Abstract
Hoof disorders are crucial factors leading to dairy cattle culling. These disorders are difficult to detect, diagnose, and record, causing animal health problems and serious economic losses. The coronet score is a new hoof health indicator developed by the Dairy Herd Improvement Program of Japan. This score is assigned on a 5‐point scale and can easily be determined by examining the degree of redness and swelling around the hooves. To determine whether coronet scores can be used to genetically improve hoof health, we investigated non‐genetic factors in the score, developed a genetic analysis model, and estimated genetic parameters. Coronet scores were collected from 1280 herds in 28 prefectures once a month from 2014 to 2021. Furthermore, 1,598,878 test‐day records of 94,951 cows from the first‐ to third‐parity and pedigree information of 216,416 individuals were used in the analysis. Results revealed that herd size, parity, age, test month, and lactation stage influenced the coronet score. Additionally, the heritability of the coronet score estimated via the herd‐test‐day model was 0.027, and genetic trends deteriorated in sires and cows. Overall, we demonstrated that coronet score is a heritable trait, suggesting that hoof health could be genetically improved by selection for coronet score.
Keywords: coronet score, dairy cow, genetic parameter, hoof health
1. INTRODUCTION
Hoof health of dairy cows is one of the serious concerns in dairy farming. Genetic improvements in dairy cows have contributed to significant increase in milk production; however, milk yield have unfavorable genetic correlations with various nonproduction traits, and the decline in health associated with selection for highly productive animals has become a major problem (Abdelsayed et al., 2017; Egger‐Danner et al., 2015). Hoof disorders have the highest incidence and culling frequency, following mastitis and reproductive disorders in dairy cows (Abdelsayed et al., 2017; Shabalina et al., 2020). Mastitis and reproductive disorders are the most prominent diseases directly linked to productivity; they have been incorporated into the selection index in various countries (Brito et al., 2021; Miglior et al., 2017). In contrast, hoof diseases tend to be overlooked and underestimated until they become severe (Bennett et al., 2014; Espejo et al., 2006). Hoof‐related disorders lead to reduced milk production and fertility (Buch et al., 2011; Enting et al., 1997; Huxley, 2013). Economic losses due to these disorders are estimated at $76 to $533 per case, including treatment and culling (Dolecheck & Bewley, 2018). Additionally, hoof‐related disorders lead to long‐term pain and stress in cows (Whay & Shearer, 2017). Therefore, it is a serious concern that requires immediate attention from both economic and animal welfare perspectives.
Hoof disorders are heritable, which implies that genetic improvements can reduce their incidence. Previous studies have used linear model analysis to estimate the heritability of hoof disorders and revealed heritability ranging from <0.01 to 0.14 (Pérez‐Cabal & Charfeddine, 2015; van der Linde et al., 2010; van der Spek et al., 2013; van der Waaij et al., 2005). Collecting high‐quality data on a large scale is necessary to obtain reliable estimated breeding values (EBVs) for effective genetic improvement. Hence, systems documenting hoof health have been established in several countries, including the Nordic countries and the Netherlands, and the records are used for genetic evaluation (Heringstad et al., 2018).
In Japan, records of hoof disease treatment are collected by veterinarians. However, the data cannot be used for genetic evaluation because the aggregation methods and formats used are not standardized for each region, making direct selection of hoof disorders difficult. In such cases, indirect selection methods are considered. For example, indirect genetic improvement methods through locomotion or lameness scoring have been investigated in other countries. The heritability of these traits is estimated to range between 0.03 and 0.14, and a favorable genetic correlation with hoof disorder can be estimated (Chapinal et al., 2013; van der Linde et al., 2010; Weber et al., 2013). However, these methods have some limitations, such as the inability to record the data of individual animals repeatedly and labor‐intensive procedures (Heringstad et al., 2018; Weber et al., 2013). Therefore, establishing a system that frequently collects large‐scale data about hoof health status and developing evaluation indicators that can be easily recorded are necessary (Abdelsayed et al., 2017).
We focused on the “coronet score,” which can easily determine the health status of a hoof. The coronet score has been used as a hoof health indicator in the Japan Dairy Herd Improvement Program. The scale ranges from 1 (normal) to 5 (severe) and is assigned on the basis of the degree of redness and swelling around the hooves. Coronet scores are recorded at a monthly test day, and these large‐scale data could be utilized in genetic improvement programs. However, the genetic characteristics of the coronet scores and the possibility of genetic evaluation of hoof health using test‐day (TD) records have not yet been investigated. Therefore, the objectives of this study were to develop a model for genetic analysis of coronet scores by clarifying nongenetic factors and to estimate genetic parameters. The findings of this study could provide novel insights into strategies for improving hoof health.
2. MATERIALS AND METHODS
2.1. Ethics statement
Institutional approval for animal experiments was not required for this study because the data used for analysis were obtained from existing databases.
2.2. Data collection
Monthly TD records of Holstein cows collected by the Livestock Improvement Association of Japan (LIAJ) from April 2014 to November 2021 were used in this study. Coronet scores were recorded by the milk‐recording supervisor or farmer. Coronet scores were determined on a scale of 1 to 5 (1 = no skin redness or swelling; 2 = mild skin redness; 3 = mild skin redness and swelling; 4 = severe skin redness and large swelling; 5 = severe skin redness, large swelling, and disorders such as hunched posture), considering the degree of redness and swelling around the coronary band, dew claw, and heel bulb of the hind hoof. Hind limbs were scored because hoof disease occurs more often in the hind than in the fore limbs (Chapinal et al., 2013; Pérez‐Cabal & Charfeddine, 2015). If the scores differed between the left and right feet, the higher score was used. In this study, scores 4 and 5 were combined with score 3, which indicates the need for attention to hoof health, because the proportion of scores ≥4 was extremely low. Thus, we defined the coronet score as an ordered categorical trait with three categories (scores 1, 2, and ≥3). To ensure that records were collected appropriately, records were extracted from herds with at least 3 years of recording for first‐to third‐parity cows and with at least one record of a score ≥3. TD records from 6 to 305 days in milk (DIM) of the first, second, and third parities were included, with ages at calving of 18–35, 30–55, and 42–75 months, respectively. Each lactation period had at least three records, including at least one record for early (6–105 DIM), mid (106–205 DIM), and late (206–305 DIM) lactation. There were at least two cows for each herd‐test‐day (HTD). Cows with unknown parents and two or more different herd records for the same cow were removed. The final dataset comprised 1,598,878 records of 94,951 cows collected from 1280 herds in 28 prefectures. Table 1 shows herd classification by herd size and housing system. Significant changes were not observed in the frequency of the coronet scores compared with those in the dataset before editing. Pedigree information used for the genetic analysis was provided by the Holstein Cattle Association of Japan, included 216,416 individuals going back three generations from animals with coronet score records.
TABLE 1.
Cross‐tabulation of herd size and housing system for the 1280 herds used in the analysis.
| Herd size | |||
|---|---|---|---|
| ≤100 cows | >100 cows | ||
| Housing system | Tie stall | 864 | 111 |
| Free stall | 96 | 89 | |
| Free barn | 58 | 58 | |
| Other | 2 | 2 | |
2.3. Statistical analysis
The following mathematical model was used to examine environmental effects on coronet scores,
where is the observation of a three category coronet score (score 1, 2, and ≥3), is the overall mean, is the th herd size effect ( = 1 or 2; ≤100 and >100 cows), is the th housing system effect ( = 1 to 4; tie stall, free stall, free barn, and other systems), is the th parity‐age of calving group effect ( = 1–82; 18–22, 23, 24, … 41, 42, and 43–45 months for first parity; 30–34, 35, 36, … 59, 60, and 61–65 months for second parity; and 42–46, 47, 48, … 75, 76–79, and 80–85 months for third parity), is the th test month effect ( = 1 to 12; January to December), is the th lactation stage effect ( = 1 to 10; 6 to 305 DIM were classified every 30 days), and is the random error. Least square analysis based on sum of squares (Type III) and calculation of least square means were performed in R Studio® (R version 4.1.2) using the “car” and “lsmeans” packages, respectively. Based on the results, two models for genetic analysis were generated. The herd effect explains not only the contemporary group effect but also the herd size and housing system effect. A linear model that considered HTD or herd‐test‐year (HY) as a fixed effect was developed. The variance components were estimated by applying these two models. The models are as follows:
HTD model
HY model
where and are the observations of a three‐category coronet score (score 1, 2, and ≥3), is the fixed effect of the th HTD ( = 1–86339) for the HTD model, is the fixed effect of the th HY ( = 1–8535) for the HY model, is the fixed effect of the th parity‐age group ( = 1–82), is the fixed effect of the th test month ( = 1–12), is the fixed effect of the th lactation stage ( = 1–10), is the random additive genetic effect, is the random permanent environmental effect, and and is the random error. Goodness of fit was assessed using the deviance information criterion (DIC) (Spiegelhalter et al., 2002).
Estimates of the variance components were calculated using the Gibbs sampling method in the GIBBSF90+ program (http://nce.ads.uga.edu/wiki/doku.php?id=readme.gibbsf90plus). The number of iterations was set to 100,000, and the first 50,000 samples were discarded as burn‐in. The latter 50,000 samples were kept at 10 intervals, and the posterior mean, standard deviation, and 95% highest probability density interval for each parameter were calculated. Convergence was visually confirmed using trace plots (Figure 1). Heritability () and repeatability () were calculated using the following formulae:
where is the posterior mean of additive genetic variance, is the posterior mean of permanent environmental variance, and is the random residual variance. The HTD model was a better fit than the HY model and, therefore, estimates of genetic parameters based on the HTD model were used to calculate the EBV using the BLUPF90+ program (http://nce.ads.uga.edu/wiki/doku.php?id=readme.blupf90plus). Furthermore, the EBVs of 94,951 cows with records and 739 sires with ≥15 daughters with records were averaged by birth year to determine genetic trends.
FIGURE 1.
Time‐series plots for estimating additive genetic variance using the herd‐test‐day model. The number of iterations was set to 100,000, and the first 50,000 samples were discarded as burn‐in. The arrows indicate 5,000 samples stored at every 10 intervals of the latter 50,000 samples.
3. RESULTS
3.1. Frequency of coronet scores
The frequency of the coronet scores used in this study was highest for score 1 (healthy) and decreased as the score increased (Figure 2). The frequency of score 1 decreased as the number of parities increased, and the frequency of score 2 was similar for all parities. For higher scores (scores 3–5), the frequency was higher for later parities. This indicates that hoof health worsens as parity progresses. The frequency of each score was approximately 45%–51% for score 1, 35% for score 2, 12%–17% for score 3, 0.5%–2.5% for score 4, and 0.1%–0.5% for score 5. In this study, scores from 3 to 5, which require attention to hoof health, were combined and analyzed as a three‐level categorical trait—scores 1, 2, and ≥3.
FIGURE 2.
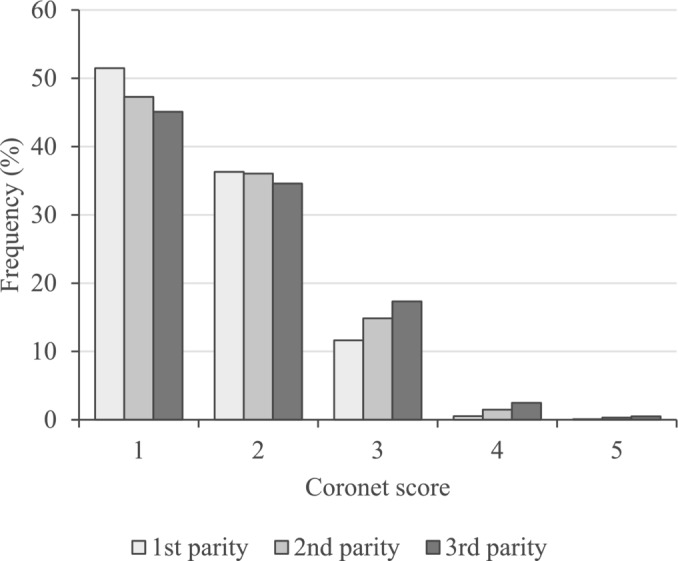
Frequency of the coronet scores. The frequencies of coronet scores of 4 and 5 were very low. In subsequent analysis, scores ≥3 were combined and defined as 3‐scale (score 1, 2, and ≥3) categorical traits.
3.2. Analysis of environmental factors and model selection
All factors considered by the least square analysis were significant (p < 0.001). In terms of herd size, the scores were higher in small than in large herds (Figure 3a). Among the housing systems, the scores were higher in tie stalls than in free stalls or barns (Figure 3b). In the parity‐age group, the scores increased with age for each parity (Figure 3c). In terms of the test months, the scores were significantly higher from August to November than from January to May and December, although the difference between the levels was marginal (Figure 3d). In terms of the lactation stage, the differences between levels were small but were significantly higher from early to mid‐lactation (Figure 3e).
FIGURE 3.
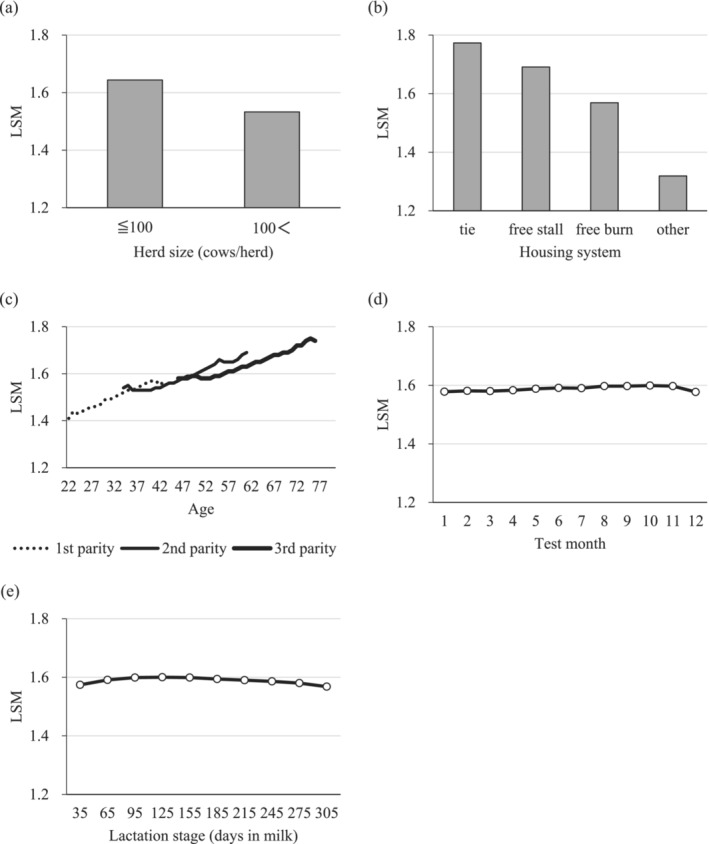
Least square mean (LSM) of each environmental factor: (a) herd size, (b) housing system, (c) parity‐age, (d) test month, and (e) lactation stage.
The estimated variance components, heritability, repeatability, and DIC values for each model are presented in Table 2. Estimates of additive genetic and permanent environmental variances were similar between the HTD and HY models. Contrastingly, the estimated residual variance and DIC were smaller in the HTD than in the HY model. Additionally, the correlation coefficient between EBV based on the HTD and HY models was high (0.98) for both sires with ≥15 daughters and cows with records (Figure 4). Based on these results, breeding values were estimated using the HTD model that can comprehensively consider nongenetic effects on the TD.
TABLE 2.
Posterior mean (mean), standard deviation (SD), and 95% highest posterior density interval (95% HPD) of parameters for the coronet score and deviance information criterion (DIC) from the herd‐test‐day (HTD) and herd‐year (HY) models.
| 95% HPD | ||||||
|---|---|---|---|---|---|---|
| Parameters | Mean | SD | Lower | Upper | DIC | |
| HTD model | 759,888 | |||||
|
|
0.0036 | 0.0003 | 0.0030 | 0.0041 | ||
|
|
0.0458 | 0.0004 | 0.0452 | 0.0465 | ||
|
|
0.0848 | 0.0001 | 0.0846 | 0.0849 | ||
|
|
0.027 | 0.002 | 0.022 | 0.031 | ||
|
|
0.368 | 0.002 | 0.366 | 0.371 | ||
| HY model | 1,198,532 | |||||
|
|
0.0030 | 0.0003 | 0.0025 | 0.0035 | ||
|
|
0.0416 | 0.0003 | 0.0410 | 0.0422 | ||
|
|
0.1174 | 0.0001 | 0.1171 | 0.1176 | ||
|
|
0.018 | 0.002 | 0.015 | 0.021 | ||
|
|
0.275 | 0.001 | 0.272 | 0.277 | ||
Note: Models applying HTD or HY model as contemporary group effects were compared.
Abbreviations: , estimates of additive genetic variance; , estimates of permanent environmental variance; , estimates of residual variance; , estimates of heritability; , estimates of repeatability.
FIGURE 4.
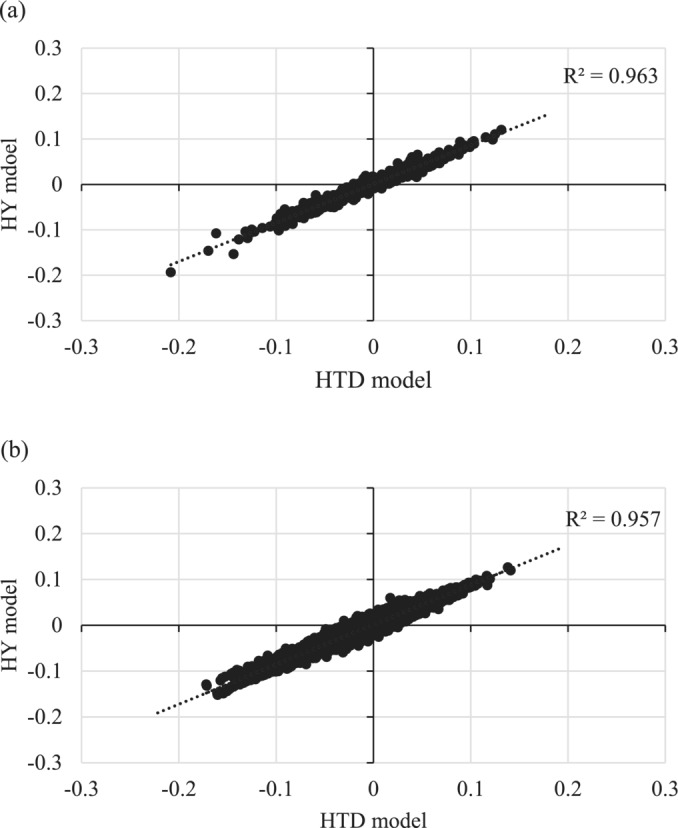
Correlation between estimated breeding values (EBVs) from the herd‐test‐day (HTD) and herd‐year (HY) models: (a) in 739 sires that had ≥15 daughters with coronet score records, and (b) in 94,951 cows with coronet score records.
3.3. Genetic parameters and trends
In the analysis using the HTD model, the heritability of the coronet score was estimated to be 0.027, and the repeatability was estimated to be 0.368 (Table 2). Genetic trends in sires and cows were determined by plotting average EBV of coronet scores by year of birth (Figure 5). The genetic trends of sires exhibited a gradual decrease until 2008, followed by an increase from 2009 onwards. Similarly, the genetic trend of cows showed a slight decrease from 2008 to 2014, followed by an increase from 2015 onwards. These results indicate that coronet score is a heritable trait, with a recent trend of genetic deterioration.
FIGURE 5.
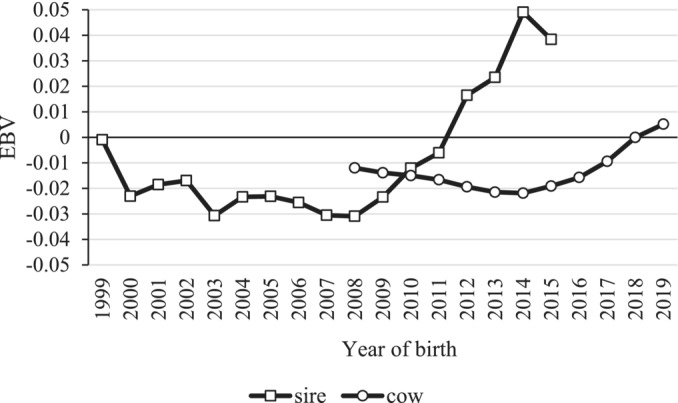
Mean estimated breeding value (EBV) coronet score by year of birth for 739 sires that had ≥15 daughters with coronet score records, and 94,951 cows with coronet score records.
4. DISCUSSION
The coronet score can be used as an effective indicator for the incidence of hoof disorders and lameness. The frequency of the coronet scores was consistent with that of the 5‐scale lameness score reported by Weber et al. (2013). In this study, the frequency of score ≥4, which usually indicates the requirement of veterinary treatment, was approximately 0.6%–3.0%. This incidence was consistent with the hoof disorder prevalence of 2.0% observed by farmers in Holstein first‐calving cows in the Hokkaido region of Japan (Hagiya et al., 2014). In contrast, previous studies have reported that the incidence of hoof disorders detected by hoof trimmers can be as high as 70% (Pérez‐Cabal & Charfeddine, 2015; van der Linde et al., 2010; van der Waaij et al., 2005). It has been highlighted that compared to hoof trimmers, farmers, and veterinarians are able to detect only serious cases of hoof disorders and that mild cases may be overlooked until they become serious (Heringstad et al., 2018). In fact, hoof disorders may occur more frequently than farmers and veterinarians diagnose. In this study, the combined frequency of scores 3–5, which require attention for hoof health through treatment and improving diet management, was approximately 13%–20%. This result indicates that the coronet score can also be used to detect milder hoof disorders. Coronet score is a useful indicator because it can effectively indicate even milder hoof disorders and allows for data collection on a larger scale.
Coronet scores were high in herds of ≤100 cows and in tie stalls. A relationship between herd size and housing system was identified (Table 1); herd size tended to be smaller in tie stalls. Cook and Nordlund (2009) stated that tethered cows are more likely to be restricted in their lying behavior than free‐ranging cows, which is one of the risk factors that leads to an increase in lameness. In addition, when measuring coronet scores, it is relatively easy to observe individual cows in small herds or tie stalls but not in large herds or free stalls and barns, which may have resulted in an underestimation of the scores. Contrastingly, in the free‐range conditions, coronet scores were lower in the free barns than in the free stalls. Free barns can reportedly contribute to a reduction in the incidence of hoof disorders compared to free stalls in terms of the floor surface (hardness, slipperiness, etc.) and ease of lying behavior (Solano et al., 2015; Somers et al., 2003; Webster, 2002).
Coronet score increased with parity and age. The effects of parity and age on the occurrence of hoof disorders can be explained by age‐related changes in body shape and accumulated damage to the hoof (Koenig et al., 2005; Sogstad et al., 2005). Hoof damage is irreversible, and cows that experience hoof disorder at a young age are more likely to show recurrence in later parities (Hirst et al., 2002; Koenig et al., 2005). Previous studies have reported that cows with three or more parities exhibit higher incidence rates of hoof diseases (sole ulcers and white‐line disease) and lameness than do younger cows (Chapinal et al., 2013; Koenig et al., 2005; Weber et al., 2013).
Analysis of test month results showed that coronet scores were higher from August to November, but the effect was less than that of other factors. Seasonal environmental changes are risk factors for hoof diseases (Pérez‐Cabal & Charfeddine, 2014). Particularly, extended standing time and acidosis due to heat stress negatively impact hoof health, and the incidence of hoof diseases increases from summer to autumn (Cook et al., 2007). To clarify the effect of season on coronet score, more detailed studies are necessary, including seasonal management and heat stress.
The effect of lactation stage was also small, but coronet scores increased from the early‐ to mid‐lactation stage. The incidence of certain hoof disorders has been reported to be highest during early lactation (Buch et al., 2011; van der Waaij et al., 2005; Weber et al., 2013). Collard et al. (2000) indicated that a negative energy balance during early lactation affects hoof health in later stages. Our results show that the hoof health tend to be serious from the early‐ to mid‐lactation stage because of the negative energy balance and recover in the late lactation stage as the energy balance become positive.
Generally, HTD model is used to analyze TD records, such as milk production traits. In the HTD model, a small number of records per HTD class may result in an inappropriate estimation of the HTD effect. Because the HY class contains more records compared to HTD, we conducted an analysis using the HTD and HY models and compared the results. Estimates of additive genetic and permanent environmental variances were similar between the HTD and HY models. The estimated residual variance, and DIC were lower in the HTD model. For TD records, it has been reported that the residual variance was reduced by applying the HTD effect (Strabel & Szwaczkowski, 1999), consistent with the results of this study. The correlation coefficient between the EBV estimated by the two models was high (0.98) for both sires with ≥15 daughters and cows with records. Based on these results, we applied a HTD model to the TD record of coronet scores, because the model better accounts for nongenetic effects at the TD. It should be noted that, the data we used in this study included both repeated records within and across lactations. We conducted the analysis with repeatability model assuming genetic and permanent environmental correlations within and across lactations to be one. Actually, it is possible that the genetic correlation between lactations was <1 or that there was variation in genetic variance during lactation. In the genetic analysis of coronet score, there is potential to consider more advanced TD models, such as random regression models that could explain the changes in variance within and between lactations.
The heritability of the coronet score estimated using the HTD model was 0.027, indicating that the coronet score is a heritable trait. Although there are no reports on the heritability of the coronet score, reports on hoof disorders and lameness and locomotion scores are available. Previous studies have revealed an estimated heritability of hoof disorders ranging from <0.01 to 0.14 using a linear model (Pérez‐Cabal & Charfeddine, 2015; van der Linde et al., 2010; van der Spek et al., 2013; van der Waaij et al., 2005). Other studies have reported an estimated heritability ranging from 0.03 to 0.14 for locomotion score or lameness studied as auxiliary traits for indirect evaluation of hoof disorders (Chapinal et al., 2013; van der Linde et al., 2010; Weber et al., 2013). The estimated heritability of the coronet score is considered reasonable, as it is within this range.
Direct records, such as diagnostic records of hoof disorders, are important for efficient genetic improvement of hoof health (Koenig et al., 2005; van der Linde et al., 2010). Nevertheless, it is generally difficult to widely collect diagnostic records, and locomotion or lameness scores have been studied as auxiliary traits for indirect evaluation. However, because the locomotion score is part of the conformation assessment, which is generally measured only once after the first calving, it is not possible to repeatedly record each individual. In Japan, locomotion scoring is limited to free‐stall herds, and it is not possible to repeatedly record each individual within a single lactation. Lameness scoring also has the issue of requiring space and effort to make each animal walk a certain distance when measuring (Heringstad et al., 2018; Weber et al., 2013). By contrast, the coronet score has advantages in that it is easy to determine by simply observing the external appearance. Furthermore, as the coronet score is collected by the Japan Dairy Herd Improvement Program, it allows large‐scale data collection and repeated records collection within and across lactations. We conclude that the use of coronet score for genetic evaluation is effective in Japan, owing to lack of records on hoof health.
Genetic trends in coronet scores suggested a genetic deterioration in coronet scores. The genetic trends of the coronet score in bulls exhibited a slight decline until 2008 but increased from 2009 onwards. Similarly, cows exhibited a slight decline in coronet score from 2008 to 2014 but an increase from 2015 onwards. A genetic response was confirmed, as cows followed the genetic trends of bulls. This suggests the importance of genetic improvement in enhancing hoof health. In this analysis, more than half of the sires were imported bulls (data not shown). The imported bulls might have contributed to the genetic deterioration of the coronet scores, but due to the composition of the dataset used in this analysis, the ratio of Japanese bulls is very low in recent years, so it is difficult to compare and discuss in detail about the effect of imported and Japanese bulls. Concerns have been raised about the impact of the increased frequency of imported bulls on genetic improvement in Japan, and we need to continue to collect data and monitor closely. In Japan, the Nippon Total Profit (NTP) selection index is used for genetic selection to improve lifetime productivity. Since 2000, the NTP has included feet and legs as conformation traits, which may have contributed to genetic improvements in hoof robustness and therefore health. If this is correct, genetic improvement of hoof health will continue through selection with NTP. However, previous studies have suggested that indirect selection for hoof health using only conformation traits is inefficient (Chapinal et al., 2013; Heringstad et al., 2018; van der Linde et al., 2010). The coronet score provides more direct information about hoof health; accordingly, the use of the coronet score may be effective in improving hoof health in breeding. To improve hoof health more effectively, including both coronet score and conformation traits in the NTP might be useful. The genetic relationship between coronet score and conformation traits is unclear and requires further investigation.
In this study, we investigated nongenetic factors that influence the coronet score and developed a genetic analysis model. We also estimated genetic parameters of the coronet scores and found them to be heritable. The coronet scores indicated a genetically deteriorating trend, suggesting the importance of genetic improvement in hoof health. Genetic improvement of hoof health requires a large‐scale system for collecting data on hoof health. Coronet score is an indicator of hoof health, and extensive data collection has been conducted. Additionally, the coronet score can easily be used to determine hoof health and can be recorded repeatedly; therefore, it is more useful than other hoof disease‐related traits (such as locomotion or lameness scores) for genetic improvement of hoof health. Overall, we demonstrated the potential of using coronet scores for the genetic improvement of hoof health in Japan, where a large‐scale record system for hoof diseases has not been available in the breeding program in dairy cattle.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
We thank Yoshinobu Uemoto of Tohoku University for providing advice during the study and Shinichiro Ogawa of Kyoto University for carefully proofreading the manuscript.
Saito, Y. , Nishiura, A. , Yamazaki, T. , Yamaguchi, S. , Tatebayashi, R. , Sasaki, O. , & Satoh, M. (2024). Genetic characteristics of the coronet scores related to Holstein cattle hoof health in Japan. Animal Science Journal, 95(1), e70014. 10.1111/asj.70014
REFERENCES
- Abdelsayed, M. , Haile‐Mariam, M. , & Pryce, J. E. (2017). Genetic parameters for health traits using data collected from genomic information nucleus herds. Journal of Dairy Science, 100, 9643–9655. 10.3168/jds.2017-12960 [DOI] [PubMed] [Google Scholar]
- Bennett, R. M. , Barker, Z. E. , Main, D. C. J. , Whay, H. R. , & Leach, K. A. (2014). Investigating the value dairy farmers place on a reduction of lameness in their herds using a willingness to pay approach. The Veterinary Journal, 199, 72–75. 10.1016/j.tvjl.2013.09.068 [DOI] [PubMed] [Google Scholar]
- Brito, L. , Bedere, N. , Douhard, F. , Oliveira, H. , Arnal, M. , Peñagaricano, F. , Schinckel, A. P. , Baes, C. F. , & Miglior, F. (2021). Review: Genetic selection of high‐yielding dairy cattle toward sustainable farming systems in a rapidly changing world. Animal, 15(Suppl 1), 100292. 10.1016/j.animal.2021.100292 [DOI] [PubMed] [Google Scholar]
- Buch, L. H. , Sorensen, A. C. , Lassen, J. , Berg, P. , Eriksson, J. , Jakobsen, J. H. , & Sorensen, M. K. (2011). Hygiene‐related and feed‐related hoof diseases show different patterns of genetic correlations to clinical mastitis and female fertility. Journal of Dairy Science, 94, 1540–1551. 10.3168/jds.2010-3137 [DOI] [PubMed] [Google Scholar]
- Chapinal, N. , Koeck, A. , Sewalem, A. , Kelton, D. F. , Mason, S. , Cramer, G. , & Miglior, F. (2013). Genetic parameters for hoof lesions and their relationship with feet and leg traits in Canadian Holstein cows. Journal of Dairy Science, 96, 2596–2604. 10.3168/jds.2012-6071 [DOI] [PubMed] [Google Scholar]
- Collard, B. , Boettcher, P. , Dekkers, J. , Petitclerc, D. , & Schaeffer, L. (2000). Relationships between energy balance and health traits of dairy cattle in early lactation. Journal of Dairy Science, 83, 2683–2690. 10.3168/jds.S0022-0302(00)75162-9 [DOI] [PubMed] [Google Scholar]
- Cook, N. B. , Mentink, R. L. , Bennett, T. B. , & Burgi, K. (2007). The effect of heat stress and lameness on time budgets of lactating dairy cows. Journal of Dairy Science, 90, 1674–1682. 10.3168/jds.2006-634 [DOI] [PubMed] [Google Scholar]
- Cook, N. B. , & Nordlund, K. V. (2009). The influence of the environment on dairy cow behavior, claw health and herd lameness dynamics. Veterinary Journal, 179, 360–369. 10.1016/j.tvjl.2007.09.01 [DOI] [PubMed] [Google Scholar]
- Dolecheck, K. , & Bewley, J. (2018). Animal board invited review: Dairy cow lameness expenditures, losses and total cost. Animal, 12, 1462–1474. 10.1017/S1751731118000575 [DOI] [PubMed] [Google Scholar]
- Egger‐Danner, C. , Cole, J. B. , Pryce, J. E. , Gengler, N. , Heringstad, B. , Bradley, A. , & Stock, K. F. (2015). Invited review: Overview of new traits and phenotyping strategies in dairy cattle with a focus on functional traits. Animal, 9, 191–207. 10.1017/S1751731114002614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enting, H. , Kooij, D. , Dijkhuizen, A. A. , Huirne, R. B. M. , & Noordhuizen‐Stassen, E. N. (1997). Economic losses due to clinical lameness in dairy cattle. Livestock Production Science, 49, 259–267. 10.1016/S0301-6226(97)00051-1 [DOI] [Google Scholar]
- Espejo, L. A. , Endres, M. I. , & Salfer, J. A. (2006). Prevalence of lameness in high‐producing Holstein cows housed in freestall barns in Minnesota. Journal of Dairy Science, 89, 3052–3058. 10.3168/jds.S0022-0302(06)72579-6 [DOI] [PubMed] [Google Scholar]
- Hagiya, K. , Yamazaki, T. , Nagamine, Y. , Togashi, K. , Yamaguchi, S. , Gotoh, Y. , Kawahara, T. , Masuda, Y. , & Suzuki, M. (2014). Genetic correlations between production and disease traits during first lactation in Holstein cows. Animal, 8, 217–223. 10.1017/S1751731113002048 [DOI] [PubMed] [Google Scholar]
- Heringstad, B. , Egger‐Danner, C. , Charfeddine, N. , Pryce, J. E. , Stock, K. F. , Kofler, J. , Sogstad, A. M. , Holzhauer, M. , Fiedler, A. , Müller, K. , Nielsen, P. , Thomas, G. , Gengler, N. , de Jong, G. , Ødegård, C. , Malchiodi, F. , Miglior, F. , Alsaaod, M. , & Cole, J. B. (2018). Invited review: Genetics and claw health: Opportunities to enhance claw health by genetic selection. Journal of Dairy Science, 101, 4801–4821. 10.3168/jds.2017-13531 [DOI] [PubMed] [Google Scholar]
- Hirst, W. M. , Murray, R. D. , Ward, W. R. , & French, N. P. (2002). A mixed‐effects time‐to‐event analysis of the relationship between first‐lactation lameness and subsequent lameness in dairy cows in the UK. Preventive Veterinary Medicine, 54, 191–201. 10.1016/S0167-5877(02)00021-1 [DOI] [PubMed] [Google Scholar]
- Huxley, J. N. (2013). Impact of lameness and claw lesions in cows on health and production. Livestock Science, 156, 64–70. 10.1016/j.livsci.2013.06.012 [DOI] [Google Scholar]
- Koenig, S. , Sharifi, A. R. , Wentrot, H. , Landmann, D. , Eise, M. , & Simianer, H. (2005). Genetic parameters of claw and foot disorders estimated with logistic models. Journal of Dairy Science, 88, 3316–3325. 10.3168/jds.S0022-0302(05)73015-0 [DOI] [PubMed] [Google Scholar]
- Miglior, F. , Fleming, A. , Malchiodi, F. , Brito, L. , Martin, P. , & Baes, C. (2017). A 100‐year review: Identification and genetic selection of economically important traits in dairy cattle. Journal of Dairy Science, 100, 10251–10271. 10.3168/jds.2017-12968 [DOI] [PubMed] [Google Scholar]
- Pérez‐Cabal, M. A. , & Charfeddine, N. (2014). Claw lesions and risk factors in Spanish dairy cows. Proceedings of 10th WCGALP, Vancouver, Canada, August 17‐22.
- Pérez‐Cabal, M. A. , & Charfeddine, N. (2015). Models for genetic evaluations of claw health traits in Spanish dairy cattle. Journal of Dairy Science, 98, 8186–8194. 10.3168/jds.2015-9562 [DOI] [PubMed] [Google Scholar]
- Shabalina, T. , Yin, T. , & König, S. (2020). Influence of common health disorders on the length of productive life and stayability in German Holstein cows. Journal of Dairy Science, 103, 583–596. 10.3168/jds.2019-16985 [DOI] [PubMed] [Google Scholar]
- Sogstad, Å. M. , Fjeldaas, T. , & Osterås, O. (2005). Lameness and claw lesions of the Norwegian red dairy cattle housed in free stalls in relation to environment, parity and stage of lactation. Acta Veterinaria Scandinavica, 46, 203–217. 10.1186/1751-0147-46-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano, L. , Barkema, H. W. , Pajor, E. A. , Mason, S. , LeBlanc, S. J. , Zaffino Heyerhoff, J. C. , Nash, C. G. , Haley, D. B. , Vasseur, E. , Pellerin, D. , Rushen, J. , de Passillé, A. M. , & Orsel, K. (2015). Prevalence of lameness and associated risk factors in Canadian Holstein‐Friesian cows housed in freestall barns. Journal of Dairy Science, 98, 6978–6991. 10.3168/jds.S0022-0302(03)73797-7 [DOI] [PubMed] [Google Scholar]
- Somers, J. G. C. J. , Frankena, K. , Noordhuizen‐Stassen, E. N. , & Metz, J. (2003). Prevalence of claw disorders in Dutch dairy cows exposed to several floor systems. Journal of Dairy Science, 86, 2082–2093. 10.3168/jds.2015-9652 [DOI] [PubMed] [Google Scholar]
- Spiegelhalter, D. J. , Best, N. G. , Carlin, B. R. , & van der Linde, A. (2002). Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society, Series B: Statistical Methodology, 64, 583–616. 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- Strabel, T. , & Szwaczkowski, T. (1999). The use of test day models with small size of contemporary groups. Journal of Animal Breeding and Genetics, 116, 379–386. 10.1046/j.1439-0388.1999.00216.x [DOI] [Google Scholar]
- van der Linde, C. , de Jong, G. , Koenen, E. P. C. , & Eding, H. (2010). Claw health index for Dutch dairy cattle based on claw trimming and conformation data. Journal of Dairy Science, 93, 4883–4891. 10.3168/jds.2010-3183 [DOI] [PubMed] [Google Scholar]
- van der Spek, D. , van Arendonk, J. A. M. , Vallée, A. A. A. , & Bovenhuis, H. (2013). Genetic parameters for claw disorders and the effect of preselecting cows for trimming. Journal of Dairy Science, 96, 6070–6078. 10.3168/jds.2013-6833 [DOI] [PubMed] [Google Scholar]
- van der Waaij, E. H. , Holzhauer, M. , Ellen, E. , Kamphuis, C. , & de Jong, G. (2005). Genetic parameters for claw disorders in Dutch dairy cattle and correlations with conformation traits. Journal of Dairy Science, 88, 3672–3678. 10.3168/jds.S0022-0302(05)73053-8 [DOI] [PubMed] [Google Scholar]
- Weber, A. , Stamer, E. , Junge, W. , & Thaller, G. (2013). Genetic parameters for lameness and claw and leg diseases in dairy cows. Journal of Dairy Science, 96, 3310–3318. 10.3168/jds.2012-6261 [DOI] [PubMed] [Google Scholar]
- Webster, A. J. F. (2002). Effects of housing practices on the development of foot lesions in dairy heifers in early lactation. Veterinary Record, 151, 9–12. 10.1136/vr.151.1.9 [DOI] [PubMed] [Google Scholar]
- Whay, H. R. , & Shearer, J. K. (2017). The impact of lameness on welfare of the dairy cow. The Veterinary Clinics of North America. Food Animal Practice, 33, 153–164. 10.1016/j.cvfa.2017.02.008 [DOI] [PubMed] [Google Scholar]


