Abstract
Although systemic immunotherapy has achieved durable responses and improved survival for certain patients and cancer types, low response rates and immune system-related systemic toxicities limit its overall impact. Intratumoral (intralesional) delivery of immunotherapy is a promising technique to combat mechanisms of tumor immune suppression within the tumor microenvironment and reduce systemic drug exposure and associated side effects. However, intratumoral injections are prone to variable tumor drug distribution and leakage into surrounding tissues, which can compromise efficacy and contribute to toxicity. Controlled release drug delivery systems such as in situ-forming hydrogels are promising vehicles for addressing these challenges by providing improved spatio-temporal control of locally administered immunotherapies with the goal of promoting systemic tumor-specific immune responses and abscopal effects. In this review we will discuss concepts, applications, and challenges in local delivery of immunotherapy using controlled release drug delivery systems with a focus on intratumorally injected hydrogel-based drug carriers.
Graphical Abstract
1. Introduction
Immunotherapy has transformed the treatment landscape of oncology by harnessing the body’s immune system to combat cancer. This is exemplified by the success of immune checkpoint inhibitors (ICI), which have achieved durable responses in a subset of patients with advanced cancers. However, many tumors have limited immunogenicity and possess numerous mechanisms of immune suppression that contribute to poor response rates or eventual failure of immunotherapy [1]. Moreover, systemic immunotherapies commonly cause immune-related adverse events [2], which can be severe [3]. Therefore, strategies are needed to increase the percentage of patients that benefit from immunotherapy and improve its tolerability.
Intratumoral (intralesional) immunotherapy aims to potentiate systemic anti-tumor effects via local delivery of therapeutic agents that stimulate tumor antigen-specific immune responses or promote immuno-permissive conditions that increase a tumor’s susceptibility to systemic ICI or other therapies. Immunotherapeutic agents delivered directly into a tumor via percutaneous injection dramatically increase intratumoral drug concentrations and reduce systemic drug exposure compared to systemic delivery. There are a variety of intratumoral immunotherapies undergoing clinical evaluation including Toll-like receptor (TLR) agonists [4, 5], stimulator of interferon gene (STING) agonists [6, 7], cytokines [8], and oncolytic viruses [9, 10]. However, leakage into adjacent tissues, rapid clearance, and variable distribution following intratumoral injection can contribute to toxicity and undermine treatment efficacy, potentially confounding analyses of treatment outcomes and jeopardizing clinical translation of intratumoral immunotherapies.
Hydrogels are a promising platform for enhancing the intratumoral delivery of immunotherapy. These biomaterials-based drug delivery systems consist of a semi-solid network of polymers with high water content that provide localized, sustained release of a variety of encapsulated drugs [11, 12]. Stimuli-responsive, in situ-forming hydrogels (ISFH) are of particular interest due to their ability to undergo a transition from liquid to semi-solid gel in response to physiologic stimuli (e.g., pH or temperature). This feature enables ISFH to be delivered via a syringe or catheter directly into a tumor, averting the need for surgical implantation. Encapsulation of immune-modulating agents within ISFH provides enhanced spatial and temporal control of local immunotherapy. In addition, ISFH can provide a three-dimensional scaffold-like microenvironment that attracts immune cells and fosters interactions between the immune system and entrapped tumor antigens or immunomodulators. Other local immunomodulation strategies include catheter-based intraarterial infusions with co-administration of embolics to induce tissue ischemia and necrosis as well as reduce washout of drug [13–15]. Recently, drug-eluting beads containing immunomodulators have been developed to provide sustained, local delivery of immunotherapy during transarterial immunoembolization [16, 17]. The extent to which these therapeutic paradigms impact the dynamic balance between treatment efficacy and systemic toxicity remains central to determining the success of local immunotherapy delivery strategies.
In this review we provide an overview of the determinants of drug distribution following intratumoral injection, highlight key properties of injectable ISFH, and define pathways, hurdles, and emerging opportunities for intratumoral, hydrogel-based delivery of immunotherapy.
1.1. The tumor immune microenvironment (TIME)
The tumor immune microenvironment (TIME) harbors multifaceted interactions between cancer cells, immune cells, stromal cells, and extracellular matrix components [18]. This heterogeneous intratumoral milieu possesses both anti-tumor immune activity and mechanisms of immune suppression that promote tumor development [19]. The hallmark of anti-tumor immunity is the presence of tumor-specific CD8+ T lymphocytes within the tumor microenvironment. So-called “inflamed” tumors are characterized by high levels of cytotoxic CD8+ T cells, while immune- “excluded” or “desert” phenotypes are characterized by T cells confined to the border of the tumor or absent from the tumor, respectively [20]. Immune-inflamed tumors are more likely to respond to ICI compared to immune-excluded or desert tumor phenotypes and are predictive of improved survival in some cancer types [21, 22].
Upon encountering specific tumor antigens, activated cytotoxic CD8+ and helper CD4+ Th1 T cells undergo extensive clonal proliferation and differentiation into various effector subtypes which can determine a tumor’s fate. Tumors can induce a state of T cell exhaustion or anergy, characterized by reduced functionality and diminished cytokine production, limiting their ability to control tumor growth. Additionally, regulatory T cells (Tregs) may accumulate within the tumor, suppressing effector T-cell responses and promoting immune tolerance [23]. Manipulating the balance of T-cell phenotypes may be an effective approach to enabling the immune system to combat tumors. Other cytotoxic cells, including NK cells, can promote tumor cell killing and immune cell recruitment. By contrast, myeloid derived suppressor cells (MDSC) are essential contributors to immunosuppressive conditions in the TIME [24].
Non-cellular components are crucial for the homeostasis of the TIME. Collagenous extracellular matrix (ECM), promoted by cancer-associated fibroblasts, can influence the migration, phenotype, and function of T cells [25, 26]. Moreover, dysfunctional tumor vasculature and connective tissue may reduce the infiltration of effector T cells and inhibit delivery of systemically administered therapeutic agents. Intratumoral delivery of ECM-modifying agents, e.g., collagenase or hyaluronidase, or antiangiogenic agents for vascular normalization, could alter the composition of the tumor microenvironment, improving distribution and penetration of drugs and immune cells, and reducing immunosuppression directly.
1.2. Rationale for intratumoral delivery of immunotherapy
Intratumoral drug delivery is a well-established technique that has generally been used to treat localized, superficial cancers, with limited rationale for treating locally advanced and metastatic tumors. However, the emergence of cancer immunotherapy and the recognition that local therapies can activate systemic adaptive immune responses has expanded the possibilities and potential indications for intratumoral drug delivery. In addition, advancements in interventional imaging guidance and minimally invasive devices have provided exquisite access to nearly all tissues within the body, which presents vast opportunities for selective tumor-localized delivery of immunotherapy [27].
Intratumoral delivery of immunotherapy has several advantages over systemic delivery. Since many immunotherapeutic agents, such as proinflammatory agents and adjuvants, possess a narrow systemic therapeutic index, local intratumoral delivery is appealing as it can substantially reduce systemic drug exposure and limit associated toxicities. This route of administration also results in higher drug concentrations in the tumor compared to an equivalent systemic dose. Moreover, intratumoral injections can circumvent some of the transport barriers associated with systemic drug delivery that impede drugs from reaching sequestered tumor cells distant from blood vessels [28]. Intratumoral injections can also enable precise targeting of spatially discrete tumor compartments using X-ray fluoroscopy, computed tomography (CT), cone beam CT, or ultrasound guidance (with or without magnetic resonance imaging (MRI) or positron emission tomography (PET) fusion imaging) for needle placement. Delivery of immunotherapy to cells within microenvironments not adequately reached by systemic therapy, such as hypoxic regions with limited blood supply, could enhance the robustness of the immune response.
1.3. Determinants of drug distribution following intratumoral injection
1.3.1. Injection parameters
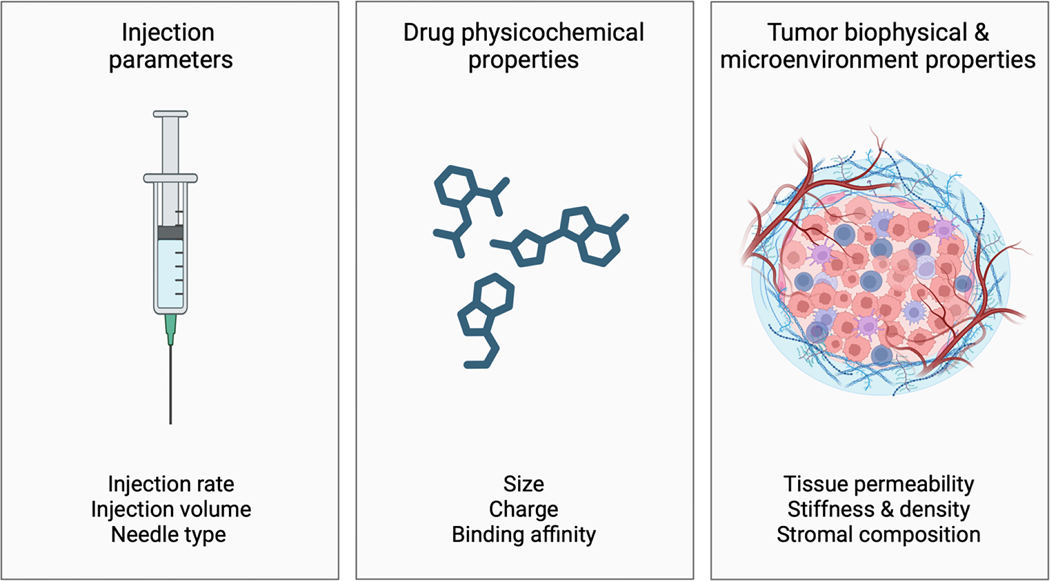
When a drug is injected into tissue, the tissue deforms around the needle tip creating a fluid-filled cavity [29, 30]. Fluid flows through the extracellular space at a velocity that increases with injection rate (pressure) and tissue permeability (hydraulic conductivity). When the applied pressure surpasses a threshold, the tissue may rupture [31, 32], forming low-resistance channels that can result in non-uniform or off-target drug distribution, or rapid intravasation. Slower injection rates reduce the applied pressure, possibly mitigating the risk of tissue rupture and leakage [33, 34]. Similarly, multi-hole, or multi-pronged needles can reduce the applied pressure by distributing it over several points of fluid egress [35, 36]. Immediate outflow of injected drugs into systemic circulation has been observed even at injection volumes below the theoretical “hold-up” or fluid volume capacity of the tumor [37, 38]. This highlights a need for adequate calibration of injection parameters to maximize tumor coverage and minimize systemic drug exposure. However, optimal injection techniques and parameters, such as injection rate and volume as well as needle type, have yet to be defined, and are not universal. Indeed, various temporal, spatial, organ, and histological heterogeneities may influence this optimization across various clinical scenarios (Figure 1).
Figure 1.
Factors influencing intratumoral drug transport and distribution (i.t. injection). These include injection parameters, physicochemical properties of the injected drug and delivery system (e.g., hydrogels), and biophysical and microenvironment properties of the tumor.
1.3.2. Drug physicochemical properties
The physiochemical properties (e.g., size, charge, and binding affinity) of a drug (or nanomedicine) influence its tissue transport and ultimate distribution following intratumoral injection [39–43]. Larger macromolecules or drug carriers have lower diffusivity, in accordance with the Stokes-Einstein relationship, and may be entrapped entirely within the ECM if they are large enough. Therefore, modification of the ECM, for example by delivery of enzymes that degrade hyaluronan or collagen, can increase diffusive transport [44]. Binding affinity to ECM components or cellular targets can also affect drug transport and retention in the tumor interstitium. Macromolecules with low binding affinity may penetrate further yet are cleared more rapidly compared to macromolecules with high binding affinity, such as targeted monoclonal antibodies [45–47]. For example, a preclinical study evaluated the effects of intratumoral injections of IL-2 fused to proteins of different sizes and collagen-binding affinity. The study demonstrated that higher molecular weight and collagen affinity resulted in greater tumor exposure and efficacy of IL-2 in a mouse tumor model [37].
1.3.3. Tumor biophysical and microenvironment properties
Drug distribution following intratumoral injection is influenced by the mechanical and hydraulic properties of the tissue (Figure 1). Tissue elasticity (stiffness) can influence its deformation during injection, which can affect convection and diffusion, and resistance to stress before mechanical failure [29, 48]. Tumors may be more susceptible to crack formation during injection into regions of necrosis, abnormal ECM, or other tissue defects [49, 50]. Tissue permeability affects the flow of fluid containing dissolved drug through tissue, with dense ECM and cellular compartments or desmoplasia contributing to reduced permeability and fluid flow. Since tumor properties can differ between tumor types or within different regions of the same tumor, use of a single universal injection strategy is therefore likely to yield inconsistent results.
1.4. Limitations of intratumoral drug delivery
Leakage of injected fluid into tissues surrounding the tumor or along the needle track (“backflow”) [51] may compromise treatment efficacy or incur toxicity. A recent clinical study using CT imaging following injection of an iodinated immunotherapeutic agent in patients revealed heterogeneous intratumoral distribution and high inter-patient variability [36] (Figure 2). In some cases, significant off-target drug deposition was observed requiring repeated injections. Moreover, multiple periodic injections may be needed due to rapid clearance of drug following a single injection, increasing bleeding risks and costs. Technical guidelines for optimal intratumoral injection, ideally developed using imaging confirmation of drug delivery, may expedite clinical translation of intratumoral immunotherapies. Unfortunately, most drugs cannot be seen using clinical imaging. Incorporation of immunotherapeutic agents into image-able controlled release drug delivery systems (DDS), such as image-able ISFH, may help to ensure adequate on-target drug delivery following intratumoral injection.
Figure 2.
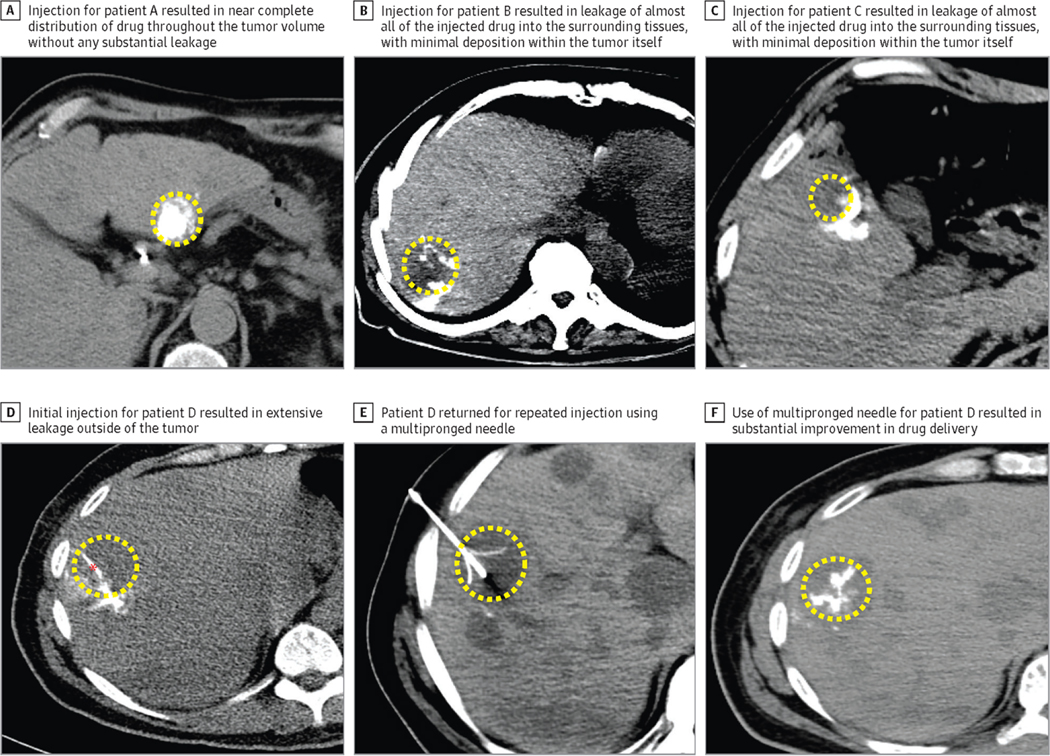
(A-C) Example of variability in the distribution of a radiopaque drug, PV-10, following intratumoral injection into three patients as seen using intra-procedural non-contrast enhanced axial computed tomography. (D) Example of leakage of intratumorally injected drug from the tumor (yellow dashed ellipse) using a single end hole needle (red asterisk). (E) Deployment of a multipronged needle inside the tumor. (E, F) Intratumoral injection using multipronged needle demonstrating on-target delivery and heterogeneous intratumoral distribution [36]. Reproduced with permission from Elsevier.
2. Hydrogel drug delivery systems
DDS may be classified by the scale of the carrier (e.g., nano, micro, or macro), delivery route (e.g., intravenous (i.v.), intraarterial (i.a.), or intratumoral (i.t)), or pharmacologic paradigm (e.g., cell-cycle specific chemotherapy, or immunotherapy). Hydrogels are crosslinked macromolecular networks of hydrophilic polymers that contain a large proportion of water. These biomaterials are of particular interest for intratumoral drug delivery due to their ability to provide sustained localized release of entrapped drugs [11, 12, 52]. Hydrogels can be generated from natural (e.g., alginate) or synthetic (e.g., poloxamers) polymers that are chemically or physically crosslinked via covalent or non-covalent bonds, respectively, and may be permanent or degradable at predictable or calibrated rates [11, 12]. Drug-loaded hydrogels can be delivered by surgical implantation of preformed gels, or by intratumoral or intraarterial injection using a syringe or catheter, respectively, followed by gelation in situ. In the following sections we will focus on injectable hydrogel-based DDS for minimally invasive intratumoral drug delivery.
2.1. In situ-forming hydrogels
Unlike preformed hydrogels that must be surgically implanted, in situ-forming hydrogels can be injected with a standard needle and syringe prior to undergoing a phase transition (sol-gel) in response to specific endogenous stimuli [53]. Common stimuli to initiate gelation are changes in temperature [54, 55], pH [56, 57], or ion concentration [58]. Alternatively, polymers that are water-insoluble may be injected as a liquid dissolved in a solvent and subsequently precipitate forming a semi-solid depot [59]. Numerous natural and synthetic materials have been investigated for generating in situ-forming hydrogels including alginate [60–62], chitosan [63], polypeptides [64], PEG-conjugates [55], and hyaluronic acid [65]. For example, alginate can be injected as a liquid and undergo ionic crosslinking in the presence of physiologic concentrations of calcium ions (Ca2+) which can interact with carboxylate groups on different polymer chains resulting in the formation of an insoluble gel [66]. In another example, poloxamers, comprised of a central hydrophobic chain of polyoxypropylene (poly(propylene oxide)) and two hydrophilic chains of polyoxyethylene (poly(ethylene oxide), possess temperature dependent self-assembling behavior [67]. Aqueous solutions of poloxamers are liquid at room temperature and form a gel in a reversible manner at elevated temperatures [68]. Alternatively, shear-thinning, or “self-healing”, materials can be used that undergo a reduction in viscosity when passed through a needle, and regain their original viscosity once the applied shear stress has ceased [52, 69].
2.2. Drug loading and release
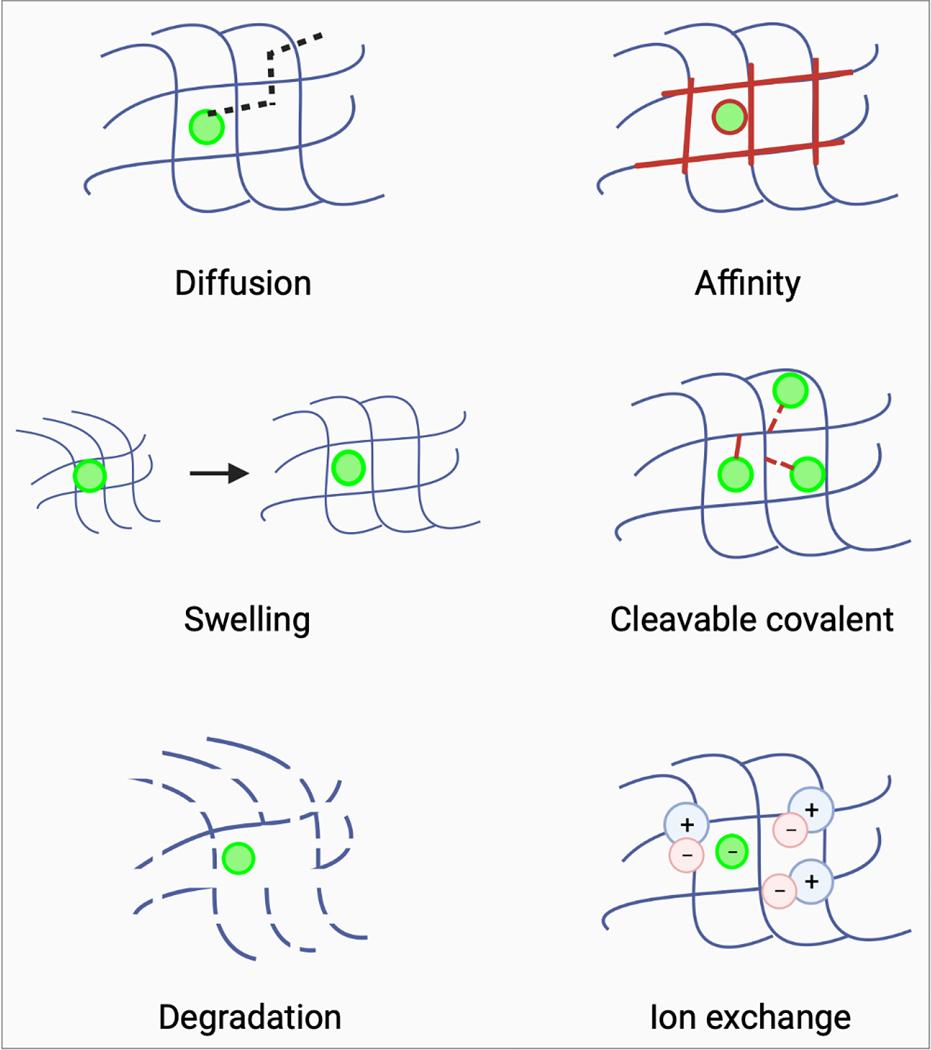
A variety of drugs can be loaded into hydrogels by solubilization and mixing with the hydrogel precursor solution. Upon gelation, the drug is physically entrapped within the hydrogel and is released by diffusion through the hydrogel polymer network (Figure 3). In general, larger macromolecular drugs, such as proteins, have lower diffusion coefficients and permeate more slowly through the hydrogel compared to small-molecule drugs [70]. The rate of release is determined in part by the size of the space between polymer chains, known as the hydrogel “mesh size”, which can be influenced by polymer concentration or degree of crosslinking, and hydrodynamic radius of the drug [71–73]. Hydrogel degradation or swelling can alter the mesh size, triggering or altering the rate of drug release [74, 75] and may be initiated under normal physiologic conditions or by environmental changes (e.g., change in pH). Non-covalent interactions between the drug and the polymer network, for example electrostatic interactions, can influence the rate and extent of drug release with stronger affinity between drug and polymer resulting in slower or reduced release from the hydrogel [76]. For rapidly diffusing small-molecule drugs, burst release may be mitigated by tethering the drug to the polymer via a covalent bond that is cleavable by hydrolysis or specific enzymes [77]. Alternatively, drug release can be controlled by entrapment or formation of drug-loaded nanoparticles within the hydrogel. In this scenario, drug release from the hydrogel may depend on the rate of drug release from the nanoparticles, or release of the nanoparticles from the hydrogel [78]. Therefore, hydrogels may be designed to obtain desired rates of drug release through careful tailoring of their physical and chemical properties.
Figure 3.
Mechanisms of drug release from hydrogels. Drug release from hydrogels is governed by diffusion. However, interactions between drug and hydrogel (drug-hydrogel affinity) can alter the rate and extent of drug release. Similarly, hydrogel swelling or degradation may trigger or alter the kinetics of drug release. Drugs can be loaded into hydrogels and released using cleavable covalent bonds between drug and hydrogel or by ionic interaction between drug and hydrogel and subsequent displacement by ions in tissues.
2.3. Determinants of intratumoral distribution and drug delivery
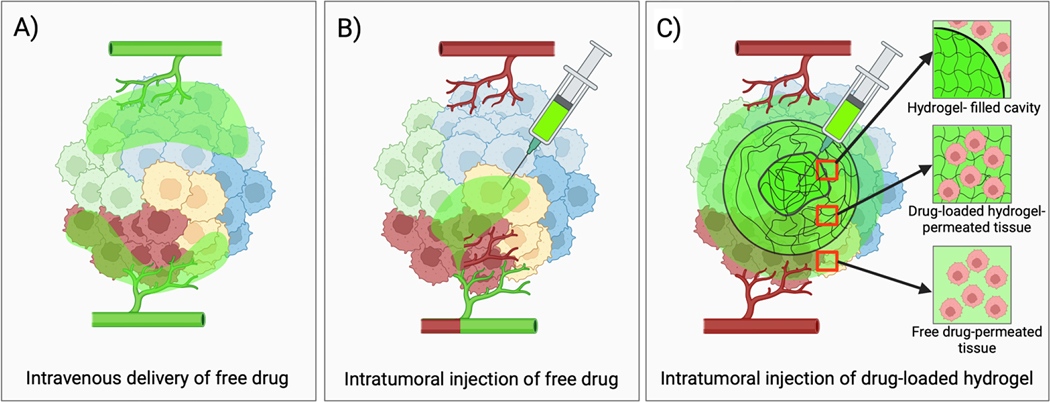
Intravenous drug delivery is limited by poor tumor penetration caused by tumor hypoperfusion, dense ECM, and elevated interstitial fluid pressure, which may leave substantial regions of tumor insufficiently treated (Figure 4A) [79]. Direct intratumoral drug delivery may circumvent some of these barriers, however drug distribution can be heterogeneous and prone to leakage (Figure 4 B). Tailoring hydrogel properties can enable spatial and temporal control of intratumoral drug delivery including more predictable localization at the injection site and controlled, prolonged drug release kinetics (Figure 4C). Several hydrogel characteristics, described below, are important determinants of hydrogel intratumoral distribution and drug delivery.
Figure 4.
Intratumoral drug distribution following systemic and intratumoral drug delivery. A) Systemic drug delivery is limited by poor penetration of the tumor interstitium resulting in insufficient treatment of cells distant from blood vessels. B) Intratumoral injections of free drug are prone to leakage, rapid clearance, and intravasation leading to incomplete and heterogenous tumor drug coverage. C) In contrast, intratumoral injections using in situ-forming hydrogels can improve on-target drug delivery and retention resulting in prolonged tumor drug exposure [59].
2.3.1. Viscosity
The injectability of a hydrogel-drug precursor solution depends on how much pressure is required to push it through a syringe or catheter within a given timeframe. The injection pressure depends on the solution viscosity, needle or catheter geometry (i.e. length and diameter), and flow rate, in accordance with the Hagen-Poiseuille equation, which describes flow through a cylinder [80]. Low viscosity or shear-thinning solutions require less injection pressure and are desirable for their ease of delivery [81]. A low viscosity solution may permeate through tissue to a greater extent than high viscosity solutions, according to Darcy’s Law, resulting in more dispersed depot formation. However, if the viscosity is very low, the resulting hydrogel distribution will approximate that of an injection of free drug. By contrast, high viscosity solutions may permeate tissue to a lesser extent, resulting in depot formation more proximal to the needle tip [29]. Therefore, rheological characterization is critical during the development of injectable in situ-forming hydrogels including testing of handling (e.g., injection pressure) and tissue distribution studies in bench and animal models using needles and catheters of relevant geometry to ensure translation of results to humans.
2.3.2. Gelation rate
Rapidly gelling hydrogels may result in gel formation that is proximal to the needle tip compared to slow gelling hydrogels that have more time to permeate through tissue, given the same viscosity, tissue properties, and injection parameters. Rapidly gelling temperature-responsive materials may be prone to gel formation inside the needle resulting in a blockage depending on the residence time of the precursor solution within the indwelling portion of the needle. Therefore, the gelation rate should be optimized for handling to provide adequate ease of delivery and desired tissue distribution.
2.3.3. Drug release kinetics
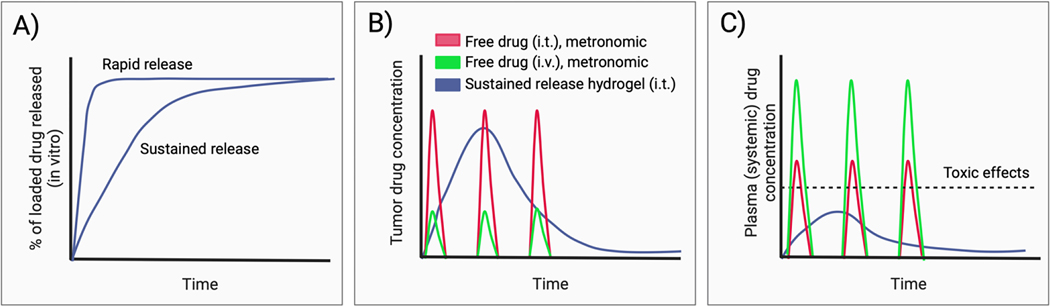
The rate of drug release is an important consideration in the design of in situ-forming hydrogels for drug delivery. Rapid drug release from the hydrogel will result in tissue and plasma pharmacokinetics that approximate those of free drug. This may necessitate repeat injections to compensate for rapid clearance of drug from the tumor, and dose adjustments depending on the extent of systemic exposure and toxicity. In general, prolonged drug release from the hydrogel is desired to reduce peak plasma drug concentrations and associated systemic toxicity (Figure 5). Moreover, sustained drug delivery with a single intratumoral injection of drug-loaded hydrogel may alleviate the need for repeat or metronomic dosing, common to clinical immunotherapy dosing algorithms [82]. Biphasic and triggered drug release have also been studied, which may have special significance for the timelines inherent to the cancer immunity cycle [83, 84].
Figure 5.
Hydrogel drug release kinetics and the influence of hydrogels on tumor and plasma pharmacokinetics. A) Representation of sustained and rapid drug release kinetic profiles for drug-loaded hydrogels measured using an in vitro drug elution assay. B) Sustained drug release from hydrogels can achieve more prolonged exposure of the injected tumor to therapeutic drug concentrations, compared to free drug administered by intratumoral (i.t.) or intravenous (i.v.) injection. C) Sustained drug release from hydrogels reduces peak plasma concentrations and associated systemic toxicity compared to free drug following i.t. or i.v. administration.
To assess drug release (elution) from hydrogels, the hydrogel precursor solution containing a known amount of drug is placed into a mold prior to gelation to achieve consistent sample morphology as the size and shape of the drug-loaded hydrogel may influence the rate of drug release. Subsequently the drug-loaded hydrogel is submerged in a release medium, such as a buffer solution, and incubated under controlled conditions that mimic the intended physiological environment. Alternatively, in situ-forming hydrogel precursor solutions may be placed inside a dialysis membrane cassette of fixed morphology and submerged in an environment with conditions appropriate to stimulate gelation. At predetermined time points, samples are collected from the release medium and the drug concentration measured using appropriate analytical techniques like high-performance liquid chromatography (HPLC) or UV-visible spectrophotometry. Sink conditions should be maintained throughout the experiment to avoid the influence of release medium saturation on the rate of drug release.
3. Hydrogel-mediated immunomodulation
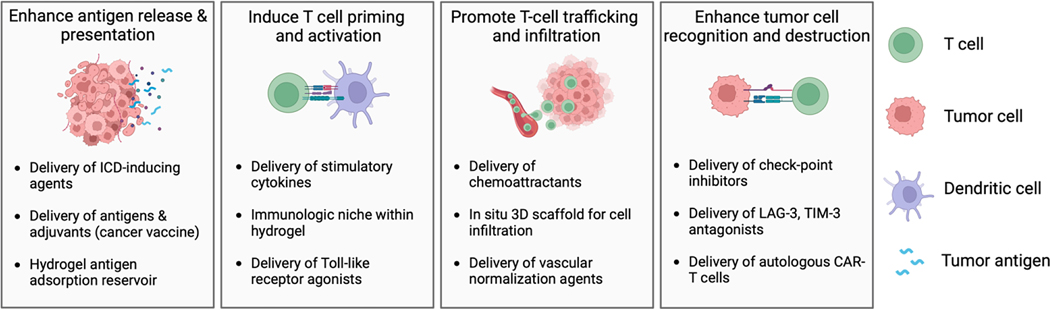
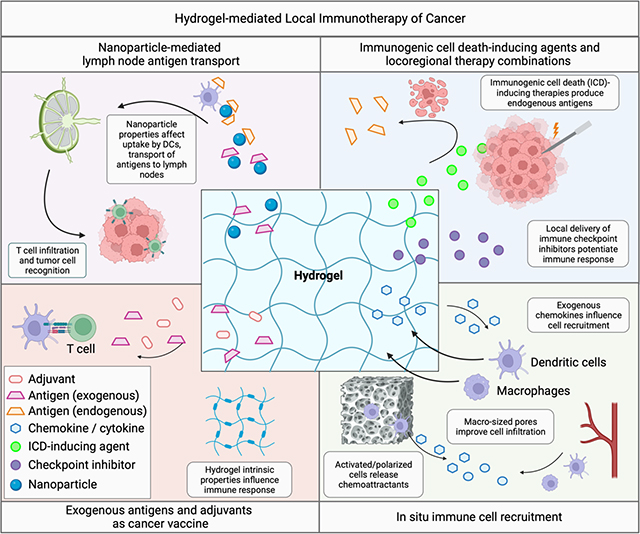
The goal of immunotherapy is to generate sustained anti-tumor immunity by potentiating the steps of the cancer immunity cycle [85]. Local delivery of immunotherapy using hydrogels can effectively promote various stages of the cancer immunity cycle (Figure 6), which starts with the release of tumor antigens and begins anew upon immune system recognition and destruction of cancer cells. Hydrogels can encapsulate tumor-associated antigens or neoantigens and release them gradually, providing a steady supply to antigen-presenting cells (APCs), such as dendritic cells (DC). Alternatively, hydrogel-mediated delivery of cytotoxic agents that induce ICD can initiate antigen release in situ, whereby the hydrogel may additionally serve as an antigen-adsorbing “reservoir”, prolonging immune stimulation and enhancing DC recruitment. Hydrogels can also encapsulate immunomodulating agents, including adjuvants and cytokines, and release them in a controlled manner, priming the immune system via activation and maturation of APCs. The three-dimensional structure of hydrogels supports the infiltration of T cells and other immune effectors, enabling creation of an immunologic niche to support immunomodulation of immune cells lured by chemoattractants released from the hydrogel. In addition, hydrogel-mediated local delivery of immune checkpoint inhibitors or other co-inhibitory molecules may combat tumor immunosuppression and promote T cell function.
Figure 6.
Potential applications of in situ-forming hydrogels for local immunotherapy. ICD: immunogenic cell death.
The following sections describe emerging applications for intratumoral delivery of immunotherapy, which commonly consists of small molecules, nucleic acids, proteins, antibodies, oncolytic viruses, or immune cells, using injectable hydrogels (Figure 7). Nano- and micro-particle DDS for immunotherapy have been recently reviewed [86–88], as have endovascular hydrogel embolics, with relevance to local drug delivery [89, 90]. A selection of preclinical studies demonstrating the use of in situ-forming hydrogels for delivery of intratumoral immunotherapy can be found in Table 1.
Figure 7.
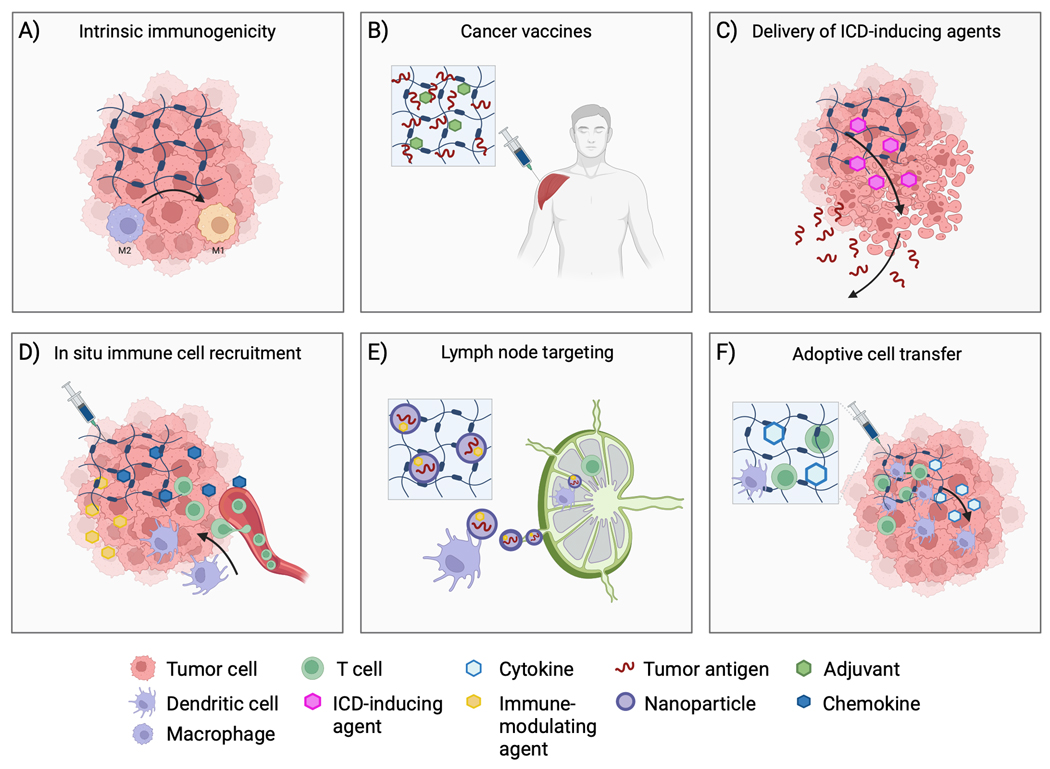
Applications of hydrogels for local delivery of immunotherapy. A) Hydrogel properties, such as charge and surface topography, can influence immune responses including by converting tumor-associated macrophages from immunosuppressive (M2) to antitumor (M1) phenotypes. B) Hydrogels may be employed for delivery of antigens and adjuvants in the form of cancer vaccines that generate tumor-specific immune responses. C) Release of ICD-inducing agents (with or without ICI) from hydrogels delivered via intratumoral injection can result in release of tumor antigens that can potentiate tumor-specific immune responses. D) Hydrogels can be used to increase tumor immune cell infiltration by releasing chemoattractants. Inclusion of immune-modulating agents within the hydrogel may provide an immunologic niche for immune cell integration and immunomodulation within macroporous hydrogels. E) Nanoparticles loaded with antigens and immunomodulators may be released from hydrogels and shuttled to lymph nodes via dendritic cells. F) Hydrogels can also be used for autologous transfer of immune cells, such as CAR-T cells, providing protection during injection and potentially improving cell localization and viability compared to free injection of cells.
Table 1.
Preclinical study examples demonstrating the use of in injectable hydrogels for delivery of immunotherapy.
| Hydrogel | Injection location | Gelation-stimuli | Immunotherapy | Other loaded molecules | Drug release stimulus | Tumor model | Citation |
|---|---|---|---|---|---|---|---|
| Alginate | peritumoral | Ionic interactions | R837 | Doxorubicin | None | B16F 10 | [123] |
| Alginate | i.t. | Ionic interactions | R837; anti-PD-L1 (i.v. or i.t.) | Doxorubicin or Oxaliplatin | None | CT26; 4T1 | [119] |
| Alginate | peritumoral | Ionic interactions | GM-CSF; epacadostat | None | None | 4T1 | [60] |
| F127 (Pluronic)/polyethylene glycol (PEG) | intradermal (i.d.) | In situ crosslinking; body temperature | anti- CTLA-4 and anti-PD-1 | None | None | 4T1 | [193] |
| Hyaluronic acid (modified) | i.t. | In situ mixing of components | OX40; anti-PD-1 (nanocomplex) | None | ROS-triggered depolymerization and hydrolysis | B16F 10 | [194] |
| PEG-b-PLA (nanoparticle) + HPMC | peritumoral | Shear thinning | CD40 agonist antibody | None | None | B16F 10 | [52] |
| Self-assembling camptothecin prodrug - nanotubes | s.c. | Ionic interactions | Anti-PD-1 | Camptothecin | MMP activity | GL-261; CT 26 | [195] |
| PCLA-PEG-PCLA PAMAM dendritic nanoparticle | i.t. | Temperature | Indoximod | Doxorubicin | pH (drug release from nanoparticle) | HeLa | [120] |
| PLGA-PEG-PLGA | i.t. | Temperature | CA4P (vascular disruptive agent) | Epirubicin | None | 4T1 | [124] |
| Silk-chitosan composite hydrogel with dibenzaldehyde-functionalized polyethylene glycol (DF-PEG) | peritumoral | Crosslinking in situ (two syringe compartments) | JQ1 | Doxorubicin | pH (degradation of hydrogel) | 4T1 | [57] |
| Hyaluronic acid and poly(N-(3-aminopropyl)methacrylamide)-co-(N-[Tris(hydroxymethyl)methyl] a crylamide) (p(AMPA-THMA))-based hydrogel | peritumoral | Shear thinning | kynureninase | Doxorubicin | Degradation of hydrogel | 4T1; B16F10 | [74] |
| poly(ethylene glycol)-block-poly(γ-ethyl-L-glutamate) (mPEG-b-PELG) | i.t | Temperature | Anti-PD-L1 | Doxorubicin | None | B16F10 | [121] |
| Hyaluronic acid-based supramolecular hydrogel | i.t | Temperature | DPPA-1 peptide (high binding affinity to PD-L1) | Doxorubicin | None | CT26 | [109] |
| Alginate | i.t | Ionic interactions | CpG | catalase labelled with the therapeutic 131I radioisotope | None | 4T1; CT26; PDX; VX2 | [62] |
| Chitosan-based hydrogel | Injection at site of tumor resection | Temperature | Liposome/protamine/RNA | None | None | KPC | [63] |
| poly(ethylene glycol)-block-polylactide (PEG-b-PLA) nanoparticles + HPMC | s.c. | Shear-thinning | DC cytokine CCL21 | None | None | Tumor-free mice | [69] |
| Hyaluronic acid | s.c. | Reduction-responsive | PD-L1 inhibitor | rod-like nanohydroxyapatite | None | B16 | [65] |
| Multidomain peptide (MDP) | Oral cavity injection | Self-assembling nanofiber matrix | Cyclic dinucleotide (CDN) (STING agonist) | None | None | MOC2-E6E7 tumor cells in maxillary oral vestibule | [196] |
| functional triblock copolymer comprising a central polyethylene glycol (PEG) block flanked by two polypeptide blocks | i.t. | temperature | aPD-L1 | None | ROS-responsive hydrogel degradation | B16F 10 | [197] |
| Low molecular weight polyethylenimine (LPEI) mixed with graphene oxide | s.c. | None | Ovalbuminen-coding mRNA; R848 (resiquimod) | None | Hydrogel degradation (transformation into nanoparticles for lymph node targeting) | B16-OVA | [110] |
| Poly-L-lysine-PEG | peritumoral | Shear thinning | R848 (resiquimod); | PP2 (Polyphyllin II) | None | MFC gastric tumors (s.c.) | [198] |
| melittin-(RADA)n hybrid peptide sequences | i.t. | Self-assembly | None | Ca2+/calmodulin-dependent protein kinase II (CAMKII) inhibitor, KN93 | None | B16F10; H22 hepatoma i.p ascites model | [199] |
| Pluronic F-127 | i.t. | temperature | None | NaHCO3 | None | MC38 | [200] |
| Poly vinyl alcohol (PVA) + TSPBA, ROS- responsive hydrogel | peritumoral | Mixing of hydrogel precursors | IPI549 (PI3 kinase inhibitor); anti-PD-L1 | None | None at early timepoints, ROS-induced polymer degradation | CT26, 4T1 | [190] |
| Nanofiber hydrogel (betamethasone phosphate + calcium ions) | i.t. | Exposure of betamethasone phosphate (anti-inflammatory steroid drug) to Ca2+ | Anti-PD-L1 antibody | None | Hydrogel disassembly in vivo | Mouse CT26 | [201] |
| mPEG-b-poly (γ-ethyl-l-glutamate) (mPEG-b-PELG) | peritumoral | Temperature | Interleukin-15 | Cisplatin | None | C57BL/6 mice B16F0-RFP | [202] |
3.1. Intrinsic immunogenicity
When a biomaterial, such as a hydrogel, is injected or implanted in the body it triggers a series of responses collectively known as a foreign body reaction that is influenced by the biomaterial's composition, surface properties, size, shape, and the specific tissue or organ in which it resides. Proteins that become adsorbed to the biomaterial surface influence subsequent interactions with the immune system [91, 92]. The composition of this protein layer is affected by biomaterial properties including hydrophilicity/hydrophobicity (wettability) [93], charge [94], and topography [95].
Biomaterials with intrinsic immunomodulating properties possess structural or chemical features that can activate or suppress specific immune responses without pharmacologic agents [96, 97]. For example, cationic polymers such as polyethylenimine (PEI) and cationic dextran were reported to convert tumor associated macrophages (TAM) and MDSC polarization from immunosuppressive to antitumor phenotypes via TLR-4 dependent pathway, promote proinflammatory cytokines, and achieve anti-tumor immunity in murine tumor models [98, 99]. Soluble PEI was also found to increase the expressions of CD86 and MHC II on bone-marrow-derived dendritic cells as well as the production of pro-inflammatory cytokines TNF-alpha and IL-6 [100]. DCs grown on different polymer films demonstrated differences in the relative expression of various costimulatory molecules and MHC class II molecules suggestive of inherent adjuvant effects of some biomaterials [101]. Moreover, DCs exposed to different biomaterials and subsequently cultured with autologous T-cells differentially affected T cell phenotype and polarization, depending on the biomaterial [102]. Degradation of biomaterials can also influence their immunogenicity since polymer properties may change during the degradation process [103] including by formation of particles that are known to have immunostimulatory effects that depend on particle size [104] and shape [105].
Immune reactions to foreign materials such as hydrogels can result in excessive inflammation and fibrotic encapsulation. Judicious tailoring of hydrogel properties may be required to strike a balance between surface passivation, for example by attachment of hydrophilic, protein-repelling polymer chains (e.g., polyethylene oxide), and bioactivity via specific interactions with proteins, antigens, and immune cells that promote anti-tumor immune responses.
3.2. Cancer vaccines
Cancer vaccines typically consist of tumor antigens and adjuvants that induce tumor-specific immune responses and anti-tumor immunologic memory [106]. A selection of recent studies demonstrating the use of ISFH for delivery of cancer vaccines can be found in Table 2. After injection (usually into subcutaneous tissue), tumor antigens are captured by endogenous DCs, processed, and presented to T cells in the lymph nodes resulting in T cell priming and activation [85, 107]. Activated T cells circulate throughout the body, infiltrate tumors, and recognize and destroy tumor cells, ultimately propagating the cancer immunity cycle. Successful therapeutic vaccination requires tumor antigens that possess sufficient immunogenicity (variety and tumor specificity) and a strategy to overcome mechanisms of tumor immunosuppression. Hydrogels can provide prolonged simultaneous delivery of antigens – including peptides [108, 109], nucleic acids [63, 110], and whole-tumor cell lysates [55, 64, 111] – and adjuvants, mimicking priming and boosting of conventional multi-injection protocols [112, 113]. They can also be administered in conjunction with systemic ICI or other immune-modulating agents to combat immune suppression (ideal timing and sequence has yet to be established). In a representative example, a preclinical study reported incorporation of a Hepa1−6 liver cancer-specific neoantigen, a toll-like receptor 9 (CpG-ODN) agonist, and stimulator of interferon genes (STING) agonist (2, 3-cyclic-GMP-AMP (cGAMP)) into a silk-hydrogel with sustained release kinetics [113]. In contrast to intradermal injection of free components, subcutaneous inoculation with immunotherapeutic-hydrogels achieved long-term prophylactic and therapeutic activity against Hepa1−6 orthotopic tumors in mice. Combining immunotherapeutic-hydrogel delivery and i.p. injection of TIM-3 antibody injection prevented pulmonary metastasis in orthotopic liver tumors and established long-term survival and protection against tumor rechallenge. Other cancer vaccination strategies include delivery of autologous DCs [114] or intratumoral delivery of immunogenic cell death (ICD)-inducing chemical agents or other locoregional ablative therapies (LRT) to increase antigen availability in situ [115].
Table 2.
Preclinical study examples demonstrating the use of in situ-forming hydrogels for delivery of cancer vaccines.
| Hydrogel | Injection location | Gelation-stimuli | Antigen | Other loaded drugs/immunotherapy | Tumor model | Citation |
|---|---|---|---|---|---|---|
| PEG crosslinked melittin-peptide | i.t. | Self-assembly | Ultrafiltered retentate from irradiated cells | Doxorubicin, melittin | B16F10; LLC; MC38 | [64] |
| self-assembled poly(L-valine) | s.c. | Self-assembly | Tumor cell lysates | TLR3 agonist (poly(I:C) | B16 | [203] |
| PEG-b-poly(L-alanine) | s.c. | Self-assembly | Tumor cell lysates | GM-CSF; Anti-CTL-A-4; anti-PD-1 (encapsulated in hydrogel) | B16F10; 4T-1 | [111] |
| polymerized phenylboronic acid (pPBA)-based | Contralateral flank | Mixture of components from two syringes | Tumor cell lysate | Mannan | 4T1 | [204] |
| Silk hydrogel | s.c. | Ultrasound | Hepa1−6 liver cancer-specific neoantigen | TLR9 agonist (CPG-ODN); STING; | Hepa1−6 | [113] |
| mPEG-OVApeptide-AuNPs + ⍺-cyclodextrin | s.c. | Complexation between alpha-CD and PEG before injection. Shear thinning | OVApeptide | CPG | B16-OVA | [108] |
| PDLLA-PEG-PDLLA | s.c. | Temperature | Tumor cell lysates | GM-CSF, CpG-ODN | B16F10; CT26 | [55] |
3.3. Delivery of ICD-inducing agents
Certain chemotherapeutic agents, such as doxorubicin, can induce ICD resulting in release of tumor neoantigens and DAMPs which may potentiate tumor-specific immune responses [116–118]. To minimize systemic toxicities, ICD-inducing chemotherapy and immune adjuvants can be delivered locally using in situ-forming hydrogels to form an in situ cancer vaccine that may be combined with other immunomodulatory agents including checkpoint inhibitors [57, 74, 75, 109, 119–122]. For example, in situ-forming hydrogels have been used for sustained local delivery of chemotherapy (e.g. doxorubicin, epirubicin) with immune-modulating agents such as Toll-Like receptor agonists [123], vascular disrupting agents [124], enzymes [74], and systemic ICI, an approach that has been shown to control the growth of treated and distant tumors in mouse models [119, 123]. Moreover, hydrogels may be designed as antigen “sponges” or “reservoirs” that adsorb antigens following ICD-inducing therapies to prolong antigen residence time, provide protection from degradation, and recruit DCs. Similarly, nanoparticles released from hydrogels may adsorb and traffic tumor antigens to lymph nodes after ICD-inducing ablative procedures [125].
3.4. Lymph node targeting
Tumor-draining lymph nodes are the primary location for DC antigen presentation and T-cell activation [126], although tertiary lymphatic structures adjacent to a tumor ablation zone or antigen source may also function as surrogate lymphoid organs [127]. As such, there is great interest in developing minimally invasive drug delivery systems that target lymph nodes for delivery of antigens and immune-modulating agents. Nanoparticles containing immune-modulating agents and antigens may be encapsulated within hydrogels (nanogels) and slowly released into the tumor as a means for increasing lymph node localization [63, 108, 125]. Antigen stability is preserved within the nanoparticle and transport to the lymph node is ameliorated compared to free antigens that can be degraded or quickly cleared from tumors [63, 108, 110, 125]. Nanoparticles with small diameters (~ < 200 nm) enter lymph nodes via lymphatic drainage while larger particles generally remain entrapped within the ECM and may be degraded or trafficked to lymph nodes by DCs [128–130]. Other physicochemical properties, including charge [131] and composition [132], can also affect the route and extent of lymph node accumulation [133]. Established needle- and catheter-based techniques that deliver ethiodized oil to map the lymph system by lymphangiography have potential implications for delivery of immunotherapy [134]. For example, intratumoral injection of a Pickering emulsion of ethiodized oil and PLGA nanoparticles containing anti-CTLA-4 has been demonstrated in a preclinical model [135].
3.5. In situ immune cell recruitment
Macroporous biomaterial scaffolds containing chemoattractants or inflammatory cytokines can serve as local microenvironments within which DC function may be modulated in situ [136–138]. These “cell-homing” hydrogels are designed to release chemokines, such as GM-CSF, TNF-alpha, and CCL21 to attract immature DCs [69, 139, 140]. Recruited DCs can infiltrate the porous hydrogels gaining access to a reservoir of antigens and adjuvants that initiate DC maturation, antigen-presentation, and priming of antigen-specific T cells. In one example, injectable porous alginate cryogels were loaded with irradiated melanoma tumor cells as antigen source, GM-CSF, a chemoattractant for recruiting dendritic cells, and CpG-ODN, a danger signal. Subcutaneous injection of the loaded hydrogel solution resulted in recruitment, hydrogel infiltration, and activation of DCs, and a strong antitumor response in a B16F10 mouse melanoma model [141]. In another example, injectable pore-forming alginate hydrogels enabled controlled release of GM-CSF, and epacadostat, an inhibitor of indoleamine 2, 3-dioxygenase for activating T cells. Peritumoral injection of the loaded hydrogel resulted in recruitment of DCs, and significant DC infiltration of the hydrogels, as well as an increased CD8+/Treg ratio in the tumor [60].
3.6. Adoptive cell transfer
During adoptive cell transfer, a patient’s immune cells are activated or genetically modified in vitro to improve their ability to recognize tumor cells once infused back into the patient. The efficacy of chimeric antigen receptor T (CAR-T) cells in treating hematologic cancers has raised the prospect of using this therapy to treat solid tumors, though several barriers remain. After systemic administration, CAR-T cells have difficulty localizing, infiltrating, proliferating, and maintaining adequate function within the immunosuppressive tumor microenvironment [142]. Locoregional delivery of immune cells using ISFH may increase intratumoral localization and retention of CAR-T cells by providing a cell scaffold capable of controlled cell deployment, as previously demonstrated using hydrogel implants [143, 144]. Moreover, ISFH could serve to protect cells during injection and subsequently provide an immunologic niche to improve cell viability via co-encapsulation of immune-modulating agents [145]. However, challenges such as achieving adequate viability and intratumoral distribution of CAR-T cells and potential role of local cell and drug delivery systems require further investigation.
4. Applications in interventional oncology
Interventional oncology employs medical imaging and interventional tools, such as needles, probes, and catheters, to provide direct, minimally invasive access to tumors using imaging guidance. The goal is to navigate selectively towards an identified therapeutic target and deliver therapy using intraprocedural feedback to make necessary adjustments to the treatment in real-time. Ultimately, this information may be used to optimize treatment in a tumor- and patient-specific fashion. In light of advancements in modern interventional oncology tools, almost every tissue in the human body can be reached via percutaneous needle placement or endovascular catheter for the purpose of LRT including drug delivery. Imaging guidance is crucial to achieving precise needle or catheter placement and is usually performed using computed tomography (CT), fluoroscopy, cone beam-CT (CBCT), and/or ultrasound (US). The ability to visualize or predict the spatial distribution of immunotherapeutic agents in vivo would also be beneficial for treatment guidance and assessment of treatment adequacy including early visualization of off-target drug deposition. However, most drugs cannot be seen using clinical imaging modalities such as CT or US. Therefore, image-able DDS such as ISFH could serve as surrogate markers of in vivo drug distribution enabling imaging confirmation of local delivery of immunotherapy.
4.1. Image-guided drug delivery
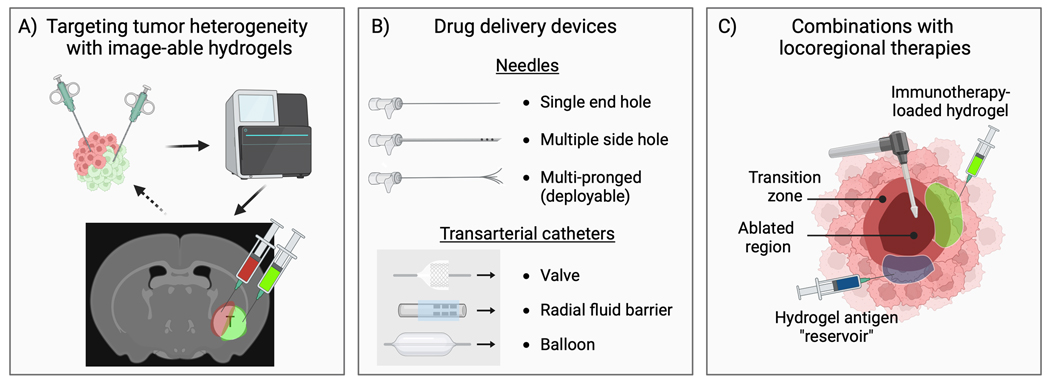
Soluble contrast agents can be incorporated into ISFH by mixing with the hydrogel precursor solution. The resulting image-able hydrogels could enable imaging confirmation of on-target delivery and tumor coverage. Radiopaque materials have been incorporated into injectable hydrogels for imaging with fluoroscopy or CT, including bismuth [146], iodinated compounds [147, 148], and gold nanoparticles [146, 149–151]. Micro- or nano-bubbles provide contrast on ultrasound due to the intrinsic hyperechogenic nature of air and may be incorporated into hydrogels to impart US-image-ability or US-triggered drug delivery [152, 153]. Contrast agents entrapped within hydrogels are usually released over a period of hours to days, providing adequate time for assessment of delivery. Interestingly, it may be possible to correlate changes in image-ability, such as the diminution of X-ray attenuation over time on CT, with drug concentration remaining in the hydrogel, raising the potential for calibrated or prescribed drug dosimetry. Alternatively, contrast agents can be conjugated directly to the hydrogel polymer backbone if persistent image-ability is desired. For example, iodinated moieties have been incorporated into embolic drug-eluting microspheres thus enabling visualization of microsphere localization during transarterial chemoembolization [154–156], and correlation of radiopacity with drug concentration in embolized tissues [157, 158]. Image-able hydrogels may facilitate targeting of regions with identified susceptibilities to specific therapeutic agents or spatially discrete microenvironments using intraprocedural imaging for confirmation of delivery (Figure 8A).
Figure 8.
Opportunities for innovation and advancements in intratumoral delivery of immunotherapy-loaded hydrogels (“immunogels”). A) Improvements in next-generation sequencing and computational tools are enabling more detailed insights into the mutational landscape of tumors. Image-able hydrogels may thus enable targeting of identified regions of clonal heterogeneity or discrete microenvironments within the tumor using intraprocedural imaging for dose estimation and verification of on-target delivery of multiple drugs. B) Innovation and evaluation of advanced needles and catheters may enable greater control of intratumoral drug delivery including immunogels. C) The ability to deliver immunogels to regions of increased immunologic activity stimulated by locoregional therapies (e.g., the transition zone following thermal ablation) may potentiate local and systemic (abscopal) anti-tumor effects. Injection of hydrogels with or without entrapped immunotherapy could serve as antigen “sponges” or “reservoirs” that adsorb tumor antigens released during ICD-inducing locoregional therapy, prolonging antigen tumor residence time and promoting uptake by antigen presenting cells.
4.2. Intratumoral immunotherapy
4.2.1. Percutaneous
Recently, new needle devices have been developed including multiple side hole needles (MSHN) (e.g., Profusion, Cook®, Regentec) and multipronged (deployable) injection needles (MPIN) (e.g., Quadra-Fuse, Rex Medical) (Figure 8B). A preclinical study demonstrated that intratumoral injections of soluble contrast into a rat flank model of hepatocellular carcinoma, viewed under live fluoroscopy, resulted in greater intravasation using a single end hold needle compared with MSHN, which demonstrated greater tumor localization. In addition, greater intratumoral retention of contrast on CT was observed when delivered with a hydrogel [35]. In another study, distribution of an X-ray and CT imageable thermosensitive hydrogel loaded with doxorubicin was evaluated using different needle types and injection techniques for intratumoral injection into ex vivo bovine liver [159]. More research is needed to better understand the influence of needle type, hydrogel properties, and injection parameters on intratumoral drug distribution and its implications for local delivery of immunotherapy.
4.2.2. Transcatheter
Catheter-based intraarterial infusions can achieve higher tumor drug concentrations, or improved localization of cellular immunotherapy, compared to intravenous administration. Yet, limited “first pass” extravasation and rapid washout may limit tumor drug concentrations and contribute to elevated systemic drug levels. Transarterial immunoembolization consists of targeted, catheter-based delivery of embolics and immunomodulators. This dual approach serves to obstruct blood flow to the tumor, leading to ischemia and necrosis as well as reduced washout of immunotherapy. In an early example, a low-virulence pathogen, thrombin, and fibrinogen were mixed with ethiodized oil (Lipiodol®) and injected into tumor arteries to treat hepatocellular carcinoma [15]. Immunoembolization was later performed by delivering GM-CSF emulsified in Lipiodol into hepatic arteries to treat uveal melanoma prior to injection of gelatin sponge particles [160]. Recently, immunomodulating drugs have been loaded into drug-eluting “immunobeads” to exploit combined and potential synergistic effects of embolization and sustained local delivery of immunotherapy [16, 17]. In situ-forming hydrogels are an intriguing delivery platform for combined embolization and controlled intraarterial delivery of immune-modulating agents due to their potential distal vascular penetration. However, optimization of hydrogel properties such as the rate of gelation is required to achieve a balance between adequate handling (e.g., ease of flow through the catheter) and desired vascular distribution.
New microcatheters for endovascular therapies have been developed that may further enhance tumor-selective targeting of intra-arterial immunotherapy including multiple catheter designs intended to minimize reflux of drugs or embolics leading to off target delivery. Microcatheters can also be used in the systemic and portal venous systems as well as lymphatics. Microcatheters with small side holes near the tip create a fluid barrier that helps prevent non-target embolization that can occur with reflux [161, 162]. A catheter delivery system with an anti- reflux valve (TriSalus/Surefire) has also been used to deliver CAR-T cells for hepatic infusions with the ability to increase forward delivery pressure while minimizing reflux [163]. Additionally, inflatable balloon catheters and microcatheters (Occlusafe Terumo; Sniper balloon occlusion microcatheter, Embolx) are designed to prevent reflux but also modulate pressure in target vasculature potentially increasing deposition or vascular penetration of embolic materials and drugs in target lesions [164–167]. A clinical study found increased delivery of microspheres to tumors using microvalve infusion catheters compared to standard end hole catheters [168]. However, more research is needed to better understand the performance implications of different catheter designs for intraarterial delivery of embolics and drugs including hydrogels and immunotherapeutic agents, respectively.
4.3. Locoregional therapy – immunotherapy combinations
Minimally invasive LRT such as chemical, thermal, or non-thermal ablative therapies have demonstrated an ability to induce the release of tumor antigens and DAMPs via induction of ICD [169–172]. Consequently, LRT can lead to the activation of innate and adaptive immune responses, attracting immune cells such as dendritic cells, macrophages, and T lymphocytes into the tumor microenvironment. Antigens captured by APCs following LRT can potentiate systemic anti-tumor immune responses [173, 174]. A variety of tumor ablation modalities have demonstrated the ability to generate tumor-specific immune responses including radiation [175], chemoembolization [176, 177], RFA [169–172], MWA [178], cryoablation [179, 180], therapeutic thermal or mechanical ultrasound [181], photothermal therapy [182, 183], and pulsed electrical fields or irreversible electroporation (IRE) [184]. Unfortunately, the abscopal effect induced by ablative therapies is often too weak to achieve a detectable or significant therapeutic response. Thus, there is growing interest in combining immunotherapy with LRT to enhance systemic antitumor effects [185, 186]. For thermal therapies, a region of sublethal hyperthermia at the rim of the ablation zone, called the transitional zone, is host to immune activity that may be an ideal target for local delivery of immunotherapy (Figure 8C). A summary of preclinical studies demonstrating combined locoregional ablative therapies and in situ-forming hydrogels for delivery of immunotherapy can be found in Table 3.
Table 3.
Preclinical study examples demonstrating combinations of locoregional ablative therapies and local delivery of immunotherapy using in situ-forming hydrogels.
| Hydrogel | Injection location | Gelation-stimuli | Immunotherapy | Ablative modality | Other loaded molecules | Tumor model | Citation |
|---|---|---|---|---|---|---|---|
| Alginate/collagen | i.t. | Temperature | Poly I:C | Photothermal therapy | ICG | CT26 | [182] |
| Alginate | i.t. | Ionic interactions | R837 (imiquimod) | MWA | None | 4T1 | [189] |
| Alginate | i.t. | Ionic interactions | CPG ODNs | Photothermal therapy | NIR-II photothermal nanoagent | 4T1 | [61] |
| α-cyclodextrin (CD)–based | i.t. | Time-dependent | Mannose (attached by disulfide bond) CPG | Photothermal therapy | Doxorubicin ICG | B16F10 | [125] |
| PLGA-PEG-PLGA | i.t. | temperature | ROCK inhibitor, Y27632 | RFA | None | B16F1x0 | [188] |
Locally administered hydrogels may be used to carry LRT sensitizers in combination with immune-modulating agents to promote immune responses following LRT. For example, several studies demonstrated the use of hydrogels to deliver photo-thermal sensitizers via intratumoral injection, including gold nanoparticles and indocyanine green, and immune adjuvants that were released upon heating with laser irradiation resulting in effective inhibition of tumor growth [125, 182, 183]. In an intriguing example, hydrogels with high protein adsorption capability containing manganese dioxide nanoparticles for enhancement of photothermal therapy were injected into tumors forming a “reservoir” of autologous antigens in situ. The authors found enhanced CD8+ T cell-mediated immune responses, inhibition of distant tumor growth, and protection against tumor rechallenge compared to a formulation with lower protein binding [187]. Hydrogels containing immune adjuvants and microenvironment modulators have also been injected into tumors prior to or following radiofrequency or microwave thermal ablation resulting in greater anti-tumor immune effects compared to ablation alone in mouse models [188–190]. However, the relative timing of LRT and delivery of immunotherapy, as well as the location and coverage of intratumoral immunotherapy injections, may be important determinants of local and systemic anti-tumor responses and necessitate further investigation.
5. Conclusions
Immunotherapy has demonstrated remarkable promise in its ability to harness the immune system to recognize and destroy tumors. Intratumoral immunotherapy may be used to boost the effects of systemic checkpoint inhibition, for example by converting “cold” ICI-unresponsive tumors to “hot”, and as an alternative route of drug delivery with reduced toxicity and improved tumor targeting. Reaching the full potential of cancer immunotherapy will require maximizing anti-tumor immune activity and overcoming mechanisms of resistance, without significant systemic toxicity, across a diverse array of cancer types.
Intratumoral delivery of immunotherapy can reduce systemic drug exposure and potentiate systemic anti-tumor immunity, particularly when combined with systemic ICI. Intratumoral immunotherapy could also provide benefits as neoadjuvant therapy in patients with locally advanced and resectable cancers, potentially at lower cost and with fewer treatment cycles than systemic immunotherapy. However, intratumoral injections have encountered limitations stemming from rapid egress or washout of drugs from the injection site, which necessitates frequent dosing and can result in adverse systemic inflammatory effects. Hydrogels are well-suited to meet formulation and delivery challenges for a broad repertoire of immunotherapeutic agents and emerging delivery paradigms due to their adjustable physico-chemical properties, tunable drug release characteristics, and potential use for image-guided drug delivery.
Early hydrogels were engineered to be biologically inert, minimizing interactions with tissues, proteins, and immune cells to mitigate inflammation and prolong their longevity and function in the body. In some respects, the emergence of immunotherapy requires revisiting these past goals to generate hydrogels that serve not only as inert implants or drug carriers, but rather as active agents or adjuvants with intrinsic immunogenic properties. These biomaterials-based “immunogels” could themselves be tailored to modulate specific immune pathways or microenvironments, while simultaneously delivering immunotherapy with optimized timecourses, which may have important implications and advantages for stimulating immune responses with known temporal dependencies.
Improvements in next-generation sequencing and computational tools are enabling more detailed insights into the mutational landscape of tumors, providing improved strategies for immunomodulation. In situ forming-hydrogels are promising delivery platforms for cancer vaccines, providing enhanced localization and prolonged delivery of entrapped antigens and adjuvants. A more robust immune response may be achieved by in situ vaccination, whereby the immune system is exposed to a broad array of endogenous neoantigens via ICD-inducing locoregional ablative therapies. Under this framework, hydrogels could be used to deliver chemotherapy, or sensitizers to locoregional therapies, while simultaneously serving as antigen “sponges” or “reservoirs” [187]. These antigen-saturated hydrogels could also recruit DCs or other immune cells by releasing loaded chemoattractants and serving as tunable cell scaffolds, or immunomodulatory niches, for promoting immune cell integration, activation, or repolarization [60, 141]. Furthermore, nanoparticles entrapped within the hydrogel polymer network and released over time could increase lymph node drainage or uptake of encapsulated or adsorbed antigens by APCs for subsequent trafficking to lymph nodes or tertiary lymphoid structures and enhanced T cell priming [63, 108, 125].
Recent studies have mapped extensive intratumoral spatial variations in clonality using spatially resolved transcriptomics and in situ sequencing technologies [191, 192]. Intratumoral immunotherapy using image-able hydrogels may enable greater tumor coverage of immunotherapy with delivery confirmation or enable precise spatial targeting of regions with distinct immunologic activity or clonality. In the future, these regions may be identified using AI-enhanced predictive imaging or pre-treatment biopsies and analysis of transcriptomics and subsequently targeted for intratumoral immunotherapy under imaging guidance. However, much progress remains to be made towards optimizing injection techniques and parameters for various clinical scenarios to achieve more consistent and predictable intratumoral delivery of immunotherapy, a process likely to be facilitated by image-able injectable hydrogel DDS. Progress in these areas will require the use of translational tumor-bearing animal models to evaluate novel interventional tools and treatment algorithms. Importantly, multi-disciplinary collaborations in materials science, immunology, engineering, and medicine, will help ensure expeditious clinical translation of innovative intratumoral immunotherapies and drug delivery systems.
Acknowledgments
Financial Support Statement and Declaration of Interest Statement
This work was supported by the Center for Interventional Oncology in the Intramural Research Program of the National Institutes of Health (NIH) by intramural NIH Grants NIH Z01 1ZID BC011242 and CL040015.
The NIH has a Cooperative Research and Development Agreement with the following: Philips Research, Biocompatibles UK Ltd-Boston Scientific Corporation, Celsion Corp, and Siemens Medical Systems.
The content of this manuscript does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services. The mention of commercial products, their source, or their use in connection with material reported herein is not to be construed as an actual or implied endorsement of such products by the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Haslam A, Prasad V, Estimation of the Percentage of US Patients with Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs, JAMA Network Open, 2 (2019) e192535-e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Postow MA, Sidlow R, Hellmann MD, Immune-Related Adverse Events Associated with Immune Checkpoint Blockade, New England Journal of Medicine, 378 (2018) 158–168. [DOI] [PubMed] [Google Scholar]
- [3].Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL, Effects of Single-Dose Interleukin-12 Exposure on Interleukin-12-Associated Toxicity and Interferon-Gamma Production, Blood, 90 (1997) 2541–2548. [PubMed] [Google Scholar]
- [4].Haymaker C, Johnson DH, Murthy R, Bentebibel SE, Uemura MI, Hudgens CW, Safa H, James M, Andtbacka RHI, Johnson DB, Shaheen M, Davies MA, Rahimian S, Chunduru SK, Milton DR, Tetzlaff MT, Overwijk WW, Hwu P, Gabrail N, Agrawal S, Doolittle G, Puzanov I, Markowitz J, Bernatchez C, Diab A, Tilsotolimod with Ipilimumab Drives Tumor Responses in Anti-Pd-1 Refractory Melanoma, Cancer Discov, 11 (2021) 1996–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cohen EEW, Nabell L, Wong DJ, Day T, Daniels GA, Milhem M, Deva S, Jameson M, Guntinas-Lichius O, Almubarak M, Strother M, Whitman E, Chisamore M, Obiozor C, Bagulho T, Gomez-Romo J, Guiducci C, Janssen R, Gamelin E, Algazi AP, Intralesional Sd-101 in Combination with Pembrolizumab in Anti-Pd-1 Treatment-Naïve Head and Neck Squamous Cell Carcinoma: Results from a Multicenter, Phase Ii Trial, Clinical Cancer Research, 28 (2022) 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meric-Bernstam F, Sweis RF, Hodi FS, Messersmith WA, Andtbacka RHI, Ingham M, Lewis N, Chen X, Pelletier M, Chen X, Wu J, McWhirter SM, Müller T, Nair N, Luke JJ, Phase I Dose-Escalation Trial of Miw815 (Adu-S100), an Intratumoral Sting Agonist, in Patients with Advanced/Metastatic Solid Tumors or Lymphomas, Clinical Cancer Research, 28 (2022) 677–688. [DOI] [PubMed] [Google Scholar]
- [7].Harrington KJ, Brody J, Ingham M, Strauss J, Cemerski S, Wang M, Tse AN, Khilnani AD, Marabelle A, Golan T, Preliminary Results of the First-in-Human (Fih) Study of Mk-1454, an Agonist of Stimulator of Interferon Genes (Sting), as Monotherapy or in Combination with Pembrolizumab (Pembro) in Patients with Advanced Solid Tumors or Lymphomas, Annals of oncology : official journal of the European Society for Medical Oncology, 29 Suppl 8 (2018) viii712. [Google Scholar]
- [8].Nguyen KG, Vrabel MR, Mantooth SM, Hopkins JJ, Wagner ES, Gabaldon TA, Zaharoff DA, Localized Interleukin-12 for Cancer Immunotherapy, Frontiers in Immunology, 11 (2020) 575597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li K, Zhao Y, Hu X, Jiao J, Wang W, Yao H, Advances in the Clinical Development of Oncolytic Viruses, Am J Transl Res, 14 (2022) 4192–4206. [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang T, Jou TH, Hsin J, Wang Z, Huang K, Ye J, Yin H, Xing Y, Talimogene Laherparepvec (T-Vec): A Review of the Recent Advances in Cancer Therapy, Journal of Clinical Medicine, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li J, Mooney DJ, Designing Hydrogels for Controlled Drug Delivery, Nature Reviews Materials, 1 (2016) 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peppas NA, Hilt JZ, Khademhosseini A, Langer R, Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology, Advanced Materials, 18 (2006) 1345–1360. [Google Scholar]
- [13].Valsecchi ME, Terai M, Eschelman DJ, Gonsalves CF, Chervoneva I, Shields JA, Shields CL, Yamamoto A, Sullivan KL, Laudadio M, Berd D, Mastrangelo MJ, Sato T, Double-Blinded, Randomized Phase Ii Study Using Embolization with or without Granulocyte-Macrophage Colony-Stimulating Factor in Uveal Melanoma with Hepatic Metastases, Journal of Vascular and Interventional Radiology, 26 (2015) 523–532.e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yamamoto A, Chervoneva I, Sullivan KL, Eschelman DJ, Gonsalves CF, Mastrangelo MJ, Berd D, Shields JA, Shields CL, Terai M, Sato T, High-Dose Immunoembolization: Survival Benefit in Patients with Hepatic Metastases from Uveal Melanoma, Radiology, 252 (2009) 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yoshida T, Sakon M, Umeshita K, Kanai T, Miyamoto A, Takeda T, Gotoh M, Nakamura H, Wakasa K, Monden M, Appraisal of Transarterial Immunoembolization for Hepatocellular Carcinoma: A Clinicopathologic Study, J Clin Gastroenterol, 32 (2001) 59–65. [DOI] [PubMed] [Google Scholar]
- [16].Mikhail AS, Mauda-Havakuk M, Negussie AH, Hong N, Hawken NM, Carlson CJ, Owen JW, Franco-Mahecha O, Wakim PG, Lewis AL, Pritchard WF, Karanian JW, Wood BJ, Evaluation of Immune-Modulating Drugs for Use in Drug-Eluting Microsphere Transarterial Embolization, International Journal of Pharmaceutics, 616 (2022) 121466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Negussie AH, Mikhail AS, Owen JW, Hong N, Carlson CJ, Tang Y, Carrow KP, Mauda-Havakuk M, Lewis AL, Karanian JW, Pritchard WF, Wood BJ, In Vitro Characterization of Immune Modulating Drug-Eluting Immunobeads Towards Transarterial Embolization in Cancer, Scientific Reports, 12 (2022) 21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Giraldo NA, Sanchez-Salas R, Peske JD, Vano Y, Becht E, Petitprez F, Validire P, Ingels A, Cathelineau X, Fridman WH, Sautès-Fridman C, The Clinical Role of the Tme in Solid Cancer, British Journal of Cancer, 120 (2019) 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ, Natural Innate and Adaptive Immunity to Cancer, Annual Review of Immunology, 29 (2011) 235–271. [DOI] [PubMed] [Google Scholar]
- [20].Liu YT, Sun ZJ, Turning Cold Tumors into Hot Tumors by Improving T-Cell Infiltration, Theranostics, 11 (2021) 5365–5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cherkassky L, Oshi M, Abdelfatah E, Wu R, Takabe Y, Yan L, Endo I, Takabe K, An Immune-Inflamed Tumor Microenvironment as Defined by Cd8 Score Is Associated with Favorable Oncologic Outcomes in Hepatocellular Carcinoma Independent of Measures of Tumor Mutational Burden, American Journal of Cancer Research, 12 (2022) 3099–3110. [PMC free article] [PubMed] [Google Scholar]
- [22].Echarti A, Hecht M, Büttner-Herold M, Haderlein M, Hartmann A, Fietkau R, Distel L, CD8+ and Regulatory T Cells Differentiate Tumor Immune Phenotypes and Predict Survival in Locally Advanced Head and Neck Cancer, Cancers (Basel), 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Itahashi K, Irie T, Nishikawa H, Regulatory T-Cell Development in the Tumor Microenvironment, European Journal of Immunology, 52 (2022) 1216–1227. [DOI] [PubMed] [Google Scholar]
- [24].Haist M, Stege H, Grabbe S, Bros M, The Functional Crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the Immunosuppressive Tumor Microenvironment, Cancers (Basel), 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Monteran L, Erez N, The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment, Frontiers in Immunology, 10 (2019) 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rømer AMA, Thorseth ML, Madsen DH, Immune Modulatory Properties of Collagen in Cancer, Frontiers in Immunology, 12 (2021) 791453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Erinjeri JP, Fine GC, Adema GJ, Ahmed M, Chapiro J, den Brok M, Duran R, Hunt SJ, Johnson DT, Ricke J, Sze DY, Toskich BB, Wood BJ, Woodrum D, Goldberg SN, Immunotherapy and the Interventional Oncologist: Challenges and Opportunities—a Society of Interventional Oncology White Paper, Radiology, 292 (2019) 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Trédan O, Galmarini CM, Patel K, Tannock IF, Drug Resistance and the Solid Tumor Microenvironment, Journal of the National Cancer Institute, 99 (2007) 1441–1454. [DOI] [PubMed] [Google Scholar]
- [29].Netti P, Travascio F, Jain R, Coupled Macromolecular Transport and Gel Mechanics: Poroviscoelastic Approach, AIChE journal, 49 (2003) 1580–1596. [Google Scholar]
- [30].Barry SI, Aldis GK, Flow-Induced Deformation from Pressurized Cavities in Absorbing Porous Tissues, Bulletin of Mathematical Biology, 54 (1992) 977–997. [DOI] [PubMed] [Google Scholar]
- [31].Hutchens SB, Fakhouri S, Crosby AJ, Elastic Cavitation and Fracture Via Injection, Soft Matter, 12 (2016) 2557–2566. [DOI] [PubMed] [Google Scholar]
- [32].Morhard R, Mueller JL, Tang Q, Nief C, Chelales E, Lam CT, Alvarez DA, Rubinstein M, Katz DF, Ramanujam N, Understanding Factors Governing Distribution Volume of Ethyl Cellulose-Ethanol to Optimize Ablative Therapy in the Liver, IEEE Transactions on Biomedical Engineering, 67 (2020) 2337–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Netti PA, Travascio F, Jain RK, Coupled Macromolecular Transport and Gel Mechanics: Poroviscoelastic Approach, AIChE Journal, 49 (2003) 1580–1596. [Google Scholar]
- [34].McGuire S, Zaharoff D, Yuan F, Nonlinear Dependence of Hydraulic Conductivity on Tissue Deformation During Intratumoral Infusion, Annals of Biomedical Engineering, 34 (2006) 1173–1181. [DOI] [PubMed] [Google Scholar]
- [35].Muñoz NM, Williams M, Dixon K, Dupuis C, McWatters A, Avritscher R, Manrique SZ, McHugh K, Murthy R, Tam A, Naing A, Patel SP, Leach D, Hartgerink JD, Young S, Prakash P, Hwu P, Sheth RA, Influence of Injection Technique, Drug Formulation and Tumor Microenvironment on Intratumoral Immunotherapy Delivery and Efficacy, Journal of Immunotherapy of Cancer, 9 (2021) e001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sheth RA, Murthy R, Hong DS, Patel S, Overman MJ, Diab A, Hwu P, Tam A, Assessment of Image-Guided Intratumoral Delivery of Immunotherapeutics in Patients with Cancer, JAMA Network Open, 3 (2020) e207911. [DOI] [PubMed] [Google Scholar]
- [37].Momin N, Palmeri JR, Lutz EA, Jailkhani N, Mak H, Tabet A, Chinn MM, Kang BH, Spanoudaki V, Hynes RO, Wittrup KD, Maximizing Response to Intratumoral Immunotherapy in Mice by Tuning Local Retention, Nature Communications, 13 (2022) 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang Y, Wang H, Li C-Y, Yuan F, Effects of Rate, Volume, and Dose of Intratumoral Infusion on Virus Dissemination in Local Gene Delivery, Molecular Cancer Therapy, 5 (2006) 362–366. [DOI] [PubMed] [Google Scholar]
- [39].Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A, Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers, Journal of the National Cancer Institute, 98 (2006) 335–344. [DOI] [PubMed] [Google Scholar]
- [40].He X, Yang Y, Han Y, Cao C, Zhang Z, Li L, Xiao C, Guo H, Wang L, Han L, Qu Z, Liu N, Han S, Xu F, Extracellular Matrix Physical Properties Govern the Diffusion of Nanoparticles in Tumor Microenvironment, Proceedings of the National Academy of Sciences, 120 (2023) e2209260120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mikhail AS, Eetezadi S, Ekdawi SN, Stewart J, Allen C, Image-Based Analysis of the Size- and Time-Dependent Penetration of Polymeric Micelles in Multicellular Tumor Spheroids and Tumor Xenografts, International Journal of Pharmaceutics, 464 (2014) 168–177. [DOI] [PubMed] [Google Scholar]
- [42].Dewhirst MW, Secomb TW, Transport of Drugs from Blood Vessels to Tumour Tissue, Nat Rev Cancer, 17 (2017) 738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mikhail AS, Allen C, Block Copolymer Micelles for Delivery of Cancer Therapy: Transport at the Whole Body, Tissue and Cellular Levels, Journal of Controlled Release, 138 (2009) 214–223. [DOI] [PubMed] [Google Scholar]
- [44].Alexandrakis G, Brown EB, Tong RT, McKee TD, Campbell RB, Boucher Y, Jain RK, Two-Photon Fluorescence Correlation Microscopy Reveals the Two-Phase Nature of Transport in Tumors, Nature Medicine, 10 (2004) 203–207. [DOI] [PubMed] [Google Scholar]
- [45].Momin N, Mehta NK, Bennett NR, Ma L, Palmeri JR, Chinn MM, Lutz EA, Kang B, Irvine DJ, Spranger S, Wittrup KD, Anchoring of Intratumorally Administered Cytokines to Collagen Safely Potentiates Systemic Cancer Immunotherapy, Science Translational Medicine, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schmidt MM, Wittrup KD, A Modeling Analysis of the Effects of Molecular Size and Binding Affinity on Tumor Targeting, Molecular Cancer Therapy, 8 (2009) 2861–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fujimori K, Covell DG, Fletcher JE, Weinstein JN, A Modeling Analysis of Monoclonal Antibody Percolation through Tumors: A Binding-Site Barrier, Journal of Nuclear Medicine, 31 (1990) 1191. [PubMed] [Google Scholar]
- [48].Stylianopoulos T, Munn LL, Jain RK, Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside, Trends in Cancer, 4 (2018) 292–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang Y, Wang H, Li CY, Yuan F, Effects of Rate, Volume, and Dose of Intratumoral Infusion on Virus Dissemination in Local Gene Delivery, Molecular Cancer Therapy, 5 (2006) 362–366. [DOI] [PubMed] [Google Scholar]
- [50].McGuire S, Yuan F, Quantitative Analysis of Intratumoral Infusion of Color Molecules, American Journal of Physiology - Heart and Circulatory Physiology, 281 (2001) H715–721. [DOI] [PubMed] [Google Scholar]
- [51].Raghavan R, Mikaelian S, Brady M, Chen ZJ, Fluid Infusions from Catheters into Elastic Tissue: I. Azimuthally Symmetric Backflow in Homogeneous Media, Physics in Medicine and Biology, 55 (2010) 281–304. [DOI] [PubMed] [Google Scholar]
- [52].Correa S, Meany EL, Gale EC, Klich JH, Saouaf OM, Mayer AT, Xiao Z, Liong CS, Brown RA, Maikawa CL, Grosskopf AK, Mann JL, Idoyaga J, Appel EA, Injectable Nanoparticle-Based Hydrogels Enable the Safe and Effective Deployment of Immunostimulatory Cd40 Agonist Antibodies, Advanced Science, 9 (2022) e2103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fakhari A, Anand Subramony J, Engineered in-Situ Depot-Forming Hydrogels for Intratumoral Drug Delivery, Journal of Controlled Release, 220 (2015) 465–475. [DOI] [PubMed] [Google Scholar]
- [54].Lacroce E, Rossi F, Polymer-Based Thermoresponsive Hydrogels for Controlled Drug Delivery, Expert Opinion on Drug Delivery, 19 (2022) 1203–1215. [DOI] [PubMed] [Google Scholar]
- [55].Yang F, Shi K, Jia Y, Hao Y, Peng J, Yuan L, Chen Y, Pan M, Qian Z, A Biodegradable Thermosensitive Hydrogel Vaccine for Cancer Immunotherapy, Applied Materials Today, 19 (2020) 100608. [Google Scholar]
- [56].Rizwan M, Yahya R, Hassan A, Yar M, Azzahari AD, Selvanathan V, Sonsudin F, Abouloula CN, pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications, Polymers, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gu J, Zhao G, Yu J, Xu P, Yan J, Jin Z, Chen S, Wang Y, Zhang LW, Wang Y, Injectable pH-Responsive Hydrogel for Combinatorial Chemoimmunotherapy Tailored to the Tumor Microenvironment, Journal of Nanobiotechnology, 20 (2022) 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhao J, Wang L, Zhang H, Liao B, Li Y, Progress of Research in in Situ Smart Hydrogels for Local Antitumor Therapy: A Review, Pharmaceutics, 14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nief C, Morhard R, Chelales E, Adrianzen Alvarez D, Bourla Bs I, Lam CT, Sag AA, Crouch BT, Mueller JL, Katz D, Dewhirst MW, Everitt JI, Ramanujam N, Polymer-Assisted Intratumoral Delivery of Ethanol: Preclinical Investigation of Safety and Efficacy in a Murine Breast Cancer Model, PLoS One, 16 (2021) e0234535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Han J, Bhatta R, Liu Y, Bo Y, Wang H, In Situ Dendritic Cell Recruitment and T Cell Activation for Cancer Immunotherapy, Frontiers in Pharmacology, 13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shen J, Lin M, Ding M, Yu N, Yang C, Kong D, Sun H, Xie Z, Tumor Immunosuppressive Microenvironment Modulating Hydrogels for Second near-Infrared Photothermal-Immunotherapy of Cancer, Materials Today Bio, 16 (2022) 100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chao Y, Xu L, Liang C, Feng L, Xu J, Dong Z, Tian L, Yi X, Yang K, Liu Z, Combined Local Immunostimulatory Radioisotope Therapy and Systemic Immune Checkpoint Blockade Imparts Potent Antitumour Responses, Nature Biomedical Engineering, 2 (2018) 611–621. [DOI] [PubMed] [Google Scholar]
- [63].Gao C, Cheng K, Li Y, Gong R, Zhao X, Nie G, Ren H, Injectable Immunotherapeutic Hydrogel Containing Rna-Loaded Lipid Nanoparticles Reshapes Tumor Microenvironment for Pancreatic Cancer Therapy, Nano Letters, 22 (2022) 8801–8809. [DOI] [PubMed] [Google Scholar]
- [64].Wan C, Sun Y, Hu Y, Huang J, Lu L, Gao Y, Zi H, He Q, Sun J, Lovell JF, Yang K, Jin H, Peptide Hydrogels Loaded with Irradiated Tumor Cell Secretions Enhance Cancer Immunotherapy, Nano Today, 41 (2021) 101323. [Google Scholar]
- [65].Yang Y, Cui Y, Cao W, Zhao M, Lin W, Xu R, Xu Y, Chen Y, Li H, Liang J, Lin Y, Fan Y, Zhang X, Sun Y, Nanohydroxyapatite Stimulates Pd-L1 Expression to Boost Melanoma Combination Immunotherapy, ACS nano, 16 (2022) 18921–18935. [DOI] [PubMed] [Google Scholar]
- [66].Lee KY, Mooney DJ, Alginate: Properties and Biomedical Applications, Prog Polym Sci, 37 (2012) 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shriky B, Kelly A, Isreb M, Babenko M, Mahmoudi N, Rogers S, Shebanova O, Snow T, Gough T, Pluronic F127 Thermosensitive Injectable Smart Hydrogels for Controlled Drug Delivery System Development, Journal of Colloid and Interface Science, 565 (2020) 119–130. [DOI] [PubMed] [Google Scholar]
- [68].Brambilla E, Locarno S, Gallo S, Orsini F, Pini C, Farronato M, Thomaz DV, Lenardi C, Piazzoni M, Tartaglia G, Poloxamer-Based Hydrogel as Drug Delivery System: How Polymeric Excipients Influence the Chemical-Physical Properties, Polymers, 14 (2022) 3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fenton OS, Tibbitt MW, Appel EA, Jhunjhunwala S, Webber MJ, Langer R, Injectable Polymer–Nanoparticle Hydrogels for Local Immune Cell Recruitment, Biomacromolecules, 20 (2019) 4430–4436. [DOI] [PubMed] [Google Scholar]
- [70].Johnson EM, Berk DA, Jain RK, Deen WM, Hindered Diffusion in Agarose Gels: Test of Effective Medium Model, Biophysical Journal, 70 (1996) 1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Axpe E, Chan D, Offeddu GS, Chang Y, Merida D, Hernandez HL, Appel EA, A Multiscale Model for Solute Diffusion in Hydrogels, Macromolecules, 52 (2019) 6889–6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hadjiev NA, Amsden BG, An Assessment of the Ability of the Obstruction-Scaling Model to Estimate Solute Diffusion Coefficients in Hydrogels, Journal of Controlled Release, 199 (2015) 10–16. [DOI] [PubMed] [Google Scholar]
- [73].Lin CC, Metters AT, Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling, Advanced Drug Delivery Reviews, 58 (2006) 1379–1408. [DOI] [PubMed] [Google Scholar]
- [74].Wang B, Chen J, Caserto JS, Wang X, Ma M, An in Situ Hydrogel-Mediated Chemo-Immunometabolic Cancer Therapy, Nature Communications, 13 (2022) 3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Li J, Luo G, Zhang C, Long S, Guo L, Yang G, Wang F, Zhang L, Shi L, Fu Y, Zhang Y, In Situ Injectable Hydrogel-Loaded Drugs Induce Anti-Tumor Immune Responses in Melanoma Immunochemotherapy, Materials Today Bio, 14 (2022) 100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Abraham BL, Toriki ES, Tucker NDJ, Nilsson BL, Electrostatic Interactions Regulate the Release of Small Molecules from Supramolecular Hydrogels, Journal of Materials Chemistry B, 8 (2020) 6366–6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ashley GW, Henise J, Reid R, Santi DV, Hydrogel Drug Delivery System with Predictable and Tunable Drug Release and Degradation Rates, Proceedings of the National Academy of Sciences, 110 (2013) 2318–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jiang Y, Krishnan N, Heo J, Fang RH, Zhang L, Nanoparticle–Hydrogel Superstructures for Biomedical Applications, Journal of Controlled Release, 324 (2020) 505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Minchinton AI, Tannock IF, Drug Penetration in Solid Tumours, Nature reviews. Cancer, 6 (2006) 583–592. [DOI] [PubMed] [Google Scholar]
- [80].Hedstrm BOA, Flow of Plastic Materials in Pipes, Industrial & Engineering Chemistry, 44 (1952) 651–656. [Google Scholar]
- [81].Saporito A, Quadri C, Kloth N, Capdevila X, The Effect of Rate of Injection on Injection Pressure Profiles Measured Using in-Line and Needle-Tip Sensors: An in-Vitro Study, Anaesthesia, 74 (2019) 64–68. [DOI] [PubMed] [Google Scholar]
- [82].Franke V, Berger DMS, Klop WMC, van der Hiel B, van de Wiel BA, Ter Meulen S, Wouters M, van Houdt WJ, van Akkooi ACJ, High Response Rates for T-Vec in Early Metastatic Melanoma (Stage Iiib/C-Ivm1a), International Journal of Cancer, 145 (2019) 974–978. [DOI] [PubMed] [Google Scholar]
- [83].Xu C, Xu J, Xiao L, Li Z, Xiao Y, Dargusch M, Lei C, He Y, Ye Q, Double-Layered Microsphere Based Dual Growth Factor Delivery System for Guided Bone Regeneration, RSC Advances, 8 (2018) 16503–16512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhang X, Battig MR, Wang Y, Programmable Hydrogels for the Controlled Release of Therapeutic Nucleic Acid Aptamers Via Reversible DNA Hybridization, Chemical Communications, 49 (2013) 9600–9602. [DOI] [PubMed] [Google Scholar]
- [85].Chen DS, Mellman I, Oncology Meets Immunology: The Cancer-Immunity Cycle, Immunity, 39 (2013) 1–10. [DOI] [PubMed] [Google Scholar]
- [86].Mikhail AS, Negussie AH, Mauda-Havakuk M, Owen JW, Pritchard WF, Lewis AL, Wood BJ, Drug-Eluting Embolic Microspheres: State-of-the-Art and Emerging Clinical Applications, Expert Opinion on Drug Delivery, 18 (2021) 383–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R, Engineering Precision Nanoparticles for Drug Delivery, Nature Reviews Drug Discovery, 20 (2021) 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Huang P, Wang X, Liang X, Yang J, Zhang C, Kong D, Wang W, Nano-, Micro-, and Macroscale Drug Delivery Systems for Cancer Immunotherapy, Acta Biomaterialia, 85 (2019) 1–26. [DOI] [PubMed] [Google Scholar]
- [89].Lord J, Britton H, Spain SG, Lewis AL, Advancements in the Development on New Liquid Embolic Agents for Use in Therapeutic Embolisation, Journal of Materials Chemistry B, 8 (2020) 8207–8218. [DOI] [PubMed] [Google Scholar]
- [90].Li X, Ullah MW, Li B, Chen H, Recent Progress in Advanced Hydrogel-Based Embolic Agents: From Rational Design Strategies to Improved Endovascular Embolization, Advanced Healthcare Materials, (2023). [DOI] [PubMed] [Google Scholar]
- [91].Andorko JI, Jewell CM, Designing Biomaterials with Immunomodulatory Properties for Tissue Engineering and Regenerative Medicine, Bioengineering and Translational Medicine, 2 (2017) 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ, Mediation of Biomaterial-Cell Interactions by Adsorbed Proteins: A Review, Tissue Engineering, 11 1–2 (2005) 1–18. [DOI] [PubMed] [Google Scholar]
- [93].Thevenot P, Hu L. W Fau - Tang, L. Tang, Surface Chemistry Influences Implant Biocompatibility, Current Topics in Medicinal Chemistry, 8 (2008) 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Huang Z, Yang Y, Jiang Y, Shao J, Sun X, Chen J, Dong L, Zhang J, Zhang J, Anti-Tumor Immune Responses of Tumor-Associated Macrophages Via Toll-Like Receptor 4 Triggered by Cationic Polymers, Biomaterials, 34 (2013) 746–755. [DOI] [PubMed] [Google Scholar]
- [95].Christo SN, Bachhuka A, Diener KR, Mierczynska A, Hayball JD, Vasilev K, The Role of Surface Nanotopography and Chemistry on Primary Neutrophil and Macrophage Cellular Responses, Advanced Healthcare Materials, 5 (2016) 956–965. [DOI] [PubMed] [Google Scholar]
- [96].Jewell CM, Collier JH, Biomaterial Interactions with the Immune System, Biomaterials Science, 7 (2019) 713–714. [DOI] [PubMed] [Google Scholar]
- [97].Mariani E, Lisignoli G, Borzì RM, Pulsatelli L, Biomaterials: Foreign Bodies or Tuners for the Immune Response?, International Journal of Molecular Sciences, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Huang Z, Yang Y, Jiang Y, Shao J, Sun X, Chen J, Dong L, Zhang J, Anti-Tumor Immune Responses of Tumor-Associated Macrophages Via Toll-Like Receptor 4 Triggered by Cationic Polymers, Biomaterials, 34 (2013) 746–755. [DOI] [PubMed] [Google Scholar]
- [99].He W, Liang P, Guo G, Huang Z, Niu Y, Dong L, Wang C, Zhang J, Re-Polarizing Myeloid-Derived Suppressor Cells (MDSCs) with Cationic Polymers for Cancer Immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].He W, Liang P, Guo G, Huang Z, Niu Y, Dong L, Wang C, Zhang J, Re-Polarizing Myeloid-Derived Suppressor Cells (MDSCs) with Cationic Polymers for Cancer Immunotherapy, Scientific Reports, 6 (2016) 24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Babensee JE, Paranjpe A, Differential Levels of Dendritic Cell Maturation on Different Biomaterials Used in Combination Products, Journal of Biomedical Materials Research Part A, 74 (2005) 503–510. [DOI] [PubMed] [Google Scholar]
- [102].Park J, Gerber MH, Babensee JE, Phenotype and Polarization of Autologous T Cells by Biomaterial-Treated Dendritic Cells, Journal of Biomedical Materials Research Part A, 103 (2015) 170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Andorko JI, Hess KL, Pineault KG, Jewell CM, Intrinsic Immunogenicity of Rapidly-Degradable Polymers Evolves During Degradation, Acta Biomaterialia, 32 (2016) 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA, Chitin Is a Size-Dependent Regulator of Macrophage TNF and IL-10 Production, Journal of Immunology, 182 (2009) 3573–3582. [DOI] [PubMed] [Google Scholar]
- [105].Sunshine JC, Perica K, Schneck JP, Green JJ, Particle Shape Dependence of CD8+ T Cell Activation by Artificial Antigen Presenting Cells, Biomaterials, 35 (2014) 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Saxena M, van der Burg SH, Melief CJM, Bhardwaj N, Therapeutic Cancer Vaccines, Nature Reviews Cancer, 21 (2021) 360–378. [DOI] [PubMed] [Google Scholar]
- [107].Mempel TR, Henrickson SE, von Andrian UH, T-Cell Priming by Dendritic Cells in Lymph Nodes Occurs in Three Distinct Phases, Nature, 427 (2004) 154–159. [DOI] [PubMed] [Google Scholar]
- [108].Xu K, Wen Y, Zhang X, Liu Y, Qiu D, Li B, Zheng L, Wu Y, Xing M, Li J, Injectable Host-Guest Gel Nanovaccine for Cancer Immunotherapy against Melanoma, Materials Today Advances, 15 (2022) 100236. [Google Scholar]
- [109].Liu M, Cao Z, Zhang R, Chen Y, Yang X, Injectable Supramolecular Hydrogel for Locoregional Immune Checkpoint Blockade and Enhanced Cancer Chemo-Immunotherapy, ACS Applied Materials & Interfaces, 13 (2021) 33874–33884. [DOI] [PubMed] [Google Scholar]
- [110].Yin Y, Li X, Ma H, Zhang J, Yu D, Zhao R, Yu S, Nie G, Wang H, In Situ Transforming Rna Nanovaccines from Polyethylenimine Functionalized Graphene Oxide Hydrogel for Durable Cancer Immunotherapy, Nano Letters, 21 (2021) 2224–2231. [DOI] [PubMed] [Google Scholar]
- [111].Song H, Yang P, Huang P, Zhang C, Kong D, Wang W, Injectable Polypeptide Hydrogel-Based Co-Delivery of Vaccine and Immune Checkpoint Inhibitors Improves Tumor Immunotherapy, Theranostics, 9 (2019) 2299–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lofthouse S, Immunological Aspects of Controlled Antigen Delivery, Advanced Drug Delivery Reviews, 54 (2002) 863–870. [DOI] [PubMed] [Google Scholar]
- [113].Zhao Q, Wang Y, Zhao B, Chen H, Cai Z, Zheng Y, Zeng Y, Zhang D, Liu X, Neoantigen Immunotherapeutic-Gel Combined with Tim-3 Blockade Effectively Restrains Orthotopic Hepatocellular Carcinoma Progression, Nano Letters, 22 (2022) 2048–2058. [DOI] [PubMed] [Google Scholar]
- [114].Perez CR, De Palma M, Engineering Dendritic Cell Vaccines to Improve Cancer Immunotherapy, Nature Communications, 10 (2019) 5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Jin MZ, Wang XP, Immunogenic Cell Death-Based Cancer Vaccines, Frontiers in Immunology, 12 (2021) 697964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Opzoomer JW, Sosnowska D, Anstee JE, Spicer JF, Arnold JN, Cytotoxic Chemotherapy as an Immune Stimulus: A Molecular Perspective on Turning up the Immunological Heat on Cancer, Frontiers in Immunology, 10 (2019) 1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G, Immunological Aspects of Cancer Chemotherapy, Nature Reviews Immunology, 8 (2008) 59–73. [DOI] [PubMed] [Google Scholar]
- [118].Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G, Immunostimulation with Chemotherapy in the Era of Immune Checkpoint Inhibitors, Nature Reviews Clinical Oncology, 17 (2020) 725–741. [DOI] [PubMed] [Google Scholar]
- [119].Chao Y, Liang C, Tao H, Du Y, Wu D, Dong Z, Jin Q, Chen G, Xu J, Xiao Z, Chen Q, Wang C, Chen J, Liu Z, Localized Cocktail Chemoimmunotherapy after in Situ Gelation to Trigger Robust Systemic Antitumor Immune Responses, Science Advances, 6 (2020) eaaz4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hanurry EY, Birhan YS, Darge HF, Mekonnen TW, Arunagiri V, Chou H-Y, Cheng C-C, Lai J-Y, Tsai H-C, PAMAM Dendritic Nanoparticle-Incorporated Hydrogel to Enhance the Immunogenic Cell Death and Immune Response of Immunochemotherapy, ACS Biomaterials Science & Engineering, 8 (2022) 2403–2418. [DOI] [PubMed] [Google Scholar]
- [121].Shi Y, Li D, He C, Chen X, Design of an Injectable Polypeptide Hydrogel Depot Containing the Immune Checkpoint Blocker Anti-Pd-L1 and Doxorubicin to Enhance Antitumor Combination Therapy, Macromolecular Bioscience, 21 (2021) e2100049. [DOI] [PubMed] [Google Scholar]
- [122].Zhang H, Zhang Y, Hu H, Yang W, Xia X, Lei L, Lin R, Li J, Li Y, Gao H, In Situ Tumor Vaccine for Lymph Nodes Delivery and Cancer Therapy Based on Small Size Nanoadjuvant, Small, (2023). [DOI] [PubMed] [Google Scholar]
- [123].Li Z, Xiang J, Zhang Q, Zhao M, Meng Y, Zhong J, Li T, Jia L, Li K, Lu X, Ao Z, Han D, An Engineered Hydrogel with Low-Dose Antitumor Drugs Enhances Tumor Immunotherapy through Tumor Interstitial Wrap, Frontiers in Bioengineering and Biotechnology, 10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Li F, Shao X, Liu D, Jiao X, Yang X, Yang W, Liu X, Vascular Disruptive Hydrogel Platform for Enhanced Chemotherapy and Anti-Angiogenesis through Alleviation of Immune Surveillance, Pharmaceutics, 14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Qin L, Cao J, Shao K, Tong F, Yang Z, Lei T, Wang Y, Hu C, Umeshappa CS, Gao H, Peppas NA, A Tumor-to-Lymph Procedure Navigated Versatile Gel System for Combinatorial Therapy against Tumor Recurrence and Metastasis, Science Advances, 6 eabb3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Munn DH, Mellor AL, The Tumor-Draining Lymph Node as an Immune-Privileged Site, Immunological Reviews, 213 (2006) 146–158. [DOI] [PubMed] [Google Scholar]
- [127].Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH, Tertiary Lymphoid Structures in the Era of Cancer Immunotherapy, Nature Reviews Cancer, 19 (2019) 307–325. [DOI] [PubMed] [Google Scholar]
- [128].Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF, Nanoparticles Target Distinct Dendritic Cell Populations According to Their Size, European Journal of Immunology, 38 (2008) 1404–1413. [DOI] [PubMed] [Google Scholar]
- [129].Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA, Exploiting Lymphatic Transport and Complement Activation in Nanoparticle Vaccines, Nature Biotechnology, 25 (2007) 1159–1164. [DOI] [PubMed] [Google Scholar]
- [130].Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA, In Vivo Targeting of Dendritic Cells in Lymph Nodes with Poly(Propylene Sulfide) Nanoparticles, Journal of Controlled Release, 112 (2006) 26–34. [DOI] [PubMed] [Google Scholar]
- [131].Nakamura T, Kawai M, Sato Y, Maeki M, Tokeshi M, Harashima H, The Effect of Size and Charge of Lipid Nanoparticles Prepared by Microfluidic Mixing on Their Lymph Node Transitivity and Distribution, Molecular Pharmaceutics, 17 (2020) 944–953. [DOI] [PubMed] [Google Scholar]
- [132].De Koker S, Cui J, Vanparijs N, Albertazzi L, Grooten J, Caruso F, De Geest BG, Engineering Polymer Hydrogel Nanoparticles for Lymph Node-Targeted Delivery, Angewandte Chemie (International ed. in English), 55 (2016) 1334–1339. [DOI] [PubMed] [Google Scholar]
- [133].Schudel A, Francis DM, Thomas SN, Material Design for Lymph Node Drug Delivery, Nature Reviews Materials, 4 (2019) 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Schwartz FR, James O, Kuo PH, Witte MH, Koweek LM, Pabon-Ramos WM, Lymphatic Imaging: Current Noninvasive and Invasive Techniques, Seminars in Interventional Radiology, 37 (2020) 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Tselikas L, de Baere T, Isoardo T, Susini S, Ser-Le Roux K, Polrot M, Adam J, Rouanne M, Zitvogel L, Moine L, Deschamps F, Marabelle A, Pickering Emulsions with Ethiodized Oil and Nanoparticles for Slow Release of Intratumoral anti-CTLA4 Immune Checkpoint Antibodies, Journal of Immunotherapy of Cancer, 8 (2020) e000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ali OA, Doherty E, Mooney DJ, Emerich D, Relationship of Vaccine Efficacy to the Kinetics of DC and T-Cell Responses Induced by PLG-Based Cancer Vaccines, Biomatter, 1 (2011) 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ, Infection-Mimicking Materials to Program Dendritic Cells in Situ, Nature Materials, 8 (2009) 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ali OA, Emerich D, Dranoff G, Mooney DJ, In Situ Regulation of DC Subsets and T Cells Mediates Tumor Regression in Mice, Science Translational Medicine, 1 (2009) 8ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Verbeke CS, Mooney DJ, Injectable, Pore-Forming Hydrogels for in Vivo Enrichment of Immature Dendritic Cells, Advanced Healthcare Materials, 4 (2015) 2677–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wang H, Sobral MC, Zhang DKY, Cartwright AN, Li AW, Dellacherie MO, Tringides CM, Koshy ST, Wucherpfennig KW, Mooney DJ, Metabolic Labeling and Targeted Modulation of Dendritic Cells, Nature Materials, 19 (2020) 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Bencherif SA, Warren Sands R, Ali OA, Li WA, Lewin SA, Braschler TM, Shih TY, Verbeke CS, Bhatta D, Dranoff G, Mooney DJ, Injectable Cryogel-Based Whole-Cell Cancer Vaccines, Nature Communications, 6 (2015) 7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Martinez M, Moon EK, Car T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment, Frontiers in Immunology, 10 (2019) 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, Stephan MT, Biopolymer Implants Enhance the Efficacy of Adoptive T-Cell Therapy, Nature Biotechnology, 33 (2015) 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Hu Q, Li H, Archibong E, Chen Q, Ruan H, Ahn S, Dukhovlinova E, Kang Y, Wen D, Dotti G, Gu Z, Inhibition of Post-Surgery Tumour Recurrence Via a Hydrogel Releasing CAR-T Cells and Anti-Pdl1-Conjugated Platelets, Nature Biomedical Engineering, 5 (2021) 1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Grosskopf AK, Labanieh L, Klysz DD, Roth GA, Xu P, Adebowale O, Gale EC, Jons CK, Klich JH, Yan J, Maikawa CL, Correa S, Ou BS, d'Aquino AI, Cochran JR, Chaudhuri O, Mackall CL, Appel EA, Delivery of CAR-T Cells in a Transient Injectable Stimulatory Hydrogel Niche Improves Treatment of Solid Tumors, Science Advances, 8 (2022) eabn8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Luo K, Wu H, Chen Y, Li J, Zhou L, Yang F, Huang M, An X, Wang S, Preparation of Bi-Based Hydrogel for Multi-Modal Tumor Therapy, Colloids and Surfaces B: Biointerfaces, 200 (2021) 111591. [DOI] [PubMed] [Google Scholar]
- [147].Uman S, Wang LL, Thorn SL, Liu Z, Duncan JS, Sinusas AJ, Burdick JA, Imaging of Injectable Hydrogels Delivered into Myocardium with SPECT/CT, Advanced Healthcare Materials, 9 (2020) e2000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wu X, Wang X, Chen X, Yang X, Ma Q, Xu G, Yu L, Ding J, Injectable and Thermosensitive Hydrogels Mediating a Universal Macromolecular Contrast Agent with Radiopacity for Noninvasive Imaging of Deep Tissues, Bioactive Materials, 6 (2021) 4717–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Bouché M, Dong YC, Sheikh S, Taing K, Saxena D, Hsu JC, Chen MH, Salinas RD, Song H, Burdick JA, Dorsey J, Cormode DP, Novel Treatment for Glioblastoma Delivered by a Radiation Responsive and Radiopaque Hydrogel, ACS Biomaterials Science & Engineering, 7 (2021) 3209–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Keshavarz M, Moloudi K, Paydar R, Abed Z, Beik J, Ghaznavi H, Shakeri-Zadeh A, Alginate Hydrogel Co-Loaded with Cisplatin and Gold Nanoparticles for Computed Tomography Image-Guided Chemotherapy, Journal of Biomaterials Applications, 33 (2018) 161–169. [DOI] [PubMed] [Google Scholar]
- [151].Wu Y, Wang H, Gao F, Xu Z, Dai F, Liu W, An Injectable Supramolecular Polymer Nanocomposite Hydrogel for Prevention of Breast Cancer Recurrence with Theranostic and Mammoplastic Functions, Advanced Functional Materials, 28 (2018) 1801000. [Google Scholar]
- [152].Tzu-Yin W, Wilson KE, Machtaler S, Willmann JK, Ultrasound and Microbubble Guided Drug Delivery: Mechanistic Understanding and Clinical Implications, Current Pharmaceutical Biotechnology, 14 (2014) 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kwan JJ, Myers R, Coviello CM, Graham SM, Shah AR, Stride E, Carlisle RC, Coussios CC, Ultrasound-Propelled Nanocups for Drug Delivery, Small, 11 (2015) 5305–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ashrafi K, Tang Y, Britton H, Domenge O, Blino D, Bushby AJ, Shuturminska K, den Hartog M, Radaelli A, Negussie AH, Mikhail AS, Woods DL, Krishnasamy V, Levy EB, Wood BJ, Willis SL, Dreher MR, Lewis AL, Characterization of a Novel Intrinsically Radiopaque Drug-Eluting Bead for Image-Guided Therapy: DC Bead Lumi™, Journal of Controlled Release, 250 (2017) 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Levy EB, Krishnasamy VP, Lewis AL, Willis S, Macfarlane C, Anderson V, van der Bom IM, Radaelli A, Dreher MR, Sharma KV, Negussie A, Mikhail AS, Geschwind JH, Wood BJ, First Human Experience with Directly Image-Able Iodinated Embolization Microbeads, Cardiovascular and Interventional Radiology, 39 (2016) 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Negussie AH, Dreher MR, Johnson CG, Tang Y, Lewis AL, Storm G, Sharma KV, Wood BJ, Synthesis and Characterization of Image-Able Polyvinyl Alcohol Microspheres for Image-Guided Chemoembolization, Journal of Materials Science. Materials in Medicine, 26 (2015) 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Mikhail AS, Pritchard WF, Negussie AH, Inkiyad G, Long DJ, Mauda-Havakuk M, Wakim PG, van der Sterren W, Levy EB, Lewis AL, Karanian JW, Wood BJ, Cone-Beam Computed Tomography-Based Spatial Prediction of Drug Dose after Transarterial Chemoembolization Using Radiopaque Drug-Eluting Beads in Woodchuck Hepatocellular Carcinoma, Investigative Radiology, 57 (2022) 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Mikhail AS, Pritchard WF, Negussie AH, Krishnasamy VP, Amchin DB, Thompson JG, Wakim PG, Woods D, Bakhutashvili I, Esparza-Trujillo JA, Karanian JW, Willis SL, Lewis AL, Levy EB, Wood BJ, Mapping Drug Dose Distribution on Ct Images Following Transarterial Chemoembolization with Radiopaque Drug-Eluting Beads in a Rabbit Tumor Model, Radiology, 289 (2018) 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Delgado J, Pritchard W, Varble N, Mikhail A, Owen J, Arrichiello A, Ray T, Lopez-Silva T, Morhard R, Yang J, Abstract No. 242 Distribution of Imageable Thermosensitive Drug-Loaded Gel in Ex Vivo Bovine Liver Depends on Needle Type and Injection Technique, Journal of Vascular and Interventional Radiology, 34 (2023) S110. [Google Scholar]
- [160].Sato T, Eschelman DJ, Gonsalves CF, Terai M, Chervoneva I, McCue PA, Shields JA, Shields CL, Yamamoto A, Berd D, Mastrangelo MJ, Sullivan KL, Immunoembolization of Malignant Liver Tumors, Including Uveal Melanoma, Using Granulocyte-Macrophage Colony-Stimulating Factor, Journal of Clinical Oncology, 26 (2008) 5436–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Rizzitelli S, Holtzman N, Maleux G, De Baere T, Sun F, Comby PO, Tal M, Bazin G, Montestruc F, Viel T, Robert P, Harbater O, Miller E, Corot C, Reduced Nontarget Embolization and Increased Targeted Delivery with a Reflux-Control Microcatheter in a Swine Model, Diagnostic and Interventional Imaging, 102 (2021) 641–648. [DOI] [PubMed] [Google Scholar]
- [162].Angileri SA, Rodà GM, Arrichiello A, Signorelli G, Di Meglio L, Gurgitano M, Di Bartolomeo F, Ierardi AM, Paolucci A, Carrafiello G, Efficacy, Safety and Usability of Bronchial Artery Embolization Using a New Anti-Reflux Microcatheter in the Management of Haemoptysis, Acta Bio-medica, 91 (2020) e2020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Phase 1b Study of CAR2Anti-CEA CAR-T Cell Hepatic Infusions for Pancreatic Carcinoma Patients with CEA+ Liver Metastases (Anticea_Cart), ClinicalTrials.gov, identifier: NCT03818165. Updated January 17 2023, https://classic.clinicaltrials.gov/ct2/show/NCT03818165, 2019-.
- [164].Irie T, Kuramochi M, Takahashi N, Dense Accumulation of Lipiodol Emulsion in Hepatocellular Carcinoma Nodule During Selective Balloon-Occluded Transarterial Chemoembolization: Measurement of Balloon-Occluded Arterial Stump Pressure, Cardiovascular and Interventional Radiology, 36 (2013) 706–713. [DOI] [PubMed] [Google Scholar]
- [165].Rose SC, Halstead GD, Narsinh KH, Pressure-Directed Embolization of Hepatic Arteries in a Porcine Model Using a Temporary Occlusion Balloon Microcatheter: Proof of Concept, Cardiovascular and Interventional Radiology, 40 (2017) 1769–1776. [DOI] [PubMed] [Google Scholar]
- [166].Arepally A, Chomas J, Kraitchman D, Hong K, Quantification and Reduction of Reflux During Embolotherapy Using an Antireflux Catheter and Tantalum Microspheres: Ex Vivo Analysis, Journal of Vascular and Interventional Radiology, 24 (2013) 575–580. [DOI] [PubMed] [Google Scholar]
- [167].Rose SC, Kikolski SG, Chomas JE, Downstream Hepatic Arterial Blood Pressure Changes Caused by Deployment of the Surefire Antireflux Expandable Tip, Cardiovascular and Interventional Radiology, 36 (2013) 1262–1269. [DOI] [PubMed] [Google Scholar]
- [168].Titano JJ, Fischman AM, Cherian A, Tully M, Stein LL, Jacobs L, Rubin RA, Bosley M, Citron S, Joelson DW, Shrestha R, Arepally A, End-Hole Versus Microvalve Infusion Catheters in Patients Undergoing Drug-Eluting Microspheres-Tace for Solitary Hepatocellular Carcinoma Tumors: A Retrospective Analysis, Cardiovascular and Interventional Radiology, 42 (2019) 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ, In Situ Tumor Ablation Creates an Antigen Source for the Generation of Antitumor Immunity, Cancer Research, 64 (2004) 4024–4029. [DOI] [PubMed] [Google Scholar]
- [170].Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, Schivazappa S, Zibera C, Fagnoni FF, Ferrari C, Missale G, Radiofrequency Thermal Ablation of Hepatocellular Carcinoma Liver Nodules Can Activate and Enhance Tumor-Specific T-Cell Responses, Cancer Research, 66 (2006) 1139–1146. [DOI] [PubMed] [Google Scholar]
- [171].Hansler J, Wissniowski TT, Schuppan D, Witte A, Bernatik T, Hahn EG, Strobel D, Activation and Dramatically Increased Cytolytic Activity of Tumor Specific T Lymphocytes after Radio-Frequency Ablation in Patients with Hepatocellular Carcinoma and Colorectal Liver Metastases, World Journal of Gastroenterology, 12 (2006) 3716–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Wissniowski TT, Hänsler J, Neureiter D, Frieser M, Schaber S, Esslinger B, Voll R, Strobel D, Hahn EG, Schuppan D, Activation of Tumor-Specific T Lymphocytes by Radio-Frequency Ablation of the VX2 Hepatoma in Rabbits, Cancer Research, 63 (2003) 6496–6500. [PubMed] [Google Scholar]
- [173].Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS, Immuno-Thermal Ablations - Boosting the Anticancer Immune Response, Journal of Immunotherapy of Cancer, 5 (2017) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R, Locoregional Therapies in the Era of Molecular and Immune Treatments for Hepatocellular Carcinoma, Nature Reviews Gastroenterology & Hepatology, 18 (2021) 293–313. [DOI] [PubMed] [Google Scholar]
- [175].Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S, Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect, Trends in Immunology, 39 (2018) 644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, Burroughs AK, Meyer T, Behboudi S, Unmasking of Alpha-Fetoprotein-Specific CD4(+) T Cell Responses in Hepatocellular Carcinoma Patients Undergoing Embolization, Journal of Immunology, 178 (2007) 1914–1922. [DOI] [PubMed] [Google Scholar]
- [177].Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R, Matsumura T, Yanagawa T, Ito T, Imawari M, Strong CD8(+) T-Cell Responses against Tumor-Associated Antigens Prolong the Recurrence-Free Interval after Tumor Treatment in Patients with Hepatocellular Carcinoma, Journal of Gastroenterology, 45 (2010) 451–458. [DOI] [PubMed] [Google Scholar]
- [178].Li L, Wang W, Pan H, Ma G, Shi X, Xie H, Liu X, Ding Q, Zhou W, Wang S, Microwave Ablation Combined with OK-432 Induces Th1-Type Response and Specific Antitumor Immunity in a Murine Model of Breast Cancer, Journal of Translational Medicine, 15 (2017) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Levy MY, Sidana A, Chowdhury WH, Solomon SB, Drake CG, Rodriguez R, Fuchs EJ, Cyclophosphamide Unmasks an Antimetastatic Effect of Local Tumor Cryoablation, Journal of Pharmacology and Experimental Therapeutics, 330 (2009) 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Peng P, Hu H, Liu P, Xu LX, Neoantigen-Specific Cd4+ T-Cell Response Is Critical for the Therapeutic Efficacy of Cryo-Thermal Therapy, Journal of Immunotherapy of Cancer, 8 (2020) e000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Abe S, Nagata H, Crosby EJ, Inoue Y, Kaneko K, Liu C-X, Yang X, Wang T, Acharya CR, Agarwal P, Snyder J, Gwin W, Morse MA, Zhong P, Lyerly HK, Osada T, Combination of Ultrasound-Based Mechanical Disruption of Tumor with Immune Checkpoint Blockade Modifies Tumor Microenvironment and Augments Systemic Antitumor Immunity, J immunother Cancer, 10 (2022) e003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Hwang J, An E-K, Zhang W, Kim HJ, Eom Y, Jin J-O, Dual-Functional Alginate and Collagen–Based Injectable Hydrogel for the Treatment of Cancer and Its Metastasis, Journal of Nanobiotechnology, 20 (2022) 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Yata T, Takahashi Y, Tan M, Nakatsuji H, Ohtsuki S, Murakami T, Imahori H, Umeki Y, Shiomi T, Takakura Y, Nishikawa M, DNA Nanotechnology-Based Composite-Type Gold Nanoparticle-Immunostimulatory DNA Hydrogel for Tumor Photothermal Immunotherapy, Biomaterials, 146 (2017) 136–145. [DOI] [PubMed] [Google Scholar]
- [184].Justesen TF, Orhan A, Raskov H, Nolsoe C, Gögenur I, Electroporation and Immunotherapy-Unleashing the Abscopal Effect, Cancers (Basel), 14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF, Tremelimumab in Combination with Ablation in Patients with Advanced Hepatocellular Carcinoma, Journal of Hepatology, 66 (2017) 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Xie C, Duffy AG, Mabry-Hrones D, Wood B, Levy E, Krishnasamy V, Khan J, Wei JS, Agdashian D, Tyagi M, Gangalapudi V, Fioravanti S, Walker M, Anderson V, Venzon D, Figg WD, Sandhu M, Kleiner DE, Morelli MP, Floudas CS, Brar G, Steinberg SM, Korangy F, Greten TF, Tremelimumab in Combination with Microwave Ablation in Patients with Refractory biliary Tract Cancer, Hepatology, 69 (2019) 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Fan M, Jia L, Pang M, Yang X, Yang Y, Kamel Elyzayati S, Liao Y, Wang H, Zhu Y, Wang Q, Injectable Adhesive Hydrogel as Photothermal-Derived Antigen Reservoir for Enhanced Anti-Tumor Immunity, Advanced Functional Materials, 31 (2021) 2010587. [Google Scholar]
- [188].Chen M, Tan Y, Hu J, Jiang Y, Wang Z, Liu Z, Chen Q, Injectable Immunotherapeutic Thermogel for Enhanced Immunotherapy Post Tumor Radiofrequency Ablation, Small, 17 (2021) 2104773. [DOI] [PubMed] [Google Scholar]
- [189].Cao Y, Zhou Y, Pan J, Zhong X, Ding J, Jing X, Sun S-K, A General Strategy Towards an Injectable Microwave-Sensitive Immune Hydrogel for Combined Percutaneous Microwave Ablation and Immunotherapy, Chemical Engineering Journal, 422 (2021) 130111. [Google Scholar]
- [190].Li S, Zhu C, Zhou X, Chen L, Bo X, Shen Y, Guan X, Han X, Shan D, Sun L, Chen Y, Xu H, Yue W, Engineering ROS-Responsive Bioscaffolds for Disrupting Myeloid Cell-Driven Immunosuppressive Niche to Enhance Pd-L1 Blockade-Based Postablative Immunotherapy, Advanced Science, 9 (2022) 2104619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Lomakin A, Svedlund J, Strell C, Gataric M, Shmatko A, Rukhovich G, Park JS, Ju YS, Dentro S, Kleshchevnikov V, Vaskivskyi V, Li T, Bayraktar OA, Pinder S, Richardson AL, Santagata S, Campbell PJ, Russnes H, Gerstung M, Nilsson M, Yates LR, Spatial Genomics Maps the Structure, Nature and Evolution of Cancer Clones, Nature, 611 (2022) 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Erickson A, He M, Berglund E, Marklund M, Mirzazadeh R, Schultz N, Kvastad L, Andersson A, Bergenstråhle L, Bergenstråhle J, Larsson L, Alonso Galicia L, Shamikh A, Basmaci E, Díaz De Ståhl T, Rajakumar T, Doultsinos D, Thrane K, Ji AL, Khavari PA, Tarish F, Tanoglidi A, Maaskola J, Colling R, Mirtti T, Hamdy FC, Woodcock DJ, Helleday T, Mills IG, Lamb AD, Lundeberg J, Spatially Resolved Clonal Copy Number Alterations in Benign and Malignant Tissue, Nature, 608 (2022) 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Kim J, Francis DM, Thomas SN, In Situ Crosslinked Hydrogel Depot for Sustained Antibody Release Improves Immune Checkpoint Blockade Cancer Immunotherapy, Nanomaterials (Basel), 11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Fu Y, Huang Y, Li P, Wang L, Tang Z, Liu X, Bian X, Wu S, Wang X, Zhu B, Yu Y, Jiang J, Li C, Physical- and Chemical-Dually ROS-Responsive Nano-in-Gel Platforms with Sequential Release of Ox40 Agonist and Pd-1 Inhibitor for Augmented Combination Immunotherapy, Nano Letters, 23 (2023) 1424–1434. [DOI] [PubMed] [Google Scholar]
- [195].Wang F, Xu D, Su H, Zhang W, Sun X, Monroe MK, Chakroun RW, Wang Z, Dai W, Oh R, Wang H, Fan Q, Wan F, Cui H, Supramolecular Prodrug Hydrogelator as an Immune Booster for Checkpoint Blocker-Based Immunotherapy, Science Advances, 6 (2020) eaaz8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Leach DG, Dharmaraj N, Piotrowski SL, Lopez-Silva TL, Lei YL, Sikora AG, Young S, Hartgerink JD, Stingel: Controlled Release of a Cyclic Dinucleotide for Enhanced Cancer Immunotherapy, Biomaterials, 163 (2018) 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Yu S, Wang C, Yu J, Wang J, Lu Y, Zhang Y, Zhang X, Hu Q, Sun W, He C, Chen X, Gu Z, Injectable Bioresponsive Gel Depot for Enhanced Immune Checkpoint Blockade, Advanced Materials, 30 (2018) 1801527. [DOI] [PubMed] [Google Scholar]
- [198].Yang Y, Yang Y, Chen M, Chen J, Wang J, Ma Y, Qian H, Injectable Shear-Thinning Polylysine Hydrogels for Localized Immunotherapy of Gastric Cancer through Repolarization of Tumor-Associated Macrophages, Biomaterials Science, 9 (2021) 6597–6608. [DOI] [PubMed] [Google Scholar]
- [199].Dai X, Meng J, Deng S, Zhang L, Wan C, Lu L, Huang J, Hu Y, Zhang Z, Li Y, Lovell JF, Wu G, Yang K, Jin H, Targeting Camkii to Reprogram Tumor-Associated Macrophages and Inhibit Tumor Cells for Cancer Immunotherapy with an Injectable Hybrid Peptide Hydrogel, Theranostics, 10 (2020) 3049–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Jin HS, Choi DS, Ko M, Kim D, Lee DH, Lee S, Lee AY, Kang SG, Kim SH, Jung Y, Jeong Y, Chung JJ, Park Y, Extracellular pH Modulating Injectable Gel for Enhancing Immune Checkpoint Inhibitor Therapy, Journal of Controlled Release, 315 (2019) 65–75. [DOI] [PubMed] [Google Scholar]
- [201].Chen M, Tan Y, Dong Z, Lu J, Han X, Jin Q, Zhu W, Shen J, Cheng L, Liu Z, Chen Q, Injectable Anti-Inflammatory Nanofiber Hydrogel to Achieve Systemic Immunotherapy Post Local Administration, Nano Letters, 20 (2020) 6763–6773. [DOI] [PubMed] [Google Scholar]
- [202].Wu X, Wu Y, Ye H, Yu S, He C, Chen X, Interleukin-15 and Cisplatin Co-Encapsulated Thermosensitive Polypeptide Hydrogels for Combined Immuno-Chemotherapy, Journal of Controlled Release, 255 (2017) 81–93. [DOI] [PubMed] [Google Scholar]
- [203].Song H, Huang P, Niu J, Shi G, Zhang C, Kong D, Wang W, Injectable Polypeptide Hydrogel for Dual-Delivery of Antigen and Tlr3 Agonist to Modulate Dendritic Cells In vivo and Enhance Potent Cytotoxic T-Lymphocyte Response against Melanoma, Biomaterials, 159 (2018) 119–129. [DOI] [PubMed] [Google Scholar]
- [204].Kim S, Lee J, Im S, Kim WJ, Injectable Immunogel Based on Polymerized Phenylboronic Acid and Mannan for Cancer Immunotherapy, Journal of Controlled Release, 345 (2022) 138–146. [DOI] [PubMed] [Google Scholar]