Abstract
1. Radioactively labelled cholecalciferol was injected into the land snails Levantina hiersolyma and Theba pisana. Three metabolites (C, D and E), more polar than cholecalciferol, were found. 2. Metabolite C was found to be identical with 25-hydroxycholecalciferol. On injection of 25-hydroxy[26,27-3H]cholecalciferol, metabolite E was predominantly formed. Metabolite D was predominantly formed from cholecalciferol. Metabolites D and E differ from any known cholecalciferol metabolites. 3. The intestine was found to be the tissue capable of carrying out the transformation of 25-hydroxycholecalciferol into metabolite E. 4. 25-Hydroxycholecalciferol and metabolite E were localized in the digestive gland of the snail, the tissue responsible for the absorption of Ca2+ and its storage. Metabolite D was not localized in any specific tissue.
Full text
PDF
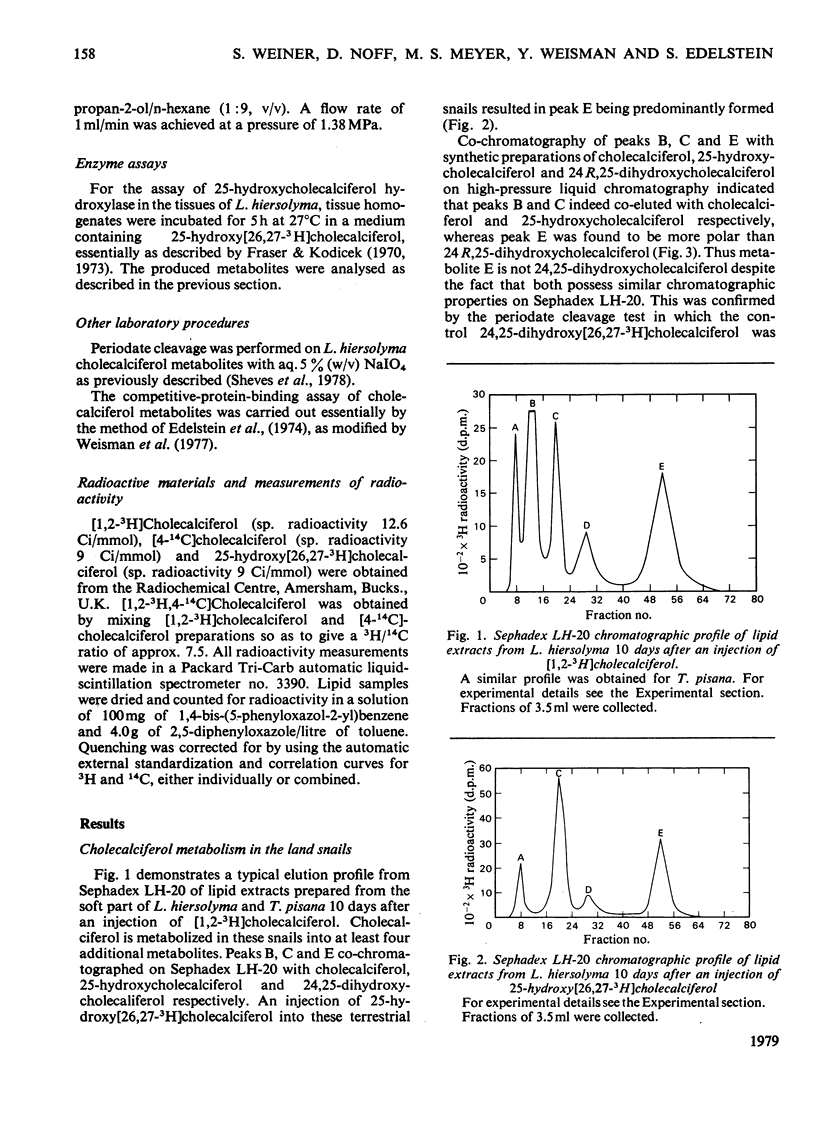
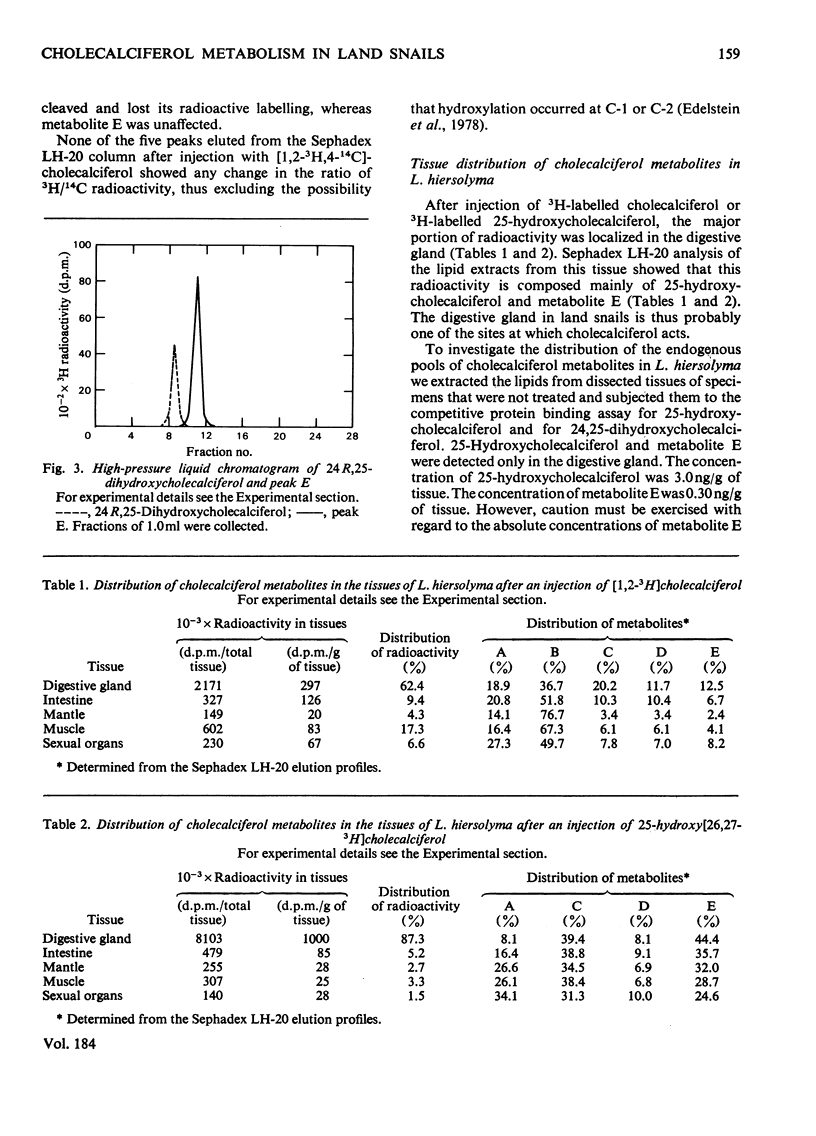


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Burton R. F. The storage of calcium and magnesium phosphates and of calcite in the digestive glands of the pulmonata (gastropoda). Comp Biochem Physiol A Comp Physiol. 1972 Nov 1;43(3):655–663. doi: 10.1016/0300-9629(72)90252-6. [DOI] [PubMed] [Google Scholar]
- Edelstein S., Charman M., Lawson D. E., Kodicek E. Competitive protein-binding assay for 25-hydroxycholecalciferol. Clin Sci Mol Med. 1974 Feb;46(2):231–240. doi: 10.1042/cs0460231. [DOI] [PubMed] [Google Scholar]
- Edelstein S., Noff D., Sinai L., Harell A., Puschett J. B., Golub E. E., Bronner F. Vitamin D metabolism and expression in rats fed on low-calcium and low-phosphorus diets. Biochem J. 1978 Feb 15;170(2):227–233. doi: 10.1042/bj1700227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol. 1973 Feb 7;241(110):163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Ornoy A., Goodwin D., Noff D., Edelstein S. 24, 25-dihydroxyvitamin D is a metabolite of vitamin D essential for bone formation. Nature. 1978 Nov 30;276(5687):517–519. doi: 10.1038/276517a0. [DOI] [PubMed] [Google Scholar]
- Sheves M., Mazur Y., Noff D., Edelstein S. Biological deactivation of the active analogue of cholecalciferol. FEBS Lett. 1978 Dec 1;96(1):75–78. doi: 10.1016/0014-5793(78)81065-5. [DOI] [PubMed] [Google Scholar]
- Van der Borght O., Van Puymbroeck S. Calcium metabolism in a freshwater mollusc: quantitative importance of water and food as supply for calcium during growth. Nature. 1966 May 21;210(5038):791–793. doi: 10.1038/210791a0. [DOI] [PubMed] [Google Scholar]
- Weisman Y., Reiter E., Rott A. Measurement of 24,25 dihydroxyvitamin D in sera of neonates and children. J Pediatr. 1977 Dec;91(6):904–908. doi: 10.1016/s0022-3476(77)80887-1. [DOI] [PubMed] [Google Scholar]


