Abstract
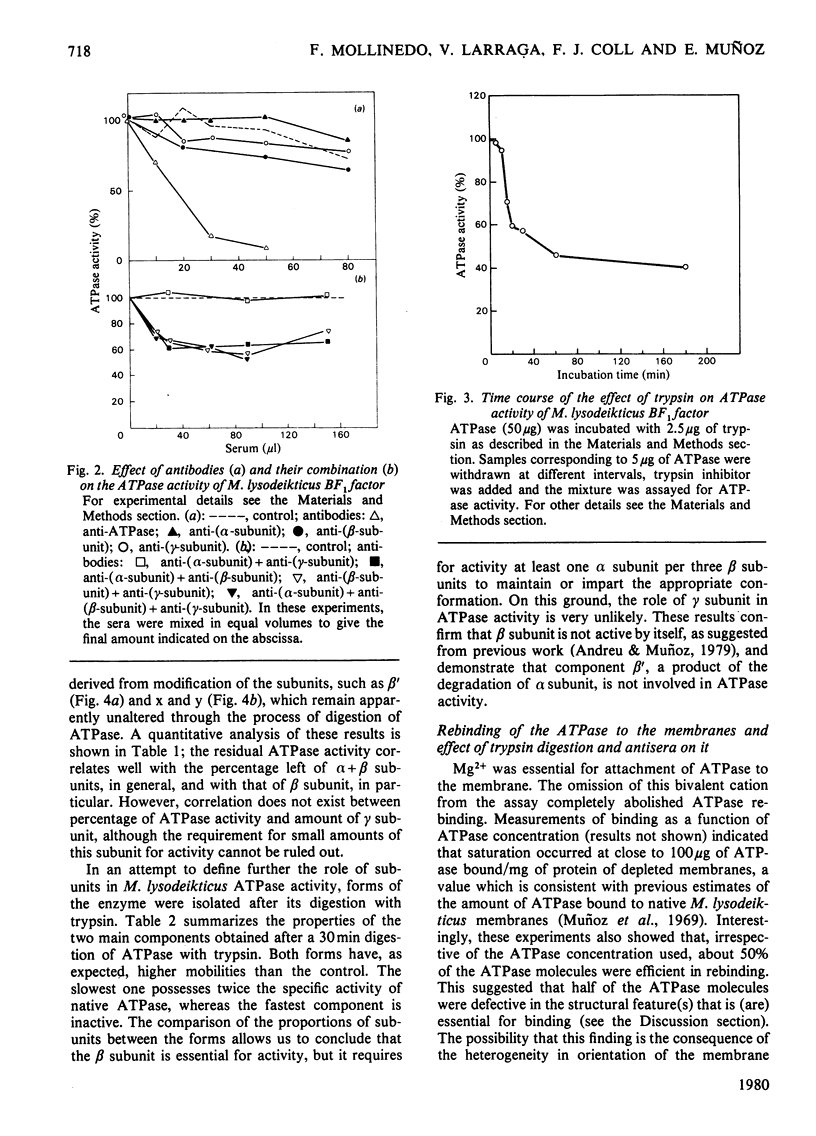
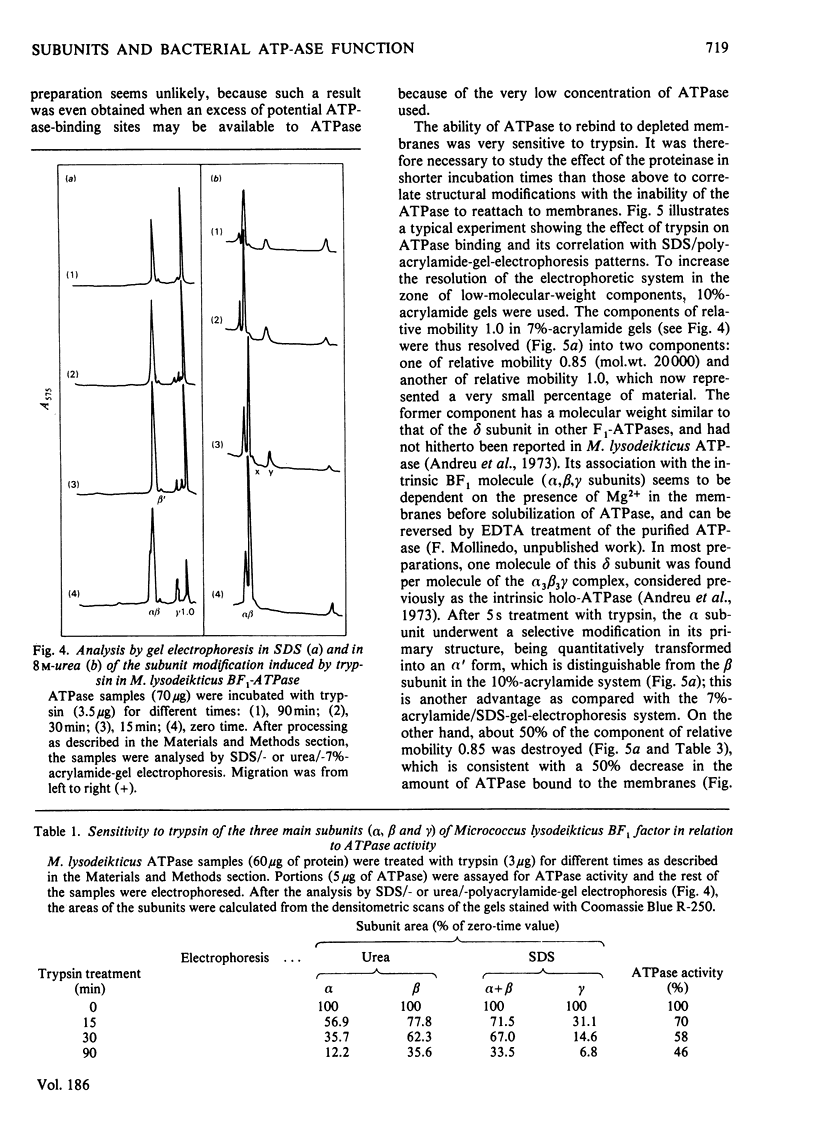
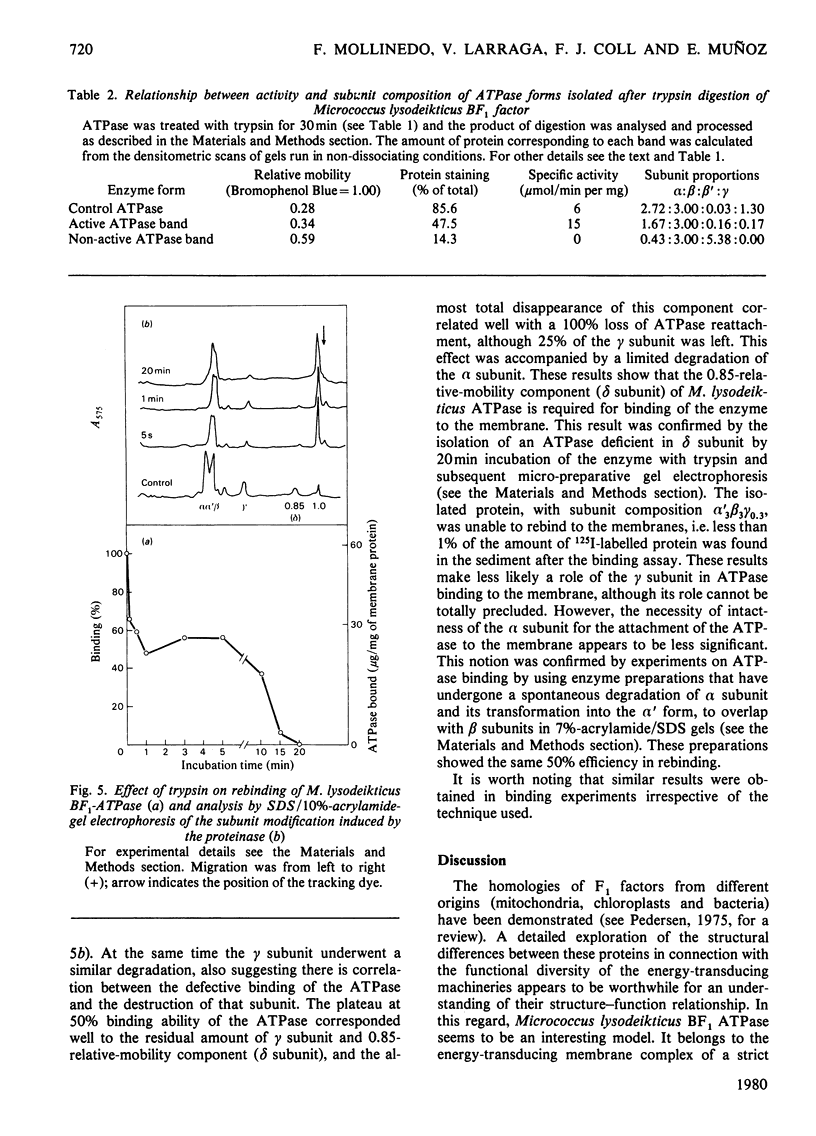
An energy-transducing adenosine triphosphatase (ATPase, EC 3.6.1.3) that contains an extra polypeptide (delta) as well as three intrinsic subunits (alpha, beta, gamma) was purified from Micrococcus lysodeikticus membranes. The apparent subunit stoichiometry of this soluble ATPase complex is alpha 3 beta 3 gamma delta. The functional role of the subunits was studied by correlating subunit sensitivity to trypsin and effect of antibodies raised against holo-ATPase and its alpha, beta and gamma subunits with changes in ATPase activity and ATPase rebinding to membranes. A form of the ATPase with the subunit proportions 1.67(alpha):3.00(beta:0.17(gamma) was isolated after trypsin treatment of purified ATPase. This form has more than twice the specific activity of native enzyme. Other forms with less relative proportion of alpha subunits and absence of gamma subunit are not active. Of the antisera to subunits, only anti-(beta-subunit) serum shows a slight inhibitory effect on ATPase activity, but its combination with either anti-(alpha-subunit) or anti-(gamma-subunit) serum increases the effect. The results suggest that beta subunit is required for full ATPase activity, although a minor proportion of alpha and perhaps gamma subunit(s) is also required, probably to impart an active conformation to the protein. The additional polypeptide not hitherto described in Micrococcus lysodeikticus ATPase had a molecular weight of 20 000 and was found to be involved in ATPase binding to membranes. This 20 000-dalton component can be equated with the delta subunit of other energy-transducing ATPases and its association with the (alpha, beta, gamma) M. lysodeikticus ATPase complex appears to be dependent on bivalent cations. The present results do not preclude the possibility that the gamma subunit also plays a role in ATPase binding, in which, however, the major subunits do not seem to play a role.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A., Jensen C., Morris D. H. Role of Mg2+ ions in the subunit structure and membrane binding properties of bacterial energy transducing ATPase. Biochem Biophys Res Commun. 1976 Apr 5;69(3):804–811. doi: 10.1016/0006-291x(76)90946-3. [DOI] [PubMed] [Google Scholar]
- Abrams A., Jensen C., Morris D. Studies of substructure and tightly bound nucleotide in bacterial membrane ATPase. J Supramol Struct. 1975;3(3):261–274. doi: 10.1002/jss.400030309. [DOI] [PubMed] [Google Scholar]
- Abrams A., Morris D., Jensen C. Chymotryptic conversion of bacterial membrane ATPase to an active form with modified alpha chains and defective membrane binding properties. Biochemistry. 1976 Dec 14;15(25):5560–5566. doi: 10.1021/bi00670a021. [DOI] [PubMed] [Google Scholar]
- Andreu J. M., Carreira J., Muñoz E. Isolation and partial characterization of the two major subunits of the BF1 factor (ATPase) from Micrococcus lysodeikticus and evidence for their glycoprotein nature. FEBS Lett. 1976 Jun 1;65(2):198–203. doi: 10.1016/0014-5793(76)80479-6. [DOI] [PubMed] [Google Scholar]
- Andreu J. M., Muñoz E. Micrococcus lysodeikticus ATPase. Purification by preparative gel electrophoresis and subunit structure studied by urea and sodium dodecylsulfate gel electrophoresis. Biochim Biophys Acta. 1975 May 15;387(2):228–233. doi: 10.1016/0005-2728(75)90105-x. [DOI] [PubMed] [Google Scholar]
- Andreu J. M., Muñoz E. Molecular properties of random coil and refolded forms of alpha and beta subunits of an energy transducing ATPase from bacterial membranes. Biochemistry. 1979 May 1;18(9):1836–1844. doi: 10.1021/bi00576a032. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Binding of the Ca2+,Mg2+-activated adenosine triphosphatase of Escherichia coli to phospholipid vesicles. Can J Biochem. 1978 Jun;56(6):559–564. doi: 10.1139/o78-085. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Subunit composition, function, and spatial arrangement in the Ca2+-and Mg2+-activated adenosine triphosphatases of Escherichia coli and Salmonella typhimurium. Arch Biochem Biophys. 1975 Mar;167(1):311–321. doi: 10.1016/0003-9861(75)90467-1. [DOI] [PubMed] [Google Scholar]
- Carreira J., Andreu J. M., Muñoz E. Differential sensitivity to trypsin digestion of purified forms of Micrococcus lysodeikticus ATPase (BFI). A study of their structural and conformational differences and mechanism of conversion. Biochim Biophys Acta. 1977 Jun 24;492(2):387–398. doi: 10.1016/0005-2795(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Carreira J., Andreu J. M., Nieto M., Muñoz E. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. ISolation of two forms of the enzyme complex and correlation between ezymatic stability, latency and activity. Mol Cell Biochem. 1976 Feb 16;10(2):67–76. doi: 10.1007/BF01742200. [DOI] [PubMed] [Google Scholar]
- Carreira J., Muńoz E., Andreu J. M., Nieto M. Micrococcus lysodeikticus membrane ATPase. Effect of trypsin on stimulation of a purified form of the enzyme and idenfification of its natural inhibitor. Biochim Biophys Acta. 1976 Jun 4;436(1):183–189. doi: 10.1016/0005-2736(76)90229-7. [DOI] [PubMed] [Google Scholar]
- Deters D. W., Racker E., Nelson N., Nelson H. Partial resolution of the enzymes catalyzing photophosphorylation. XV. Approaches to the active site of coupling factor I. J Biol Chem. 1975 Feb 10;250(3):1041–1047. [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Futai M. Reconstitution of ATPase activity from the isolated alpha, beta, and gamma subunits of the coupling factor, F1, of Escherichia coli. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1231–1237. doi: 10.1016/0006-291x(77)91138-x. [DOI] [PubMed] [Google Scholar]
- Höckel M., Hulla F. W., Risi S., Dose K. Me2+-(13 S) ATPase from Micrococcus sp. ATCC 398E. The effect of trypsin on the purified enzyme. Biochim Biophys Acta. 1976 May 13;429(3):1020–1028. doi: 10.1016/0005-2744(76)90346-6. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Nelson N., Gutnick D. L. Differentiation between mutants of Escherichia coli K defective in oxidative phosphorylation. Biochim Biophys Acta. 1975 Sep 8;396(3):347–359. doi: 10.1016/0005-2728(75)90141-3. [DOI] [PubMed] [Google Scholar]
- Kozlov I. A., Skulachev V. P. H+-Adenosine triphosphatase and membrane energy coupling. Biochim Biophys Acta. 1977 Jun 21;463(1):29–89. doi: 10.1016/0304-4173(77)90003-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larraga V., Muñoz E. Molecular organization in bacterial cell membranes. Specific labelling and topological distribution of glycoproteins and proteins in Streptomyces albus membranes. Eur J Biochem. 1975 May;54(1):207–218. doi: 10.1111/j.1432-1033.1975.tb04130.x. [DOI] [PubMed] [Google Scholar]
- Larson R. J., Smith J. B. Assembly of the catalytic unit of the Escherichia coli membrane ATPase in vitro requires the gamma chain. Biochemistry. 1977 Sep 20;16(19):4266–4270. doi: 10.1021/bi00638a021. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Barlow V. Molecular weight, amino acid composition and other properties of membrane-bound ATPase from Bacillus megaterium KM. Biochim Biophys Acta. 1973 Jan 26;291(2):480–488. doi: 10.1016/0005-2736(73)90499-9. [DOI] [PubMed] [Google Scholar]
- Munoz E., Freer J. H., Ellar D. J., Salton M. R. Membrane-associated ATPase activity from Micrococcus lysodeikticus. Biochim Biophys Acta. 1968 Apr 29;150(3):531–533. doi: 10.1016/0005-2736(68)90156-9. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Nachbar M. S., Schor M. T., Salton M. R. Adenosinetriphosphatase of Micrococcus lysodeikticus: selective release and relationship to membrane structure. Biochem Biophys Res Commun. 1968 Aug 13;32(3):539–546. doi: 10.1016/0006-291x(68)90696-7. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- Nelson N., Deters D. W., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 8. Properties of isolated subunits of coupling factor 1 from spinach chloroplasts. J Biol Chem. 1973 Mar 25;248(6):2049–2055. [PubMed] [Google Scholar]
- Nelson N., Kanner B. I., Gutnick D. L. Purification and properties of Mg2+-Ca2+ adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2720–2724. doi: 10.1073/pnas.71.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Karny O. The role of delta subunit in the coupling activity of chloroplast coupling factor 1. FEBS Lett. 1976 Nov;70(1):249–253. doi: 10.1016/0014-5793(76)80768-5. [DOI] [PubMed] [Google Scholar]
- Nelson N. Structure and function of chloroplast ATPase. Biochim Biophys Acta. 1976 Nov 30;456(3-4):314–338. doi: 10.1016/0304-4173(76)90003-3. [DOI] [PubMed] [Google Scholar]
- Nieto M., Muñoz E., Carreira J., Andreu J. M. Conformational and molecular responses to pH variation of the purified membrane adenosine triphosphatase of Micrococcus lysodeikticus. Biochim Biophys Acta. 1975 Dec 16;413(3):394–414. doi: 10.1016/0005-2736(75)90123-6. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Schor M. T. Subunit structure and properties of two forms of adenosine triphosphatase released from Micrococcus lysodeikticus membranes. Biochem Biophys Res Commun. 1972 Oct 17;49(2):350–357. doi: 10.1016/0006-291x(72)90417-2. [DOI] [PubMed] [Google Scholar]
- Senior A. E. The structure of mitochondrial ATPase. Biochim Biophys Acta. 1973 Dec 31;301(3):249–277. doi: 10.1016/0304-4173(73)90006-2. [DOI] [PubMed] [Google Scholar]
- Serrahima-Zieger M., Monteil H. Membrane ATPase of Bacillus subtilis. I. Purification and properties. Biochim Biophys Acta. 1978 Jun 8;502(3):445–457. doi: 10.1016/0005-2728(78)90077-4. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Sternweis P. C. Restoration of coupling factor activity to Escherichia coli ATPase missing the delta subunit. Biochem Biophys Res Commun. 1975 Feb 3;62(3):764–771. doi: 10.1016/0006-291x(75)90465-9. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Smith J. B. Characterization of the purified membrane attachment (beta) subunit of the proton translocating adenosine triphosphatase from Escherichia coli. Biochemistry. 1977 Sep 6;16(18):4020–4025. doi: 10.1021/bi00637a013. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C. The epsilon subunit of Escherichia coli coupling factor 1 is required for its binding to the cytoplasmic membrane. J Biol Chem. 1978 May 10;253(9):3123–3128. [PubMed] [Google Scholar]
- Vogel G., Steinhart R. ATPase of Escherichia coli: purification, dissociation, and reconstitution of the active complex from the isolated subunits. Biochemistry. 1976 Jan 13;15(1):208–216. doi: 10.1021/bi00646a032. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Salton M. R. Antibody to adenosine triphosphatase from membranes of Micrococcus lysodeikticus. Biochemistry. 1970 Jul 21;9(15):3034–3040. doi: 10.1021/bi00817a015. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. A highly stable adenosine triphosphatase from a thermophillie bacterium. Purification, properties, and reconstitution. J Biol Chem. 1975 Oct 10;250(19):7910–7916. [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. Reconstitution of adenosine triphosphatase of thermophilic bacterium from purified individual subunits. J Biol Chem. 1977 May 25;252(10):3480–3485. [PubMed] [Google Scholar]



