Abstract
Background: Food chemical antioxidants have demonstrated protective effects against reactive oxygen species and free radicals, but present in excess, harmful consequences might occur on health. Therefore, replacing these synthetic additives with nontoxic natural antioxidants is crucial.
Objective: The current study examined aroma profile, antioxidant activity, and multivariate analysis of Mentha piperita, Mentha pulegium, Thymus serpyllum, and Thymus zygis essential oils from Morocco.
Methods: GC-MS analysis was carried out to determine the chemical composition of the four oils, and their antioxidant activity was evaluated with 2,2-diphenyl-1-picrylhydrazyl (DPPH), cation radical (ABTS+), hydrogen peroxide scavenging capacity (H2O2), ferric reducing antioxidant power (FRAP), and total antioxidant capacity (TAC) methods.
Results: Isomintlactone (35.55%), pulegone (74.04%), borneol (37.87%), and borneol (30.99%) were the most abundant compounds of M. piperita, M. pulegium, T. serpyllum, and T. zygis EOs. The antioxidant activity of the four EOs was particularly notable, with an IC50 varying between 3.51 ± 0.22 mg/mL and 0.49 ± 0.08 mg/mL by the DPPH method, 1.02 ± 0.21 mg/mL and 0.4 ± 0.7 mg/mL by the ABTS method, and 0.063 ± 0.01 mg/mL and 0.009 ± 0.008 mg/mL by the H2O2 method. For the FRAP technique, the EC50 was between 0.42 ± 0.02 mg/mL and 0.09 ± 0.01 mg/mL. Finally, the equivalent concentration of ascorbic acid ranged between 10.42 ± 0.03 mg AAs/mL for M. piperita and 7.25 ± 0.19 mg AAs/mL for T. serpyllum. As determined by multivariate analysis, antioxidant activities through the DPPH, ABTS, TAC, and FRAP were mainly influenced the major compounds of M. pulegium and M. piperita EOs. However, the H2O2 method showed a stronger positive correlation with major compounds of T. zygis EO.
Conclusion: The EOs derived from M. piperita, M. pulegium, T. serpyllum, and T. zygis species might be exploited as a natural source for antioxidant activity.
Keywords: ABTS+, DPPH, food antioxidants, FRAP, H2O2, Mentha species, TAC, Thymus species
1. Introduction
Thanks to the use of various food additives during the last few years, the agricultural and food industries have undergone a significant revolution. While higher standards for sanitary and salubrity have been mandated, consumers are becoming more demanding when it comes to the variety and choice of agricultural and food products [1].
Food antioxidants are chemical compounds that are frequently manufactured. Although they have been shown to defend against free radicals and reactive oxygen species (ROS), they may also have negative impacts on consumer health [2]. Note that, a free radical is known as a chemical entity that possesses one or more unpaired electrons produced in the body during metabolism. When present in excess, harmful consequences might occur [3].
The inactivation of ROS or prevention of their cellular production by antioxidants and free radical scavengers is seen as a viable strategy for lowering the risk of diseases like cancers, cardiovascular disease, inflammations, and the aging process [4–6]. For example, sodium benzoate can cause skin reactions (eczema) and respiratory problems, and butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ) are carcinogens while having strong antioxidant properties [7]. Therefore, replacing these synthetic additives with safe and nontoxic natural antioxidants is crucial.
Essential oils (EOs) are delicate, fragrant, and volatile liquids derived from diverse plant sections as secondary metabolites. Secondary metabolites perform critical ecological and biological functions, as well as being crucial for plant defense due to their antioxidative characteristics [8]. For ages, EOs have been widely used in the medicinal, agricultural, sanitary, and cosmetic sectors, as well as in cuisine as spices or herbs [9].
On the other hand, EOs have a high potential for application as food preservatives due to their ability to minimize oxidative reactions that occur during food handling, processing, and storage [10]. Furthermore, natural antioxidants such as phenolic compounds, flavonoids, and other phytochemicals can operate as free radical scavengers [11]. They also help to postpone lipid oxidation and increase consumer acceptability of food products [12]. Many studies have found that eating foods containing flavonoids on a daily basis may reduce the risk of some malignancies, such as colon, pancreatic, and breast cancer [13].
Among the most economically significant genera having aromatic and medicinal properties, the Thymus and the Mentha species belonging to the Lamiaceae family [14] are the most extensively utilized in terms of health and medicinal purposes, mostly because of menthol, menthone, thymol, and carvacrol [15].
The objective of this study was to analyze the aroma profile and antioxidant activity using five different methods, of two Mentha species (Mentha piperita and Mentha pulegium) and two Thymus species (Thymus serpyllum and Thymus zygis) EOs from Morocco. The correlation between the antioxidant activity and the major components of the four oils was also examined using a multivariate analysis.
To the best of our knowledge, this research is the first of its kind to assess these EOs in such detail. It not only evaluates their antioxidant activities using the five methods mentioned but also delves into the correlations between antioxidant activity and their key components. The findings of this study may represent an important step toward investigating the potential applications of these EOs as antioxidants in food products.
2. Materials and Methods
2.1. Plant Material
The M. piperita, M. pulegium, T. serpyllum, and T. zygis plants were cultivated and collected from Timezgana commune located in Taounate Province, North Center of Morocco (34°33′ 02.7″ N 4°40′ 49.3″ W) in April and May 2021. These species were identified by Professor Badr Satrani, botanist at the Forestry Research Center—Rabat (FRC-Rabat), Morocco. Each plant's voucher specimen was placed at the herbarium of Morocco's National Agency of Medicinal and Aromatic Plants.
2.2. Extraction of the EOs
All of aerial parts of the plants (with the branches) were dried under shade at ambient temperature at an average of 35 ± 4°C) by dispersing the herbs on paper for 72 h (shade drying).
Hydrodistillation of each plant's aerial parts (stems, leaves, and flowers) was carried out using a Clevenger-type apparatus for 2 h and 30 min. The EOs were then kept in amber-colored vials at 4°C until usage.
2.3. Yield Calculation
The EO yield was calculated in relation to the dry matter of the plant (calculated after putting a certain amount of the plant in the pond, evaporation of the water, and stabilization of the plant's weight) using the following equation [16]:
| (1) |
where mEO = mass of EO (g), mD = mass of dry plant material (g), and Y = yield of EO (%).
2.4. The Identification of EO Chemical Compositions
The components of M. piperita, M. pulegium, T. serpyllum, and T. zygis EOs were identified using a Shimadzu gas chromatography (Trace GC Ultra, Hewlett-Packard, HP 6890) coupled with a mass spectrometry (MS, HP 5973) (Tokyo, Japan) supplied with HP-5MS column (60 m × 0.32 mm × 0.25 μm). The following requirements were made for the GC-MS analysis: At a flow rate of 1.0 mL/min, helium was employed as the carrier gas; the column temperature was planned to rise from 40°C to 280°C at a rate of 5°C/min; and at a temperature of 220°C, a sample volume of 1 μL was injected in a split mode. Before attempting to inject the sampling port, the samples of EOs were diluted (1/20 v/v) in hexane. Quantitative analysis was performed by HP ChemStation software, and the identification of the components based on their Kovats retention index (KI) calculated against n-alkanes (C8-C23) series was verified by comparing their mass spectral fractionation with NIST MS Search database 2012 and by Adams terpenes library [17].
2.5. Antioxidant Activity
2.5.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
The free radical scavenging activity of the two EOs was evaluated by assessing their capacity to scavenge DPPH stable radicals. The DPPH test was carried out accurately as specified by [18] with a few modifications. The DPPH solution was prepared by dissolving 0.005 g of DPPH in 200 mL of absolute ethanol. Then, 25 μL of the sample at various concentrations was mixed with 825 μL of DPPH. After being agitated, the tubes were placed in the dark at room temperature for 1 h.
The reading was performed using an absorbance measurement at 517 nm. Moreover, the results obtained for each tested EO were compared to those obtained for BHT, which was used as a control. The antiradical activity was calculated using the following equation:
| (2) |
where Abs control is the absorbance of the control (including all reagents except the EOs for testing) and Abs sample is the absorbance of tested EOs.
IC50 values, which reflected the EO concentration that triggered 50% scavenging, were determined graphically from a plot of I (%) vs concentration.
2.5.2. The Cation Radical 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Assay
The ability of an antioxidant agent to inhibit the radical cation ABTS was determined using a modified version of the method described by [19]. This method is based on transforming a stable radical cation, ABTS+ into ABTS in the presence of antioxidants.
The radical cation ABTS+ was regenerated by reacting an ABTS (7 mM) solution with 2.5 mM of potassium persulfate, and the mixture is kept in the dark at room temperature for 16 h before use. The mixture was then diluted with ethanol to provide a spectrophotometric absorbance of 0.70–734 nm. Subsequently, 50 μL of the sample at various concentrations was mixed with 825 μL of ABTS+.
After 6 min, the absorbance was measured at 734 nm, and the results for each tested EO were compared to those obtained for gallic acid, which was used as a control. The antiradical activity was calculated using the following equation:
| (3) |
The IC50 values were determined graphically using linear regression.
2.5.3. Hydrogen Peroxide Scavenging Capacity (H2O2) Assay
The capacity of the four EOs to scavenge hydrogen peroxide was assessed using the technique of [20] with slight modifications. In phosphate buffer (50 mM, pH = 7.4), a solution of hydrogen peroxide (40 mM) was produced. A total of 100 μL of each EO concentration was mixed with a hydrogen peroxide solution (0.6 mL and 40 mM), one mL of phosphate buffer (50 mM, pH = 7.4), and 1650 μL of distilled water. After 20 min, the absorbance of hydrogen peroxide at 230 nm was measured in comparison with a blank solution containing the phosphate buffer but no hydrogen peroxide.
The hydrogen peroxide inhibition percentage was calculated as follows:
| (4) |
IC50 values were determined graphically from a plot of % scavenging vs concentration.
2.5.4. Ferric Reducing Antioxidant Power (FRAP) Assay
The ability of an antioxidant compound to reduce iron was determined using the method described by [21], with some modifications. This method is based on the capacity of EOs to reduce the ferric ion (Fe3+) present in the potassium ferricyanide complex to ferrous ion (Fe2+). A total of 50 μL of EOs at various concentrations were mixed with 200 μL of a tampon phosphate solution 0.2 M (pH 6.6) and 200 μL of a potassium ferricyanide solution (1%). After 20 min in a water bath of 50°C, 200 μL of trichloroacetic acid (10%), 200 μL of distillate water, and 120 μL of FeCl3 (0.1%) were added.
The absorbance of the reactionary medium was measured at 700 nm. An increase in absorbance corresponds to an increase in the reductive power of the tested sample. The positive control was represented by a solution of a standard antioxidant: ascorbic acid, at the same conditions as the samples. IC50 values were determined graphically from a plot of % scavenging vs concentration.
2.5.5. Total Antioxidant Capacity (TAC)
The phosphomolybdate test was carried out using the method described by [22], with a few changes. This approach was based on converting molybdates to molybdenum in the presence of an antioxidant, which produces a green complex that absorbs at 700 nm.
The phosphomolybdate reagent was created by combining sulfuric acid (25 mL in 225 mL of distillate water), sodium phosphate (3.28 g in 250 mL of distillate water), and ammonium molybdate (3.7 g in 250 mL of distillate water). Briefly, 100 μL of sample and standard was mixed with 1 mL of phosphomolybdate reagent. After 1°h 30 of incubation at 96°C, the absorbance was measured at 700 nm.
A typical blank solution contained 1 mL of reagent solution and the appropriate amount of the same solvent used for the sample was incubated with the other samples under the same conditions. The antioxidant capacity was calculated using an established calibration range with ascorbic acid and was expressed in milligrams of ascorbic acid equivalent per milliliter of EO (mg Eq.AAs/mL).
2.6. Statistical Analysis
All tests were carried out in triplicate. The three measurements for each data point in each independent repeat were first utilized to determine the average values and standard deviations (SDs) shown in the results, as well as to perform statistical analysis. The data were analyzed using computer software Excel 2010 and represented as mean ± SD.
2.7. Principal Component Analysis (PCA), Hierarchical Cluster Analysis (HCA), and Study of Correlations Between Major Compounds and Antioxidant Activity
2.7.1. Explained Variability
To determine the number of components to retain, we will adopt Kaiser's criterion, which states that in a normalized PCA, and we retain the components whose eigenvalues are greater than 1. The table of explained variability (Table 1) showed that the first three components have eigenvalues greater than 1 and explained 100% variability, while the first two components explain 79% variability.
Table 1.
The eigenvalues of the components, the percentages of explained variability, and the cumulative percentages of the variables on the first three components.
| Number | Eigenvalue | Percentage | Cumulative percentage |
|---|---|---|---|
| 1 | 11.0074 | 47.858 | 47.858 |
| 2 | 7.3700 | 32.043 | 79.902 |
| 3 | 4.6226 | 20.098 | 100.000 |
3. Results and Discussion
3.1. The EO Yields and Chemical Composition
The EO average yields obtained in this investigation are shown in Table 2, which showed that M. piperita, M. pulegium, T. serpyllum, and T. zygis EO yields were 1.9%, 2.4%, 0.8%, and 2.7%, respectively. These results were higher than those reported in other studies (Table 2).
Table 2.
Average yields of EOs of M. piperita, M. pulegium, T. serpyllum, and T. zygis.
These variations in yields could be attributed to various factors, including climatological conditions, genetic factors, geographic distribution, collection period, and extraction method [34]. As reported by [35, 36], these changes may also be caused by the plant's maturity, its age, and interaction with the environment (soil, climate, etc.).
The results of chromatographic analysis of M. piperita, M. pulegium, T. serpyllum, and T. zygis EOs are presented in Table 3 and Figure 1. They revealed that four volatile substances made up 82.44% of M. piperita EO. Three major constituents were detected in the M. pulegium EO, accounting for 86.07% of the total EO. T. serpyllum EO analysis found 10 distinct components that account for 84.16% of its content. For T. zygis EO, nine volatile components were discovered, representing 83.46% of this EO.
Table 3.
The chemical composition of M. piperita, M. pulegium, T. serpyllum, and T. zygis EOs.
| Compounds | RI exp | RI ref | (%) | |||
|---|---|---|---|---|---|---|
| M. piperita | M. pulegium | T. serpyllum | T. zygis | |||
| α-Pinene | 948 | 949 | 1.71 | 0.39 | 2.28 | 3.22 |
| Camphene | 943 | 948 | — | — | 3.72 | 5.45 |
| 3-Methylcyclohexanone | 952 | 937 | — | 0.48 | — | — |
| Sabinene (β-thujene) | 897 | 920 | 1.13 | — | — | — |
| β-Pinene | 943 | 949 | 2.53 | — | 0.74 | 0.63 |
| 3-Octanone | 979 | 985 | — | 0.84 | — | — |
| δ-Carene | 998 | 1002 | — | — | — | 0.44 |
| o-Cymene | 1042 | 1026 | — | — | 3.26 | 3.41 |
| D-Limonene | 1018 | 1029 | 2.90 | 0.94 | 0.98 | 0.89 |
| Eucalyptol (1,8-cineole) | 1059 | 1031 | 18.04 | — | 4.06 | 1.66 |
| γ-Terpinene | 998 | 1059 | — | — | — | 1.26 |
| Linalool | 1082 | 1085 | 0.78 | — | 2.67 | 3.60 |
| Camphor | 1121 | 1135 | — | — | 1.17 | 1.48 |
| α-Terpinyl acetate | 1350 | 1352 | — | 1.17 | — | — |
| Menthone | 1148 | 1157 | 18.48 | 4.09 | 3.11 | — |
| Isomintlactone | 1142 | 1219 | 35.55 | — | 4.13 | — |
| Borneol | 1138 | 1169 | 1.08 | — | 37.87 | 30.99 |
| L-4-Terpineol | 1137 | 1131 | — | — | 1.61 | 2.20 |
| 4,7,7-Trimethylbicyclo[4.1.0]heptan-3-ol, (1α,3β,4α,6α)- | 1125 | 1127 | — | — | — | 0.36 |
| α-Fenchol | 1138 | 1100 | 2.15 | — | — | — |
| α-Terpineol | 1143 | 1143 | — | — | 12.23 | 15.89 |
| 1,6,6-Trimethyl-8-oxabicyclo[3.2.1]octan-2-one | 1230 | 1229 | — | 0.30 | — | — |
| Isoborneol formate | 1275 | 1227 | — | — | — | 0.62 |
| Bornyl formate | 1277 | 1239 | — | — | 0.47 | |
| Isothymol methyl ether | 1231 | 1238 | — | — | — | 2.37 |
| 1,3-Dimethyladamantane | 1290 | 1296 | — | — | 3.14 | — |
| Pulegone | 1212 | 1237 | 10.37 | 74.04 | — | — |
| α-Elemene | 1142 | 1177 | — | 0.90 | — | — |
| Acid methyl ester | 1372 | 1378 | — | 1.12 | — | — |
| Piperitone | 1190 | 1232 | — | 7.94 | — | — |
| Fenchyl acetate | 1277 | 1221 | — | — | 1.83 | 2.54 |
| Acetaldehyde | 1115 | 1068 | — | 2.68 | — | — |
| Thymol | 1262 | 1290 | — | — | 2.74 | 3.54 |
| Carvacrol | 1262 | 1299 | — | — | 7.58 | 9.47 |
| Menthofuran | 1368 | 1364 | 0.99 | 0.32 | — | — |
| Copaene | 1344 | 1378 | — | — | — | 0.31 |
| Caryophyllene | 1494 | 1466 | 1.65 | 0.76 | 5.06 | 7.89 |
| α-Humulene | 1494 | 1446 | — | 1.56 | — | 0.35 |
| (+)-Mintlactone | 1368 | 1368 | 0.75 | 0.31 | — | — |
| γ-Cadinene | 1435 | 1513 | — | — | 0.42 | 0.55 |
| δ-Cadinene | 1469 | 1514 | — | — | 0.37 | 0.54 |
| Benzofuranone | 1579 | 1539 | — | 0.28 | — | — |
| Caryophyllene oxide | 1368 | 1583 | — | 0.57 | 0.55 | 0.35 |
| Humulene epoxide II | 1518 | 1593 | — | 0.86 | — | — |
| p-Cresol | 1565 | 1071 | 0.68 | — | — | — |
| β-Resorcylaldehyde | 1650 | 1450 | 1.23 | 0.45 | — | — |
| Monoterpene hydrocarbons | 42.88 | 4.41 | 19.86 | 15.39 | ||
| Oxygenated monoterpenes | 14.35 | 75.28 | 50.81 | 49.36 | ||
| Sesquiterpene hydrocarbons | 1.65 | 4.65 | 6.40 | 9.99 | ||
| Esters | — | 2.29 | — | — | ||
| Keto-alcohols | 35.55 | — | 4.13 | — | ||
| Ketones | 0.75 | 9.85 | — | — | ||
| Alcohols | 3.61 | — | 16.51 | 22.05 | ||
| Aldehydes | 1.23 | 3.13 | — | — | ||
| Total | 100 | 100 | 100 | 100 | ||
Note: RI exp, retention index obtained; RI Ref, retention index of literature; monoterpene hydrocarbons, α-pinene, camphene, β-thujene, β-pinene, δ-carene, o-cymene, eucalyptol, γ-terpinene, menthone, 1,3-dimethyladamantane, fenchyl acetate, and menthofuran; oxygenated monoterpenes, D-limonene, camphor, borneol, 1,6,6-trimethyl-8-oxabicyclo[3.2.1]octan-2-one, isoborneol formate, bornyl formate, isothymol methyl ether, pulegone, thymol, and carvacrol; sesquiterpene hydrocarbons, α-elemene, copaene, caryophyllene, α-humulene, γ-cadinene, δ-cadinene, caryophyllene oxide, and humulene epoxide II; esters, α-terpinyl acetate, and acid methyl ester; keto-alcohols, isomintlactone; ketones, 3-methylcyclohexanone, 3-octanone, piperitone, mintlactone, and benzofuranone; alcohols, linalool, L-4-terpineol, 4,7,7-trimethylbicyclo[4.1.0]heptan-3-ol, (1α,3β,4α,6α)-, α-fenchol, α-terpineol, and p-cresol; aldehydes, acetaldehyde, and β-resorcylaldehyde.
Figure 1.
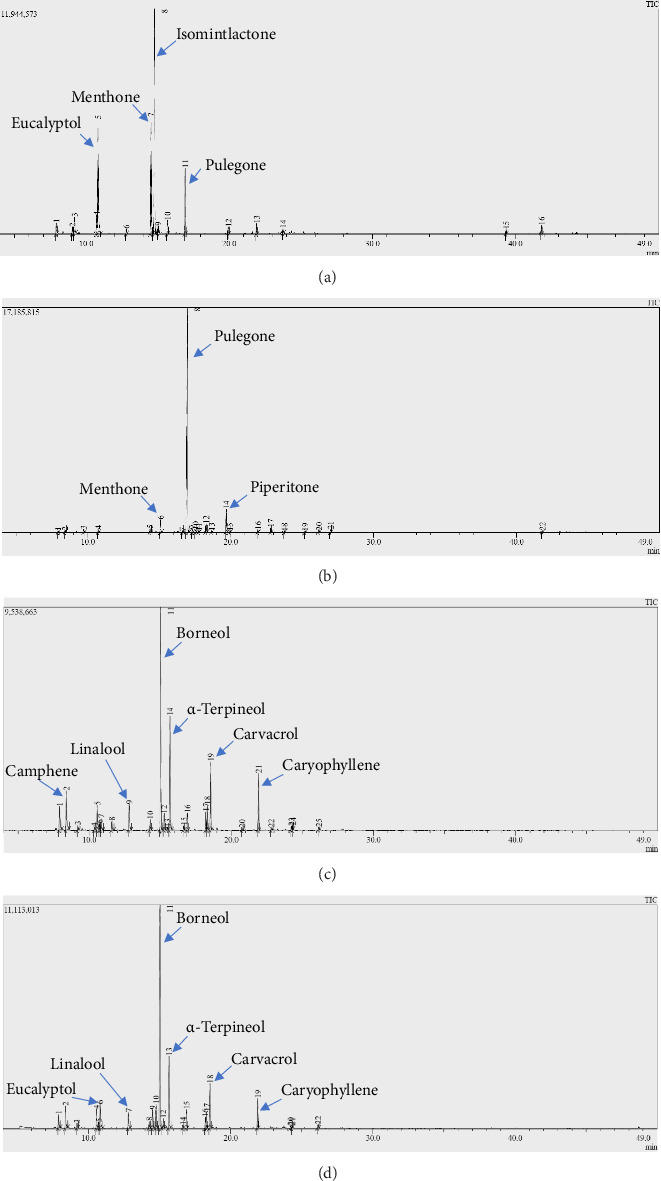
Chromatograms of essential oils: (a) M. piperita; (b) M. pulegium; (c) T. zygis; (d) T. serpyllum.
The composition of EO assessed in our investigation revealed that the main constituents were as follows: isomintlactone (35.55%), menthone (18.48%), eucalyptol (18.04%), and pulegone (10.37%). On the other part, [37] found menthol (59.73%), followed by isomenthone (18.45%) and methyl acetate (6.02%) in Brazilian EO. In the same country but in a different region, de Sousa, de Morais, and Ferreira [30] obtained carvone D (49.27%) and limonene (37.18%) as major components in M. piperita EO. Menthol (49.89%), menthone (20.84%), isomenthone (7.25%), 1,8-cineole (6.73%), and ciscarane (4.99%) characterized the Algerian oil [38]. In India, [39] obtained menthol (34.82%), carvone (19.54%), and menthone (9.10%). Reference [40] reported that menthol (4.30%), caryophyllene (5.50%), and 1,8-cineole (62.16%) were the major components of Korean M. piperita EO. Iranian and Saudi Arabian M. piperita EOs possessed almost identical components: menthol (36.9%), menthone (28.8%), menthyl acetate (4.54%), and 1,8-cineole (3.75%) for the Iranian EO by [41] and menthol (36.02%), menthone (24.56%), menthyl acetate (8.95%), and menthofuran (6.88%) for the Saudi Arabian EO by Desam et al. [42].
The EO of M. pulegium was composed of 22 components. The main components were pulegone (74.04%), piperitone (7.94%), and menthone (4.09%). It has been observed that the presence of pulegone as a major constituent of this oil indicated that it is of the pulegone chemotype. These findings were similar to the majority of previous studies on M. pulegium EO, albeit at different percentages [43–46]. Other authors have found other components as major components of M. pulegium EO: pulegone (75.48%), carvone (6.66%), and dihydrocarvone (4.64%) by Chraibi et al. [47]; isomenthone (13.4%) by [48]; menthone (21.16%) and pulegone (40.98%) by Bouyahya al. [49]; pulegone (69.8%) and piperitenone (3.1%) by [50]; and piperitone (38.00%), piperitenone (33.0%), and α-terpineol (4.7%) by [51].
Twenty-two components representing 100% of the total amount of T. serpyllum EO have been identified. The main constituents were borneol (37.87%), α-terpineol (12.23%), carvacrol (7.58%), caryophyllene (5.06%), isomintlactone (4.13%), eucalyptol (4.06%), camphene (3.72%), o-cymene (3.26%), 1,3-dimethyladamantane (3.14%), and pulegone (3.11%). Except for a few common compounds, this olfactory profile differs from that of many other authors. In fact, [52] obtained γ-terpinene (13.70%), thymol (13.70%), carvacrol (13.60%), β-bisabolene (8.50%), p-cymene (6.30%), farnesol (3.60%), elemol (3.60%), and ethyl carvacrol (3.10%). The EO studied by [53] was mostly composed of thymol (31.29 ± 1.13%), α-terpinene (14.58 ± 0.09%), carvacrol (5.11 ± 0.08%), and α-pinene (3.28 ± 0.03%). The main components obtained by [25] were carvacrol (35%–56%), thymol (11%–29%), γ-terpinene (14%–17%), and p-cymene (11%–15%). Reference [54] found carvacrol (66%) and γ-terpinene (11.5%) in Algerian EO. To compare with the EOs of Moroccan plants, [55] discovered linalyl (52.20%), (E)-nerolidol (15.10%), geranyl (5.00%), α-terpineol (4.30%), and terpinyl (3.40%) for plants harvested in the south and southeast of Morocco. Ouedrhiri et al. [24] obtained p-cymene (36.15%), γ-terpinene (18.31%), thymol (17.29%), and linalool (4.51%) from the EO of the plant harvested in Taounate (the same region as our harvest). Almost all studies on T. serpyllum showed that carvacrol and thymol are major components of their EOs [56–10].
In terms of T. zygis EO's chemical composition, the main components were borneol (30.99%), α-terpineol (15.89%), carvacrol (9.47%), caryophyllene (7.89%), camphene (5.45%), linalool (3.60%), thymol (3.54%), o-cymene (3.41%), and α-pinene (3.22). In accordance with this research, [60] reported that the major components of T. zygis EO cultured in Portugal were p-cymene (22.0%), thymol (19.5%), carvacrol (16.3%), γ-terpinene (7.4%), linalool (5.5%), and borneol (3.4%). Also, [61, 62] found thymol (68.1%), p-cymene (11.2%), γ-terpinene (4.8%), and carvacrol (3.5%) as major components in Spanish EO and carvacrol (16.07%–74.33%), thymol (1.47%–32.46%), p-cymene (6.97%–40.26%), and γ-terpinene (2.68%–22%) in Moroccan EO. However, this composition differed from that of the EO of T. zygis studied in Saudi Arabia and South Africa, where the major components were linalool (39.7%), terpinen-4-ol (11.7%), β-myrcene (8.6%), and γ-terpinene (7.6%) reported by [63], and p-thymol (46.393%) and p-cymene (22.154%) reported by [64]. References [32, 65–68] also revealed a chemical composition that differed from ours.
The quantitative and qualitative differences in the composition of EOs may be related to the origin of the plant, climate and geographical circumstances, and genetic diversity [69–70]. This variance might also be attributable to environmental factors, agronomic conditions, drying method, oil extraction methodology, and harvesting season [38, 71, 72].
3.2. Antioxidant Activity
Antioxidant activity of the obtained EOs was determined by using five different previously described methods (DPPH, ABTS, H2O2, FRAP, and TAC), because a single technique will offer basic information regarding antioxidant characteristics, but a combination of methods will characterize the sample's antioxidant qualities in greater depth [73].
3.2.1. DPPH Scavenging Activity
The IC50 value is defined as the concentration of plant EO necessary to scavenge half of the total DPPH radicals (50%) present. Scavenging of free radical DPPH was an approach utilized to investigate the antioxidant activity of the two Mentha and the two Thymus species. The T. zygis EO with IC50 = 0.42 ± 0.07 mg/mL was the most active, followed by T. serpyllum, M. piperita, and M. pulegium, with an IC50 of 1.39 ± 0.34 mg/mL, 2.99 ± 0.20 mg/mL, and 3.24 ± 0.20 mg/mL, respectively. The difference in antioxidant capacity of the four EOs might be attributed to different chemical profiles, with different functional groups, polarity, and chemical behavior [74].
The inhibitory concentration 50 determined for M. piperita (IC50 = 2.99 ± 0.20 mg/mL) was lower than that reported by other authors as an example [75], who determined an IC50 of (IC50 = 7.81 ± 0.23 mg/mL) for Iranian EO. Meanwhile, the Algerian EO of M. piperita studied by [76] showed a higher activity of radical scavenging than our result, which is on the order of 17.0 μg/mL. Other studies, such as those of [77] and Singh et al. [29], have demonstrated the significant antioxidant activity of Libyan M. piperita EO ((IC50 = 0.86 mg/mL) and (IC50 = 0.015 ± 0.009 mg/mL)), respectively. The differences in the antioxidant capacity of peppermint oil can be explained by the complex and changeable chemistry of this EO, which varies according on the climate, cultivar, and geographical location [29].
The EO of M. pulegium had an IC50 of IC50 = 3.24 ± 0.20 mg/mL. This activity was lower than that of the other three EOs. The EOs of M. pulegium studied by Bouyahya et al. [49] from Morocco [78], from Tunisia, and [76] from Algeria showed higher radical scavenging activities than our result, which were in the range of IC50 = 0.32 ± 0.002 mg/mL, IC50 = 0.14 mg/mL, and IC50 = 0.025 mg/mL, respectively. On the opposite, several researchers had reported that this EO has a lower antioxidant activity (IC50 = 14.73 ± 0.15 mg/mL) by [79] from Iran and IC50 = 6.2 ± 0.2 mg/mL by [1] from Portugal. The composition of M. pulegium's EO may be the source of its antioxidative activity; this can be mainly attributed to the concentration of the main components: pulegone, menthone, and piperitenone, or refers to the interaction of these three elements either antagonistically, synergistically, or both. In this regard, the most powerful components in this EO were monoterpene ketones [80]. According to de Sousa, de Morais, and Ferreira [30], the antioxidant activity of Mentha species is related to a higher piperitenone concentration. The antioxidant potential of M. pulegium EO was confirmed by [81], who reported that pulegone and menthone were known to have significant antioxidant activity. It is well known that the antioxidant activity of EOs is heavily dependent on their chemical composition, with special attention paid to the oxygenated compounds found in them [74].
T. serpyllum EO had demonstrated significant activity in radical scavenging (IC50 = 1.39 ± 0.34 mg/mL), and this is due to the unique chemical profile of this oil, which is devoid of oxygenated monoterpenes [82], whose have well-known antioxidant effects such as thymol, carvacrol, γ-terpinene, and linalool. According to the literature, the antioxidant activity of T. serpyllum EO as measured by the DPPH test appeared to be significantly higher than that of other authors' studies: IC50 = 34.8 mg/mL by [83] and an IC50 higher than 30,000 μg/mL by [55]. Meanwhile, the oils tested by [56, 59, 10] and [83] were more active than our EO, with an IC50 = 0.96 ± 0.05 mg/mL, IC50 = 384.60 μg/mL, IC50 = 34 µg/mL, and IC50 = 0.96 μg/mL, respectively.
T. zygis EO was the most efficient in scavenging DPPH radicals, with IC50 = 0.42 ± 0.07 mg/mL, which, however, was always superior to the positive control (BHT), with IC50 = 0.021 ± 0.001 mg/mL. This is owing to the oil's distinct chemical composition and maybe linked to the synergistic and cumulative effects of its contents. The IC50 values obtained in our study were comparable to those published by other researchers [84, 85] and much higher than those reported by [33, 86]. [32] also discovered a less effective antioxidative activity, as evidenced by a higher IC50 value (IC50 = 3.7 ± 1.6 mg/mL).
3.2.2. The Cation Radical ABTS
The results of the ABTS test supported the results of the DPPH test by classifying T. zygis as the most active EO with an IC50 of 0.40 ± 0.04 mg/mL and M. pulegium as the EO with the lowest antioxidant activity, with an IC50 of 1.02 ± 0.21 mg/mL. The other EOs had an IC50 value of 0.44 ± 0.7 mg/mL for T. serpyllum and 0.96 ± 0.29 mg/mL for M. piperita. The gallic acid had the highest activity, with an IC50 of 0.019 ± 0.001 mg/mL.
To the best of our knowledge, the number of published studies on the antioxidant activity of M. piperita, M. pulegium, and T. zygis EOs utilizing the ABTS technique is rather modest; however, it has never been tested on the EO of T. serpyllum. Previous research has revealed that both Mentha species and T. zygis EOs have varied antioxidant activities: an IC50 varying from 1.41 ± 0.01 to 6.59 ± 0.02 μg/mL for Serbian M. piperita EO [87], an IC50 = 155 μg/mL for Tunisian EO [78], IC50 = 57.4 ± 1.9 μg/mL [88], an IC50 = 5.72 ± 0.060 mg/mL [74] for Algerian M. pulegium EO, and finally an IC50 = 2.07 mg/mL for T. zygis [85].
3.2.3. Hydrogen Peroxide Scavenging Capacity (H2O2)
The antioxidant activity of EOs of M. piperita, M. pulegium, T. serpyllum, and T. zygis was evaluated by hydrogen peroxide scavenging capacity method (H2O2). The results of the H2O2 test also support the results of the DPPH and ABTS tests. T. zygis and T. serpyllum showed significant antioxidant activity compared to the ascorbic acid with an IC50 of 0.063 ± 0.01 mg/mL, 0.05 ± 0.004 mg/mL, and 0.023 ± 0.03 mg/mL, respectively, while M. piperita and M. pulegium significantly reduced H2O2 with an IC50 = 0.013 ± 0.003 mg/mL and IC50 = 0.009 ± 0.008 mg/mL, respectively. The H2O2 test findings were encouraging when compared to those obtained with ascorbic acid, which was employed as a control.
To the best of our knowledge, according to the literature, the antioxidant capacity of Thymus species (including T. serpyllum and T. zygis) and M. pulegium has not been investigated using this approach. In contrast, one H2O2 activity has been reported by [89] for M. piperita (IC50 = 34.81 ± 0.01 μg/mL), which was higher than the value obtained in our study.
3.2.4. FRAP
Table 4 displayed the findings of investigating the antioxidant activity of M. piperita, M. pulegium, T. serpyllum, and T. zygis EOs by the iron reduction method (FRAP). The iron-reducing activity of M. pulegium EO was higher than that of the other EOs, followed by T. serpyllum EO, M. piperita EO, and T. zygis EO, respectively. The iron-reducing activity of all EOs was higher than that of ascorbic acid, which was used as a control with a best iron reduction. The antioxidant activity of the four EOs was quantified by estimating the effective concentration (EC50).
Table 4.
DPPH, ABTS, H2O2, and FRAP IC50 of M. piperita, M. pulegium, T. serpyllum, and T. zygis EOs and the ascorbic acid equivalence using the TAC assay.
| Plants EO | DPPH (IC50) (mg/mL) | ABTS (IC50) (mg/mL) | H2O2 (IC50) (mg/mL) | FRAP (EC50) (mg/mL) | TAC (mg AAs/mL) |
|---|---|---|---|---|---|
| M. piperita | 2.99 ± 0.20 | 0.96 ± 0.29 | 0.013 ± 0.003 | 0.101 ± 0.01 | 10.42 ± 0.03 |
| M. pulegium | 3.24 ± 0.20 | 1.02 ± 0.21 | 0.009 ± 0.008 | 0.42 ± 0.02 | 9.94 ± 0.21 |
| T. serpyllum | 1.39 ± 0.34 | 0.44 ± 0.7 | 0.05 ± 0.004 | 0.161 ± 0.002 | 7.25 ± 0.19 |
| T. zygis | 0.42 ± 0.07 | 0.40 ± 0.04 | 0.063 ± 0.01 | 0.09 ± 0.01 | 8.36 ± 0.42 |
| BHT | 0.021 ± 0.001 | — | — | — | — |
| Gallic acid | — | 0.019 ± 0.001 | — | — | — |
| Ascorbic acid | — | — | 0.023 ± 0.03 | 0.035 ± 0.010 | — |
From these results, we can deduce that the EC50 to reduce 50% of the Fe3+ ions were 0.42 ± 0.02 mg/mL, 0.161 ± 0.002 mg/mL, 0.101 ± 0.01, 0.09 ± 0.01 mg/mL, and 0.035 ± 0.010 mg/mL, for M. pulegium, T. serpyllum, M. piperita, T. zygis, and ascorbic acid, respectively. These results were lower than those discovered by [75, 87] for Serbian and Iranian M. piperita [74, 78, 53]; for Tunisian, Moroccan, and Algerian M. pulegium; and [54, 59] for T. serpyllum. On the other hand, [33] discovered an EC50 of 2.46 ± 0.01 μg/mL for T. zygis, which was lower than the one found in our study.
There is a link between the chemical composition and the reported antioxidant activity, particularly with the high quantities of some components like carvacrol, menthone and pulegone…, which have a strong antioxidant potential. Indeed, multiple investigations have shown that EOs containing phenolic chemotypes have better antioxidant potential [84, 90]. Due to their redox characteristics, phenols act as reducing agents, hydrogen donors, and single oxygen donors. Other minor components, however, can interact directly, synergistically, antagonistically or both to produce a combination with greater action. Furthermore, the antioxidant activity of the bulk of the components evaluated independently gives lower findings when compared to the activity of the EO as a whole [91].
3.2.5. TAC
TAC assessed by the phosphomolybdate method for the four EOs ranged from 10.42 ± 0.03 mg AAs/mL in M. piperita to 7.25 ± 0.19 mg AAs/mL in T. serpyllum. The maximum concentrations of the other EOs were 9.94 ± 0.21 mg AAs/mL in M. pulegium and 8.36 ± 0.42 mg AAs/mL in T. zygis. The results of all EOs were dose-dependent, considering antioxidant activity increased with concentration.
The phosphomolybdate reduction capacity of M. piperita's EO was (10.42 ± 0.03 mg AAs/mL), and this value appeared to be greater than that provided by [92] for the same species from Serbia. The phosphomolybdate test on M. pulegium's EO (9.94 ± 0.21 mg AAs/mL) confirmed its potent antioxidant activity. As a result, our EO has a much higher activity than [93] of the same species found in Algeria, which is in the range of 159 μg AAs/mL. Similarly, the research of [74] on the chemical composition and biological activity of M. pulegium's EO yielded a value in the order of 109 μg AAs/mL that was significantly lower than ours.
To the best of our knowledge, the phosphomolybdate test has never been performed on the EOs of T. serpyllum and T. zygis. As a result, we will compare these EOs to those of other species of the same genre. In this regard, a study conducted by [93] with the goal of studying the chemical composition and antioxidative and antimicrobial activities of EOs from Algerian Lamiaceae species, and it was discovered that the antioxidant capacity of T. algeriensis and T. vulgaris EOs was evaluated using the phosphomolybdate method, showing values in the range of 220 μg AAs/mL and 107 μg AAs/mL, respectively, which were lower than our result for T. serpyllum (7.25 ± 0.19 mg AAs/mL) and T. zygis (8.36 ± 0.42 mg AAs/mL). Other studies on the Thymus species had also been conducted. References [94, 95] discovered an antioxidant activity of T. vulgaris in the order of 90 μg AAs/mL and an antioxidant activity of T. spathulifolius in the order of 502 μg AAs/mL, respectively, which were still less than our values.
3.3. Multivariate Analysis
3.3.1. PCA
3.3.1.1. Study of Variables
The loading plot analysis revealed several significant findings (Figure 2). Firstly, the compounds borneol, α-terpineol, carvacrol, caryophyllene, camphene, 1,3-dimethyladamantane, and o-cymene exhibited positive correlations with each other. Secondly, the compounds β-myrcene, linalool, terpen-4-ol, and γ-terpinene demonstrated intercorrelations. Additionally, isomintlactone and eucalyptol were found to be positively correlated. Furthermore, a positive correlation was observed between total polyphenols and TAC. Moreover, FRAP antioxidant activity displayed correlations with both DPPH and piperitone. Notably, the antioxidant activities of DPPH and ABTS were positively correlated. The Pearson correlation test (p value < 0.05) statistically confirmed all identified correlations.
Figure 2.
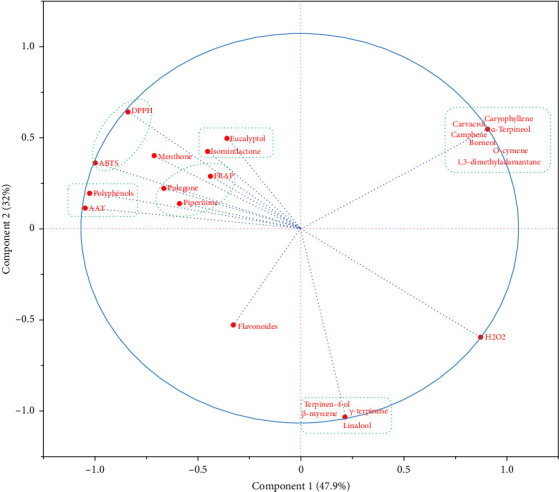
Graph of the distribution of the variables on the first and second principal components.
The loading plot analysis revealed several significant findings. Firstly, the compounds borneol, α-terpineol, carvacrol, caryophyllene, camphene, 1,3-dimethyladamantane, and o-cymene exhibited positive correlations with each other. Secondly, the compounds β-myrcene, linalool, terpen-4-ol, and γ-terpinene demonstrated intercorrelations. Additionally, isomintlactone and eucalyptol were found to be positively correlated. Furthermore, a positive correlation was observed between total polyphenols and TAC. Moreover, FRAP antioxidant activity displayed correlations with both DPPH and piperitone. Notably, the antioxidant activities of DPPH and ABTS were positively correlated. The Pearson correlation test (p value < 0.05) statistically confirmed all identified correlations.
The positive correlation between polyphenols and antioxidant activity was confirmed in vivo by the literature. Reference [96] reported that polyphenols exert cytoprotection effect by reducing the formation of oxygen free radicals. The same correlation was observed by [97].
3.3.1.2. Score Plot
The data presented in Figure 3 reveals interesting insights about the chemical composition and antioxidant activities of four different species: M. pulegium, M. piperita, T. serpyllum, and T. zygis. Upon analyzing the graph, it becomes evident that the first two species, M. pulegium and M. piperita, exhibited striking similarities in both their chemical composition and antioxidant activities. This suggests that these two species share comparable properties in terms of the compounds they contain and their ability to combat oxidative stress.
Figure 3.
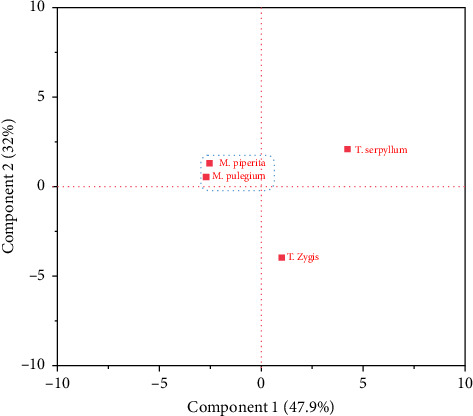
Individual graph of the four essential oils.
In contrast, the score plot (Figure 3) highlights a noticeable distinction between M. pulegium and M. piperita, and the other two species, T. serpyllum and T. zygis. The chemical composition and antioxidant activities of T. serpyllum and T. zygis differed considerably from those of M. pulegium and M. piperita, indicating unique properties exclusive to these two species.
3.3.1.3. Biplot Scores/Loadings
The biplot presented in Figure 4 highlighted that the antioxidant activities assessed using the DPPH, FRAP, ABTS, and TAC methods were predominantly associated with M. pulegium and M. piperita species. This connection can be attributed to their higher concentrations of highly active major compounds. Conversely, the influence on H2O2 activity was more pronounced in the case of T. serpyllum and T. zygis species. Furthermore, in terms of total phenolic content, the two species of Mentha exhibited the highest richness.
Figure 4.
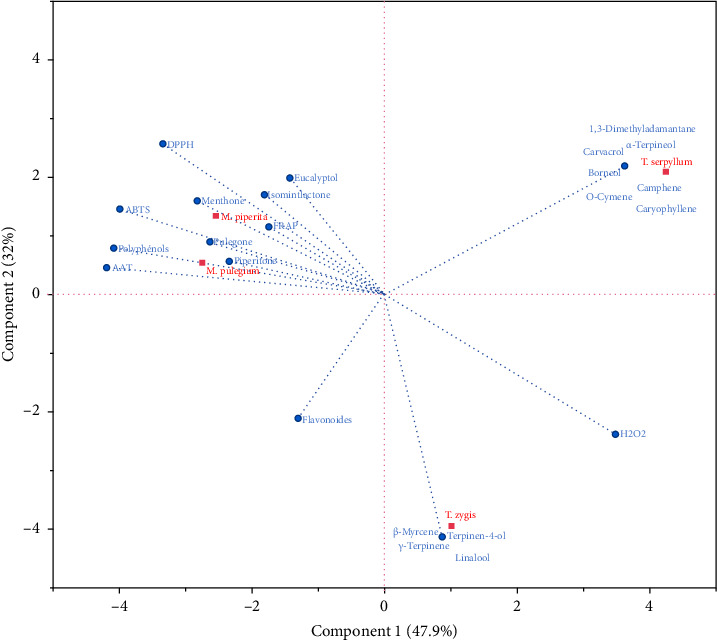
Graph of distribution of variables and individuals on the first and second principal components.
3.3.2. HCA
The results obtained by PCA were confirmed by the HCA method as shown in the graph.
Figure 5 presents a comprehensive analysis of the antioxidant activities using various methods such as DPPH, ABTS, TAC, FRAP, and H2O2. The data reveal an interesting correlation between the antioxidant activities and the major compounds found in the two species, M. pulegium and M. piperita.
Figure 5.
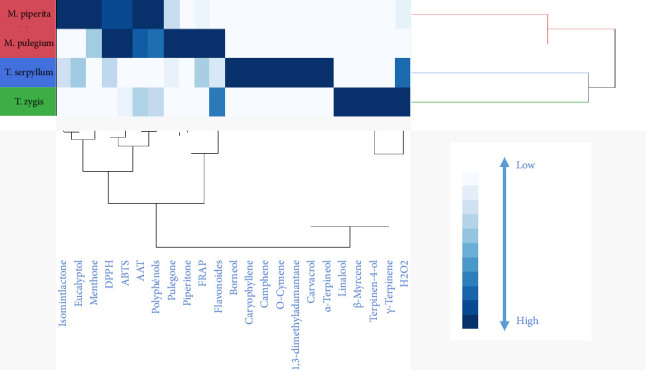
Hierarchical classification of individuals according to their major compounds and their antioxidant activities.
Specifically, when assessing the antioxidant activities through the DPPH, ABTS, TAC, and FRAP methods, it becomes evident that these activities were primarily influenced by the major compounds present in M. pulegium and M. piperita. This indicates that these two species possess significant antioxidant potential due to the presence of specific compounds that contribute to their observed activities in these methods.
In contrast, the H2O2 method demonstrated a closer association with the major compounds found in T. zygis EO. This suggests that the antioxidant activity measured through the H2O2 method is more influenced by the specific compounds present in T. zygis. These compounds likely play a crucial role in conferring antioxidant properties to T. zygis, distinguishing it from M. pulegium and M. piperita in terms of this particular method.
4. Conclusion
Based on our results, the EOs of M. piperita, M. pulegium, T. serpyllum, and T. zygis were found to be very rich in some components (e.g., menthol for M. piperita; pulegone and menthone for M. pulegium; thymol and carvacrol for T. serpyllum and T. zygis), which remain the main contributors to the biological activities of these oils. The evaluated EOs demonstrated exceptional antioxidant activity using all five techniques. For the correlations between the major compounds of the four oils and their antioxidant effect using PCA and HCA, the two Mentha species could be classified in the same group because they contained the main constituents in significant relation with the antioxidant activities by the DPPH, ABTS, TAC, and FRAP methods. The other two classes contained the other two species T. serpyllum and T. zygis with a relationship with the H2O2 method. According to the results of this investigation, the selected native species of M. piperita, M. pulegium, T. serpyllum, and T. zygis are significant natural sources with extraordinary antioxidant activity, and their use in food applications is strongly recommended. Future research on formulation studies and the potential toxicity of examined EOs is needed for safety concerns, including in vitro and in silico studies using bioactive molecules [98–99].
Acknowledgments
We express special gratitude to everyone who contributed to this study.
Data Availability Statement
The data used in this study are included within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public or commercial.
References
- 1.Teixeira B., Marques A., Ramos C., et al. European Pennyroyal (Mentha Pulegium) From Portugal: Chemical Composition of Essential Oil and Antioxidant and Antimicrobial Properties of Extracts and Essential Oil. Industrial Crops and Products . 2012;36(1):81–87. doi: 10.1016/j.indcrop.2011.08.011. [DOI] [Google Scholar]
- 2.Poljsak B., Kovač V., Milisav I. Antioxidants, Food Processing and Health. Antioxidants . 2021;10(3):p. 433. doi: 10.3390/antiox10030433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phaniendra A., Jestadi D. B., Periyasamy L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian Journal of Clinical Biochemistry . 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussain A. I., Anwar F., Chatha S. A. S., Jabbar A., Mahboob S., Nigam P. S. Rosmarinus Officinalis Essential Oil: Antiproliferative, Antioxidant and Antibacterial Activities. Brazilian Journal of Microbiology . 2010;41(4):1070–1078. doi: 10.1590/s1517-83822010000400027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivasothy Y., Chong W. K., Hamid A., Eldeen I. M., Sulaiman S. F., Awang K. Essential Oils of Zingiber Officinale Var. Rubrum Theilade and Their Antibacterial Activities. Food Chemistry . 2011;124(2):514–517. doi: 10.1016/j.foodchem.2010.06.062. [DOI] [Google Scholar]
- 6.Warraich U. e A., Hussain F., Kayani H. U. R. Aging-Oxidative Stress, Antioxidants and Computational Modeling. Heliyon . 2020;6(5):p. e04107. doi: 10.1016/j.heliyon.2020.e04107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pateiro M., Barba F. J., Domínguez R., et al. Essential Oils as Natural Additives to Prevent Oxidation Reactions in Meat and Meat Products: A Review. Food Research International . 2018;113:156–166. doi: 10.1016/j.foodres.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Tajkarimi M. M., Ibrahim S. A., Cliver D. O. Antimicrobial Herb and Spice Compounds in Food. Food Control . 2010;21(9):1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- 9.Bolouri P., Salami R., Kouhi S., et al. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules . 2022;27(24):p. 8999. doi: 10.3390/molecules27248999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tohidi B., Rahimmalek M., Arzani A. Essential Oil Composition, Total Phenolic, Flavonoid Contents, and Antioxidant Activity of Thymus Species Collected From Different Regions of Iran. Food Chemistry . 2017;220:153–161. doi: 10.1016/j.foodchem.2016.09.203. [DOI] [PubMed] [Google Scholar]
- 11.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and Other Phenolic Compounds From Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines . 2018;5(3):p. 93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baharfar R., Azimi R., Mohseni M. Antioxidant and Antibacterial Activity of Flavonoid-Polyphenol- and Anthocyanin-Rich Extracts From Thymus Kotschyanus Boiss & Hohen Aerial Parts. Journal of Food Science and Technology . 2015;52(10):6777–6783. doi: 10.1007/s13197-015-1752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-García C., Sánchez-Quesada C., Gaforio J. J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants . 2019;8(5):p. 137. doi: 10.3390/antiox8050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El amrani S., Sanae L., Ez zoubi Y., et al. Combined Antibacterial Effect of Origanum Compactum and Mentha Piperita (Lamiaceae) Essential Oils Against ATCC Escherichia coli and Staphylococcus aureus. Vegetos . 2022;35(1):74–82. doi: 10.1007/s42535-021-00276-0. [DOI] [Google Scholar]
- 15.Nabavi S. M., Marchese A., Izadi M., Curti V., Daglia M., Nabavi S. F. Plants Belonging to the Genus Thymus as Antibacterial Agents: From Farm to Pharmacy. Food Chemistry . 2015;173:339–347. doi: 10.1016/j.foodchem.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Ainane A., Khammour F., Charaf S., et al. Chemical Composition and Insecticidal Activity of Five Essential Oils: Cedrus Atlantica, Citrus Limonum, Rosmarinus Officinalis, Syzygium Aromaticum and Eucalyptus Globules. Materials Today: Proceedings . 2019;13:474–485. doi: 10.1016/j.matpr.2019.04.004. [DOI] [Google Scholar]
- 17.Adams R. P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Journal of the American Society for Mass Spectrometry . 2001;16 [Google Scholar]
- 18.Boulanouar B., Abdelaziz G., Aazza S., Gago C., Miguel M. G. Antioxidant Activities of Eight Algerian Plant Extracts and Two Essential Oils. Industrial Crops and Products . 2013;46:85–96. doi: 10.1016/j.indcrop.2013.01.020. [DOI] [Google Scholar]
- 19.De León-Zapata M. A., Pastrana-Castro L., Rua-Rodríguez M. L., Alvarez-Pérez O. B., Rodríguez-Herrera R., Aguilar C. N. Experimental Protocol for the Recovery and Evaluation of Bioactive Compounds of Tarbush Against Postharvest Fruit Fungi. Food Chemistry . 2016;198:62–67. doi: 10.1016/j.foodchem.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 20.El-Guendouz S., Aazza S., Lyoussi B., et al. Moroccan Propolis: A Natural Antioxidant, Antibacterial, and Antibiofilm Against Staphylococcus aureus With No Induction of Resistance After Continuous Exposure. Evidence-Based Complementary and Alternative Medicine: ECAM . 2018;2018(1):p. 9759240. doi: 10.1155/2018/9759240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bougandoura N., Bendimerad N. Evaluation de l’activité Antioxydante des Extraits Aqueux et Méthanolique de Satureja Calamintha ssp.Nepeta (L.) Briq. Rev Nat Technol . 2013;5:14–19. [Google Scholar]
- 22.Prieto P., Pineda M., Aguilar M. Spectrophotometric Quantitation of Antioxidant Capacity Through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Analytical Biochemistry . 1999;269(2):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 23.Šojić B., Tomović V., Kocić-Tanackov S., et al. Supercritical Extracts of Wild Thyme (Thymus Serpyllum L.) By-Product as Natural Antioxidants in Ground Pork Patties. LWT-Food Science & Technology . 2020;130:p. 109661. doi: 10.1016/j.lwt.2020.109661. [DOI] [Google Scholar]
- 24.Ouedrhiri W., Balouiri M., Bouhdid S., et al. Mixture Design of Origanum Compactum, Origanum Majorana and Thymus Serpyllum Essential Oils: Optimization of Their Antibacterial Effect. Industrial Crops and Products . 2016;89:1–9. doi: 10.1016/j.indcrop.2016.04.049. [DOI] [Google Scholar]
- 25.Kirillov V., Stikhareva T., Mukanov B., et al. Composition of the Essential Oil of Thymus serpyllum L. From Northern Kazakhstan. Journal of Essential Oil Bearing Plants . 2016;19(1):212–222. doi: 10.1080/0972060X.2015.1010600. [DOI] [Google Scholar]
- 26.Boukhebti H., Chaker A. N., Belhadj H., et al. Chemical Composition and Antibacterial Activity of Mentha Pulegium L. And Mentha Spicata L. Essential Oils . 2011. [Google Scholar]
- 27.Mollaei S., Ebadi M., Hazrati S., Habibi B., Gholami F., Sourestani M. M. Essential Oil Variation and Antioxidant Capacity of Mentha Pulegium Populations and Their Relation to Ecological Factors. Biochemical Systematics and Ecology . 2020;91:p. 104084. doi: 10.1016/j.bse.2020.104084. [DOI] [Google Scholar]
- 28.Derwich E., Benziane Z., Taouil R. GC/MS Analysis of Volatile Compounds of the Essential Oil of the Leaves of Mentha Pulegium Growing in Morocco. Chem Bull Politeh Univ Timisoara . 2010;55:103–106. [Google Scholar]
- 29.Singh R., Shushni M. A. M., Belkheir A. Antibacterial and Antioxidant Activities of Mentha Piperita L. Arabian Journal of Chemistry . 2015;8(3):322–328. doi: 10.1016/j.arabjc.2011.01.019. [DOI] [Google Scholar]
- 30.de Sousa B. A., de Morais S. M., Ferreira P. A. T., et al. Chemical Composition and Functional Properties of Essential Oils From Mentha Species. Industrial Crops & Products . 2015;76:557–564. doi: 10.1016/j.indcrop.2015.07.004. [DOI] [Google Scholar]
- 31.Bassolé I. H. N., Lamien-Meda A., Bayala B., et al. Composition and Antimicrobial Activities of Lippia Multiflora Moldenke, Mentha X Piperita L. and Ocimum Basilicum L. Essential Oils and Their Major Monoterpene Alcohols Alone and in Combination. Molecules . 2010;15(11):7825–7839. doi: 10.3390/molecules15117825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordán M. J., Martínez R. M., Martínez C., Moñino I., Sotomayor J. A. Polyphenolic Extract and Essential Oil Quality of Thymus Zygis Ssp. Gracilis Shrubs Cultivated Under Different Watering Levels. Industrial Crops and Products . 2009;29(1):145–153. doi: 10.1016/j.indcrop.2008.04.021. [DOI] [Google Scholar]
- 33.Radi F. Z., Bouhrim M., Mechchate H., et al. Phytochemical Analysis, Antimicrobial and Antioxidant Properties of Thymus Zygis L. and Thymus Willdenowii Boiss. Essential Oils. Plants . 2021;11(1):p. 15. doi: 10.3390/plants11010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouyahya A., Jamal A. Origanum Compactum Benth: A Review on Phytochemistry and Pharmacological Properties. Medicinal & Aromatic Plants . 2016;05(04):p. 252. doi: 10.4172/2167-0412.1000252. [DOI] [Google Scholar]
- 35.Chahboun N., Esmail A., Abed H., et al. Evaluation of the Bacteriostatic Activity of the Essential Oil of Lavandula Officinalis Towards of the Original Strains Resistant to Antibiotics Clinic . 2015;6 [Google Scholar]
- 36.Bey-Ould Si Said Z., Haddadi-Guemghar H., Boulekbache-Makhlouf L., et al. Essential Oils Composition, Antibacterial and Antioxidant Activities of Hydrodistillated Extract of Eucalyptus Globulus Fruits. Industrial Crops & Products . 2016;89:167–175. doi: 10.1016/j.indcrop.2016.05.018. [DOI] [Google Scholar]
- 37.de Sousa Guedes J. P., da Costa Medeiros J. A., de Souza e Silva R. S., de Sousa J. M. B., da Conceição M. L., de Souza E. L. The Efficacy of Mentha Arvensis L. and M. Piperita L. Essential Oils in Reducing Pathogenic Bacteria and Maintaining Quality Characteristics in Cashew, Guava, Mango, and Pineapple Juices. International Journal of Food Microbiology . 2016;238:183–192. doi: 10.1016/j.ijfoodmicro.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Benabdallah A., Boumendjel M., Aissi O., Rahmoune C., Boussaid M., Messaoud C. Chemical Composition, Antioxidant Activity and Acetylcholinesterase Inhibitory of Wild Mentha Species From Northeastern Algeria. South African Journal of Botany . 2018;116:131–139. doi: 10.1016/j.sajb.2018.03.002. [DOI] [Google Scholar]
- 39.Ahmad A., Khan A., Samber N., Manzoor N. Antimicrobial Activity of Mentha Piperita Essential Oil in Combination With Silver Ions. Synergy . 2014;1(2):92–98. doi: 10.1016/j.synres.2014.11.001. [DOI] [Google Scholar]
- 40.Park Y. J., Baskar T. B., Yeo S. K., et al. Composition of Volatile Compounds and In Vitro Antimicrobial Activity of Nine Mentha Spp. SpringerPlus . 2016;5(1):p. 1628. doi: 10.1186/s40064-016-3283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahboubi M., Kazempour N. Chemical Composition and Antimicrobial Activity of Peppermint (Mentha Piperita L.) Essential Oil. Songklanakarin Journal of Science and Technology . 2014;36:83–87. [Google Scholar]
- 42.Desam N. R., Al-Rajab A. J., Sharma M., Mylabathula M. M., Gowkanapalli R. R., Albratty M. Chemical Constituents, In Vitro Antibacterial and Antifungal Activity of Mentha × Piperita L. (Peppermint) Essential Oils. Journal of King Saud University-Science . 2019;31(4):528–533. doi: 10.1016/j.jksus.2017.07.013. [DOI] [Google Scholar]
- 43.Beghidja N., Bouslimani N., Benayache F., Benayache S., Chalchat J. C. Composition of the Oils From Mentha Pulegium Grown in Different Areas of the East of Algeria. Chemistry of Natural Compounds . 2007;43(4):481–483. doi: 10.1007/s10600-007-0170-6. [DOI] [Google Scholar]
- 44.Benahmed A., Harfi B., Belkhiri A. Biological Activity of Essential Oils of Mentha pulegium From Field-Grown and Acclimated In Vitro Plants. Current Science . 2019;116(11):p. 1897. doi: 10.18520/cs/v116/i11/1897-1904. [DOI] [Google Scholar]
- 45.Derwich E., Benziane Z., Taouil R., Senhaji O., Touzani M. Comparative Essential Oil Composition of Leaves of Mentha rotundifolia and Mentha pulegium a Traditional Herbal Medicine in Morocco . 2010. [Google Scholar]
- 46.Casiglia S., Bruno M., Fontana G., Senatore F. Chemical Composition of the Essential Oil of Mentha Pulegium Growing Wild in Sicily and its Activity on Microorganisms Affecting Historical Art Crafts. Natural Product Communications . 2017;12(8):p. 1934578X1701200840. doi: 10.1177/1934578X1701200840. [DOI] [PubMed] [Google Scholar]
- 47.Chraibi M., Farah A., Lebrazi S., El Amine O., Iraqui Houssaini M., Fikri-Benbrahim K. Antimycobacterial Natural Products From Moroccan Medicinal Plants: Chemical Composition, Bacteriostatic and Bactericidal Profile of Thymus Satureioides and Mentha Pulegium Essential Oils. Asian Pacific Journal of Tropical Biomedicine . 2016;6(10):836–840. doi: 10.1016/j.apjtb.2016.08.002. [DOI] [Google Scholar]
- 48.El Asbahani A., Jilale A., Voisin S. N., et al. Chemical Composition and Antimicrobial Activity of Nine Essential Oils Obtained by Steam Distillation of Plants From the Souss-Massa Region (Morocco) Journal of Essential Oil Research . 2015;27(1):34–44. doi: 10.1080/10412905.2014.964426. [DOI] [Google Scholar]
- 49.Bouyahya A., Et-Touys A., Bakri Y., et al. Chemical Composition of Mentha Pulegium and Rosmarinus Officinalis Essential Oils and Their Antileishmanial, Antibacterial and Antioxidant Activities. Microbial Pathogenesis . 2017;111:41–49. doi: 10.1016/j.micpath.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Ait-Ouazzou A., Lorán S., Arakrak A., et al. Evaluation of the Chemical Composition and Antimicrobial Activity of Mentha Pulegium, Juniperus Phoenicea, and Cyperus Longus Essential Oils From Morocco. Food Research International . 2012;45(1):313–319. doi: 10.1016/j.foodres.2011.09.004. [DOI] [Google Scholar]
- 51.Mahboubi M., Haghi G. Antimicrobial Activity and Chemical Composition of Mentha Pulegium L. Essential Oil. Journal of Ethnopharmacology . 2008;119(2):325–327. doi: 10.1016/j.jep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 52.Schött G., Liesegang S., Gaunitz F., et al. The Chemical Composition of the Pharmacologically Active Thymus Species, its Antibacterial Activity Against Streptococcus Mutans and the Antiadherent Effects of T. vulgaris on the Bacterial Colonization of the In Situ Pellicle. Fitoterapia . 2017;121:118–128. doi: 10.1016/j.fitote.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Kačániová M., Terentjeva M., Vukovic N., et al. The Antioxidant and Antimicrobial Activity of Essential Oils Against Pseudomonas Spp. Isolated From Fish. Saudi Pharmaceutical Journal . 2017;25(8):1108–1116. doi: 10.1016/j.jsps.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madouni N., Meddah B., Aicha T. T., et al. Chemical Profile, Antioxidant and Photoprotective Activities of Essential Oil and Crude Extracts of Algerian Thymus Serpyllum. Nova Biotechnol Chim . 2021;20:p. e916. doi: 10.36547/nbc.916. [DOI] [Google Scholar]
- 55.Jamali C. A., El Bouzidi L., Bekkouche K., et al. Chemical Composition and Antioxidant and Anticandidal Activities of Essential Oils From Different Wild Moroccan Thymus Species. Chemistry and Biodiversity . 2012;9(6):1188–1197. doi: 10.1002/cbdv.201200041. [DOI] [PubMed] [Google Scholar]
- 56.Hussain A. I., Anwar F., Chatha S. A. S., et al. Chemical Composition and Bioactivity Studies of the Essential Oils From Two Thymus Species From the Pakistani Flora. LWT-Food Science Technology . 2013;50(1):185–192. doi: 10.1016/j.lwt.2012.06.003. [DOI] [Google Scholar]
- 57.Kloucek P., Smid J., Frankova A., Kokoska L., Valterova I., Pavela R. Fast Screening Method for Assessment of Antimicrobial Activity of Essential Oils in Vapor Phase. Food Research International . 2012;47(2):161–165. doi: 10.1016/j.foodres.2011.04.044. [DOI] [Google Scholar]
- 58.Kulisic T., Radonic A., Milos M. Antioxidant Properties of Thyme (Thymus vulgaris L.) and Wild Thyme (Thymus Serpyllum L.) Essential Oils . 2005. [Google Scholar]
- 59.Nikolić M., Glamočlija J., Ferreira I. C. F. R., et al. Chemical Composition, Antimicrobial, Antioxidant and Antitumor Activity of Thymus Serpyllum L., Thymus Algeriensis Boiss. and Reut and Thymus Vulgaris L. Essential Oils. Industrial Crops and Products . 2014;52:183–190. doi: 10.1016/j.indcrop.2013.10.006. [DOI] [Google Scholar]
- 60.Rodrigues V., Cabral C., Évora L., et al. Chemical Composition, Anti-Inflammatory Activity and Cytotoxicity of Thymus Zygis L. Subsp. Sylvestris (Hoffmanns. & Link) Cout. Essential Oil and its Main Compounds. Arabian Journal of Chemistry . 2019;12(8):3236–3243. doi: 10.1016/j.arabjc.2015.08.026. [DOI] [Google Scholar]
- 61.Rota M. C., Herrera A., Martínez R. M., Sotomayor J. A., Jordán M. J. Antimicrobial Activity and Chemical Composition of Thymus Vulgaris, Thymus Zygis and Thymus Hyemalis Essential Oils. Food Control . 2008;19(7):681–687. doi: 10.1016/j.foodcont.2007.07.007. [DOI] [Google Scholar]
- 62.Yakoubi S., Cherrat A., Diouri M., El Hilali F., Zair T. Chemical Composition and Antibacterial Activity of Thymus Zygis Subsp. Gracilis (Boiss.) R. Morales Essential Oils From Morocco. Mediterr J Chem . 2014;3(1):746–758. doi: 10.13171/mjc.3.1.2014.01.04.18. [DOI] [Google Scholar]
- 63.Lagha R., Ben Abdallah F., AL-Sarhan B. O., Al-Sodany Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated From UTI Patients. Molecules . 2019;24(6):p. 1161. doi: 10.3390/molecules24061161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sedikelo G. K., Lenetha G. G., Malebo N. J. Chromatography-Mass Spectrometry and Chemical Characteristics of Thymus Zygis and Cymbopogon Winterianus Essential Oils: Possible Insect Repellents. Scientific African . 2022;15:p. e01095. doi: 10.1016/j.sciaf.2022.e01095. [DOI] [Google Scholar]
- 65.Ballester-Costa C., Sendra E., Fernández-López J., Pérez-Álvarez J., Viuda-Martos M. Chemical Composition and In Vitro Antibacterial Properties of Essential Oils of Four Thymus Species From Organic Growth. Industrial Crops and Products . 2013;50:304–311. doi: 10.1016/j.indcrop.2013.07.052. [DOI] [Google Scholar]
- 66.Gonçalves M. J., Cruz M. T., Cavaleiro C., Lopes M. C., Salgueiro L. Chemical, Antifungal and Cytotoxic Evaluation of the Essential Oil of Thymus Zygis Subsp. Sylvestris. Industrial Crops and Products . 2010;32(1):70–75. doi: 10.1016/j.indcrop.2010.03.005. [DOI] [Google Scholar]
- 67.Jaafari A., Mouse H. A., Rakib E. M., et al. Chemical Composition and Antitumor Activity of Different Wild Varieties of Moroccan Thyme. Rev Bras Farmacogn . 2007;17(4):477–491. doi: 10.1590/S0102-695X2007000400002. [DOI] [Google Scholar]
- 68.Lemrhari A., Zouhair R., Elidrissi M., Amechrouq E. M., El Kahkahi R. Chemical Composition and Differentiation of Essential Oils of Morocco’s Different Varieties of Thyme. Glob J Pure Appl Chem Res . 2015;3:24–34. [Google Scholar]
- 69.Amiri H. Essential Oils Composition and Antioxidant Properties of Three Thymus Species. Evidence-Based Complementary and Alternative Medicine . 2012;2012:1–8. doi: 10.1155/2012/728065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Satmi F. R. S., Hossain M. A. In Vitro Antimicrobial Potential of Crude Extracts and Chemical Compositions of Essential Oils of Leaves of Mentha Piperita L Native to the Sultanate of Oman. Pacific Science Review A: Natural Science and Engineering . 2016;18(2):103–106. doi: 10.1016/j.psra.2016.09.005. [DOI] [Google Scholar]
- 71.Bouyahya A., Dakka N., Talbaoui A., et al. Correlation Between Phenological Changes, Chemical Composition and Biological Activities of the Essential Oil From Moroccan Endemic Oregano (Origanum Compactum Benth) Industrial Crops and Products . 2017;108:729–737. doi: 10.1016/j.indcrop.2017.07.033. [DOI] [Google Scholar]
- 72.El amrani S., El Ouali Lalami A., Ez zoubi Y., Moukhafi K., Bouslamti R., Lairini S. Evaluation of Antibacterial and Antioxidant Effects of Cinnamon and Clove Essential Oils From Madagascar. Materials Today: Proceedings . 2019;13:762–770. doi: 10.1016/j.matpr.2019.04.038. [DOI] [Google Scholar]
- 73.Číž M., Čížová H., Denev P., Kratchanova M., Slavov A., Lojek A. Different Methods for Control and Comparison of the Antioxidant Properties of Vegetables. Food Control . 2010;21(4):518–523. doi: 10.1016/j.foodcont.2009.07.017. [DOI] [Google Scholar]
- 74.Baali F., Boumerfeg S., Napoli E., et al. Chemical Composition and Biological Activities of Essential Oils From Two Wild Algerian Medicinal Plants: Mentha Pulegium L. and Lavandula stoechas L. Journal of Essential Oil Bearing Plants . 2019;22(3):821–837. doi: 10.1080/0972060X.2019.1642800. [DOI] [Google Scholar]
- 75.Raeisi M., Hashemi M., Aminzare M., Afshari A., Zeinali T., Jannat B. An Investigation of the Effect of Zataria Multiflora Boiss and Mentha Piperita Essential Oils to Improve the Chemical Stability of Minced Meat. Veterinary World . 2018;11(12):1656–1662. doi: 10.14202/vetworld.2018.1656-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benabdallah A., Rahmoune C., Boumendjel M., Aissi O., Messaoud C. Total Phenolic Content and Antioxidant Activity of Six Wild Mentha Species (Lamiaceae) From Northeast of Algeria. Asian Pacific Journal of Tropical Biomedicine . 2016;6(9):760–766. doi: 10.1016/j.apjtb.2016.06.016. [DOI] [Google Scholar]
- 77.Stringaro A., Colone M., Angiolella L. Antioxidant, Antifungal, Antibiofilm, and Cytotoxic Activities of Mentha Spp. Essential Oils. Medicines . 2018;5(4):p. 112. doi: 10.3390/medicines5040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sebai E., Abidi A., Serairi R., et al. Essential Oil of Mentha Pulegium Induces Anthelmintic Effects and Reduces Parasite-Associated Oxidative Stress in Rodent Model. Experimental Parasitology . 2021;225:p. 108105. doi: 10.1016/j.exppara.2021.108105. [DOI] [PubMed] [Google Scholar]
- 79.Kamkar A., Javan A. J., Asadi F., Kamalinejad M. The Antioxidative Effect of Iranian Mentha Pulegium Extracts and Essential Oil in Sunflower Oil. Food and Chemical Toxicology . 2010;48(7):1796–1800. doi: 10.1016/j.fct.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Gharib F. A., Mansour K. H., Ahmed E. Z., Galal T. M. Heavy Metals Concentration, and Antioxidant Activity of the Essential Oil of the Wild Mint (Mentha longifolia L.) in the Egyptian Watercourses. International Journal of Phytoremediation . 2021;23(6):641–651. doi: 10.1080/15226514.2020.1847035. [DOI] [PubMed] [Google Scholar]
- 81.Yakoubi R., Megateli S., Hadj Sadok T., Bensouici C., Bağci E. A Synergistic Interactions of Algerian Essential Oils of Laurus Nobilis L., Lavandula Stoechas L. and Mentha Pulegium L. on Anticholinesterase and Antioxidant Activities. Biocatalysis and Agricultural Biotechnology . 2021;31:p. 101891. doi: 10.1016/j.bcab.2020.101891. [DOI] [Google Scholar]
- 82.Vitali L. A., Dall’Acqua S., Maggi F., et al. Antimicrobial and Antioxidant Activity of the Essential Oil From the Carpathian Thymus alternans Klokov. Natural Product Research . 2017;31(10):1121–1130. doi: 10.1080/14786419.2016.1224874. [DOI] [PubMed] [Google Scholar]
- 83.Jarić S., Mitrović M., Pavlović P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evidence-Based Complementary and Alternative Medicine . 2015;2015:1–10. doi: 10.1155/2015/101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dandlen S. A., Lima A. S., Mendes M. D., et al. Antioxidant Activity of Six Portuguese Thyme Species Essential Oils. Flavour and Fragrance Journal . 2010;25(3):150–155. doi: 10.1002/ffj.1972. [DOI] [Google Scholar]
- 85.Ballester-Costa C., Sendra E., Fernández-López J., Pérez-Álvarez J., Viuda-Martos M. Assessment of Antioxidant and Antibacterial Properties on Meat Homogenates of Essential Oils Obtained From Four Thymus Species Achieved From Organic Growth. Foods . 2017;6(8):59–11. doi: 10.3390/foods6080059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amarti F., El Ajjouri M., Ghanmi M., et al. Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oils of Thymys Zygis of Morocco. Phytothérapie . 2011;9(3):149–157. doi: 10.1007/s10298-011-0625-6. [DOI] [Google Scholar]
- 87.Đurović S., Micić D., Pezo L., et al. Influence of the Mowing and Drying on the Quality of the Peppermint (Mentha X Piperita L.) Essential Oil: Chemical Profile, Thermal Properties, and Biological Activity. Industrial Crops and Products . 2022;177:p. 114492. doi: 10.1016/j.indcrop.2021.114492. [DOI] [Google Scholar]
- 88.Brahmi F., Abdenour A., Bruno M., et al. Chemical Composition and In Vitro Antimicrobial, Insecticidal and Antioxidant Activities of the Essential Oils of Mentha Pulegium L. and Mentha Rotundifolia (L.) Huds Growing in Algeria. Industrial Crops and Products . 2016;88:96–105. doi: 10.1016/j.indcrop.2016.03.002. [DOI] [Google Scholar]
- 89.Hamad Al-Mijalli S., Elsharkawy E. R., Abdallah E. M., et al. Determination of Volatile Compounds of Mentha Piperita and Lavandula Multifida and Investigation of Their Antibacterial, Antioxidant, and Antidiabetic Properties. Evidence-Based Complementary and Alternative Medicine . 2022;2022:1–9. doi: 10.1155/2022/9306251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boudid S., Idaomar M., Zhiri A., Baudoux D., Skali N. S., Abrini J. Thymus Essential Oils: Chemical Composition and In Vitro Antioxidant and Antibacterial Activities. Congrs Int Biochim . 2006;324 [Google Scholar]
- 91.Safaei-Ghomi J., Ebrahimabadi A. H., Djafari-Bidgoli Z., Batooli H. GC/MS Analysis and In Vitro Antioxidant Activity of Essential Oil and Methanol Extracts of Thymus Caramanicus Jalas and its Main Constituent Carvacrol. Food Chemistry . 2009;115(4):1524–1528. doi: 10.1016/j.foodchem.2009.01.051. [DOI] [Google Scholar]
- 92.Pavlić B., Teslić N., Zengin G., et al. Antioxidant and Enzyme-Inhibitory Activity of Peppermint Extracts and Essential Oils Obtained by Conventional and Emerging Extraction Techniques. Food Chemistry . 2021;338:p. 127724. doi: 10.1016/j.foodchem.2020.127724. [DOI] [PubMed] [Google Scholar]
- 93.Benabed K., Gourine N., Ouinten M., Bombarda I., Yousfi M. Chemical Composition, Antioxidant and Antimicrobial Activities of the Essential Oils of Three Algerian Lamiaceae Species. Current Nutrition & Food Science . 2017;13(2):97–109. doi: 10.2174/1573401313666170104105521. [DOI] [Google Scholar]
- 94.Ramadan K. M. A., Ashoush I. S., El-Batawy O. I. Comparative Evaluation of Three Essential Oils as Functional Antioxidants and Natural Flavoring Agents in Ice Cream. World Applied Sciences Journal . 2013:159–166. doi: 10.5829/idosi.wasj.2013.23.02.13065. [DOI] [Google Scholar]
- 95.Ceylan R., Zengin G., Uysal S., et al. GC-MS Analysis and In Vitro Antioxidant and Enzyme Inhibitory Activities of Essential Oil From Aerial Parts of Endemic Thymus spathulifolius Hausskn. et Velen. Journal of Enzyme Inhibition and Medicinal Chemistry . 2015;31(6):983–990. doi: 10.3109/14756366.2015.1077822. [DOI] [PubMed] [Google Scholar]
- 96.Dos Santos K. P., Sedano-Partida M. D., Sala-Carvalho W. R., Loureiro B. O. S. J., da Silva-Luz C. L., Furlan C. M. Biological Activity of Hyptis Jacq. (Lamiaceae) is Determined by the Environment. Industrial Crops and Products . 2018;112:705–715. doi: 10.1016/j.indcrop.2017.12.065. [DOI] [Google Scholar]
- 97.Rahimmalek M., Afshari M., Sarfaraz D., Miroliaei M. Using HPLC and Multivariate Analyses to Investigate Variations in the Polyphenolic Compounds as Well as Antioxidant and Antiglycative Activities of Some Lamiaceae Species Native to Iran. Industrial Crops and Products . 2020;154:p. 112640. doi: 10.1016/j.indcrop.2020.112640. [DOI] [Google Scholar]
- 98.Abdelli M., Moghrani H., Aboun A., Maachi R. Algerian Mentha Pulegium L. Leaves Essential Oil: Chemical Composition, Antimicrobial, Insecticidal and Antioxidant Activities. Industrial Crops and Products . 2016;94:197–205. doi: 10.1016/j.indcrop.2016.08.042. [DOI] [Google Scholar]
- 99.Ouakouak H., Chohra M., Denane M. Chemical Composition, Antioxidant Activities of the Essential Oil of Mentha Pulegium L, South East of Algeria. International Letters of Natural Sciences . 2015;39:49–55. doi: 10.18052/www.scipress.com/ilns.39.49. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are included within the article.


