Abstract
Abrocitinib is a novel oral small molecule which acts as a selective JAK-1 inhibitor. Currently approved for use in moderate-to-severe cases of atopic dermatitis, this drug is gaining a rapid impetus for its use across various inflammatory dermatoses for its selective downstream action on cytokines of the JAK-1 pathway. Its efficacy and safety in atopic dermatitis has been established in phase 3 clinical trials. The future implication of this drug will depend largely on feasibility and practical use in Indian scenario.
Keywords: Abrocitinib, Janus Kinase, JAK-STAT inhibitor, oral small molecule
Abrocitinib is an oral Janus kinase (JAK) 1-selective inhibitor that is currently authorized for use (UK, EU, and Japan) in moderate-to-severe atopic dermatitis (AD) that is not adequately controlled with other systemic drugs/biologics or if these are not feasible, for adults and adolescents 12 years and above [Table 1].[1,2,3,4] It is a newer oral small molecule after baricitinib (JAK1/2 inhibitor) and upadacitinib (selective JAK1 inhibitor) to be used in AD with encouraging results. JAK1 inhibition is considered crucial for mediating a variety of downstream signaling pathways crucial to AD pathogenesis, including thymic stromal lymphopoietin (TSLP), interleukin (IL)-4, IL-13, IL-22, and IL-31.[1] This abrogates the clinical signs and symptoms of AD, including pruritus, dryness, eczema, and lichenification, thereby also improving the quality of life of patients with AD. The present review aims to consolidate the available literature on abrocitinib in AD and other dermatoses as a ready reckoner for dermatologists. In this review, abrocitinib is being discussed as a molecule in detail, including its pharmacokinetics, mechanism of action, efficacy, and safety with the objective of providing comprehensive outlook on this new JAK inhibitor on the block.
Table 1.
| Indication | Agency | Age group | Approval month and year |
|---|---|---|---|
| Treatment of moderate-to-severe AD not adequately controlled with other systemic drugs/biologics, or when those therapies are inadvisable[1] | US-FDA | ≥18 years age ≥12 years age | January 2022 February 2023 |
| Treatment of moderate-to-severe AD in adults and adolescents who are candidates for systemic therapy[2] | EU | ≥12 years age | October 2021 |
| Treatment of moderate to severe AD in adults and adolescents who are candidates for systemic therapy[3] | UK | ≥12 years age | September 2021 |
| Treatment of moderate to severe AD in adults and adolescents with inadequate response to existing therapies[4] | Japan | ≥12 years age | September 2021 |
Search Strategy
“Abrocitinib” was the search term used in the title of the PubMed database. With an emphasis on publications relating to dermatology, abstracts were filtered to include studies written in English and those concerning the use of abrocitinib for both dermatological and non-dermatological purposes with the last search date of March 3, 2024. Randomized control trials and case reports in English were included in this review. Relevant articles and Food and Drug Administration (FDA) abrocitinib label data were selected from a total of 118 search results and included in the review.
Mechanism of Action
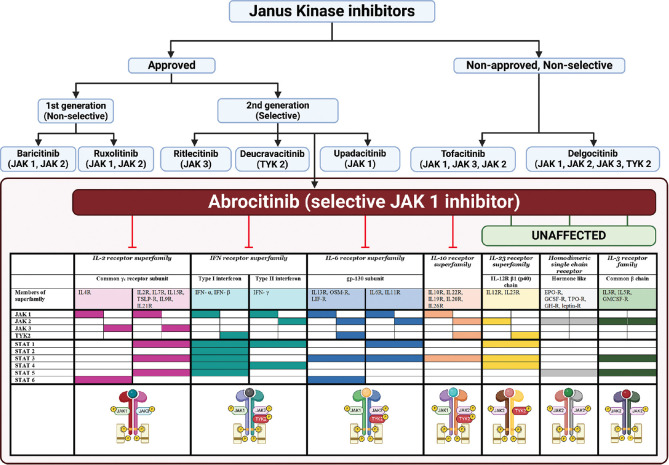
Abrocitinib, a reversible inhibitor of JAK1, functions by obstructing the adenosine triphosphate binding site of JAK1, which consequently fails to recruit and phosphorylate the signal transducer and activator of transcription (STAT) proteins. In experiments using cell-free isolated enzymes, abrocitinib is seen to have JAK1 selectivity more than JAK2, JAK3, and TYK2 by 28, >340, and 43 folds, respectively. Both the original medication and its active metabolites selectively inhibit JAK1 activity in vitro. The prevention of STAT phosphorylation results in the inhibition of signaling of various Th2 cytokines, including IL-4, IL-13, IL-31, IL-22, and TSLP.[5] Detailed mechanism of action along with other JAK inhibitors in dermatology is depicted in Figure 1.
Figure 1.
Janus Kinase inhibitors in dermatology: Selective inhibition of JAK1 by abrocitinib. “Created with BioRender.com”. JAK inhibitors can be classified as first-generation (non-selective) and second-generation (selective), depending upon the JAK(s) they inhibit. (top) JAK inhibitors approved by the US-FDA in dermatology for use include: first generation- baricitinib (alopecia areata) and ruxolitinib (vitiligo); and among second generation- ritlecitinib (alopecia areata), deucravacitinib (psoriasis), Upadacitinib, and abrocitinib (atopic dermatitis). Abrocitinib (bottom) is a selective JAK1 inhibitor that inhibits various downstream mediators in atopic dermatitis, as depicted. All are systemic molecules, except ruxolitinib which is US-FDA approved for topical use in vitiligo, and delgocitinib which is approved in Japan for topical use in atopic dermatitis
Abrocitinib treatment is linked to a dose-dependent reduction in serum inflammatory markers such as IL-31, thymus and activation-regulated chemokine, and hsCRP high-sensitivity C-reactive protein. In 4 weeks, these alterations recover to almost baseline levels upon treatment cessation.[6]
Pharmacokinetics [Table 2]
Table 2.
Summary of pharmacokinetic parameters
| Pharmacokinetic parameter | Details |
|---|---|
| Dose-dependent rise | Dose-dependent rise in plasma Cmax and AUC up to 200 mg, indicating proportional escalation. |
| Steady-state plasma | Reaches steady-state plasma concentrations in 48 hours with daily treatment. |
| Absorption | Oral bioavailability: ~60% Peak plasma concentration: Within 1 hour |
| Distribution | Volume of distribution: ~100 L (after intravenous injection) Plasma protein binding: Abrocitinib (64%), Metabolites M1 (37%) and M2 (29%) Binding: Similar amounts in red blood cells and plasma, mostly to albumin |
| Elimination | Cleared mainly through metabolic processes Half-lives: Abrocitinib and its active metabolites (M1 and M2) - 3–5 h |
| Metabolism | Major enzymes involved: CYP2C19 (~53%), CYP3A4 (~11%), CYP2C9 (~30%), CYP2B6 (~6%) Active metabolites: M1 (less active), M2 (similar activity to abrocitinib) Unbound exposure: Abrocitinib (~60%), M2 (~30%), M1 (~10%) |
| Excretion | <1% of a single dose eliminated as unchanged drug in urine Main metabolites (M1 and M2) eliminated in urine, act as substrates for the OAT3 transporter |
Up to the dose of 200 mg, there is a dose-dependent rise in the plasma Cmax (maximum plasma concentration) and area under curve (AUC) of abrocitinib, indicating proportional escalation. After daily treatment, abrocitinib reaches steady-state plasma concentrations in 48 hours.[7]
Absorption
Abrocitinib has a total oral bioavailability of around 60%. Within an hour, peak plasma concentrations are reached.
Distribution
After intravenous injection, around 100 L is the volume of distribution for abrocitinib.[8] The proportion of free abrocitinib in blood and its metabolites M1 (3-hydroxypropyl) and M2 (2-hydroxypropyl), which are plasma protein bound, is approximately 64%, 37%, and 29%, respectively.[7] Red blood cells and plasma get similar amounts of binding from abrocitinib and its active metabolites, which are mostly bound to albumin.
Metabolism
A large number of cytochrome P (CYP) enzymes play key roles in the metabolism of abrocitinib, including CYP2C19 (~53%), CYP3A4 (~11%), CYP2C9 (~30%), and CYP2B6 (~6%). In a recent study, the major circulating molecule, abrocitinib, created two active polar mono-hydroxylated metabolites, M1 and M2. Metabolite M2 has activity comparable to that of the parent, but metabolite M1 showed lesser activity as compared to abrocitinib. Because of exposure of the unbound parent molecule (~60%), M2 (~30%) and M1 (~10%), in the systemic circulation, abrocitinib has a pharmacologic effect.[7]
Excretion
The main processes via which abrocitinib is cleared are metabolic ones. Typically, abrocitinib and its active metabolites, M1 and M2, have half-lives of 3–5 hours.[7] Less than 1% of a single dosage of radio-labeled abrocitinib is eliminated as unmodified medication in the urine. M1 and M2, two of the metabolites of abrocitinib, are mostly eliminated in urine and act as substrates for the OAT3 transporter.[7]
Efficacy
Abrocitinib in atopic dermatitis
More insights into AD pathogenesis led to the exploration of selective JAK1 inhibition for targeted therapy of AD. Abrocitinib has been developed to be a second-generation selective JAK1 inhibitor to be used in moderate-severe AD, which remains refractory despite available systemic therapies and biologics, including dupilumab. Apart from AD, JAK1 inhibition has been explored off-label across various immune-mediated dermatoses. Table 3 summarizes a few of the important trials of abrocitinib in AD,[9,10,11,12] while Table 4 illustrates the uses of abrocitinib in dermatology beyond AD.[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]
Table 3.
| Study Name | ClinicalTrials.gov Identifier | Randomized Trial Type | Patient Age | Treatment Arms | Treatment Duration | Topical Therapy (medicated) | Topical emollients (Non- medicated) | Primary Endpoint(s) - Skin Clearance | Key secondary Endpoint - Pruritus | Select secondary endpoints | Other Select Efficacy endpoints | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TEEN[9] | NCT03796676 | Phase 3, double-blind, placebo- controlled | Adolescents (12–17 years) | Placebo (n=78) Abrocitinib 100 mg QD (n=158) Abrocitinib 200 mg QD (n=155) | 12 weeks | Required | Required | IGA 0/1 at week 12 | PP-NRS4 at weeks 2, 4, and 12 | PP-NRS4 at weeks 2, 4, and 12 | EASI-90 | □ 12-week time frame limits long-term safety research on abrocitinib in teens. □ Trial lacked power to compare 200-mg and 100-mg abrocitinib dosages. □ Concurrent topical treatment may have influenced dose-response findings. |
| COMPARE[10] | NCT03720470 | Phase 3, double-blind, double- dummy, placebo- controlled | Adults (≥18 years) | Abrocitinib 200 mg QD (n=154) Dupilumab 300 mg Q2W (n=242) Placebo (n=131) | 16 weeks | Required | Required | IGA 0/1 at week 12 | PP-NRS4 at week 2 | EASI-90 | • Not designed to show abrocitinib’s superiority over dupilumab on primary endpoints. • Inferences on secondary endpoints limited due to lack of confidence interval adjustment for multiple comparisons. • Bias against placebo due to handling of missing responses for withdrawn patients. • Trial included only adults, limiting generalizability to other age groups. | |
| MONO-1[11] | NCT03349060 | Phase 3, double-blind, placebo- controlled | Adolescents and adults (≥12 years) | Placebo (n=77) Abrocitinib 200 mg QD (n=154) | 12 weeks | Not permitted | Permitted | IGA 0/1 at week 12 | PP-NRS4 at weeks 2, 4, and 12 | PP-NRS4 at week 12 | EASI-90 | □ Limited by a 12-week treatment period, not addressing long-term efficacy and safety of abrocitinib. □ Did not compare abrocitinib efficacy against current standard care for moderate-to-severe atopic dermatitis. □ Study did not allow concomitant topical therapies, which are common in clinical practice. |
| MONO-2[12] | NCT03575871 | Phase 3, double-blind, placebo- controlled | Adolescents and adults (≥12 years) | Abrocitinib 100 mg QD (n=156) Placebo (n=78) | 12 weeks | Not permitted | Permitted | IGA 0/1 at week 12 | PP-NRS4 at weeks 2, 4, and 12 | PP-NRS4 at week 12 | EASI-90 | □ Short treatment and follow-up periods limited assessment of long-term efficacy and safety of abrocitinib. □ Longer treatment duration might have increased the number of patients achieving IGA and/or EASI-75 responses. □ Small adolescent and nonwhite patient populations limit generalizability of abrocitinib’s applicability. □ Exclusion of patients with platelet dysfunction restricts the applicability of study results in this patient group. |
Table 4.
Summary of uses of abrocitinib in other INDICATIONS (off-label)[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]
| Dermatosis | Study | Duration | Cases/Sex/Age (Years) | Abrocitinib dose | Results | Previous Treatment |
|---|---|---|---|---|---|---|
| Alopecia areata[13] | 2023 Huang et al. | >6 years | 1/M/11 | 100 mg QD | Great improvement after 4 months | Corticosteroids, minoxidil, glycyrrhizic acid, traditional Chinese medicine |
| Alopecia areata after DRESS[14] | 2023: Zhang et al. | >4 months | 1/F/30 | 100 mg QD for 2 months, 200 mg QD for 2 months, 100 mg QD for 2 months | Significant improvement after 6 months | Betamethasone, tofacitinib (5 mg daily for 2 months) |
| Eruptive pruritic papular porokeratosis[15] | 2023 Xia et al. | 60 years | 1/M/75 | 100 mg QD | Completely subsided after 1 month | Ketotifen fumarate, levocetirizine dihydrochloride, ebastine, prednisone acetate, tripterygium wilfordii, cyclosporine A |
| Hailey-Hailey disease[16] | 2023 Li et al. | 12 years | 1/M/41 | 100 mg QD | Evident clinical response after 4 weeks | Corticosteroids, antibiotics |
| Lichen sclerosus[17] | 2023 Bao et al. | 14–52 months | 10/7F, 3M/22-48 | 100 mg QD | All achieved disease control after 12 weeks | Corticosteroids, calcineurin inhibitors |
| Occupational airborne allergic contact dermatitis[18] | 2022 Baltazar et al. | 2 years | 1/M/37 | 100 mg QD | Complete clearance in 8 weeks | Desonide 0.05% cream, mometasone furoate 0.1% cream, dupilumab (300 mg every 2 weeks for 11 months) |
| Prurigo nodularis[19] | 2023 Vander Does et al. | 2 years | 1/F/62 | 100 mg QD | Complete resolution after 2 months | Dupilimab (300 mg every 2 weeks), ruxolitinib 1.5% cream, triamcinolone, crisaborole 2% ointment |
| Netherton syndrome[20] | 2023 Zheng et al. | 20 years | 1/F/28 | 200 mg QD for 1 week, 100 mg QD | Significant improvement after 3 weeks | Emollients, corticosteroids, methotrexate, cyclosporine, secukinumab, dupilumab |
| Oral lichen planus[21] | 2023 Solimani et al. | NR | 1/M/58 | 200 mg QD for 12 weeks 100 mg QD | Good clinical response after 12 weeks | NR |
| Pyoderma gangrenosum[22] | 2023 Chen et al. | >1 years | 1/M/16 | 100 mg QD | Significant improvement after 4 weeks | Doxycycline, isotretinoin, glucocorticoids, cyclosporine A |
| Livedoid vasculopathy[22] | 2023 Chen et al. | >2 years | 1/F/31 | 100 mg QD for 4 weeks, 100 mg every 2 days | Complete remission after 6 weeks | Glucocorticoids, thalidomide, hydroxychloroquine, doxycycline, cyclosporine A, vitamin C |
| Cutaneous lichen amyloidosis[23] | 2023 Bai et al. | 3 years/5 years | 2/F, M/53, 59 | 100 mg QD for 4 months, once 3 days/100 mg QD for 9 weeks, once 2 days | Improved noticeably after 4 months/improved markedly after 8 weeks | Antihistamines, corticosteroids, clobetasol propionate and all-trans retinoic acid/antihistamines, corticosteroid, cryotherapy, UVB |
| Hidradenitis suppurativa[22] | 2023 Chen et al. | 3 years | 1/M/17 | 100 mg QD for 4 weeks, 100 mg once every 2 days | Complete remission after 6 weeks | Glucocorticoids, doxycycline |
| Granulomatous Rosacea[24] | 2023 Ren et al. | 1 month | 1/F/53 | 100 mg QD for 20 weeks | Within a week of starting the medication, the patient’s burning sensations markedly subsided. After 20 weeks of treatment and subsequent follow-ups, she experienced significant improvements in erythema, swelling, and capillary dilatation | |
| Nipple and areola eczema[25] | 2023 Teng et al. | >10 years | 1/M/28 | 100 mg QD for 12 weeks | Remarkable cure and no relapse 20 weeks after stopping treatment | Moisturizers, corticosteroids, calcineurin inhibitors, UVA1 phototherapy |
| Necrobiosis lipoidica[26] | 2023 Arnet et al. | >10 years | 1/F/53 | 200 mg QD for 11 weeks, 100 mg QD | Improvement after 11 months | Steroids, calcineurin inhibitors, psoralen-UVA, fumarate, hydroxychloroquine |
| Non- Segmental Vitiligo[27] | 2024 Satkunanathan et al. | 3 months | 1/M/61 | Abrocitinib 100 mg QD for 2 months | Significant repigmentation, no noted side effects, and no recurrence or progression of vitiligo patches | Tacrolimus 0.1% ointment to be applied twice a day (BID) oral mini-pulse prednisone 10 mg 2 consecutive days per week |
| Mucous Membrane Pemphigoid[28] | 2024 Teng et al. | 6 months | 1/F/62 | Abrocitinib 100 mg QD for 1 month then alternate day for 1 month | Two weeks later, the oral erosions had mostly subsided; 4 weeks later, they had disappeared without any accompanying pain and discomfort and the skin blisters were mostly dry | High-potency topical corticosteroid and topical tacrolimus |
| Perioral Dermatitis[29] | 2023 Teng et al. | 1 year | 1/M/26 | Abrocitinib 100 mg QD for 12 weeks | Rapid alleviation of pruritus and the complete disappearance of skin lesions after 2 weeks | Moisturizers, topical calcineurin inhibitors, oral tetracycline antibiotics (like doxycycline and minocycline), and hydroxychloroquine |
| Dupilumab-associated head and neck dermatitis[30] | 2024 Santosa et al. | 2 51/F 41/M | Abrocitinib 200 mg QD for 4 weeks | Improvement likely attributed to both discontinuation of dupilumab as well as effect of abrocitinib | Mometasone 0.1% cream and protopic 0.1% ointment to the face whilst continued on dupilumab, baricitinib Topical miconazole did not lead to any improvement. Dupilumab was discontinued and he was switched to baricitinib 4 mg | |
| Steroid-induced Rosacea[31] | 2023 Xu et al. | 1–2 years | 4 1–55/F 2,3,4-35/F | Abrocitinib 100 mg QD | After 2 weeks of treatment, the papules and facial erythema had improved significantly in all cases | Hydroxychloroquine, macrolide antibiotics, and a small dose of betamethasone intramuscularly |
| Granuloma Annulare[32] | 2024 Liu et al. | 1 year | 1/29/F | Abrocitinib at 150 mg QD | Two weeks, the patient experienced thinning of the plaques, and after 6 weeks, substantial improvement was observed with no new lesions | Oral cyclosporine (50 mg twice daily) and hydroxychloroquine (0.2 g twice daily) |
| Chronic Actinic Dermatitis[33] | 2023 Jin et al. | 18 years | 1/70/M | Abrocitinib 100 mg QD | Remarkable cleating of hypertrophic lesions at 6 weeks | NR |
| Bullous Pemphigoid[34] | 2024 Jiang et al. | 2 52/F 83/M | Abrocitinib 100 mg QD | Complete resolution | Minocycline, glucocorticoids, cyclophosphamide 60 mg methylprednisolone and cyclosporine | |
| Post-Hyaluronic Acid Filler Reaction[35] | 2024 Lopez et al. | 6 weeks | 1/55/F | Abrocitinib 100 mg QD | Marked improvement in itch and reduction in swelling within 14 days of starting the abrocitinib. At the 2-month follow-up, the edema had resolved with further improvement in some of the nodules as well as pruritus | Cephalexin, amoxicillin, clavulanate, and clarithromycin as well as two separate week-long courses of a methylprednisolone taper |
| Alopecia areata and AD[36] | 2022 Bennett et al. | NR/NR | 2/M, F/33, 46 | 100 mg or 200 mg QD/100 mg or 200 mg QD | Complete remission after 14 weeks/complete remission after 24 weeks | NR/NR |
| Alopecia areata[37] | 2022 Zhao et al. | 3 years | 1/F/14 | 200 mg QD | Completely relieved after 2 years | Steroids, antihistamines, Chinese acupuncture treatments |
| Plasma cell balinitis[38] | 2024: Xiong et al. | 8 months | 1/M/50 | 100 mg QD | Complete remission was achieved within the following 1 month | 0.03% tacrolimus ointment, 0.05% fluticasone propionate ointment, and 0.1% esacridine solution |
| Pityriasis Rubra Pilaris[39] | 2024 Li et al. | 0.5-11 years | 5 (2 F,3M) 27–78 years | 100 mg QD | Following a 12-week course of treatment, almost all patients attained complete clearance of the lesions | One patient had previously been treated with secukinumab and ixekizumab, while another patients received treatment with apremilast or acitretin |
Abrocitinib, tralokinumab, and upadacitinib are positioned for use after systemic immunosuppressants in treating moderate-to-severe AD. These medications are authorized for adults and adolescents aged 12 years and older, except for tralokinumab, which is approved for adults only. They are considered alternatives to dupilumab and baricitinib. Clinical experts generally agree with this positioning due to the established effectiveness and lower costs of systemic immunosuppressants such as methotrexate, although some suggest that upadacitinib and abrocitinib could precede dupilumab and baricitinib. These treatments are likely to be used in combination with topical corticosteroids rather than as monotherapy, reflecting real-world clinical practice.
As a part of JADE REGIMEN (NCT03627767), a phase 3 induction-randomized withdrawal trial of abrocitinib was conducted. Clinical responses in adult and adolescent patients with moderate-to-severe AD were assessed for recovery after flare-ups and maintenance of clinical responses following an initial response to the 200-mg dosage.[40] The group of patients who responded to treatment had a 12-week course of open-label induction monotherapy, followed by a 40-week double-blind treatment. A placebo, 200 mg QD, or 100 mg QD of abrocitinib was administered to them. In an open-label 12-week rescue phase, patients who had flare-ups throughout the 40-week treatment phase were given medicated topical therapy along with 200 mg QD of abrocitinib.
Adult and adolescent patients with moderate-to-severe AD who finished the entire treatment period of a qualifying phase 3 study and who were still eligible to receive abrocitinib could enroll to receive 200 mg or 100 mg of abrocitinib QD in a long-term phase 3 extension trial (JADE EXTEND; NCT03422822).[41] Patients who fulfilled the protocol’s response criteria at week 12 and finished either the 12-week open-label induction stage of JADE REGIMEN or the 12-week open-label rescue therapy phase of that research were also qualified to take part in JADE EXTEND. Patients who took abrocitinib 200 mg and 100 mg for 12 weeks demonstrated improvements of at least 75% in the Eczema Area and Severity Index (EASI), and at least 4 points in the Peak Pruritus Numerical Rating Scale (PPNRS), and improvements of 89.7% and 81.6% in the 200-mg and 100-mg treatment arms, respectively. In the 200-mg and 100-mg arms with abrocitinib therapy in prior dupilumab nonresponder patients, the EASI improved by ≥75% in 80.0% and 67.7%, respectively, and the PPNRS by ≥4 points in 77.3% and 37.8%, respectively.
Abrocitinib versus dupilumab
A phase 3 randomized, double-blind, active-controlled, parallel-treatment clinical study called JADE DARE randomly assigned patients with moderate-to-severe AD to receive either dupilumab 300 mg or abrocitinib 200 mg (every 2 weeks) for a total of 26 weeks. After 2 weeks, the trial demonstrated that abrocitinib was superior to dupilumab in terms of its ability to relieve itching. Furthermore, compared to those on dupilumab, those taking abrocitinib for 4 or 16 weeks showed a higher improvement in the visible skin symptoms of AD such as eczema, lichenification, and xerosis. The number of patients who had side effects while taking abrocitinib was comparable to those of patients receiving dupilumab. The majority of these adverse events were non-serious [Table 5].[42]
Table 5.
Comparative effectiveness and safety profiles of abrocitinib versus dupilumab[42]
| Aspect | Abrocitinib | Dupilumab |
|---|---|---|
| EASI-75 Response | Higher response rates at week 2 (RR 1.92), week 12 (RR 1.14), and end (RR 1.12) | Lower response rates at week 2, 12, and end |
| EASI-90 Response | Higher response rates at week 2 (RR 2.04), week 12 (RR 1.60), and end (RR 1.32) | Lower response rates at week 2, 12, and end |
| IGA Response | Faster response at week 2 (RR 2.57), week 12 (RR 1.39), and end (RR 1.13) | Slower response at week 2, week 12, and end |
| PP-NRS4 Response | Faster itch relief at week 2 (RR 1.87), week 12 (RR 1.10), end and (RR 1.20) | Slower itch relief at week 2, week 12, end |
| Adverse Events | Higher incidence of nausea (RR 6.45), acne (RR 5.21), blood CPK increased (RR 2.27); Lower incidence of conjunctivitis (RR 0.19), nasopharyngitis (RR 0.54) | Lower incidence of nausea, acne; blood CPK increased; higher incidence of conjunctivitis, nasopharyngitis |
Abrocitinib versus tofacitinib
There are no head-on trials comparing abrocitinib with tofacitinib, although the latter is deemed to be a non-selective JAK inhibitor. Theoretically, abrocitinib is known to better inhibit the cytokine profile involved in AD by selective JAK1 inhibition. Table 6 compares the two JAK inhibitors in the context of AD as per available literature.
Table 6.
Differences between tofacitinib and abrocitinib[43]
| Tofacitnib | Abrocitnib | |
|---|---|---|
| Generation | First-generation JAK inhibitor, non-selective | Second-generation JAK inhibitor, selective |
| Mechanism of action | JAK 1/3 inhibitor | JAK 1 inhibitor |
| Approval in AD | Off-label use in AD status in respect to the US FDA | US-FDA-approved in moderate-to-severe AD (year/age group) |
| Laboratory side effects | Neutropenia, lymphocytopenia Liver enzyme elevations Elevations in lipids | Thrombocytopenia, lymphocytopenia Elevations in lipids |
| Primary metabolizing enzyme | CYP3A4 | CYP2C19 and CYP2C9 |
| Primary clearance mechanism | Hepatic metabolism | Urinary excretion |
| Renal impairment | Mild-moderate: no dose adjustment Severe: half the recommended dose | Mild: no dose adjustment Moderate: 50 or 100 mg QD Severe: 50 mg QD |
| Hepatic impairment | Mild (Child-Pugh A): No dose adjustment Moderate (Child-Pugh B): Half the recommended dose Severe: Contraindicated | Mild-moderate (Child-Pugh A/B): No dose adjustment Severe (Child-Pugh C): Contraindicated |
Pre-Treatment Evaluation
Complete blood count (CBC), liver and renal function tests, lipid profile, and viral hepatitis screening are recommended before starting abrocitinib.
Immunizations, including herpes zoster vaccinations, are recommended.
It is important to screen for infections, particularly chronic or recurrent infections and especially tuberculosis, for which yearly screening should be done.
It is recommended to treat prior untreated latent tuberculosis before starting therapy. Concurrent drug history should be taken as abrocitinib is contraindicated in patients on antiplatelet therapies except for low-dose aspirin defined as ≤81 mg per day during the initial 3 months of therapy. Coadministration of abrocitnib with antiplatelet drugs may increase the risk of bleeding with thrombocytopenia.
Recommended Dosage
The available forms of abrocitinib include film-coated 50 mg, 100 mg, and 200 mg tablets. Abrocitinib is initiated as 100 mg orally once daily.
Starting dosage recommendation for teenagers (12–17 years old) weighing 25–59 kg is 100 mg once a day.
The dose may be raised to 200 mg once a day if the patient does not respond appropriately to 100 mg once daily.
Once-daily dosage of 100 mg or 200 mg is the recommended beginning dose for teenagers who weigh at least 59 kg.
For maintenance, the lowest effective dosage needs to be taken into account.
If there is inadequate response after 12 weeks, the dose may be increased to 200 mg once daily and discontinued if no response is obtained after the dosage increase.
Age, sex, body weight, and ethnicity have no clinically significant impact on dosing.
The safety results have been consistent for up to 72 weeks of treatment, and it is suggested that 100 mg once daily can be considered for long-term maintenance treatment of AD.
Patients on initial 200-mg dosing can be maintained on a 100-mg dose after the initial response, with the option of increasing the dose to 200 mg during expected seasonal flares or during disease exacerbations.
-
In case of impairment of renal function, the dose is modified as per the creatinine clearance:
- mild impairment (60–89 mL/min): 100 mg once daily;
- moderate impairment (30–59 mL/min): 50 mg once daily;
- and severe impairment (<15 mL/min or ESRD): abrocitinib is not recommended.[44]
Abrocitinib is not recommended in cases of severe hepatic impairment.[45]
It is not known if abrocitinib is safe and effective in children <12 years of age.
The drug should be avoided in pregnancy and lactation.
Drug Interactions
CYP2C19 inhibitors
Patients on drugs that interfere with CYP2C19 may require dose modifications.
Dosage adjustment:
Dosage for CYP2C19 poor metabolizers is 50 mg once daily, titrating to 100 mg once daily.
Patients using strong CYP2C19 inhibitors should have their dosage reduced to 50 mg once daily. titrating to 100 mg once daily if the response is not sufficient.
Adverse effects
A total of 1623 patients with moderate-to-severe AD participated in four randomized, placebo-controlled studies and one long-term extension study, all of which carefully assessed abrocitinib’s safety profile.[43,46,47,48,49,50,51]
Adverse responses seen in treatment groups in conducted studies were at a rate of ≥1%, which was greater than that of placebo.[46]
Common side effects
Herpes simplex, headaches, nausea, nasopharyngitis, and elevated blood creatinine phosphokinase were among the notable side effects. Both monotherapy and combination studies showed the same safety profile. Furthermore, 5.1% of patients receiving abrocitinib had adverse events that resulted in the termination of the trial.[46] Assessments were conducted on significant adverse cardiovascular events, thrombosis, herpes zoster, serious infections, cancer, and numerous laboratory abnormalities. It should be noted that direct comparisons with other medications could not fully represent real-world rates owing to differences in trial circumstances.
Laboratory Abnormalities: Treatment with abrocitinib is seen to be linked to a higher frequency of lymphopenia and thrombocytopenia. CBC should be monitored after 4 weeks of therapy initiation and after 4 weeks of dose increment. Table 7 summarizes the hematological abnormalities with abroctinib and their management. The elevations in lipid parameters are often dose-related, and it is advised to repeat lipid profile after 4 weeks of therapy. Hyperlipidemia, if any, is managed in accordance with standard clinical recommendations. The STAT1 pathway is essential for the synthesis of cholesterol, and hence, JAK inhibitors are expected to alter lipid levels.[46] Erythropoietin (EPO), thrombopoietin (TPO), and other hematopoietic growth factors such as IL-6 and IL-11 utilize the JAK2 signaling.[47] JAK2 may raise TPO levels and, in theory, this may have an impact on thromboembolic events.[48] Theoretically, JAK1 inhibitors such as abrocitinib should not result in thrombocytopenia, but studies have shown that in the first month of treatment, there is a risk of low-grade thrombocytopenia in patients with low baseline platelet counts.
Table 7.
Suggestions regarding abrocitinib dosing due to hematologic aberrations[52]
| Laboratory Measure | Recommendation |
|---|---|
| Platelet Count <50000/mm3 | Discontinue abrocitinib and follow with CBC until >100,000/mm3 |
| ALC <500/mm3 | Treatment should be temporarily discontinued if ALC is less than 500 cells/mm3 and may be restarted once ALC returns above this value |
| ANC <1000/mm3 | Treatment should be temporarily discontinued if ANC is less than 1000 cells/mm3 and may be restarted once ANC returns above this value |
| Hb value <8 g/dL | Treatment should be temporarily discontinued if Hb is less than 8 g/dL and may be restarted once Hb returns above this value |
Hb=Hemoglobin, ALC=Absolute lymphocyte count, ANC=Absolute neutrophil count
Serious side effects
Serious Infections: The most frequent severe infections were found to be pneumonia, herpes zoster, and herpes simplex in abrocitnib’s clinical research for AD. Abrocitinib should not be used by patients who have significant active infections, including even localized infections. It is necessary to elicit a history pertaining to chronic or recurrent infections, exposure to tuberculosis, serious or opportunistic infections, immunodeficient states, or history of travel to areas endemic for mycoses or tuberculosis.[43] It is essential to be watchful for infection symptoms and indicators both during and after treatment. In the event of a significant or opportunistic infection, stopping abrocitinib is advised.
Mortality: According to a post-marketing safety analysis, individuals treated with another JAK inhibitor for rheumatoid arthritis who were 50 years of age or older and had at least one cardiovascular risk factor also had greater all-cause mortality, including sudden cardiovascular death.[48] Abrocitinib was associated with two fatal cardiovascular events: one cardio-respiratory arrest after treatment discontinuation in the JADE-Mono 2 study, and one death from a hemorrhagic stroke during treatment in the JADE-DARE trial. There were two other cases of myocardial infarction and two transient ischemic attacks (TIAs) related to abrocitinib in the integrated safety analysis study. However, when compared with the placebo group, these were non-significant.[49]
Malignancy and Lymphoproliferative Disorders: Clinical trials linked the use of abrocitinib treating AD to malignancies, particularly non-melanoma skin cancer (NMSC).[43] For individuals who are more susceptible to skin cancer, regular skin examination is advised. Abrocitinib has been linked to lymphomas, especially among smokers. Risk-benefit ratios need to be weighed carefully in such patients.
Major Adverse Cardiovascular Events: Clinical trials on abrocitinib demonstrated serious adverse cardiovascular events. One should evaluate each patient’s unique risks, especially if they smoke or have smoked in the past and have cardiovascular risk factors.[49] It is crucial to educate patients about the signs of cardiovascular events, and for individuals who are having a myocardial infarction or stroke, cessation is advised.
Thrombosis Clinical research linked the usage of abrocitinib to pulmonary embolism (PE) and deep vein thrombosis (DVT).[50] When treating patients who have a higher risk of thrombosis, abrocitinib should be avoided and stopped if symptoms appear. All clinical studies, including the long-term extension study that used abrocitinib 200 mg treatment, reported three cases of pulmonary embolism (0.4 per 100 patient-years). Among patients treated with abrocitinib 200 mg, two cases (0.3 per 100 patient-years) had DVT documented. In patients receiving 100 mg of abrocitinib, there was no thrombosis.[2]
Black box warning for major adverse cardiovascular events (MACE), thrombosis, cancer, infections, and death is present with abrocitinib. Patients who have smoked in the past or now are more vulnerable. Abrocitinib should be stopped in individuals who have had a stroke or myocardial infarction. Table 8 summarizes the side effects and contraindications.[51,52]
Table 8.
Summary of side effects and contraindications
| Parameter | Abrocitinib |
|---|---|
| Common side effects | Herpes simplex, headaches, nausea, nasopharyngitis, elevated blood creatinine phosphokinase |
| Serious side effects | MACE, thrombosis, serious infections, cancer |
| Laboratory abnormalities | Lymphopenia, thrombocytopenia, elevated lipid parameters |
| Risk factors | Smoking, cardiovascular history |
| Monitoring requirements | CBC and lipid profile after 4 weeks |
| Contraindications | Active infections, significant hepatic impairment, pregnancy, breastfeeding, hypersensitivity |
Contraindications
Contraindications include hypersensitivity to the active substance or any excipients (lactose monohydrate and sodium), severe systemic infections such as tuberculosis (TB), significant hepatic impairment, and pregnancy or breastfeeding. Furthermore, contraindications extend to patients undergoing antiplatelet therapy during the first 3 months of treatment.
Conclusion
Abrocitinib promises an addition to the armamentarium of moderate-severe AD by selective JAK1 inhibition and downstream inhibition of Th2 cytokines. The available literature seems promising as it is shown to be efficacious and safe as well as approved in moderate-to-severe AD. Theoretically, the drug holds good potential, but the future implications will rely on its practical, real-time use in Indian AD patients. The feasibility of its use in the Indian scenario, along with its efficacy and safety in larger cohorts, is for time to tell. Its cost-effectiveness and long-term safety profile in AD and other emerging indications will govern the future of its placement versus other Jak inhibitors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pfizer. U. S. FDA Approves Pfizer's CIBINQO®(abrocitinib) for Adults with Moderate-to-Severe Atopic Dermatitis. [[Last accessed on 2024 Apr 28]]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults .
- 2.Cibinqo. European Medicines Agency. [[Last accessed on 2024 Apr 28]]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo .
- 3.Pfizer. UK's MHRA Grants Marketing Authorisation for Pfizer's CIBINQO®(abrocitinib) for adults and adolescents with moderate to severe atopic dermatitis. [[Last accessed on 2024 Apr 28]]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/uks-mhra-grants-marketing-authorisation-pfizers-cibinqor .
- 4.Pfizer. Japan's MHLW Approves Pfizer's CIBINQO®(abrocitinib) for Adults and adolescents with moderate to severe atopic dermatitis. [[Last accessed on 2024 Apr 28]]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/japans-mhlw-approves-pfizers-cibinqor-abrocitinib-adults .
- 5.Tsiogka A, Kyriazopoulou M, Kontochristopoulos G, Nicolaidou E, Stratigos A, Rigopoulos D, et al. The JAK/STAT pathway and its selective inhibition in the treatment of atopic dermatitis: A systematic review. J Clin Med. 2022;11:4431. doi: 10.3390/jcm11154431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guttman-Yassky E, Facheris P, Gomez-Arias PJ, Del Duca E, Da Rosa JC, Weidinger S, et al. Effect of abrocitinib on skin biomarkers in patients with moderate-to-severe atopic dermatitis. Allergy. 2024;79:1258–70. doi: 10.1111/all.15969. [DOI] [PubMed] [Google Scholar]
- 7.Bauman JN, Doran AC, King-Ahmad A, Sharma R, Walker GS, Lin J, et al. The pharmacokinetics, metabolism, and clearance mechanisms of abrocitinib, a selective janus kinase inhibitor, in humans. Drug Metab Dispos Biol Fate Chem. 2022;50:1106–18. doi: 10.1124/dmd.122.000829. [DOI] [PubMed] [Google Scholar]
- 8.Wojciechowski J, Malhotra BK, Wang X, Fostvedt L, Valdez H, Nicholas T. Population pharmacokinetics of abrocitinib in healthy individuals and patients with psoriasis or atopic dermatitis. Clin Pharmacokinet. 2022;61:709–23. doi: 10.1007/s40262-021-01104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichenfield LF, Flohr C, Sidbury R, Siegfried E, Szalai Z, Galus R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: The JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157:1165–73. doi: 10.1001/jamadermatol.2021.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101–12. doi: 10.1056/NEJMoa2019380. [DOI] [PubMed] [Google Scholar]
- 11.Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255–66. doi: 10.1016/S0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020;156:863–73. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Liu O. Effective treatment of refractory alopecia areata in pediatric patients with oral abrocitinib. J Cosmet Dermatol. 2024;23:348–9. doi: 10.1111/jocd.15896. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Zuo YG. Successful treatment of alopecia universalis with abrocitinib: A case report. J Dermatol Treat. 2023;34:2242706. doi: 10.1080/09546634.2023.2242706. [DOI] [PubMed] [Google Scholar]
- 15.Xia J, Jiang G. A report of eruptive pruritic papular porokeratosis treated with abrocitinib. Clin Cosmet Investig Dermatol. 2023;16:2223–7. doi: 10.2147/CCID.S424310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Jiang Y, Sun J. Improvement of Hailey–Hailey disease with abrocitinib. Clin Exp Dermatol. 2023;48:532–3. doi: 10.1093/ced/llad023. [DOI] [PubMed] [Google Scholar]
- 17.Bao C, Xu Q, Xiao Z, Wang H, Luo R, Cheng B, et al. Abrocitinib as a novel treatment for lichen sclerosus. Br J Dermatol. 2023;189:136–8. doi: 10.1093/bjd/ljad129. [DOI] [PubMed] [Google Scholar]
- 18.Baltazar D, Shinamoto SR, Hamann CP, Hamann D, Hamann CR. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: A case report. Contact Dermatitis. 2022;87:542–4. doi: 10.1111/cod.14204. [DOI] [PubMed] [Google Scholar]
- 19.Vander Does A, Yosipovitch G. Failure of dupilimab with severe prurigo nodularis that responded well to abrocitinib. Dermatitis. 2023;34:567. doi: 10.1089/derm.2022.0065. [DOI] [PubMed] [Google Scholar]
- 20.Zheng CC, Chen XX, Zou RT, Cai GY, Chen RY. Treatment of Netherton syndrome with abrocitinib. JAMA Dermatol. 2023;159:791–3. doi: 10.1001/jamadermatol.2023.0561. [DOI] [PubMed] [Google Scholar]
- 21.Solimani F, Mesas-Fernández A, Dilling A, Nast A, Hilke FJ, Ghoreschi FC, et al. The Janus kinase 1 inhibitor abrocitinib for the treatment of oral lichen planus. J Eur Acad Dermatol Venereol. 2023:37. doi: 10.1111/jdv.19069. [DOI] [PubMed] [Google Scholar]
- 22.Chen P, Liang J, Li C, Li Q, Liu W, Zhu J, et al. Abrocitinib as a novel treatment for multiple skin disorders:3 case reports and a scoping review. Clin Cosmet Investig Dermatol. 2024;17:35–40. doi: 10.2147/CCID.S446369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai J, Su W, Fang H, Qiao J. Treatment of primary cutaneous lichenoid amyloidosis with abrocitinib: A pilot study in two cases. Int J Dermatol. 2023;62:e480–3. doi: 10.1111/ijd.16698. [DOI] [PubMed] [Google Scholar]
- 24.Ren M, Yang X, Teng Y, Lu W, Ding Y, Tao X. Successful treatment of granulomatous rosacea by JAK inhibitor abrocitinib: A case report. Clin Cosmet Investig Dermatol. 2023;16:3369–74. doi: 10.2147/CCID.S440138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng Y, Tang H, Yu Y, Fan Y, Tao X, Xu D. Successful treatment of atopic dermatitis with a predominant nipple involvement by abrocitinib during COVID-19 pandemic: A Case Report. J Asthma Allergy. 2023;16:789–92. doi: 10.2147/JAA.S422836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnet L, Erfurt-Berge C. Effect of abrocitinib in a patient with extensive necrobiosis lipoidica. J Eur Acad Dermatol Venereol. 2023;37:e1208–10. doi: 10.1111/jdv.19189. [DOI] [PubMed] [Google Scholar]
- 27.Satkunanathan S, Boshra M, Chang J, Bose R. Rapid resolution of non-segmental vitiligo in a patient treated with abrocitinib: A case report. SAGE Open Med Case Rep. 2024;12:2050313X241231527. doi: 10.1177/2050313X241231527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng Y, Ren M, Yang X, Lu W, Tao X. Real-time experience of abrocitinib for the treatment of mucous membrane pemphigoid: A case report. Patient Prefer Adherence. 2024;18:503–6. doi: 10.2147/PPA.S451007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teng Y, Ren M, Ding Y, Yang X, Fan Y, Tao X. A case of perioral dermatitis successfully treated with abrocitinib. Clin Cosmet Investig Dermatol. 2023;16:3035–8. doi: 10.2147/CCID.S433561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santosa A, Yew YW. Dupilumab-associated head and neck dermatitis: Rapid response with abrocitinib treatment. Skin Health Dis. 2024;4:e312. doi: 10.1002/ski2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu B, Xu Z, Ye S, Sun H, Zhao B, Wu N, et al. JAK1 inhibitor abrocitinib for the treatment of steroid-induced rosacea:case series. Front Med. 2023;10:1239869. doi: 10.3389/fmed.2023.1239869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W, Chen W, Tian X, Yu Y, Zhu J, Liang J, et al. Oral abrocitinib in the treatment of granuloma annulare: A case report. J Dermatol Treat. 2024;35:2313090. doi: 10.1080/09546634.2024.2313090. [DOI] [PubMed] [Google Scholar]
- 33.Jin X, Qiao J. Effectiveness of abrocitinib in a patient with chronic actinic dermatitis. Am J Ther. 2024;31:e463–4. doi: 10.1097/MJT.0000000000001671. [DOI] [PubMed] [Google Scholar]
- 34.Jiang W, Ma X, Guo T, Song M, Zhang J. Abrocitinib-A promising option for patients with refractory bullous pemphigoid. J Eur Acad Dermatol Venereol. 2024;38:e119–21. doi: 10.1111/jdv.19475. [DOI] [PubMed] [Google Scholar]
- 35.Lopez MHP, Guenin SH, Laborada J, Lebwohl MG. Post-hyaluronic acid filler reaction treated with abrocitinib: A case report. J Drugs Dermatol. 2024;23:1355–6. doi: 10.36849/JDD.7271. [DOI] [PubMed] [Google Scholar]
- 36.Bennett M, Moussa A, Sinclair R. Successful treatment of chronic severe alopecia areata with abrocitinib. Australas J Dermatol. 2022;63:274–6. doi: 10.1111/ajd.13836. [DOI] [PubMed] [Google Scholar]
- 37.Zhao J, Liu L. A case of atopic dermatitis with alopecia universalis in a patient treated with abrocitinib. JAAD Case Rep. 2022;22:99–100. doi: 10.1016/j.jdcr.2022.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong X, Chen R, Wang L, Huang N, Huang L, Wang C, et al. Treatment of plasma cell balanitis associated with male genital lichen sclerosus using abrocitinib. JAAD Case Rep. 2024;46:85–8. doi: 10.1016/j.jdcr.2024.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Zhong X, Huang D, Shi Y, Ding Y. Efficacy and safety of oral abrocitinib monotherapy in pityriasis rubra pilaris. J Eur Acad Dermatol Venereol. 2024 Mar 30; doi: 10.1111/jdv.19992. doi:10.1111/jdv.19992. Epub ahead of print. PMID:38553894. [DOI] [PubMed] [Google Scholar]
- 40.Gooderham MJ, Pink AE, Simpson EL, Silverberg JI, Güler E, Watkins M. Abrocitinib 100 mg once daily for moderate-to-severe atopic dermatitis: A review of efficacy and safety, and expert opinion on use in clinical practice. Dermatol Ther. 2023;13:1893–907. doi: 10.1007/s13555-023-00948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi VY, Bhutani T, Fonacier L, Deleuran M, Shumack S, Valdez H, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND) J Am Acad Dermatol. 2022;87:351–8. doi: 10.1016/j.jaad.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Reich K, Thyssen JP, Blauvelt A, Eyerich K, Soong W, Rice ZP, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: A randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400:273–82. doi: 10.1016/S0140-6736(22)01199-0. [DOI] [PubMed] [Google Scholar]
- 43.Yoon S, Kim K, Shin K, Kim HS, Kim B, Kim MB, et al. The safety of systemic Janus kinase inhibitors in atopic dermatitis: A systematic review and meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2024;38:52–61. doi: 10.1111/jdv.19426. [DOI] [PubMed] [Google Scholar]
- 44.Wang EQ, Le V, Winton JA, Tripathy S, Raje S, Wang L, et al. Effects of renal impairment on the pharmacokinetics of abrocitinib and its metabolites. J Clin Pharmacol. 2022;62:505–19. doi: 10.1002/jcph.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang EQ, Le V, O’Gorman M, Tripathy S, Dowty ME, Wang L, et al. Effects of hepatic impairment on the pharmacokinetics of abrocitinib and its metabolites. J Clin Pharmacol. 2021;61:1311–23. doi: 10.1002/jcph.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke B, Yates M, Adas M, Bechman K, Galloway J. The safety of JAK-1 inhibitors. Rheumatology (Oxford) 2021;60:ii24–30. doi: 10.1093/rheumatology/keaa895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajasimhan S, Pamuk O, Katz JD. Safety of janus kinase inhibitors in older patients: A focus on the thromboembolic risk. Drugs Aging. 2020;37:551–8. doi: 10.1007/s40266-020-00775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elmariah SB, Smith JS, Merola JF. JAK in the [Black] Box: A dermatology perspective on systemic JAK inhibitor safety. Am J Clin Dermatol. 2022;23:427–31. doi: 10.1007/s40257-022-00701-3. [DOI] [PubMed] [Google Scholar]
- 49.Ertus C, Scailteux LM, Lescoat A, Berthe P, Auffret V, Dupuy A, et al. Major adverse cardiovascular events in patients with atopic dermatitis treated with oral Janus kinase inhibitors: A systematic review and meta-analysis. Br J Dermatol. 2023;189:368–80. doi: 10.1093/bjd/ljad229. [DOI] [PubMed] [Google Scholar]
- 50.Berthe P, Scailteux LM, Lescoat A, Staumont D, Coiffier G, Guéret P, et al. Oral Janus kinase inhibitors and venous thromboembolic events in atopic dermatitis: Protocols for a case-time control study and a nested case-control study based on the French national health insurance (SNDS) cohort. BMJ Open. 2022;12:e059979. doi: 10.1136/bmjopen-2021-059979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simpson EL, Silverberg JI, Nosbaum A, Winthrop KL, Guttman-Yassky E, Hoffmeister KM, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and Phase III clinical trial program. Am J Clin Dermatol. 2021;22:693–707. doi: 10.1007/s40257-021-00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.CIBINQO™ (abrocitinib). A Once-Daily Pill. Safety Info. [[Last accessed on 2024 Apr 14]]. Available from: https://www.cibinqo.com/