | 0.817 | |
| Minimally invasive | 100 (74.1) | ||
| Open | 35 (25.9) | ||
| Operative approach | 0.744 | 0.242 | |
| Right thoracic | 111 (82.2) | ||
| Left thoracic | 24 (17.8) | ||
| Number of lesions | 0.728 | 0.558 | |
| 2 | 121 (89.6) | ||
| ≥ 2 | 14 (10.4) | ||
| Site of main lesion | 0.014 | 0.011 | |
| Upper thoracic portion | 10 (7.4) | ||
| Middle thoracic portion | 47 (34.8) | ||
| Lower thoracic portion | 78 (57.8) | ||
| Length of main lesion (mm) | 0.053 | 0.071 | |
| ≤ 15 | 23 (17.0) | ||
| >15 | 112 (83.0) | ||
| Differentiation of main lesion | 0.860 | 0.541 | |
| High differentiation | 23 (17.0) | ||
| Moderate differentiation | 77 (57.0) | ||
| Poor differentiation | 35 (26.0) | ||
| pT of main lesion | 0.014 | 0.016 | |
| T1 | 27 (20.0) | ||
| T2 | 24 (17.8) | ||
| T3 | 84 (62.2) | ||
| Site of second lesion | 0.140 | 0.213 | |
| Upper thoracic portion | 48 (35.6) | ||
| Middle thoracic portion | 44 (32.6) | ||
| Lower thoracic portion | 43 (31.8) | ||
| Differentiation of second lesion | 0.089 | 0.064 | |
| High differentiation | 64 (47.4) | ||
| Moderate differentiation | 45 (33.3) | ||
| Poor differentiation | 26 (19.3) | ||
| pT of second lesion | 0.016 | 0.019 | |
| Tis/T1 | 101 (74.8) | ||
| T2 | 24 (17.8) | ||
| T3 | 10 (7.4) | ||
| Lymph node metastasis | <0.001 | <0.001 | |
| Yes | 82 (60.7) | ||
| No | 53 (39.3) | ||
| TNLD | 0.015 | 0.093 | |
| ≤ 25 | 61 (45.2) | ||
| >25 | 74 (54.8) | ||
| Postoperative adjuvant therapy | 0.925 | 0.388 | |
| No | 91 (67.4) | ||
| Yes | 44 (32.6) | ||
| Extent of lymph node dissection | 0.070 | 0.116 | |
| Two fields | 117 (86.7) | ||
| Three fields | 18 (13.3) | ||
A/G: albumin/globulin ratio; ALP: alkaline phosphatase; BMI: body mass index; DFS: disease-free survival; LDH: lactate dehydrogenase; LMR: lymphocyte-to-monocyte ratio; NLR: neutrophil-to-lymphocyte ratio; OS: overall survival; PLR: platelet-to-lymphocyte ratio; SMPESCs: synchronous multiple primary esophageal squamous carcinomas; TNLD: total number of lymph nodes dissected.
There were 121 patients (89.6%) who had double lesions, 12 patients (8.9%) who had triple lesions and 2 patients (1.5%) who had quadruple lesions. Of the 286 lesions, 62 were located in the upper thoracic esophagus, 94 in the mid thoracic esophagus and 130 in the lower thoracic esophagus. More than half of the main lesions were in the lower thoracic esophagus (57.8%) and were moderately differentiated (57.0%). Regarding the tumor length and pT stage of the main lesion, 83.0% of lesions were larger than 15 mm, and 62.2% of lesions were in pT3 stage. With regard to the second lesions, 48 cases (35.6%) were in the upper thoracic esophagus, 64 cases (47.4%) were highly differentiated, and 101 cases (74.8%) were pTis/T1 stage.
Most patients underwent minimally invasive esophagectomy (74.1%) and the most common approach was McKeown’s or Ivor Lewis esophagectomy (82.2%). A total of 117 (86.7%) patients underwent two-field lymphadenectomy, and 74 (54.8%) patients had more than 25 resected lymph nodes. In addition, 44 (32.6%) patients underwent adjuvant therapy (including 39 adjuvant chemotherapy and 5 adjuvant chemoradiotherapy). The pretreatment hematological indicators of the patients, such as the hemoglobin level, lactate dehydrogenase (LDH) level, alkaline phosphatase (ALP) level, NLR, PLR, LMR, and A/G, are also shown in Table 1.
Univariate and multivariate analyses of survival
As shown in Table 1, univariate analysis was performed to identify prognostic factors for DFS and OS. There were ten variables, including BMI (p = 0.032), site of the main lesion (p = 0.014), pT of the main lesion (p = 0.014), pT of the second lesion (p = 0.016), lymph node metastasis (p < 0.001), TNLD (p = 0.015), hemoglobin (p = 0.023), A/G (p = 0.041), LDH (p = 0.011) and LMR (p = 0.013), that were significantly related to DFS. Variables with p < 0.20 were included in the multivariate analysis model. As a result, only age (p = 0.043), site of the main lesion (p = 0.001), lymph node metastasis (p < 0.001), TNLD (p = 0.009), LDH (p = 0.013) and LMR (p = 0.004) were independent risk factors for DFS (Table 2).
Table 2.
Multivariate analyses of the DFS and OS of SMPESCs patients.
| Variables | DFS |
OS |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, years | ||||
| ≤ 67 | Reference | Reference | ||
| >67 | 1.914 (1.020–3.593) | 0.043 | 3.094 (1.573–6.083) | 0.001 |
| BMI (kg/m²) | ||||
| ≤ 18.5 | Reference | Reference | ||
| 18.5–22.9 | 1.247 (0.370–4.203) | 0.722 | 2.314 (0.664–8.063) | 0.188 |
| ≥ 23.0 | 1.055 (0.292–3.810) | 0.935 | 1.908 (0.506–7.189) | 0.340 |
| Family history | ||||
| No | Reference | |||
| Yes | 0.822 (0.366–1.842) | 0.633 | ||
| Hemoglobin (g/L) | ||||
| ≤ 125.5 | Reference | Reference | ||
| >125.5 | 0.537 (0.268–1.073) | 0.078 | 0.744 (0.330–1.677) | 0.475 |
| A/G | ||||
| ≤ 1.71 | Reference | |||
| >1.71 | 0.658 (0.341–1.270) | 0.212 | ||
| LDH (U/L) | ||||
| ≤ 183.7 | Reference | Reference | ||
| >183.7 | 1.844 (1.137–2.990) | 0.013 | 1.767 (1.041–2.998 | 0.035 |
| ALP (U/L) | ||||
| ≤ 92.0 | Reference | |||
| >92.0 | 0.642 (0.249–1.655) | 0.360 | ||
| PLR | ||||
| ≤ 144.9 | Reference | |||
| >144.9 | 0.921 (0.506–1.678) | 0.789 | ||
| LMR | ||||
| ≤ 4.00 | Reference | Reference | ||
| >4.00 | 0.478 (0.288–0.793) | 0.004 | 0.422 (0.238–0.747) | 0.003 |
| Site of main lesion | ||||
| Upper thoracic portion | Reference | Reference | ||
| Middle thoracic portion | 0.240 (0.100–0.575) | 0.001 | 0.295 (0.120–0.725) | 0.008 |
| Lower thoracic portion | 0.187 (0.081–0.433 | <0.001 | 0.162 (0.067–0.393) | <0.001 |
| Length of main lesion (mm) | ||||
| ≤ 15 | Reference | Reference | ||
| >15 | 0.975 (0.378–2.516) | 0.959 | 1.241 (0.452–3.406) | 0.675 |
| pT of main lesion | ||||
| T1 | Reference | Reference | ||
| T2 | 1.318 (0.499–3.481) | 0.578 | 1.849 (0.582–5.868) | 0.297 |
| T3 | 1.649 (0.672–4.042) | 0.275 | 2.643 (0.903–7.739) | 0.076 |
| Site of second lesion | ||||
| Upper thoracic portion | Reference | |||
| Middle thoracic portion | 0.899 (0.510–1.582) | 0.711 | ||
| Lower thoracic portion | 1.582 (0.836–2.990) | 0.158 | ||
| Differentiation of second lesion | ||||
| High differentiation | Reference | Reference | ||
| Moderate differentiation | 0.899 (0.510–1.582) | 0.711 | 0.941 (0.497–1.778) | 0.850 |
| Poor differentiation | 1.582 (0.836–2.990) | 0.158 | 1.707 (0.852–3.422) | 0.132 |
| pT of second lesion | ||||
| Tis/T1 | Reference | Reference | ||
| T2 | 0.729 (0.341–1.559) | 0.416 | 0.508 (0.230–1.121) | 0.093 |
| T3 | 1.551 (0.670–3.592) | 0.305 | 0.702 (0.287–1.712) | 0.436 |
| Lymph node metastasis | ||||
| No | Reference | Reference | ||
| Yes | 4.538 (2.580–7.982) | <0.001 | 5.373 (2.808–10.282) | <0.001 |
| TNLD | ||||
| ≤25 | Reference | Reference | ||
| >25 | 0.514 (0.313–0.844) | 0.009 | 0.527 (0.309–0.900) | 0.019 |
| Extent of lymph node dissection | ||||
| Two fields | Reference | Reference | ||
| Three fields | 0.626 (0.253–1.551) | 0.312 | 0.555 (0.210–1.471) | 0.237 |
| Reoperation | ||||
| No | Reference | |||
| Yes | 0.279 (0.064–1.213) | 0.089 | ||
A/G: albumin/globulin ratio; ALP: alkaline phosphatase; BMI: body mass index; DFS: disease-free survival; LDH: lactate dehydrogenase; LMR: lymphocyte-to-monocyte ratio; OS: overall survival; PLR: platelet-to-lymphocyte ratio; SMPESCs: synchronous multiple primary esophageal squamous carcinomas; and TNLD: total number of lymph nodes dissected.
Similarly, the results of the univariate analyses showed that age (p = 0.040), BMI (p = 0.019), site of the main lesion (p = 0.011), pT of the main lesion (p = 0.016), pT of the second lesion (p = 0.019), lymph node metastasis (p < 0.001), hemoglobin (p = 0.015), LDH (p = 0.026) and LMR (p = 0.003) were significantly related to OS (Table 1). In addition, some factors, such as length of the main lesion (p = 0.071), differentiation of the second lesion (p = 0.064), TNLD (p = 0.093), extent of lymph node dissection (p = 0.116) and ALP (p = 0.156), were also included in multivariate Cox analyses. The results showed that age (p = 0.001), site of the main lesion (p = 0.008), lymph node metastasis (p < 0.001), TNLD (p = 0.019), LDH (p = 0.035) and LMR (p = 0.003) were independent risk factors for OS (Table 2).
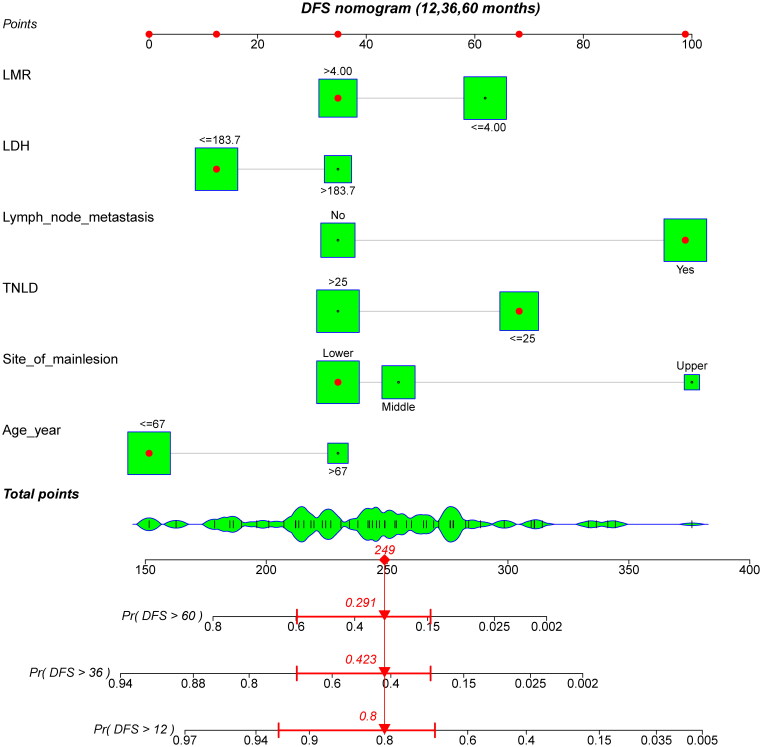
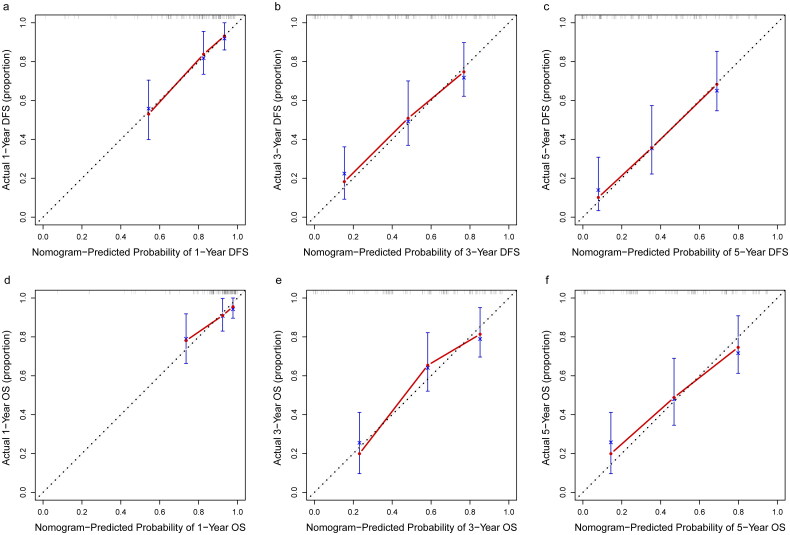
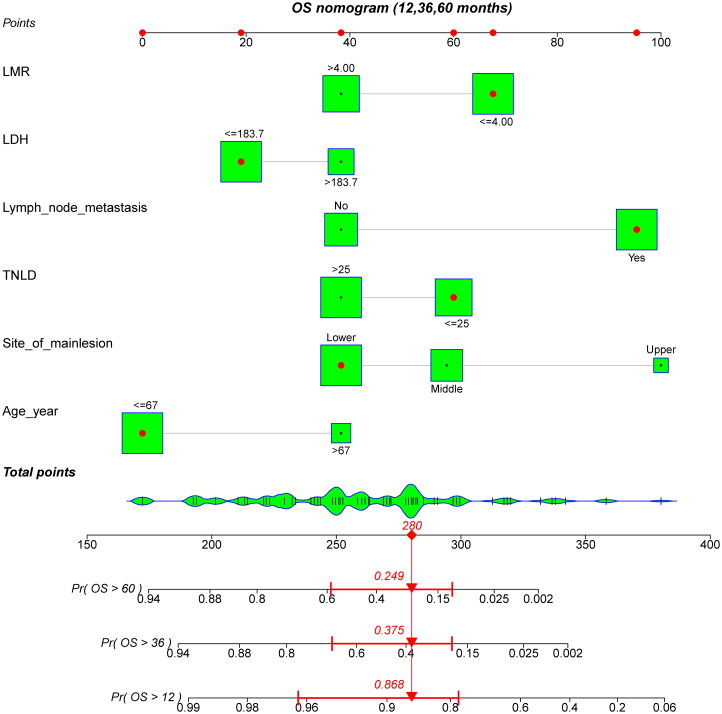
All independent risk factors determined by multivariate analyses were integrated into nomograms for predicting the 1-year, 3-year and 5-year DFS and OS of patients with SMPESCs. Figures 1 and 2 shows an example of using the nomogram to predict survival probability of a given patient. The total score was determined based on the individual scores calculated using the nomogram. For the internal validation of the nomograms of DFS and OS, the C-indices were 0.752 and 0.756, respectively. The calibration plots showed well consistency between the nomogram prediction and the actual survival (Figure 3).
Figure 1.
A constructed nomogram for DFS prediction of a patient. The patient was 58 years old with the lower third esophageal tumor lesion, had 23 lymph nodes dissected and 2 pathologically positive lymph nodes. The sum (249) of these points is located on the total points axis, and a line is drawn downward to the survival axes to determine the probability of 5-year (29.1%), 3-year (42.3%) and 1-year (80.0%) DFS. DFS: disease-free survival; LDH: lactate dehydrogenase; LMR: lymphocyte-to-monocyte ratio; TNLD: total number of lymph nodes dissected.
Figure 3.
Calibration curves of the nomogram for predicting OS and DFS (a) 1-year DFS, (b) 3-year DFS, (c) 5-year DFS, (d) 1-year OS, (e) 3-year OS, and (f) 5-year OS.
Figure 2.
A constructed nomogram for OS prediction of a patient. The patient was 60 years old with the lower third esophageal tumor lesion, had 21 lymph nodes dissected and 1 pathologically positive lymph nodes. The sum (280) of these points is located on the total points axis, and a line is drawn downward to the survival axes to determine the probability of 5-year (24.9%), 3-year (37.5%) and 1-year (86.8%) OS. LDH: lactate dehydrogenase; LMR: lymphocyte-to-monocyte ratio; OS: overall survival; TNLD: total number of lymph nodes dissected.
As shown in decision curve analysis (Supplementary Figure S1), compared with the model based on TNM stage, the models based on nomograms could bring more predictive benefit to survival outcomes of patient with SMPESCs.
Subgroup analysis
In the whole cohort, postoperative adjuvant therapy was not a significant prognostic predictor in patients with SMPESCs. However, in univariate analysis, our result showed that adjuvant therapy was significantly beneficial for prolonging DFS (p = 0.039) and OS (p = 0.003) for patients with lymph node metastasis (Supplementary Table S1 and Supplementary Figure S2). Furthermore, result from multivariate analysis showed that adjuvant therapy remained an independent prognostic factor for OS (p = 0.013) (Supplementary Table S2) but not for DFS (p = 0.124) (Supplementary Table S3). In the subgroup without lymph node metastasis, there was no difference in DFS or OS between those with and without adjuvant therapy (p = 0.973 and p = 0.619, Supplementary Figure S3).
Discussion
According to the results of our previously published article [10], the 5-year and 10-year cumulative overall survival rate of patients with SMPESCs were worse than those of patients with single lesion. Therefore, it is urgent need to improve the survival of patients with SMPESCs. As far as we know, this study is the first to develop and validate prognostic nomograms for predicting the prognosis of SMPESCs patients with the largest surgical sample, which may help us to identify those patients with the highe risk for recurrence and death inclinical practice.
It is generally believed that age is associated with the survival outcome in several previous studies [11–13]. Decreased OS may be related to comorbidities or postoperative complications; shorter DFS may be attributed to impaired immunosurveillance in elderly individuals or the surgeon may intend to reduce the degree of surgery when considering the poor physical condition of elderly patients. Our study showed that main tumor lesions located at the upper third of the esophagus predicted poorer outcomes than their counterparts. According to other studies [14,15], due to the proximity of the trachea and recurrent laryngeal nerves, radical resection of such tumors may be compromised by surgeons. Moreover, Li et al. found that 72% (126/175) of patients with upper thoracic carcinoma had locoregional recurrences in the upper mediastinum [16]. Taken together, those patients with upper third esophageal cancers have worse survival than others.
As multimodality therapy for esophageal cancer have become more important, the emphasis has focused on how and when chemotherapy, radiation therapy and even immunotherapy therapy should be administered, rather than the details of surgical technique. The prognostic value of the number of lymph nodes dissected for esophageal cancer remains controversial [17,18]. We found that more than 25 lymph nodes dissected was beneficial to the DFS and OS of patients with SMPESCs. In our opinion, an increased number of lymph nodes dissected is not only conducive to the accuracy of staging diagnosis but also enables the local lymph node dissection to be more thorough, thus reducing the risk of postoperative residue. In addition, lymph node metastasis is more likely to occur due to the presence of lesions in different segments of the thoracic esophagus in SMPESCs patients, therefore, more lymph nodes need to be dissected to ensure R0 resection. The number of dissected lymph nodes is not only important for patients with multiple lesions, but also for patients wih single lesion. However, the specific number of harvested lymph nodes taht would benefit patients with SMPESCs still needs further study.
LDH is a pivotal kinase in the interconversion of pyruvate to lactate in anaerobic glycolysis. Rapid progression of cancer cells leads to hypoxic conditions in the tumor microenvironment [19]. Moreover, elevated serum LDH levels have been suggested to be a marker of immune suppression in cancer patients [20]. Overall, serum LDH levels may reflect hypoxia in tumor cells and immune suppression in patients, which lead to poor prognosis. Several studies on cancers have reported that elevated levels of LDH are significantly associated with poor prognosis [21,22]. Similarly, in this study, we found that a relatively high level of pretreatment serum LDH was negatively correlated with DFS and OS after resection of SMPESCs. Consistent with the findings of previous studies on single lesion of esophageal carcinoma, a low LMR was also found to be associated with poorer prognosis than a high LMR in SMPESCs. Although the mechanism by which LMR affects the prognosis of cancer remains unclear, there are some speculations. On the one hand, monocytes are known to promote the tumorigenesis, angiogenesis and metastasis of tumors [23]. On the other hand, lymphocytes play an important role in suppressing cancer cell proliferation by enhancing tumor apoptosis [24].
The role of adjuvant therapy in patients with lymph node-positive esophageal cancer has not reached a consensus [25,26]. In regard to SMPESCs, we found that adjuvant therapy significantly improved OS for patients with positive lymph nodes, while it was not significant for DFS. Therefore, adjuvant therapy is a recommendable choice to improve the prognosis of such patients.
Our model had several limitations. First, it was established using a retrospective database in a single center, and selection bias was inevitable in this study population. Second, due to the relatively small sample size of the study, these results must still be further validated by randomized controlled trials and large-scale prospective analyses in multiple institutes. In addition, the efficacy of these hematological biomarkers aforementioned in identifying patients with multiple lesions from those with single lesion has not been investigated in the present study. This analysis will be explored in the future study. Finally, the C-index is considered as a parameter for internal verification, but combined with external verification is more reliable to evaluate the efficiency of this model, which will be scheduled in our future study.
Conclusion
We developed prognostic nomograms to provide individual survival predictions for patients with SMPESCs. These nomograms had good discrimination and calibration and could help identify the high-risk population after surgery. Moreover, for patients with lymph node metastasis, adjuvant therapy is a recommended choice to prolong the survival time.
Supplementary Material
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant number 81972614).
Author contributions
Study conception/design: Kexi Wang, Jian Zhong, Qianwen Liu; Data acquisition: Danting Su, Jian Zhong; Data analysis and model construction: Kexi Wang; Changsen Leng; Interpreting results: Kexi Wang, Jian Zhong, Jianhua Fu, Qianwen Liu; Initial drafting of manuscript: Jian Zhong, Kexi Wang, Danting Su; Final revision of manuscript: Kexi Wang, Jianhua Fu, Qianwen Liu.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author (Qianwen Liu, liuqw@sysucc.org.cn), upon reasonable request.
References
- 1.Strong MS, Incze J, Vaughan CW.. Field cancerization in the aerodigestive tract—its etiology, manifestation, and significance. J Otolaryngol. 1984;13(1):1–6. [PubMed] [Google Scholar]
- 2.Desai RS, Shirsat PM, Bansal S, et al. Oral field cancerization: a critical appraisal. Oral Oncol. 2021;118:105304. doi: 10.1016/j.oraloncology.2021.105304. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Lin Z-X.. Characteristics and prognostic factors of synchronous multiple primary esophageal carcinoma: a report of 52 cases. Thorac Cancer. 2014;5(1):25–30. doi: 10.1111/1759-7714.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Li S, He Z, et al. Clinical analysis of 117 cases with synchronous multiple primary esophageal squamous cell carcinomas. Korean J Intern Med. 2021;36(6):1356–1364. doi: 10.3904/kjim.2017.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pesko P, Rakic S, Milicevic M, et al. Prevalence and clinicopathologic features of multiple squamous cell carcinoma of the esophagus. Cancer. 1994;73(11):2687–2690. doi: . [DOI] [PubMed] [Google Scholar]
- 6.Kuwano H, Ohno S, Matsuda H, et al. Serial histologic evaluation of multiple primary squamous cell carcinomas of the esophagus. Cancer. 1988;61(8):1635–1638. doi: . [DOI] [PubMed] [Google Scholar]
- 7.Li Q-W, Zhu Y-J, Zhang W-W, et al. Chemoradiotherapy for synchronous multiple primary cancers with esophageal squamous cell carcinoma: a case-control study. J Cancer. 2017;8(4):563–569. doi: 10.7150/jca.17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren S, Gates O.. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932;16:1358–1414. [Google Scholar]
- 9.Rice TW, Gress DM, Patil DT, et al. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):304–317. doi: 10.3322/caac.21399. [DOI] [PubMed] [Google Scholar]
- 10.Chen J-Y, Zhang S-S, Fu X-Y, et al. The characteristics and prognostic significance of esophageal squamous cell carcinoma with synchronous multiple lesions: over 10-year experience. Esophagus. 2021;18(4):851–860. doi: 10.1007/s10388-021-00856-8. [DOI] [PubMed] [Google Scholar]
- 11.Tapias LF, Muniappan A, Wright CD, et al. Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann Thorac Surg. 2013;95(5):1741–1748. doi: 10.1016/j.athoracsur.2013.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markar SR, Karthikesalingam A, Thrumurthy S, et al. Systematic review and pooled analysis assessing the association between elderly age and outcome following surgical resection of esophageal malignancy. Dis Esophagus. 2013;26(3):250–262. doi: 10.1111/j.1442-2050.2012.01353.x. [DOI] [PubMed] [Google Scholar]
- 13.Huscher CGS, Bretagnol F, Corcione F.. Laparoscopic colorectal cancer resection in high-volume surgical centers: long-term outcomes from the LAPCOLON group trial. World J Surg. 2015;39(8):2045–2051. doi: 10.1007/s00268-015-3050-4. [DOI] [PubMed] [Google Scholar]
- 14.Law S, Kwong DLW, Kwok K-F, et al. Improvement in treatment results and long-term survival of patients with esophageal cancer: impact of chemoradiation and change in treatment strategy. Ann Surg. 2003;238(3):339–348. discussion 347-8. doi: 10.1097/01.sla.0000086545.45918.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y-F, Chen H-S, Wu S-C, et al. Esophageal squamous cell carcinoma and prognosis in Taiwan. Cancer Med. 2018;7(9):4193–4201. doi: 10.1002/cam4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Zhang Q, Xu L, et al. Factors predictive of prognosis after esophagectomy for squamous cell cancer. J Thorac Cardiovasc Surg. 2009;137(1):55–59. doi: 10.1016/j.jtcvs.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg. 2008;248(2):221–226. doi: 10.1097/SLA.0b013e31817bbe59. [DOI] [PubMed] [Google Scholar]
- 18.van der Schaaf M, Johar A, Wijnhoven B, et al. Extent of lymph node removal during esophageal cancer surgery and survival. J Natl Cancer Inst. 2015;107(5):djv043. doi: 10.1093/jnci/djv043. [DOI] [PubMed] [Google Scholar]
- 19.Biswas S, Lunec J, Bartlett K.. Non-glucose metabolism in cancer cells—is it all in the fat? Cancer Metastasis Rev. 2012;31(3–4):689–698. doi: 10.1007/s10555-012-9384-6. [DOI] [PubMed] [Google Scholar]
- 20.Ding J, Karp JE, Emadi A.. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19(4):353–363. doi: 10.3233/CBM-160336. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Fu X, Su X, et al. Elevated pretreatment serum lactate dehydrogenase level predicts inferior overall survival and disease-free survival after resection of thymic carcinoma. J Thorac Dis. 2017;9(11):4550–4560. doi: 10.21037/jtd.2017.10.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B, Dai D, Tang H, et al. Pre-treatment serum alkaline phosphatase and lactate dehydrogenase as prognostic factors in triple negative breast cancer. J Cancer. 2016;7(15):2309–2316. doi: 10.7150/jca.16622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh YW, Kang HJ, Park C, et al. The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin’s lymphoma: correlation with tumor-associated macrophages. Oncologist. 2012;17(6):871–880. doi: 10.1634/theoncologist.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 25.Pasquer A, Gronnier C, Renaud F, et al. Impact of adjuvant chemotherapy on patients with lymph node-positive esophageal cancer who are primarily treated with surgery. Ann Surg Oncol. 2015;22: 1340–9. doi: 10.1245/s10434-015-4658-1. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Zhao L, Lin B, et al. Adjuvant therapeutic modalities following three-field lymph node dissection for stage II/III esophageal squamous cell carcinoma. J Cancer. 2017;8(11):2051–2059. doi: 10.7150/jca.18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Qianwen Liu, liuqw@sysucc.org.cn), upon reasonable request.