Abstract
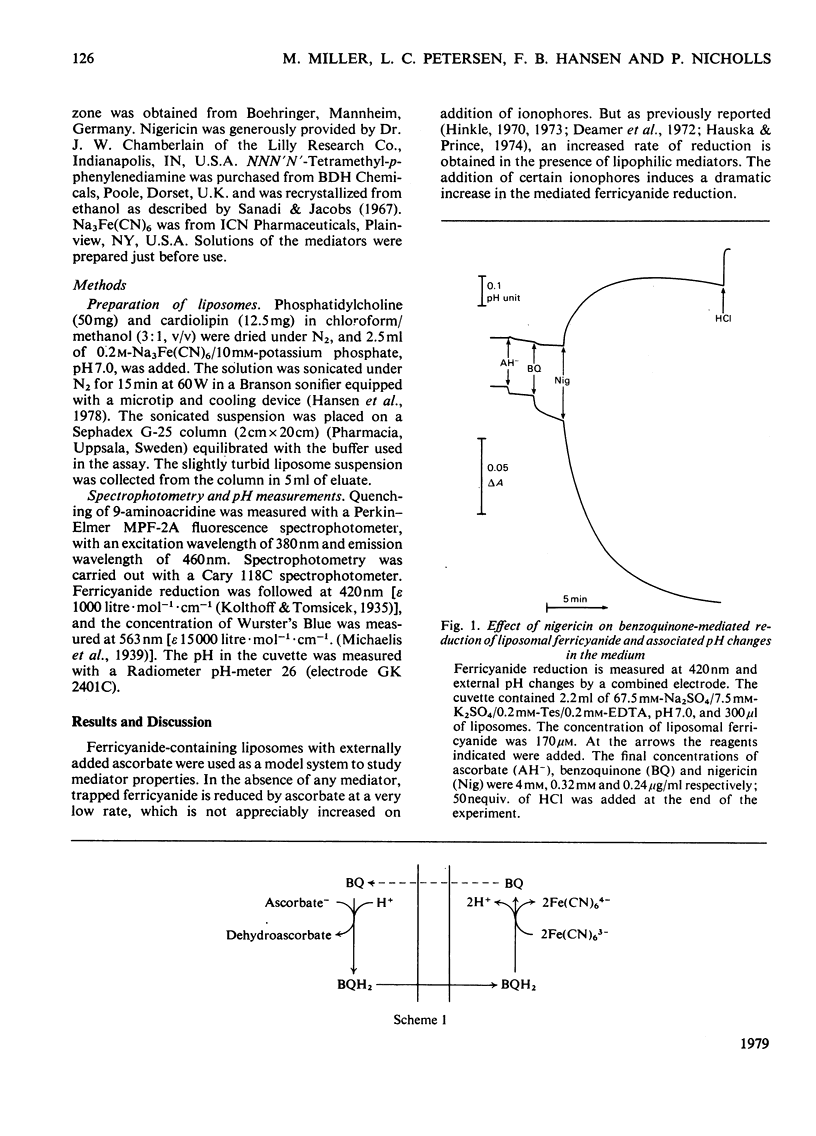
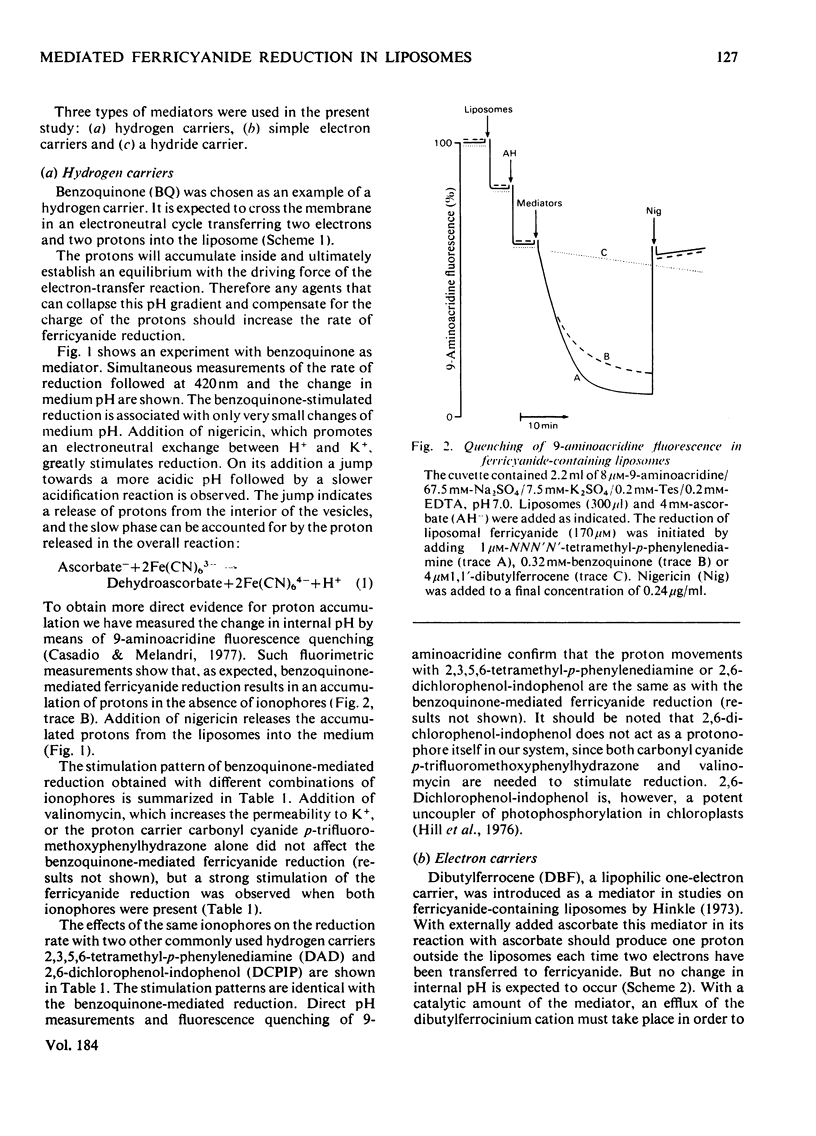
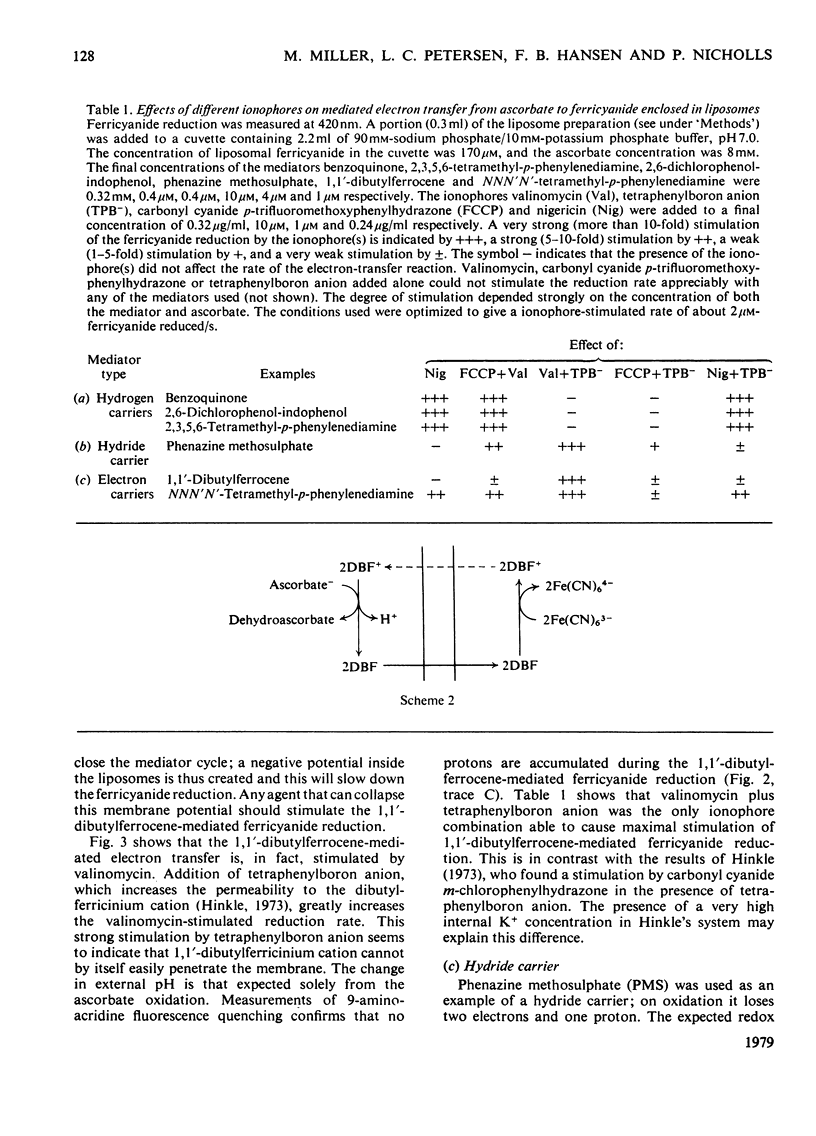
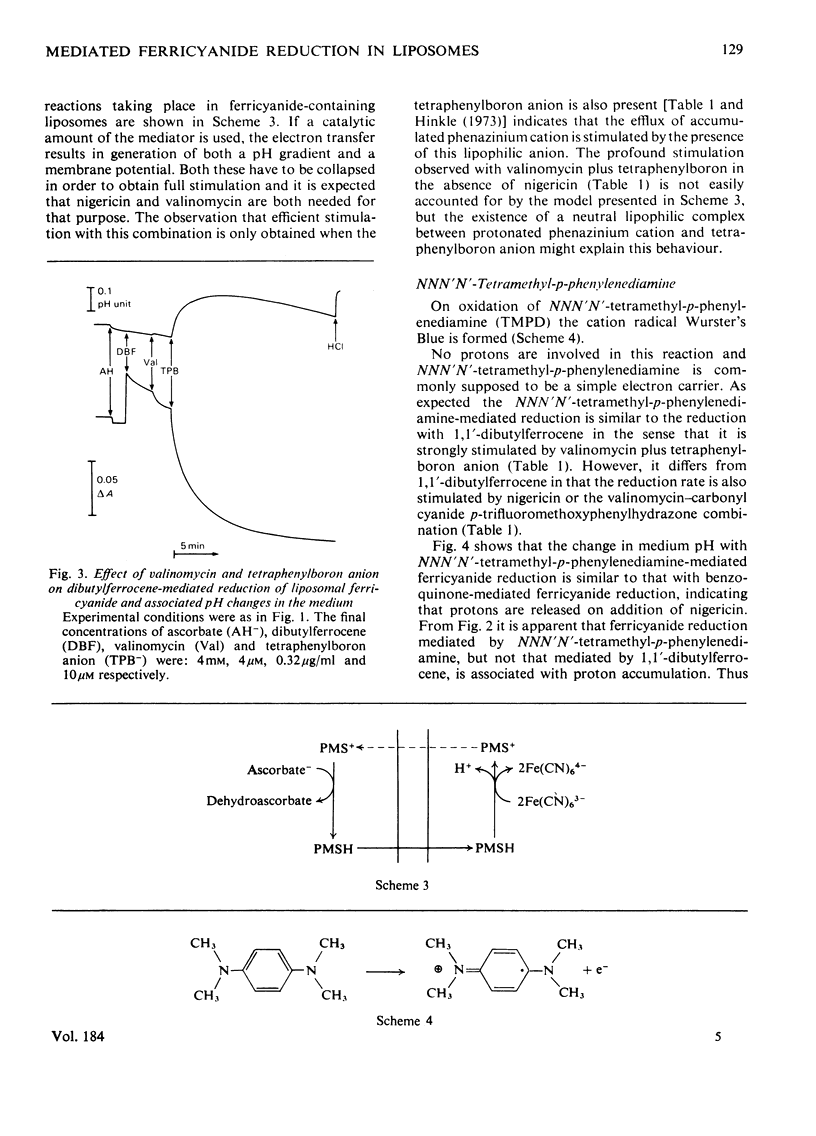
Ferricyanide-containing liposomes were used as a system to compare the electron- and proton-translocating properties of six redox reagents commonly used as electron donors for biochemical systems. The effects of different ionophore combinations on the ferricyanide-reduction rate were generally consistent with the expected proton- and electron-translocating properties of the mediators. The transmembrane pH gradient produced by hydrogen carriers was demonstrated. Nigericin or valinomycin plus carbonyl cyanide p-trifluoromethoxyphenylhydrazone are capable of collapsing this gradient and of stimulating ferricyanide reduction mediated by this type of carrier. No pH gradient is produced with the electron carrier 1,1′-dibutylferrocene. In the presence of tetraphenylboron anion, which is needed for this carrier to act as an efficient mediator, addition of valinomycin alone is sufficient to obtain full stimulation of ferricyanide reduction. NNN′N′-Tetramethyl-p-phenylenediamine does not behave as a simple electron carrier. During NNN′N′-tetramethyl-p-phenylenediamine-mediated ferricyanide reduction protons are translocated across the membrane and accumulated in the vesicles. This is not due to the presence of demethylated impurities in the NNN′N′-tetramethyl-p-phenylenediamine sample, but may be the result of an accumulation of oxidation products other than the Wurster's Blue radical. These results suggest a reconsideration of studies on protonmotive forces across membranes where NNN′N′-tetramethyl-p-phenylenediamine is used as a mediator.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casadio R., Melandri B. A. The behavior of 9-aminoacridine as an indicator of transmembrane pH difference in liposomes of natural bacterial phospholipids. J Bioenerg Biomembr. 1977 Feb;9(1):17–29. doi: 10.1007/BF00745040. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Prince R. C., Crofts A. R. The response of fluorescent amines to pH gradients across liposome membranes. Biochim Biophys Acta. 1972 Aug 9;274(2):323–335. doi: 10.1016/0005-2736(72)90180-0. [DOI] [PubMed] [Google Scholar]
- Hansen F. B., Miller M., Nicholls P. Control of respiration in proteoliposomes containing cytochrome aa3. I. Stimulation by valinomycin and uncoupler. Biochim Biophys Acta. 1978 Jun 8;502(3):385–399. doi: 10.1016/0005-2728(78)90072-5. [DOI] [PubMed] [Google Scholar]
- Hauska G. A., Prince R. C. Lipophilicity and catalysis of photophosphorylation. Artificial proton translocation by lipophilic, quinoid hydrogen carriers in chloroplasts and liposomes. FEBS Lett. 1974 Apr 15;41(1):35–39. doi: 10.1016/0014-5793(74)80947-6. [DOI] [PubMed] [Google Scholar]
- Hauska G., Trebst A. Artificial energy conservation in the respiratory chain. No native coupling site between cytochrome c and oxygen. FEBS Lett. 1977 Feb 1;73(2):257–262. doi: 10.1016/0014-5793(77)80994-0. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C. Electron transfer across membranes and energy coupling. Fed Proc. 1973 Sep;32(9):1988–1992. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Hildebrandt V. Redox state of the partially reduced cytochrome aa3- cyanide complex. Biochim Biophys Acta. 1978 Dec 7;504(3):457–460. doi: 10.1016/0005-2728(78)90067-1. [DOI] [PubMed] [Google Scholar]
- Sigel E., Carafoli E. The proton pump of cytochrome c oxidase and its stoichiometry. Eur J Biochem. 1978 Aug 15;89(1):119–123. doi: 10.1111/j.1432-1033.1978.tb20903.x. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J. Measurements of the components of the protonmotive force generated by cytochrome oxidase in submitochondrial particles. FEBS Lett. 1978 Jun 1;90(1):178–182. doi: 10.1016/0014-5793(78)80324-x. [DOI] [PubMed] [Google Scholar]
- Wikström M. K., Saari H. T. The mechanism of energy conservation and transduction by mitochondrial cytochrome c oxidase. Biochim Biophys Acta. 1977 Nov 17;462(2):347–361. doi: 10.1016/0005-2728(77)90133-5. [DOI] [PubMed] [Google Scholar]


