Abstract
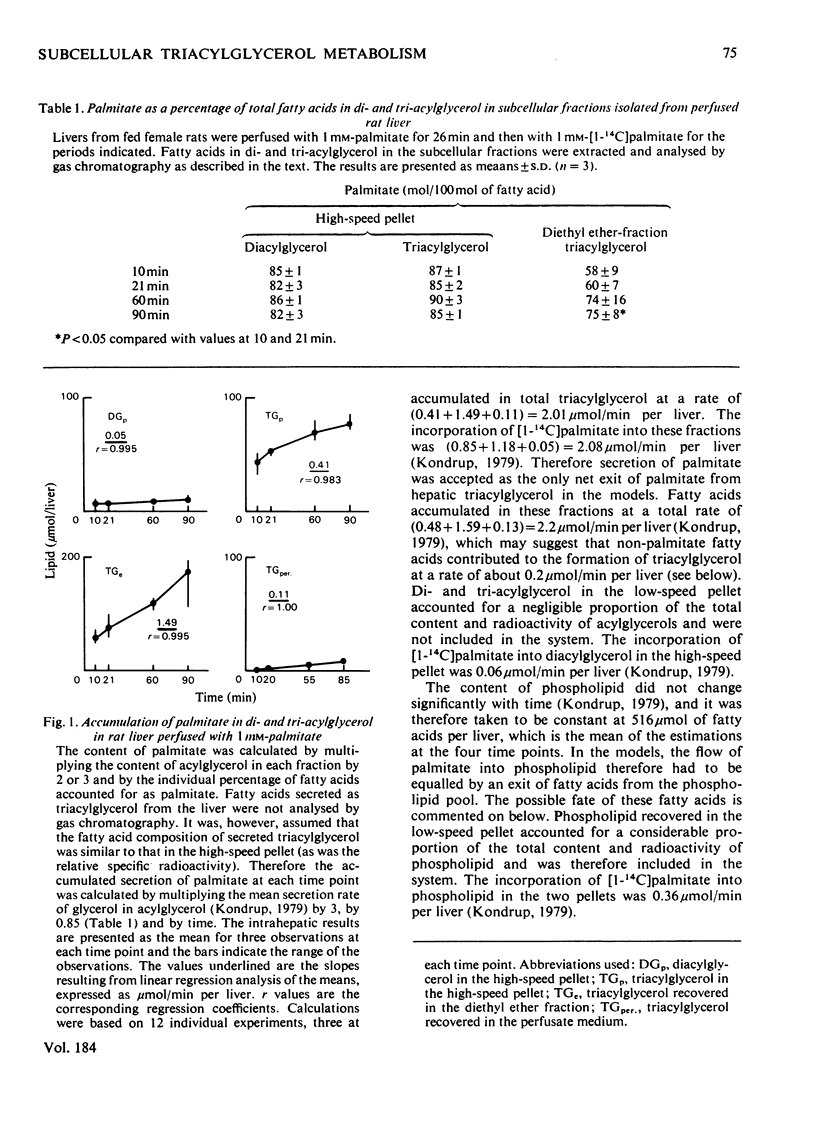
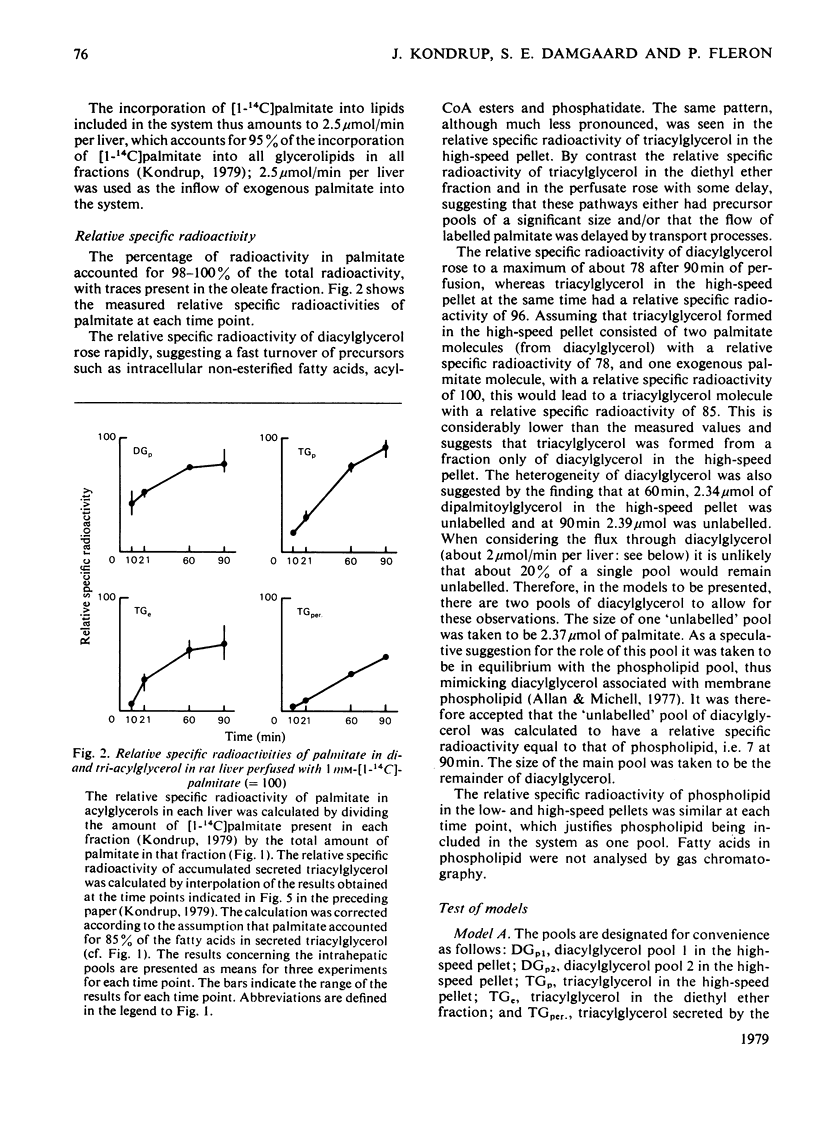
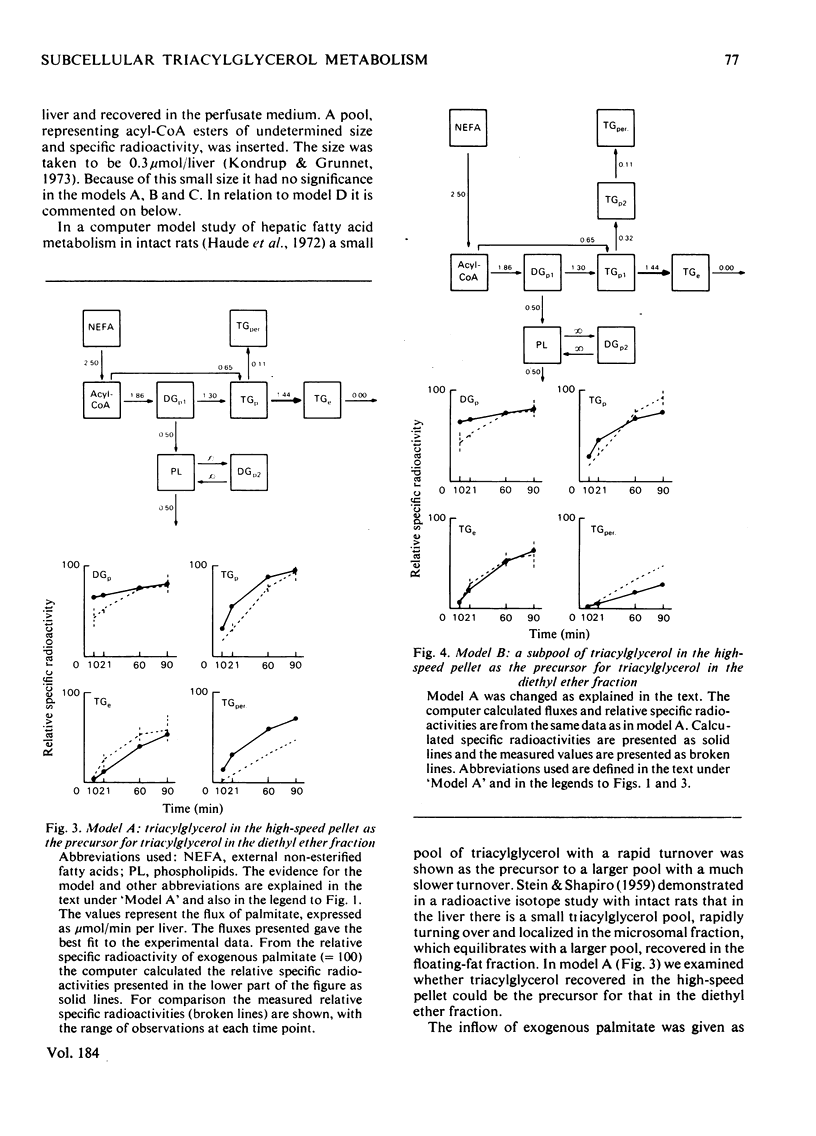
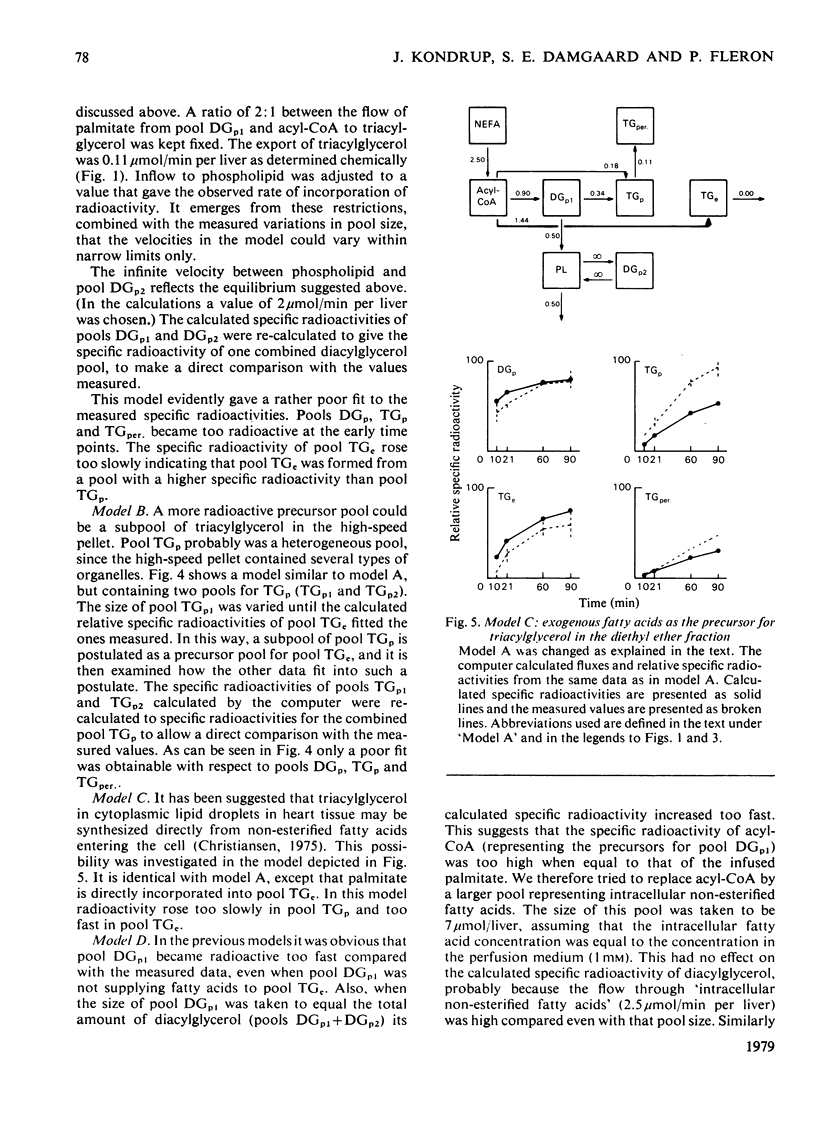
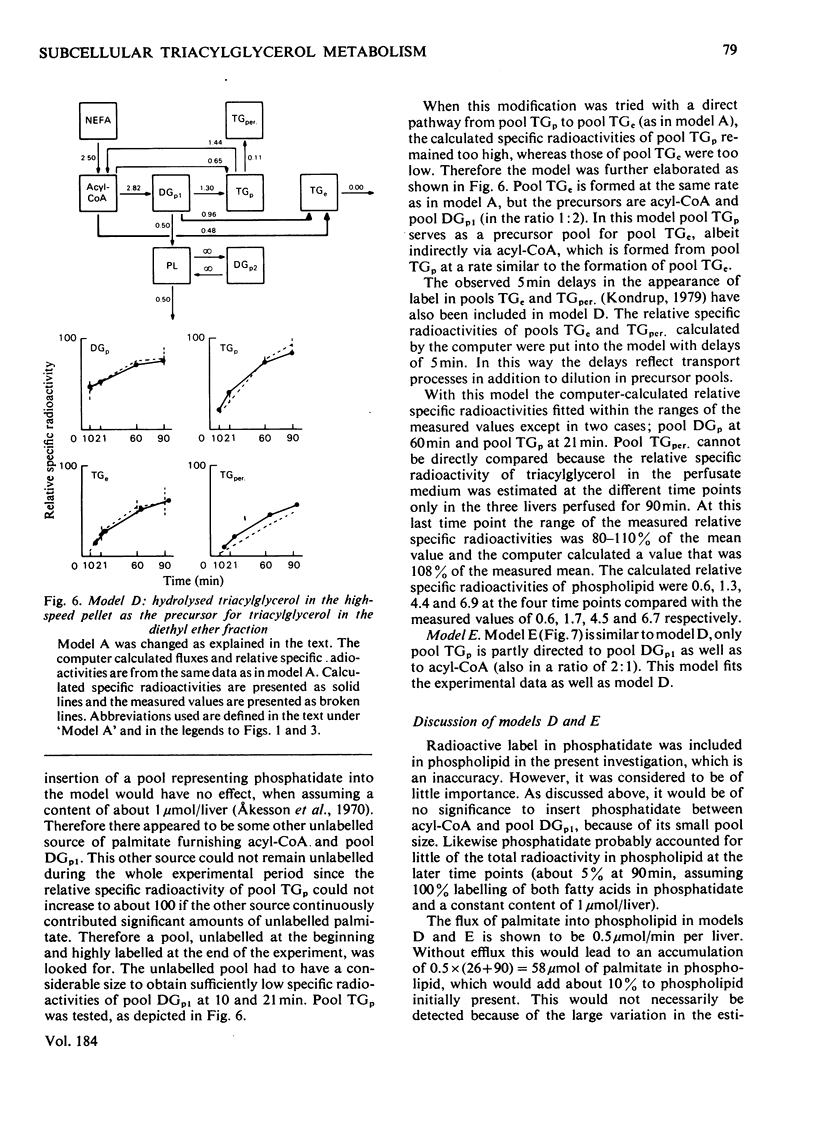
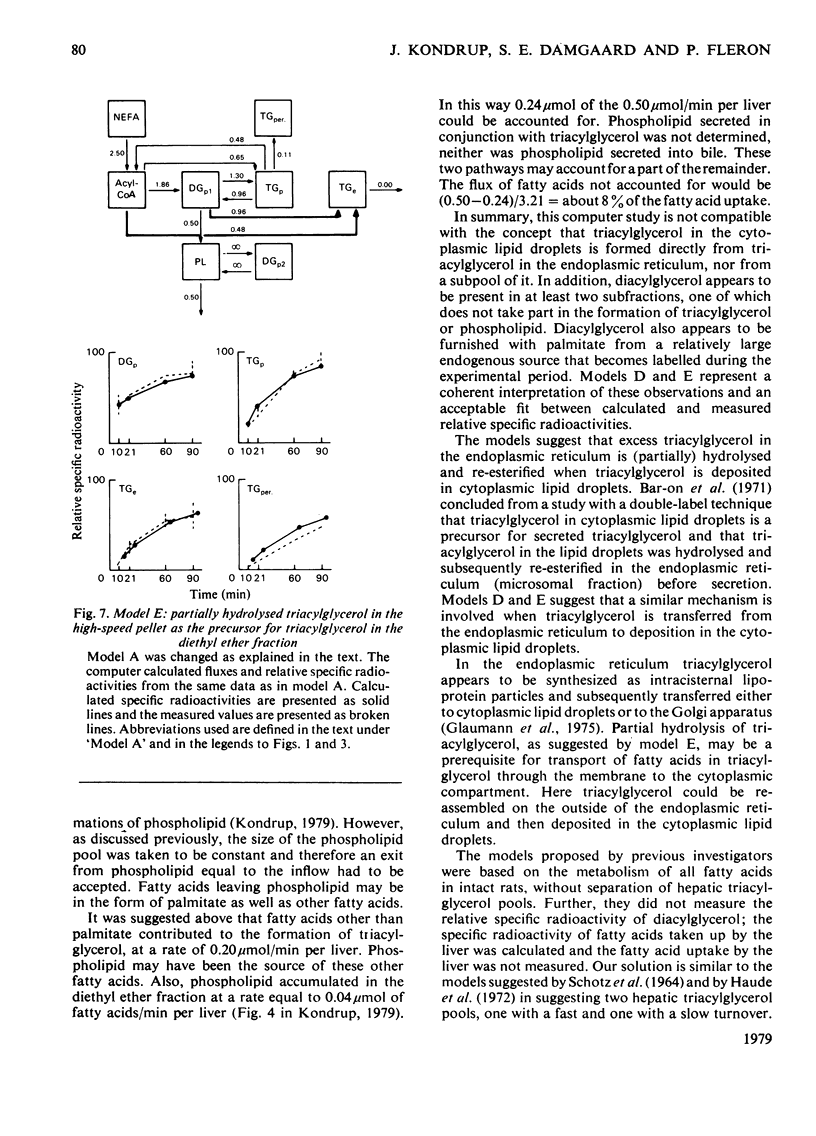
1. In the preceding paper [Kondrup (1979) Biochem. J. 184, 63–71] the separation of two major fractions of hepatic triacylglycerol was described. One fraction contained triacylglycerol from the endoplasmic reticulum and from the Golgi apparatus. The other fraction contained triacylglycerol from the cytoplasmic lipid droplets. In the present paper possible precursor–product relationships between the two fractions were investigated by means of computer models. 2. The fatty acids present in di- and tri-acylglycerol in the fractions isolated in the time studies were analysed by gas chromatography. From this analysis the relative specific radioactivities, and contents, of palmitate in acylglycerols in the two fractions at the various time points were calculated. 3. A computer was used to predict relative specific radioactivities of pools in defined models of hepatic triacylglycerol metabolism. The acceptability of the models was evaluated by comparing predicted with measured relative specific radioactivities. 4. It is suggested that triacylglycerol in cytoplasmic lipid droplets does not originate (a) directly from triacylglycerol in the endoplasmic reticulum, (b) from a sub-pool of it or (c) directly from non-esterified fatty acids entering the cell. Rather, it is formed from diacylglycerol (and acyl-CoA) in the endoplasmic reticulum. Diacylglycerol, on the other hand, is furnished in part by hydrolysis of triacylglycerol in the endoplasmic reticulum. 5. This suggestion is discussed in relation to previous models of hepatic fatty acid metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akesson B., Elovson J., Arvidson G. Initial incorporation into rat liver glycerolipids of intraportally injected (3H)glycerol. Biochim Biophys Acta. 1970 Jun 9;210(1):15–27. doi: 10.1016/0005-2760(70)90057-3. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. Metabolism of phosphatidate at the plasma membrane. Biochem Soc Trans. 1977;5(1):55–59. doi: 10.1042/bst0050055. [DOI] [PubMed] [Google Scholar]
- Baker N., Schotz M. C. Quantitative aspects of free fatty acid metabolism in the fasted rat. J Lipid Res. 1967 Nov;8(6):646–660. [PubMed] [Google Scholar]
- Bar-On H., Roheim P. S., Stein O., Stein Y. Contribution of floating fat triglyceride and of lecithin towards formation of secretory triglyceride in perfused rat liver. Biochim Biophys Acta. 1971 Oct 5;248(1):1–11. doi: 10.1016/0005-2760(71)90068-3. [DOI] [PubMed] [Google Scholar]
- Christiansen K. Membrane-bounded lipid particles from beef heart acylglycerol synthesis. Biochim Biophys Acta. 1975 Mar 24;380(3):390–402. doi: 10.1016/0005-2760(75)90107-1. [DOI] [PubMed] [Google Scholar]
- Glaumann H., Bergstrand A., Ericsson J. L. Studies on the synthesis and intracellular transport of lipoprotein particles in rat liver. J Cell Biol. 1975 Feb;64(2):356–377. doi: 10.1083/jcb.64.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haude W., Wagner H., Theil S., Haase H., Hünicke G., Goetze E. Bestimmung der Umsätze und Flussraten von Fettsäuren und Cholesterin in Serum und Leber der Ratte mit Hilfe der mathematischen Simulierung von Isotopenverdünnungskurven am Analogrechner. Acta Biol Med Ger. 1972;28(6):963–975. [PubMed] [Google Scholar]
- Jones A. L., Ruderman N. B., Herrera M. G. Electron microscopic and biochemical study of lipoprotein synthesis in the isolated perfused rat liver. J Lipid Res. 1967 Sep;8(5):429–446. [PubMed] [Google Scholar]
- Kondrup J., Grunnet N. The effect of acute and prolonged ethanol treatment on the contents of coenzyme A, carnitine and their derivatives in rat liver. Biochem J. 1973 Mar;132(3):373–379. doi: 10.1042/bj1320373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrup J. Metabolism of palmitate in perfused rat liver. Isolation of subcellular fractions containing triacylglycerol. Biochem J. 1979 Oct 15;184(1):63–71. doi: 10.1042/bj1840063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkilä E. A., Kekki M., Ojala K. Kinetic analysis of triglyceride metabolism in single rats. Ann Med Exp Biol Fenn. 1966;44(2):348–355. [PubMed] [Google Scholar]
- SCHOTZ M. C., BAKER N., CHAVEZ M. N. EFFECT OF CARBON TETRACHLORIDE INGESTION ON LIVER AND PLASMA TRIGLYCERIDE TURNOVER RATES. J Lipid Res. 1964 Oct;5:569–577. [PubMed] [Google Scholar]
- STEIN Y., SHAPIRO B. Assimilation and dissimilation of fatty acids by the rat liver. Am J Physiol. 1959 Jun;196(6):1238–1241. doi: 10.1152/ajplegacy.1959.196.6.1238. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. Lipid synthesis, intracellular transport, storage, and secretion. I. Electron microscopic radioautographic study of liver after injection of tritiated palmitate or glycerol in fasted and ethanol-treated rats. J Cell Biol. 1967 May;33(2):319–339. doi: 10.1083/jcb.33.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler R., Akesson B., Nilsson A. Effect of different fatty acids on glycerolipid synthesis in isolated rat hepatocytes. J Biol Chem. 1974 Aug 25;249(16):5102–5107. [PubMed] [Google Scholar]


