Dear Editor,
Hibernomas are extremely rare and benign soft tissue tumors derived from fetal brown fat. The name hibernoma was proposed by Gery in 1914 because of its similarity to brown fat in hibernating animals.[1] Hibernomas mainly occur in adults, are slightly predominant in women, and are commonly seen in the subcutaneous regions of the back, especially the periscapular and interscapular areas, neck, axillae, shoulders, thighs, and retro-peritoneum.[2] The typical age at presentation is between 30 and 50 years. A hibernoma usually presents as a slowly growing painless soft-tissue mass. They may be single or multiple. The treatment consists of complete surgical resection, and the post-operative prognosis is good. A 24-year-old male, a known case of sputum positive pulmonary tuberculosis on anti-tubercular drugs [isoniazid (H), rifampicin (R), pyrazinamide (Z), and ethambutol (E)] for 2 months, presented with complaints of multiple painless slow-growing masses on his upper chest and bilateral arms of 2 weeks duration. The patient first noticed a painless nodule on the right side of his chest while bathing. Two weeks later, he noticed similar nodules over the other parts of his chest, upper back, and upper arms. The number of nodules increased in size as well as extent till he presented to us. However, there was no history of fever, weight loss, rash, mucosal lesions, or any history suggestive of tender lymphadenopathy. The general physical and systemic examinations were within normal limits. Dermatological examination revealed multiple non-tender subcutaneous, rubbery nodules palpated under the affected areas. The largest nodule measured approximately 4 cm in its maximum diameter on the right upper chest. However, the nodules were not appreciated on inspection [Figure 1a]. Histopathology from the largest nodule revealed focal aggregates of hibernoma-like cells which were large and polygonal, with an abundant multi-vacuolated cytoplasm and centrally placed small, round to oval scalloped nuclei with a moderate degree of surrounding inflammatory infiltrates [Figure 1b]. PET-CT scan showed generalized mildly F-fluorodeoxyglucose (FDG)-avid subcutaneous nodules involving nearly the entire torso and part of bilateral upper and lower extremities till the level of bilateral thighs with a maximum standardized uptake value (SUV) of 2.65 in the right upper chest [Figures 2a-d]. Cytogenetic analysis was normal [Figure 3]. Based on the histopathology as well as PET-CT findings, the patient was diagnosed as a case of hibernoma. Since the hibernoma involved extensive areas of the trunk as well as proximal extremities without any complications, a wait-and-watch approach was opted for. The patient was shifted from an intensive phase of ATT (HRZE) to a continuation phase (HRE) after 2 months of ATT. The nodules started to regress on their own after a month of stopping pyrazinamide. All the lesions resolved after 4 months of stopping pyrazinamide. As per Naranjo adverse drug reaction probability score, it was classified as ‘Possible’ due to pyrazinamide.[3] The patient is under follow-up, and there has been no relapse of such nodules.
Figure 1.
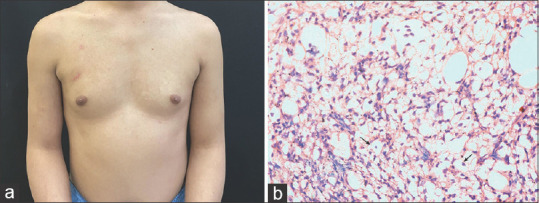
(a) Multiple subcutaneous rubbery nodules over chest and upper arms. (b) histopathological image showing focal aggregates of hibernoma-like cells in the subcutaneous tissue, which are large, polygonal cells with an abundant multi-vacuolated cytoplasm and centrally placed small, round to oval scalloped nuclei. A moderate degree of surrounding lymphomononuclear inflammatory infiltrate is seen (H & E, 40x)
Figure 2.
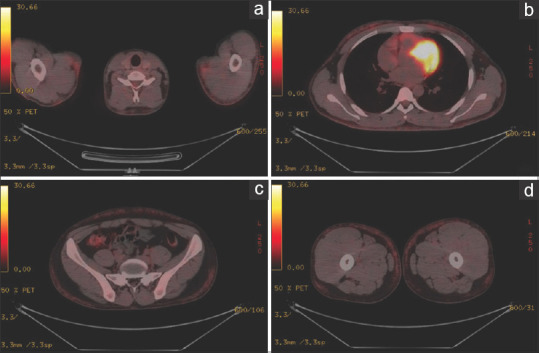
(a-d) PET-CT scan images showing generalized mildly FDG-avid subcutaneous nodules involving nearly the entire torso and part of bilateral upper and lower extremities till the level of bilateral thighs
Figure 3.
Cytogenetic analysis, normal for the proband
Hibernomas are rare, non-cancerous tumors that develop in the soft tissues and are composed of brown fat. They are named after their resemblance to the brown fat found in hibernating animals. The brown adipose tissue is believed to play a role in regulating body temperature. These tumors typically arise from remnants of brown fetal fat that persist beyond birth, commonly occurring in areas such as the neck, armpit, back, and chest.[4] Hibernomas grow slowly and typically present as painless lumps under the skin. Grossly, they appear as fatty, well-vascularized masses resembling lipomas, with colors ranging from tan to red-brown depending on the amount of fat present. The tumor size of hibernomas may range from 1 to greater than 20 cm with an average diameter of 9.3 cm.[5] Symptoms related to compression of nearby structures are uncommon. Hibernomas are typically depicted as heterogeneous masses with significant contrast enhancement on CT and MRI scans. They appear as well-defined masses with intermediate signal intensity between subcutaneous fat and muscle, enhancing after the administration of contrast. Microscopically, hibernomas display cells at various stages of development, including multi-vacuolar adipocytes and brown fat cells with a granular cytoplasm, interspersed with univacuolar adipocytes. The tumor’s characteristic color is attributed to their abundant mitochondria and high vascularity. There are four histologic variants of hibernomas (typical, myxoid, lipoma-like, and spindle cell), all of which have a benign course. PET-CT scan can demonstrate FDG-avid subcutaneous nodules.[6] Characteristic cytogenetic abnormalities described in hibernoma include structural rearrangements of 11q13 and 11q21.[7] The primary treatment for hibernomas is complete surgical removal, and local recurrence is rare. There have been no reports of metastasis or malignant transformation. Drugs as a cause of hibernomas have been demonstrated in animal studies.[8,9] Our case is unique and rare as no cases of drug-induced hibernomas in humans have been reported in the literature.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We sincerely thank the patient who allowed us to share his information by providing consent.
References
- 1.Klevos G, Jose J, Pretell-Mazzini J, Conway S. Hibernoma. Am J Orthop (Belle Mead NJ) 2015;44:284–7. [PubMed] [Google Scholar]
- 2.Pol FJ, Flucke U, Jafari K. [Intrathoracic hibernoma. Ned Tijdschr Geneeskd. 2014;158:A6610. [PubMed] [Google Scholar]
- 3.Shukla AK, Jhaj R, Misra S, Ahmed SN, Nanda M, Chaudhary D. Agreement between WHO-UMC causality scale and the Naranjo algorithm for causality assessment of adverse drug reactions. J Family Med Prim Care. 2021;10:3303–8. doi: 10.4103/jfmpc.jfmpc_831_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valejo Coelho MM, João A, Fernandes C. Hibernoma: Case report of a rare lipomatous tumor. An Bras Dermatol. 2019;94:626–8. doi: 10.1016/j.abd.2019.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pothen L, D’Abadie P, Kozyreff A, Mourin A, Coubeau L. Hibernoma mimicking liposarcoma. Lancet. 2018;392:244. doi: 10.1016/S0140-6736(18)31436-3. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Ogura K, Fujiwara T, Nagano A, Numoto K, Terauchi T, et al. The values and limitations of FDG-PET/CT for diagnosis of hibernoma. Case Rep Orthop 2015. 2015 doi: 10.1155/2015/958690. 958690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dadone B, Refae S, Lemarié-Delaunay C, Bianchini L, Pedeutour F. Molecular cytogenetics of pediatric adipocytic tumors. Cancer Genet. 2015;208:469–81. doi: 10.1016/j.cancergen.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Radi Z, Bartholomew P, Elwell M, Vogel WM. Comparative pathophysiology, toxicology, and human cancer risk assessment of pharmaceutical-induced hibernoma. Toxicol Appl Pharmacol. 2013;273:456–63. doi: 10.1016/j.taap.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Radi ZA, Vogel WM, Bartholomew PM, Koza-Taylor P, Papanikolaou A, Wisialowski T, et al. Cellular and functional actions of tofacitinib related to the pathophysiology of hibernoma development. Regul Toxicol Pharmacol. 2017;91:93–102. doi: 10.1016/j.yrtph.2017.10.020. [DOI] [PubMed] [Google Scholar]


