Abstract
Addressing bone defects is a complex medical challenge that involves dealing with various skeletal conditions, including fractures, osteoporosis (OP), bone tumours, and bone infection defects. Despite the availability of multiple conventional treatments for these skeletal conditions, numerous limitations and unresolved issues persist. As a solution, advancements in biomedical materials have recently resulted in novel therapeutic concepts. As an emerging biomaterial for bone defect treatment, graphene oxide (GO) in particular has gained substantial attention from researchers due to its potential applications and prospects. In other words, GO scaffolds have demonstrated remarkable potential for bone defect treatment. Furthermore, GO-loaded biomaterials can promote osteoblast adhesion, proliferation, and differentiation while stimulating bone matrix deposition and formation. Given their favourable biocompatibility and osteoinductive capabilities, these materials offer a novel therapeutic avenue for bone tissue regeneration and repair. This comprehensive review systematically outlines GO scaffolds’ diverse roles and potential applications in bone defect treatment.
Cite this article: Bone Joint Res 2024;13(12):725–740.
Keywords: Graphene oxide, Nanocomposites, Bone defect, Bone defect repair, Bone tissue engineering, Scaffold, biomaterials, bone defects, osteoblasts, Osteoporotic bone, bone infections, tumours and bone, bone tissue regeneration, bone matrix, composites, bone regeneration
Article focus
The potential and prospects of graphene oxide (GO) as an emerging biomaterial in the treatment of bone defects.
The various biological effects of GO in the treatment of bone defects and its application in different orthopaedic conditions.
Key messages
GO-loaded scaffolds exhibit significant biological benefits in the treatment of bone defects, promoting osteoblast adhesion, proliferation, and differentiation while stimulating bone matrix deposition and formation.
GO-loaded composites possess excellent biocompatibility and osteoinductive capabilities, offering a novel therapeutic approach for bone tissue regeneration and repair.
As a functional carrier, GO demonstrates remarkable drug-controlled release capabilities, facilitating the delivery of bioactive substances in the treatment of bone defects.
Strengths and limitations
This article provides a comprehensive and systematic review of the various roles and potential applications of GO in the treatment of bone defects, offering a detailed analysis of the current state of research.
Despite the authors’ efforts to gather recent relevant literature, some important studies and content may still be missing from this review, potentially leading to an incomplete understanding of the topic.
This article primarily relies on in vitro and in vivo experimental data, with limited discussion on clinical trials or clinical applications, which may affect the practical relevance of the findings.
Introduction
Bone defects are common skeletal disorders caused by various factors, including trauma, bone tumours, infection, osteomyelitis, and osteoporosis (OP).1-3 In addition to significantly impacting patients’ quality of life (QoL), bone defects pose substantial health risks.4,5 In clinical practice, orthopaedic surgeons have consistently encountered a formidable challenge in repairing bone defects. Surgical interventions, implantation of artificial bone or metal scaffolds, and use of bone grafts or allografts are some of the primary conventional approaches for repairing bone defects.6,7 However, these interventions have limitations and challenges, including surgical trauma, limited donor bone availability, and potential graft rejection.8,9 As a result, developing more innovative materials for bone defect repair is imperative.
Graphene oxide (GO) is an emerging nanobiomaterial with vast potential for diverse biomedical applications.10 Its distinctive 2D structure, high specific surface area, and remarkable physicochemical qualities distinguish it as a promising bioscaffold material with numerous advantages for bone defect treatment.11 As innovative therapeutic compounds, GO scaffolds have recently gained substantial attention in the biomaterials domain. Moreover, GO scaffolds have demonstrated significant biological benefits in bone defect therapy.12-14 This review centres on the impact and potential mechanisms of GO-loaded biomaterials in bone defect treatment, as well as the effects of applying GO composite bioscaffolds in various orthopaedic conditions. Specifically, we aim to foster innovation and advancements in the field of bone defect repair, with the ultimate goal of enhancing patient recovery and QoL, through comprehensive literature analysis and novel insights.
Role and characteristics of GO scaffolds in bone defect treatment
Enhancement of scaffold biocompatibility and physicochemical properties
The potential of GO in the realm of composite biomaterial development is well documented. Multiple studies have revealed that GO composites could yield notable enhancements in both the physical and biological attributes of scaffolds.15,16 For example, GO proved to be a performance booster for Poly(ε-caprolactone) (PCL) scaffolds in a rabbit tibial defect model, significantly augmenting new bone regeneration.17 Furthermore, the incorporation of GO into polymethyl methacrylate (PMMA) bone cement (PBC) contributed to its improved biocompatibility, mechanical properties, and physicochemical traits, rendering it an efficacious implantable biomaterial.18 Additionally, GO was used as a reinforcing agent for polypropylene fumarate (PPF) cross-linking composites, greatly enhancing their mechanical properties for bone defect repair.19 The GO composite with brushite (dicalcium phosphate dihydrate (DCPD)) cement has also shown great potential, with the GO/CPC composite demonstrating a higher compressive strength than pure DCPD cement while concurrently promoting cell adhesion and osteogenic activity.20,21 Combining GO with other nanomaterials within the electrospun bilayer nanofibre scaffolds has also demonstrated commendable biocompatibility, prompting osteochondral healing and upregulating osteogenic and chondrogenic genes.22
Furthermore, GO can be used as an effective additive in fabricating 3D-printed scaffolds, endowing composite scaffolds with robust biocompatibility and outstanding mechanical properties.23 Integrating GO further enhances the scaffolds’ ability to foster bone marrow stromal cell (BMSC) attachment, proliferation, and osteogenic differentiation, making it a promising biomaterial candidate for high-quality bone regeneration.24 Another study discovered that using GO as a bioink constituent enhances the plasticity, precision, and mechanical robustness of bioprinted scaffolds. This augmentation consequently fosters osteoblast cell proliferation and osteogenic differentiation, implying GO’s potential utility in the field of bone tissue engineering.25
Additionally, GO has exhibited remarkable properties in diverse composites. For example, its integration with various raw materials, such as arabinoxylan, β-glucan, nanohydroxyapatite, and acrylic acid, facilitates the creation of porous scaffolds, showcasing ideal morphological structures as well as exceptional swelling, degradation, and mechanical characteristics.26 Moreover, incorporating GO into organic materials like chitosan (CS) and collagen (Col) has shown favourable biocompatibility and mechanical attributes, promoting mesenchymal stem cell (MSC) differentiation into osteoblasts. This outcome opens up new possibilities for using novel bioscaffold materials in bone defect repair.27-37 Furthermore, GO imparts tunable mechanical strength and osteogenic induction capability in hydrogel materials. The mechanical properties of the composite hydrogel can be easily customized to meet specific bone regeneration requirements by adjusting the loaded GO concentration.38 Notably, GO can also confer conductive properties to the hydrogel through its electrical conductivity. In the realm of bone implants, using CS-GO-HA nanocomposite coating on titanium surfaces significantly enhances both the biological and electrochemical properties of the material.39 This composite coating can repair bone defects, thereby augmenting bone tissue regeneration. Optimal reduced GO (rGO) ratios enhance photocurrent generation efficiency in photosensitive materials, fostering BMSC proliferation and osteogenic differentiation, thus expediting bone regeneration, offering a feasible approach for rapid bone repair.40 On the other hand, the chemical combination of GO with isocyanate produces shape-memory composites with remarkable mechanical and shape-memory effects.41 Furthermore, incorporating GO as a nanofiller into shape-memory polyurethane (SMPU) improves its mechanical attributes. This innovation could repair bone defects, particularly in minimally invasive surgery.42
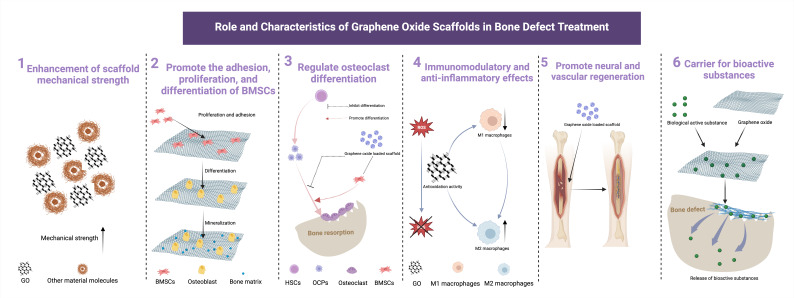
Overall, GO composites with various materials could improve bioscaffold biocompatibility as well as mechanical and physicochemical properties, providing a novel biomaterial alternative for bone tissue engineering and bone defect repair (Figure 1). Continued research and advancements within this domain hold significant potential for even broader practical implications.
Fig. 1.
The multifaceted roles and characteristics of graphene oxide scaffolds in bone defect treatment. The key attributes and functions include: 1. Enhancement of scaffold mechanical strength: the composite of graphene oxide with other materials effectively enhances the mechanical strength of the scaffold. 2. Promote the adhesion, proliferation, and differentiation of bone marrow mesenchymal stem cells (BMSCs): graphene oxide (GO) facilitates the adhesion, proliferation, and differentiation of BMSCs, crucial processes for effective bone tissue regeneration. 3. Regulate osteoclast differentiation: GO scaffolds have significant potential in regulating osteoclast differentiation and function, contributing to bone defect repair. 4. Immunomodulatory and anti-inflammatory effects: GO scaffolds exert immune modulation and anti-inflammatory effects by clearing reactive oxygen species (ROS) and promoting the transformation of macrophages into the M2 phenotype. 5. Promote neural and vascular regeneration: graphene oxide scaffolds play a vital role in promoting the regeneration of neural and vascular tissues, enhancing the overall functionality of the repaired bone. 6. Carrier for bioactive substance: serving as an effective carrier, GO scaffolds enable the controlled delivery of bioactive substances, further optimizing the bone defect treatment process. HSC, haematopoietic stem cells; OCP, osteoclast precursors. Created with BioRender.com, and the license to publish from the BioRender has been obtained.
Promotion of BMSC adhesion and proliferation
It has been reported that GO plays a crucial role in bone regeneration and repair by facilitating BMSC adhesion and proliferation.11,43,44 In vitro, GO-loaded fucoidan scaffolds showed a significant cell proliferation-promoting potential.27 The direct integration of GO into MSC-containing bioinks exhibited the ability to augment MSC proliferation and osteogenic differentiation, thereby enhancing bone regeneration.45 Furthermore, GO bolstered MSC proliferation, adhesion, and osteogenic differentiation within the bioink composite.46 Ultrasonication of GO (UGO) significantly improved the dispersion and stability of the GO solution, promoting human foetal osteoblast (hFOB) adhesion and proliferation while maintaining high biocompatibility with osteoblasts, endothelial cells (ECs), and fibroblasts. Additionally, UGO has demonstrated fibroblast migration and wound healing facilitation abilities, enhancing its potential for tissue regeneration applications, including bone and skin wound healing.47 Furthermore, rGO coatings exhibited the capacity to enhance Col scaffold performance, promoting BMSC adhesion, viability, and proliferation.29 In conclusion, GO is a remarkable cell adhesion and proliferation facilitator during tissue regeneration, offering a viable avenue for developing novel biomaterials and optimizing therapeutic strategies for effective bone tissue repair.
Regulation of osteoblast differentiation
Regulation of osteoblast differentiation is critical in both bone tissue engineering and bone defect repair. Owing to its unique structure and bioactivity, GO, an emerging nanobiomaterial, has the potential to positively influence osteoblast differentiation.48 First, applying low GO quantum dot (GOQD) concentrations effectively enhances BMSC proliferation and osteogenic differentiation by activating the Wnt/β-catenin signalling pathway, implying a potential feasibility for using GO in bone defect repair. Second, GO further stimulates MSCs to differentiate into osteoblasts through low-dose exposure.49 This process involves the activation of the JNK and FoxO1 signalling pathways, and by regulating the redox system, GO maintains intracellular redox homeostasis and exerts antioxidant effects.50
As a fundamental component in composite scaffolds, GO is critically involved in the regulation of osteoblast differentiation. In a rat cranial capitellar defect model, the composite film comprising GO and polylactic acid (PLA) offered a conducive matrix for guided bone regeneration, fostering pre-osteoblast proliferation and expediting new bone formation.51 Sharma et al52 synthesized polydopamine-reduced GO (PD-rGO) and incorporated it into 3D-printed PLA scaffolds. In vitro, these scaffolds exhibited robust pro-osteogenic differentiation of human umbilical cord-derived MSCs (hMSCs) and sustained osteoblast cellular activity post-implantation in vivo. Furthermore, introducing GO into innovative 3D nanofibre scaffolds augmented osteogenic protein expression in human adipose-derived MSCs (ADSCs), eliciting substantial bone regeneration effects in the repair of critical cranial skull defect (CSCD) in rats.53 GO-infused CS/glycerophosphate (GP) temperature-sensitive injectable hydrogels were explored for their bone defect healing and bone regeneration effects, and showed potential in inducing mouse bone marrow stem cell osteogenic differentiation.54 Additionally, surface chemical and roughness modifications of GO in nano-engineered scaffolds heightened osteogenic differentiation capabilities by enhancing extracellular matrix (ECM) interaction with β1 integrins, activating the FAK-ERK signalling pathway, and increasing osteogenic-specific protein expression.35
Jiao et al55 synthesized crafted composite structures mimicking fibrous callus by integrating an in-house-synthesized gelatin-reduced GO (GOG) with photo-crosslinked gelatin hydrogels. This approach triggered the bidirectional differentiation (osteogenesis and angiogenesis) of BMSCs via the ERK1/2 and AKT pathways, expediting bone repair.55 Furthermore, incorporating GO into gelatin and alginate nanomaterials significantly increased compressive strength in the resulting nanocomposite scaffolds, promoting cell adhesion, proliferation, and upregulation of osteoblastic transcription factors (Runx2 and osteocalcin) in BMSCs. This innovation shows the potential applicability of GO scaffolds in bone tissue engineering.56 In another related study, Sun et al57 harnessed 3D bioprinting to fabricate composite bioink infused with BMSCs and an engineered artificial periosteum which exhibited robust adhesion attributes and osteogenic effects. The infusion of GO into the composite bioink ameliorated its adhesive characteristics and amplified the osteogenic differentiation of the encapsulated stem cells, expediting new bone formation and facilitating bone defect repair.57 In addition to highlighting the efficacy of GO scaffolds in orchestrating osteoblast differentiation, these observations unveil novel prospects and opportunities in the realm of bone tissue engineering.
The composite of GO with inorganic materials has also demonstrated significant bioactivity and biological effects in the field of bone defect repair.58 First, GO enhanced the osteogenic differentiation of MSCs in engineered 2D heteronano-layers (2DHNL) created with black phosphorus (BP).59 Furthermore, GO enhanced the osteogenic induction of dexamethasone (DEX) when loaded within this structure.59 Second, rGO incorporated into natural calcium phosphate bone cement (RGO-CPC) considerably enhanced the cement’s mechanical properties and osteoinductive capabilities. Applying pulsed electromagnetic fields (PEMF) to the RGO-CPC further enhanced osteogenic differentiation, making it a promising option for bone defect repair.20 Moreover, the 3D GO foam/polydimethylsiloxane/zinc silicate (GF/PDMS/ZS) composite scaffold demonstrated robust biocompatibility in both in vitro and in vivo experiments in the field of bone tissue engineering. This scaffold effectively induced BMSC proliferation and preferential differentiation into osteogenic bone tissue when implanted in vivo.60
Incorporating GO into porous scaffolds also enhanced the osteogenic differentiation potential of rat BMSCs (rBMSCs) and stimulated inward blood vessel growth. With its remarkable osteogenic and proangiogenic properties, GO has emerged as a strong recourse for bone defect repair.61 Wang et al62 performed the functionalization of rGO on the surface of layered porous bioactive glass scaffolds, and further modified the scaffold surface with the osteoblast-specific aptamer CH6. This modification was noticeably effective in promoting osteogenic differentiation and new bone formation. In addition to enriching and differentiating osteoblasts, this functionalized coating also supported bone tissue regeneration.62 Overall, GO scaffolds can significantly influence the regulation of osteoblast differentiation, opening up new research avenues in bone tissue engineering.
Regulation of osteoclast differentiation
Osteoclasts play a critical role in bone defect repair by regulating the balance between bone resorption and new bone formation, thereby maintaining the health and strength of bone tissue.63 However, studies on the effects of GO scaffolds on osteoclasts are relatively scarce. In research conducted by Zeng et al,64 collagen sponges containing various proportions of GO were used to treat bone marrow-derived macrophages (BMMs), and the results indicated that osteoclastogenesis remained unaffected. GO may be phagocytosed and degraded by osteoclasts, which could explain why GO does not significantly impact osteoclast activity.65 Conversely, Jiao et al66 found that BMSCs stimulated by GO exhibited enhanced osteoclastogenesis when co-cultured with haematopoietic stem cells (HSCs). The results demonstrated that GO-stimulated BMSCs promoted the differentiation of osteoclast precursor cells into mature osteoclasts by increasing calcium ion concentration and reactive oxygen species (ROS) levels.66 Furthermore, the local injection of gelatin-reduced GO (GOG) accelerated bone remodelling during tooth movement in vivo, resulting in increased osteoclast-mediated bone resorption and neovascularization.66
However, research by Wang et al67 showed that GO-modified concentric microgroove structures significantly inhibited the differentiation of RAW264.7 macrophages into osteoclasts. These structures restricted the fusion of RAW264.7 macrophages, thereby suppressing osteoclast formation and the development of sealing zone.67 This modification not only increased the surface roughness of the material, but also inhibited multinucleation and functional differentiation of osteoclasts.67 Given the current studies, drawing a unified conclusion on the regulatory effects of GO on osteoclasts remains challenging, and further research is needed to elucidate the underlying mechanisms.
Although the regulatory role of GO on osteoclasts is not yet fully understood, combining GO with other materials has proven effective in modulating osteoclast function.68,69 One study has shown that GO, when combined with materials such as titanium and zinc, significantly inhibits osteoclast differentiation and function.68 These composite materials release specific ions, such as zinc ions, which block the NF-κB signalling pathway, thereby inhibiting osteoclast differentiation, maturation, and subsequent bone resorption.68 GO/Ga nanocomposites also exhibit inhibitory effects on osteoclast differentiation by modulating the BMP/Smad, mitogen-activated protein kinase (MAPK), and NF-κB signalling pathways.69 Thus, while the mechanisms of GO itself require further investigation, its combination with other materials has demonstrated substantial potential in regulating osteoclasts during bone defect repair.
Stimulation of bone matrix deposition and bone formation
It has been demonstrated that GO is significantly effective in facilitating bone matrix deposition and bone formation.16 Its effectiveness is largely based on its excellent bioactivity and biocompatibility owing to its distinctive 2D structure and abundant functional groups. Moreover, it has functional oxygen groups that can interact with calcium ions, guiding apatite formation on its surface.70-72 This mineralization activity aids in the augmentation of apatite crystal formation within the bone matrix. Devi G V et al27 designed an osteoinductive calcium cross-linked sodium alginate-nanohydroxyapatite-nanographene oxide (Alg-HA-GO-F) scaffold. This construct exhibited noteworthy pro-osteogenic differentiation of MSCs as well as mineralization activity.27 In another related study, Ding et al73 developed CS-loaded GO (CS-rGO) coatings and effectively integrated them into calcium polyphosphate (CPP) scaffolds. These composite scaffolds exhibited significantly enhanced biomineralization and antimicrobial activities, improving the CPP scaffolds’ potential for bone regeneration. Furthermore, incorporating GO into Col scaffolds yielded exceptional outcomes. Notably, GO is enriched with functional compounds like the carboxyl (-COOH) and hydroxyl (-OH) groups, which offer more active sites for biomimetic mineralization. This feature facilitates a higher Ca/P ratio and apatite formation and improves MSC adhesion and proliferation, which in turn enhances new bone formation in vivo.33
It has also been reported that GO composite synthetic polymers contribute actively to bone matrix deposition and bone formation. Applying GO to PMMA-PCL-fluorapatite (FA) bone cement effectively enhanced the apatite-forming ability on the polymer surface. This boosted the bone cement’s bioactivity and mechanical properties, as well as the osteoclast survival rate.74 Similarly, a honeycomb scaffold was fabricated by combining poly(D,L-lactic acid) (PDLLA) with graphene/multiwalled carbon nanotube oxides (MWCNTO-GO, 50% w/w) in another investigation. This composite facilitated cell adhesion and growth within the polymer, ultimately promoting the formation of mineralized matrix nodules. In vivo tests revealed that PDLLA/MWCNTO-GO scaffolds exhibited superior osteoblastic activity, increasing new bone formation. This highlights their substantial potential as an alternative for bone tissue regeneration.75
GO nanoribbons (GNR) and nanohydroxyapatite (nHAp)-based nanomaterials have attracted significant attention in the field of bone tissue engineering. According to research, composite porous materials incorporating nHAp/GNR have elevated bioactivity and a larger contact surface area. As a result, these materials can augment the expression of osteogenesis-related genes in vitro and display superior bone regeneration potential in vivo, as observed in animal models.76 At the same time, incorporating GO as the interfacial phase successfully enhanced the bonding between polyetheretherketone (PEEK) and hydroxyapatite (HAP). The GO-infused PEEK-HAP scaffolds exhibited notable compressive strength and modulus, along with the capacity to induce bone-like apatite formation. They also fostered MSC adhesion, proliferation, and osteogenic differentiation.77 In a different study, using GO and hydroxyapatite nanocomplexes (rGO/HAp NCs) promoted osteogenesis, increased alkaline phosphatase activity, and heightened mineralization levels.78 These nanocomposites further facilitated new bone formation in MC3T3-E1 osteoblasts. The evident osteogenesis-promoting potential of these GO-based composites positions them as promising candidates for facilitating expedited bone tissue repair and regeneration.78 Using GO in bone tissue engineering highlights its potential to enhance bone matrix deposition and bone formation, significantly supporting the advancement of innovative bone regeneration materials.
Immunomodulatory and anti-inflammatory effects
Immunomodulatory and anti-inflammatory effects which involve regulating inflammatory responses, protecting tissues, and influencing the fate of stem cells are critical in bone defect repair.79,80 These effects foster efficient repair and healing of bone defects by creating a conducive environment for bone regeneration. The capacity of GO to modulate immune cell activity has been highlighted in multiple studies. Notably, GO can impact the activation status of immune cells, including macrophages, lymphocytes, and dendritic cells (DCs), as well as their cytokine release. Appropriate GO treatment promotes immune cell transition to a beneficial anti-inflammatory M2 phenotype, mitigating inflammatory reactions and tissue harm. Liu et al81 developed 3D-printed scaffolds with 2D heterogeneous nanostructures comprising layers of GO and BP nanosheets. These 2D hetero-nano-layers facilitated the adsorption of immune-modulating cytokine interleukin-4 (IL-4), effectively polarizing macrophages towards the M2 phenotype. At the same time, GO layers enhanced cell adhesion, while BP nanosheets dispensed phosphate ions in a controlled manner, promoting continuous cell growth and osteogenesis. In rat models, implanted 3D scaffolds exhibited in vivo bone immunomodulation by establishing a pro-healing immune microenvironment within the defect area. This microenvironment induced angiogenesis and osteogenesis, ultimately promoting bone regeneration.81 Fu et al82 presented nanocomposite hydrogels (Alg/GO/Ser/nHAP) with distinctive immunomodulatory and osteoinductive traits for bone defect treatment. In this context, GO regulates macrophages (shifting them from the M1 to M2 phenotypes), thereby fostering a favourable bone immune microenvironment, reducing local inflammatory responses at the hydrogel-bone interface, and propelling BMSC differentiation into osteoblasts, ultimately resulting in Col matrix deposition and new bone formation.82 Furthermore, a multifaceted nanohybrid (5QCS-1GO-PDA) comprising GO, quaternized chitosan (QCS), and polydopamine (PDA), guides macrophages toward the beneficial M2 phenotype by modulating the BMP2/BMPRs/Smads/Runx2 pathway, further bolstering bone regeneration and offering an effective defect repair approach.83 Additionally, GO nanocomposite biomimetic hybrid scaffold incorporating baicalein-demineralized bone matrix (GO-BAI/DBM) exhibited unique immunomodulatory properties and promoted bone regeneration while inducing macrophage polarization to an M2 phenotype, leading to decreased activation of pro-inflammatory macrophages, which in turn increased the secretion of cytokines such as BMP-2 and transforming growth factor-β1 (TGF-β1), ultimately inducing osteogenesis during bone repair.84 Another study reported that GO-incorporated silk fibroin (SF)/nanohydroxyapatite (nHA) scaffolds could be robust platforms for tissue-engineered bone treatments. These scaffolds promote the M2-type macrophage differentiation by regulating immune function and anti-inflammatory effects, while simultaneously curbing M1-type differentiation. They also enhance osteogenesis in urogenic stem cells (USCs).85 Zhao et al86 devised a multifunctional bio-coating that facilitated the controlled release of zinc ions by combining carboxylated GO, zinc ions, and CS layers on carbon fibre-reinforced polyether ether ether ketone (CFRPEEK). This novel bio-coating exhibited the capacity to gradually release zinc ions, thereby regulating immune-inflammatory reactions, mitigating oxidative stress (OS), and stimulating both angiogenesis and osteogenic differentiation in vitro.86 Simultaneously, magnesium-enriched GO nanoscrolls (MgNPs@GNSs), a nanoplatform for combined inflammatory response modulation, showed a heightened bone regeneration potential.87 This was achieved by regulating the inflammatory response via sustained release of magnesium ions (Mg2+), facilitating the transformation of inflammatory M1 macrophages into the pro-healing M2 phenotype and stimulating angiogenesis and osteogenesis in vitro.87
Additionally, GO exerts antioxidant effects by scavenging ROS, including peroxides and free radicals. This scavenging capability effectively reduces OS and cellular damage, thereby maintaining cellular homeostasis and physiological functions, as well as facilitating tissue repair and regeneration. Zhou et al88 innovatively developed an rGO-based antioxidant hydrogel with prolonged antioxidant activity. In this context, rGO effectively lowered intracellular ROS levels, suppressed downstream miR-200c expression, and upregulated ZEB1 and Notch1, fostering BMSC proliferation and angiogenesis in vitro.88 Additionally, multifunctional polydopamine-mediated GO and hydroxyapatite nanoparticle-doped conductive scaffolds demonstrated exceptional bone regeneration properties within the periodontal microenvironment, particularly under diabetic conditions characterized by heightened inflammation.89 In addition to facilitating cellular osteogenesis through conductive pathways, these conductive scaffolds exhibited scavenging capabilities against ROS and potent immunomodulation. This multifaceted approach presents a novel strategy for managing immune-related diseases and promoting tissue regeneration.89
Along with other biomaterials, GO has been integrated into 3D bioprinted dual-channel scaffolds.90 These scaffolds exhibited a dual functionality (early immunomodulation and later osteogenic induction) by co-encapsulating rat bone marrow-derived macrophages (BMMs) and BMSCs. This novel approach synergistically promotes efficient bone defect repair.91 Relevant research has also highlighted the capabilities of GO coatings to expedite osteoblast differentiation and effectively modulate inflammatory processes.90,92 Notably, the magnitude of these effects is correlated with GO concentration, demonstrating a huge potential for immunomodulation and anti-inflammatory action.90,92 Overall, the versatility of GO composites extends to a wide range of applications encompassing immunomodulation and anti-inflammatory effects.90,92 These novel approaches offer promising avenues for advancing bone regeneration and treating immune-related conditions. Moreover, the findings present valuable insights and strategies in the realms of bone tissue engineering and immunotherapy.
Promotion of synchronized nerve regeneration in bone defect repair
Addressing nerve regeneration during bone defect repair holds significant importance due to the common co-occurrence of nerve injury with bone abnormalities, resulting in pain and functional limitations.93 The application of GO materials in nerve repair for bone defects has garnered notable attention in recent years.94-96 As earlier stated, GO, an emerging biomaterial, has distinct physicochemical properties and bioactivity, making it appropriate for nerve repair and regeneration.97 An example is the crucial role rGO plays in nerve regeneration during the repair of extensive bone defects. Specifically, rGO fosters neuronal differentiation and myelination of Schwann cells (SCs), while improving BMSC adhesion and osteogenic differentiation, resulting in synergistic nerve and bone regeneration.98 Furthermore, photoelectric stimulation can promote osteoblast differentiation and neuronal cell protrusion in novel ternary nanocoatings incorporating rGO and regenerated carbon (g-C3N4) from graphite. This innovation opens up new possibilities for targeted nerve regeneration while stimulating osteogenesis in the design of bone repair biomaterials.99 These findings offer valuable insights for developing functional nerve conduits and bone repair materials, and are poised to play a vital role in the future landscape of bone defect therapy.
Furthermore, a related investigation revealed that the rGO-GelMA-PCL nerve conduit, formed by integrating rGO with gelatin-methacryloyl (GelMA) and PCL, has high potential in promoting nerve regeneration in the repair of rat sciatic nerve injury. The conduit was loaded with BMSC-derived extracellular vesicles (EVs), offering substantial support for nerve regeneration and subsequent functional recovery. This method offers a novel approach for treating peripheral nerve defects.100 Wang et al101 developed 3D bionic nerve conduits, also known as nerve guidance conduits (NGCs), using Antheraea pernyi silk fibroin (ApF)/poly(L-lactic acid-co-caprolactone) (PLCL)/GO nanofibres, which exhibited nerve repair effects comparable to those of autografts in vivo. These findings provide valuable evidence for exploring GO composite roles in nerve regeneration during bone defect repair, laying the groundwork for advancing functional nerve conduits and bone repair materials.
Acceleration of vascular repair in bone defects
It has been reported that GO composites promote vascular regeneration through multiple mechanisms, including activation of vascular endothelial cells (VECs), stimulation of angiogenesis-related factors, and augmentation of vascular integration.102,103 Collectively, these actions critically support tissue repair and regeneration, particularly in addressing complex clinical conditions like bone defects.104,105 Research has demonstrated that nanographene oxide (NGO) induces endothelial tip cell differentiation via binding to lysophosphatidic acid (LPA), fostering angiogenesis and significantly enhancing vascular growth within cranial bone defect regions in vivo. This helps to create a microenvironment conducive for bone regeneration.105 Moreover, engineered functional spheres made up of MSCs implanted within 2DHNL comprising BP and GO promote in situ neovascularization and osseointegration in critical-sized cranial bone defects, further augmenting bone formation.59 Functionalized GO-sodium hyaluronate (GO-HY) nanohybrids can also spur in situ neovascularization, expediting tissue regeneration in rat tibial bone defects.106 Furthermore, GO-modified zinc-doped hydroxyapatite nanocomposites (G3HapZ) have demonstrated a remarkable vascularization potential in bone defects. These composites promote EC migration, attachment, and proliferation, resulting in increased bone regeneration and neovascularization.107 When applied to porous calcium phosphate scaffolds, water-soluble GO-copper nanocomposites (GO-Cu), elevate Hif-1α expression in BMSCs by activating the Erk1/2 signalling pathway and triggering the release of vascular endothelial growth factor (VEGF) and bone morphogenetic protein-2 (BMP-2) proteins. This coordinated activity significantly boosts angiogenesis and osteogenesis in vivo.108 In addition to providing useful evidence for exploring the role of GO composites in nerve regeneration during bone defect repair, these findings offer a basis for developing functional nerve conduits and bone repair materials.
Graphene oxide can serve as a functional carrier
Within the biomedical realm, GO holds immense potential as a versatile functional carrier. It is an excellent vehicle for the controlled delivery of bioactive substances primarily because of its distinctive 2D structure and highly modifiable surface properties. As a carrier, GO can achieve specific recognition and targeted transport of biomolecules via chemical modifications and the introduction of functional groups.71,109,110 In a study by Qin et al,111 GO was applied as a functionalized carrier in a nano-delivery system that successfully loaded and delivered miR-29b with osteogenesis-promoting effects, while exhibiting favourable biocompatibility and high transfection efficiency. The BMSC osteogenic differentiation and bone regeneration could be effectively promoted by preparing GO-based nanocomplexes and encapsulating them into CS hydrogels.111 A different study employed a polyethyleneimine (PEI) functionalized GO to efficiently load miR-214 inhibitors, which were incorporated into silk protein/hydroxyapatite (SF/HAP) scaffolds.112 The GO-PEI structure protected the microRNA (miRNA) inhibitor from extracellular degradation, allowing for controlled release within the complex. Besides highlighting GO’s significance as a functionalized carrier in bone defect repair, these findings offer novel prospects for bone tissue engineering and regeneration therapy.112
As a cutting-edge carrier, GO facilitates the controlled delivery of therapeutic proteins such as BMP-2.113,114 One study merged nanohydroxyapatite (nHA)-GO hybrid nanofillers with BMP-2, resulting in enhanced osteogenic differentiation of rabbit BMSCs (raBMSCs) in vitro.115 It also fostered new bone regeneration and Col deposition at bone defect sites in vivo. Interpenetrating network hydrogels incorporating GO loaded with IL-4 and BMP-2, and then integrated into carboxymethyl chitosan (CMC)/poly(ethylene glycol) diacrylate (PEGDA), significantly accelerated macrophage M2-type differentiation and BMSC osteogenesis in vitro. It also alleviated local inflammation at implantation sites, thereby promoting bone regeneration.116,117 Moreover, GO nanosheets adsorbed with BMP-2-like osteogenic peptides were fused with low-temperature 3D-printed PLGA/β-TCP composite scaffolds. This strategy effectively achieved a sustained release of BMP-2-like peptides, while maintaining high bioactivity levels. Additionally, it successfully promoted bone defect repair and tissue regeneration.118 Introducing GO into hydroxyapatite (HA)/polylactic-co-glycolic acid (PLGA) nanofibres effectively immobilized BMP-2 and basic fibroblast growth factor 2 (bFGF2) onto the nanocomposite surface, synergistically enhancing BMSC adhesion, proliferation, and osteogenic differentiation.119
The efficacy of GO as a carrier for delivering therapeutic phytomolecules like salvianolic acid B (SAB) is well documented. With abundant oxygen functional groups and active sites on its surface, GO efficiently adsorbs and forms covalent bonds with SAB, thereby enabling sustained drug release while preserving its bioactivity. This process significantly aids bone defect regeneration by promoting osteogenic differentiation and angiogenesis.120 Additionally, Sr-GO nanocomposites formed by depositing strontium nanoparticles onto the GO surface have high potential in bone defect repair. These composites ensure the prolonged release of strontium ions, working synergistically with GO to ultimately activate the MAPK signalling pathway. Moreover, cell adhesion, osteogenic differentiation, and angiogenesis are all enhanced by this concerted action.121
When employed as a functionalized carrier in bone defect repair, GO sustains the release of bioactive molecules, thereby promoting regeneration and healing within the defect areas by modulating enhanced cell signalling pathways. Given its great potential in the field of bone tissue engineering and bone defect treatment, GO provides new avenues for developing bone regeneration and bone repair treatment approaches.
Application of novel GO-loaded bioscaffolds in the repair of different types of bone defects
Repair of critical bone defects
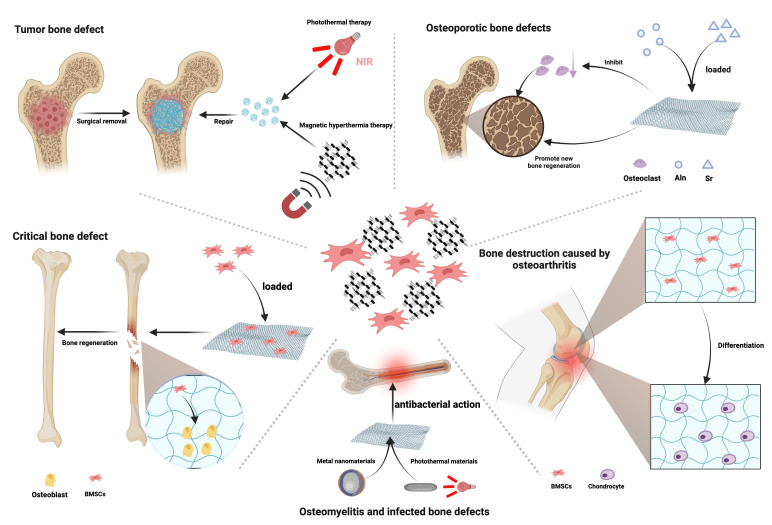
As bioscaffolds for addressing large bone defects, GO scaffolds offer a supportive structure that fosters osteoblast attachment and growth, facilitating bone tissue regeneration.13,122,123 Recent bone tissue engineering research has progressively revealed the bone formation-promoting attributes of GO nanomaterials in addressing bone defects that exceed the threshold beyond which the body cannot naturally repair the damage without external intervention (Figure 2). Various investigations have yielded multiple nanocomposite scaffolds with exceptional properties via GO incorporation into various polymers or biomaterials, advancing bone defect repair. Moreover, graphitic carbon nitride (g-C3N4) and GO nanomaterials have demonstrated substantial biocompatibility and cellular activity, both in vitro and in vivo. These nanomaterials can stimulate in vitro osteogenesis in human foetal osteoblasts (hFOB) and promote bone healing within a rabbit critical-size bone defect model in vivo.124 Another study revealed that besides bolstering bone growth, GO nanoparticles (GONP) also facilitated bone regeneration in a rat tibial bone defect model.125
Fig. 2.
This figure illustrates the versatile application of graphene oxide (GO)-loaded bioscaffolds in addressing various types of bone defects. Critical bone defect: reduced GO (rGO) has the capability to enhance the adhesion of bone marrow mesenchymal stem cells (BMSCs) and promote their proliferation and spontaneous osteogenic differentiation, thereby significantly accelerating the repair of bone defects. Tumour bone defect: GO scaffolds exhibit excellent photothermal and magnetic thermal properties, allowing for the targeted elimination of residual tumour cells surrounding the bone defect. Osteomyelitis and infected bone defects: GO/metal nanocomposites exhibit significant advantages in antibacterial efficacy. Additionally, GO composite materials demonstrate excellent photothermal conversion efficiency in photothermal/photodynamic therapy, leading to a powerful synergistic antibacterial phototherapeutic effect. Bone destruction caused by osteoarthritis: GO composite scaffolds are capable of promoting the proliferation and chondrogenic differentiation of BMSCs, effectively addressing bone destruction caused by osteoarthritis. Osteoporotic bone defects: GO scaffolds modified with strontium (Sr) or loaded with and releasing alendronate (Aln) effectively inhibit overactive osteoclasts in osteoporosis and promote new bone formation. NIR, near-infrared. Created with BioRender.com, and the license to publish from the BioRender has been obtained.
Additionally, GO has the capacity to create composite scaffolds using a range of biomaterials.126 Multifunctional hydrogels comprising rGO embedded in a gelatin methacrylate (GM) matrix, facilitated bone defect repair. In addition to enhancing osteoblast proliferation and osteogenic differentiation, these hydrogels expedited in vivo bone defect recovery within a rat cranial bone defect model.127 On the other hand, composite scaffolds predominantly comprising HA and rGO as key components also demonstrated a remarkable bone repair capability. Incorporating loaded rGO within these scaffolds enhances BMSC adhesion, proliferation, and spontaneous osteogenic differentiation, as well as gradual coverage, degradation, and arthroplasty by newly formed bone tissues. This process significantly accelerates bone tissue growth and defect repair.128 Additionally, in a rat critical-size cranial defect model, electrospun nanofibre scaffolds comprising electrospun poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/GO scaffold (P34HB/GO) exhibited an exceptional capacity for bone regeneration.129
Chitosan, gelatin, and collagen are some of the biomaterials that could be combined with GO. Incorporating GO enhances the porosity, hydrophilicity, and mechanical properties of the scaffolds, as well as cell proliferation and adhesion, and collagen fibre deposition, facilitating the repair of bone defects and surrounding tissues.36,130,131 Moreover, the use of photoacoustic effect has been explored to enhance bone defect repair. In this context, BMSCs were introduced to polylactic-co-glycolic acid/GO (PLGA-GO) scaffolds, followed by implantation into a rat bone defect model post-photoacoustic pretreatment. The scaffolds demonstrated a significant potential to enhance bone repair in vivo, presenting a novel therapeutic avenue for addressing bone defects.132
Application in the repair of tumour bone defects
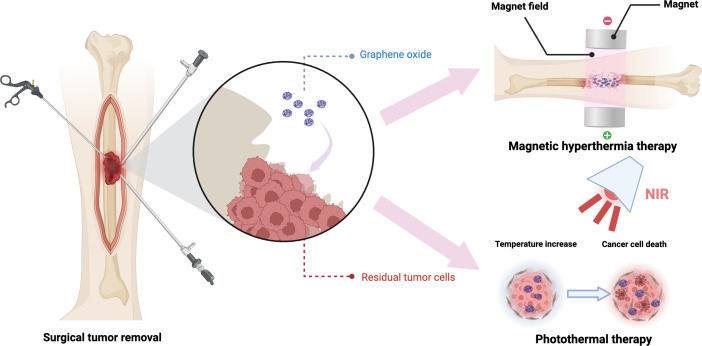
Tumour-induced bone defects contribute to poor prognosis.9,133 In recent years, developments in the field of nanomaterials have opened up new avenues for addressing and restoring tumour-associated bone defects.134 GO, an exceptional nanomaterial with distinctive physicochemical attributes and biological effects, has attracted considerable interest from researchers (Figure 3). Ge et al135 formulated a multifunctional bioscaffold by amalgamating GO nanoparticles with hydrated cerium oxide (CePO4) nanorods and bioactive chitosan. This bioscaffold possesses both photothermal tumour cell eradication capabilities and the potential to modulate macrophage polarization, thus improving vascularization and stimulating bone tissue generation. This strategy is suitable for managing bone defects arising from breast cancer bone metastasis.135 Fe3O4/GO nanocomposites were integrated into injectable α-tricalcium phosphate (α-TCP)/calcium sulphate biphasic bone cement, thereby forming magnetic bone cement (α-TCP/CS/Fe3O4/GO, αCFG). In addition to providing robust structural support for bone tissue repair, this cement harnesses its magnetothermal properties to eliminate residual tumour cells surrounding the damaged bone.136 Further research has demonstrated that 3D-printed β-tricalcium phosphate bioceramic scaffolds (β-TCP-Fe-GO), coated with surface-modified Fe3O4 nanoparticles/GO nanocomposites, exhibit remarkable magnetothermal characteristics and exceptional bone regeneration potential.137 This construct efficiently eradicated MG-63 cells (human osteosarcoma cell line MG63, obtained from the cell bank, Chinese Academy of Sciences) exposed to alternating magnetic field and significantly promoted rBMSC proliferation and osteogenesis-related gene expression.137 Furthermore, incorporation of GO into tricalcium silicate bone cement conferred high photothermal properties and controlled the cement’s hydration process, resulting in reduced setting time and enhanced compressive strength development.138 Using near-infrared laser exposure on the bone cement suppressed the growth of subcutaneous tumour tissue in nude mice, presenting a minimally invasive treatment option for both bone defects and tumours.138 Li et al139 constructed composite scaffolds by incorporating nanohydroxyapatite (nHA) and rGO sheets, demonstrating advantageous photothermal and osteogenic characteristics. In vitro experiments demonstrated that, when subjected to 808 nm near-infrared laser irradiation for 20 minutes, these scaffolds efficiently eradicated 92% of osteosarcoma cells (MG-63) and promoted the adhesion, proliferation, and osteogenic mineralization of rBMSCs.139 Furthermore, the scaffolds exhibited the capacity to enhance bone regeneration within rat cranial defects, demonstrating their potential utility in the treatment of sizable tumour-associated bone defects.139 These studies demonstrate the multifunctional role of GO in the treatment of bone tumours and bone defects, indicating that they are a promising therapeutic strategy for the repair of tumour-borne bone defects.
Fig. 3.
Graphene oxide (GO) composite materials exhibit outstanding magnetic and photothermal properties. Through magnetic hyperthermia or photothermal therapy, GO composite scaffolds can effectively eliminate residual tumour cells surrounding bone defects. NIR, near-infrared. Created with BioRender.com, and the license to publish from the BioRender has been obtained.
Treatment of osteomyelitis and infected bone defects
In recent years, bacterial infections have been a major threat to the field of bone tissue engineering due to the widespread use of bone grafting materials. To address this concern, researchers have been investigating innovative materials with both antimicrobial and osteogenic attributes.140,141 Among them, composites of GO and metal nanoparticles have demonstrated considerable advantages in terms of antimicrobial effectiveness. This combination synergistically leverages the properties of graphene oxide and metal nanoparticles, resulting in potent antimicrobial effects. A nanoplatform (GO/Ga) composed of GO and gallium nanoparticles (GaNPs) exhibited excellent antimicrobial potency and promoted the bone healing process in cases of implant-related infections.69 Moreover, this nanoplatform inhibited osteoclast differentiation by modulating BMP/Smad, MAPK, and NF-κB signalling pathways. In in vivo experiments, GO/Ga nanocomposites significantly prevented bone infections, mitigated osteolysis, and bolstered osseointegration at the implant-bone interface, with high biocompatibility.69 Furthermore, GO/copper nanocomposites (GO/Cu) exhibited a synergistic antibacterial prowess. These composite materials effectively disrupted bacterial membranes, increased the production of ROS, and hindered the activities of crucial enzymes, leading to successful antibacterial results. Moreover, GO/Cu composites exhibited positive cytocompatibility, the ability to promote osteogenic differentiation, and demonstrated in vivo characteristics of anti-infectiveness and inflammation inhibition.142 This highlights their potential applications in the treatment of infected bone defects.142 The study by Fu et al143 employed L-lysine-functionalized graphene oxide (Lys-g-GO) nanoparticles and polydopamine-assisted gold nanoparticles (AuNPs-PDA) coatings on PLGA scaffold materials, in which strong antimicrobial effects were found. Similarly, GO scaffold-modified zinc-doped hydroxyapatite nanocomposites (G3HapZ) and 3D porous scaffolds also exhibited robust antimicrobial activity and the potential to enhance bone repair.107 The bonding of rGO and Zn ions with functional protein groups (carboxyl, imidazole, thiols, and amines) in bacterial cell membranes damaged and inhibited the activity of enzymes.107 Furthermore, composite scaffolds of rGO and silver nanoparticles (AgNP) exhibited dual functionalities, i.e. antimicrobial and osteogenic attributes.144,145 Collectively, these results confirm the anti-infection potential of GO/metal nanocomposites in bone defect remediation.
GO composites exert significant photothermal conversion efficiency and biocompatibility in photothermal/photodynamic therapy.146 Li et al147 pioneered a polyetheretherketone (PEEK) implant coated with copper sulfide/graphene oxide (CuS/GO) nanocoating. The nanocoating not only possesses strong photothermal properties and exceptional thermal stability, but also enhances the photodynamic performance of the implant.147 This augmentation facilitates both photochemical and photodynamic therapeutic sterilization.147 The synergistic interactions between copper ions (Cu2+) and GO within the implant substantially improve the cell viability and osteogenic activity of osteoblasts, particularly in hyperglycaemic microenvironments.147 This introduces a novel approach to address osseointegration challenges in diabetic patients.147 GO nanosheets, polydopamine (pDA) nanomembranes, and oligopeptides were assembled onto porous sulfonated polyetheretherketone (SPEEK) surfaces, forming a multifunctional 2D nanocoating.148 This nanocoating enhances osseointegration and bone remodelling of the PEEK implant in vivo, producing a robust antimicrobial phototherapeutic effect via combined photothermal/photodynamic therapy.148 In a separate study, researchers engineered zeolitic imidazolate framework-8 (ZIF-8)/GO nanoparticles.149 This composition comprises both GO’s photothermal effect and ZIF-8-derived zinc ions’ antimicrobial ability, establishing a synergistic antimicrobial system with chemotherapeutic-photothermal functionality.149 This system not only promotes cellular growth, but also exhibits a high antimicrobial rate of up to 85% against Escherichia coli and Staphylococcus aureus. Such advancements have the potential to treat infected bone defects and promote bone repair.149
GO has been effectively integrated into injectable calcium phosphate bone cement-chitosan composites, producing pronounced antimicrobial activity.150 The multifunctionality of GO biomaterials, when incorporated into novel compositions, showcases synergistic effects for addressing osteomyelitis and infected bone defects. The amalgamation of antimicrobial and osseointegration-promoting characteristics establishes a promising basis for groundbreaking progress in the development of bone repair materials. Nevertheless, thorough and extensive investigations, coupled with rigorous clinical trials, are essential to substantiate their efficacy and safety. The ultimate goal is to attain improved therapeutic outcomes and broaden their applicability in clinical settings.
Bone destruction due to osteoarthritis
Osteoarthritis, a prevalent degenerative joint ailment, has a significant impact on global population. The available treatment strategies primarily focus on alleviating pain and managing symptoms.151 However, no effective approaches for promoting articular cartilage damage have been reported and osteolysis poses formidable challenges. In this regard, the addition of an appropriate amount of GO component has the effect of increasing the overall compressive strength of the material, and the potential application of GO materials for repairing bone damage caused by osteoarthritis has garnered significant attention.152,153
GO composite biomaterials have exhibited remarkable outcomes in osteoarthritis treatment. Xu et al153 crafted chitosan scaffolds containing sodium hyaluronate (SH) and GO, fostering in situ generation of gradient-distributed nanohydroxyapatite (NHAP) on the scaffolds to simulate the cartilage environment. This unique gradient distribution mimicked the binding of articular cartilage to subchondral bone.153 The resulting biomimetic scaffold displayed good mechanical attributes and water absorption, mirroring the stratified nature of natural joints.153 Furthermore, it promoted the proliferation of BMMSCs, showcasing an increased capacity for inducing joint regeneration.153 This novel method shows potential as a therapeutic pathway for treating osteoarthritis.153 In a separate investigation, Gong et al154 engineered 3D decellularized cartilage extracellular matrix (ECM) scaffolds with GO modifications, demonstrating remarkable effectiveness in cartilage repair. Incorporation of GO improved internal structure, mechanical properties, and enhanced adherence, proliferation, and cartilage differentiation of BMMSCs.154 These scaffolds repaired the cartilage defects in rabbit knee, suggesting that they can be applied in cartilage tissue engineering to articular cartilage injuries.154 Moreover, microplasma-assisted cross-linking formed the nanographene oxide (NGO)-enhanced gelatin hydrogels, with good mechanical and biomedical characteristics for cartilage tissue engineering.155 The hydrogels, characterized by suitable surface features, pore size, temperature-dependent viscoelasticity, and degradability, created an optimal cellular environment conducive to chondrocyte adhesion, proliferation, and the restoration of knee cartilage defects in rat models.155 This advancement has promising potential as a biocompatible platform for applications in cartilage tissue engineering.155 GO materials have diverse functions in osteoarthritis treatment.155 These include optimizing the structure and enhancing biocompatibility of bionic scaffolds, promoting the proliferation and cartilage differentiation of BMMSCs for cartilage tissue engineering, and controlling articular cartilage damage.155 These studies provide valuable insights for developing innovative therapeutic approaches and materials in the field of osteoarthritis treatment.
Repair of osteoporotic bone defects
Osteoporotic bone defects refer to the pathological condition where osteoporosis causes deterioration in bone mass and microarchitecture, leading to fragility fractures and subsequent large-scale bone defects. These defects exceed the bone tissue’s natural regenerative capacity and typically require intervention through bone tissue engineering or other surgical procedures.156-158 Over the past few decades, advancements in bone tissue engineering and biomaterials science have opened up new avenues for controlling osteoporotic bone defects. The integration of GO materials has opened up new possibilities for addressing osteoporotic bone deficiencies. GO scaffolds have demonstrated favourable biocompatibility and osteoinductive capabilities, particularly in the context of repairing osteoporotic bone defects.159,160 In the study by Mai et al,161 the development of strontium-modified 3D porous rGO/polypyrrole composite scaffolds (3D rGO/PPY/Sr) augmented cell proliferation and alkaline phosphatase (ALP) activity, along with calcified nodule formation. These scaffolds exhibited osteoconductive and osteoinductive properties, demonstrating promise for the treatment of bone defects associated with osteoporosis in the realm of bone tissue engineering.161 Moreover, the GO-modified collagen (Col-GO-Aln) sponge facilitates the controlled release of alendronate sodium (Aln), increasing the local concentration of Aln at bone defect sites. This effectively inhibits the overactive osteoclasts associated with osteoporosis-induced bone defects while promoting new bone formation.64 This delivery method circumvents the limitations associated with oral Aln administration, such as low bioavailability (0.9% to 1.8%), instability in digestive fluids, severe gastrointestinal reactions, and bisphosphonate-related osteonecrosis of the jaw.64 This approach introduces a novel strategy for addressing osteoporotic bone defects, showing the potential to provide more effective solutions to patients seeking repairs for osteoporosis-related bone defects.64
Negative effects and side effects of GO
Multiple studies have found that GO possesses potential cytotoxic and genotoxic effects.162-164 Arbo et al’s162 research demonstrated that GO significantly reduced the viability of H9c2 cardiomyoblast cells at concentrations of 20, 40, 60, 80, and 100 μg/ml, indicating marked cytotoxicity. GO induced mitochondrial hyperpolarization, suggesting a negative impact on mitochondrial function.162 This phenomenon can trigger reverse proton flow, producing ROS through the respiratory chain, which can lead to membrane peroxidation, ion loss, protein cleavage, and DNA strand breaks, ultimately causing cell death.162 Hashemi et al163 studied the effects of nano and micro GO (nGO and mGO) at concentrations of 100 and 200 μg/ml on apoptosis and the cell cycle. They found that GO induced S-phase arrest, disrupting cell division and proliferation, and triggered apoptosis, characterized by concentration-dependent cell death and sub-G1 phase apoptosis.163 Additionally, the sharp edges of GO’s sheet-like structure can cause physical damage to the cytoplasmic membrane through direct contact.164
GO can accumulate in tissues and organs, potentially leading to toxic responses in these organs. Audira et al165 investigated the effects of low and high concentrations of GO on zebrafish, revealing significant toxic effects. The group treated with 1 ppm GO showed a notable increase in ROS levels, which can lead to adverse reactions such as membrane peroxidation and DNA strand breaks.165 Zebrafish exposed to both low and high concentrations of GO exhibited significantly reduced locomotor activity, indicated by decreased average speed and a reduced proportion of fast movement time.165 Moreover, the GO-treated groups displayed significantly lower levels of serotonin (5-HT), acetylcholine (ACh), dopamine (DA), and cortisol in the brain, which may correlate with behavioral abnormalities.165 Aguado-Henche et al166 conducted intraperitoneal injections of graphene and GO nanosheets in rats to assess changes in blood and organs after 15 and 30 days. Blood analysis after 30 days showed signs of liver inflammation, including alterations in the erythrocyte series, such as increased microcytosis or higher mean corpuscular haemoglobin concentration.166 Additionally, coagulation times, including prothrombin time and thrombin time, were prolonged.166 Qu et al167 investigated the acute toxicity of intravenously injected GO in mice, finding that GO primarily accumulated in the lungs and liver. Accumulation in the lungs may affect the filtration function of the pulmonary capillary bed, while liver accumulation may involve clearance through the mononuclear phagocyte system (MPS).167 Although GO did not cause significant acute toxicity to blood cells in the short term (one day), its accumulation in the lungs and liver did not elicit acute adverse reactions.167 However, prolonged exposure might pose more potential risks, with GO possibly inducing chronic toxic effects such as long-term inflammatory responses, fibrosis, and organ damage, particularly in the lungs, leading to pulmonary fibrosis and chronic inflammation.168
GO might also have adverse effects in promoting cancer metastasis. Zhu et al169 found that GO-treated cancer cells (PC3, A549, and HepG2) exhibited statistically significant migration and invasion abilities in vitro. In a mouse breast cancer metastasis model, GO-pretreated cancer cells also showed significantly increased lung metastasis.169 The same study further revealed that low doses of GO could induce morphological and structural changes in cancer cells, triggering epithelial-mesenchymal transition (EMT).169 GO increased the levels of TGF-β receptor proteins on the cancer cell surface, leading to sustained activation of the TGF-β-Smad2/3 signalling pathway, thereby driving EMT.169
In conclusion, despite GO’s immense potential in biomedical applications, its potential adverse effects and side effects cannot be overlooked. Further in-depth studies on the toxic mechanisms and biological impacts of GO will help to optimize its applications, reduce health risks, and ensure safe use in medicine and other fields.164,170
Summary
This review described the application of GO-loaded biomaterials for treating bone defects. The incorporation of GO into novel bioscaffolds assumes multifaceted roles in therapy. First, GO scaffolds improve the biocompatibility and physicochemical properties of composite scaffolds. Second, these GO-loaded novel bioscaffolds promote cell adhesion and proliferation, thereby increasing the regeneration and repair of bone tissue. Moreover, GO scaffolds regulate osteoblast differentiation and catalyze bone matrix deposition and bone formation, while also exerting immunomodulatory and anti-inflammatory effects. These attributes improve the outcomes of bone defect repair. Furthermore, GO scaffolds stimulate nerve and blood vessel regeneration in bone defects, providing an important approach for the treatment and recovery process. Despite these benefits, the regulatory effects of GO on osteoclasts remain unclear, requiring further detailed studies to understand the specific mechanisms involved. These scaffolds have broad applicability in addressing various conditions associated with bone defects, presenting innovative treatment approaches for bone-related issues such as tumour-related, infected, and osteoporotic bone defects, osteomyelitis, and osteoarthritis. However, given the potential cytotoxicity and genotoxicity of GO observed in several studies, it is essential to investigate how GO influences osteoclast activity and its potential adverse effects, in order to optimize its therapeutic potential in bone defect repair.
Outlook
Presently, the application of GO in bone defect treatment still faces challenges and needs to address pressing concerns. Foremost, despite its potential to boost osteoblast proliferation and differentiation, the long-term in vivo biocompatibility and safety of GO necessitate deeper investigation. Researchers must thoroughly evaluate the biodegradation and in vivo metabolic behaviour of GO scaffolds to ensure they do not evoke adverse reactions or toxic effects.
Individual patients may display diverse characteristics and needs in their bone defects, underscoring the necessity of customizing the development of GO scaffolds accordingly; this tailored approach enhances outcomes in bone tissue repair. Moreover, investigating the combination of GO with other biomaterials or drugs has the potential to amplify efficacy and improve the success rate of bone defect treatment. To address this, future studies can explore adjusting the structure, morphology, and biomaterial ratios of GO, or coupling it with novel bioactive substances, ensuring the optimization of its osteoinductive and bone regenerative functions.
Moreover, despite the favourable biological effects of GO in laboratory settings, its viability and efficacy in clinical applications require further validation. In vivo animal applications of GO composite biomaterials must account for complex biological environments and clinical conditions, including immune responses and tissue tolerance. Hence, forthcoming research should pivot towards translating laboratory results of GO scaffolds into clinical settings, conducting extensive and prolonged clinical trials to gauge their actual efficacy and biosafety in treating various bone defects.
An intriguing avenue for future research is the personalized application of GO scaffolds. Given the diverse characteristics and requirements of bone defects among patients, personalized GO scaffolds tailored to specific defect types are warranted to optimize bone tissue repair outcomes. Furthermore, investigating the synergistic integration of GO with other biomaterials or therapeutic agents holds promise for enhancing treatment efficacy and improving bone defect healing success rates.
In summation, while GO has shown promise in bone defect repair and brought novel research perspectives to bone tissue engineering, biomaterials loaded with GO remain poised to tackle several challenges. In future research, a continued exploration of its biological impacts and safety, optimization of its material qualities and functionalization, and rigorous validation in clinical contexts will be instrumental in realizing the practical use of GO in bone defect treatment. Through ongoing research, GO scaffolds have the potential to bring enhanced opportunities and optimism to bone defect repair.
Author contributions
J. Xing: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Project administration
S. Liu: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing
Funding statement
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: the first batch of Young Scholars Support Program of Peking Union Medical College (Project No. 2022002; grant recipient: S. Liu), and Peking Union Medical College Hospital Research Incubation Funding for Postdoc (Project No. kyfyjj202309; grant recipient: S. Liu).
ICMJE COI statement
Both authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: the first batch of Young Scholars Support Program of Peking Union Medical College (Project No. 2022002; grant recipient: S. Liu), and Peking Union Medical College Hospital Research Incubation Funding for Postdoc (Project No. kyfyjj202309; grant recipient: S. Liu).
Data sharing
The anonymized data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The authors gratefully acknowledge all authors whose work contributed to this review, and BioRender for help with the manuscript preparation and editorial services.
Open access funding
The authors report that they received open access funding for their manuscript from the first batch of Young Scholars Support Program of Peking Union Medical College (Project No. 2022002; grant recipient: S. Liu), and Peking Union Medical College Hospital Research Incubation Funding for Postdoc (Project No. kyfyjj202309; grant recipient: S. Liu).
© 2024 Xing and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Jinyi Xing, Email: xingjinyi@126.com.
Shuzhong Liu, Email: 13585931654@163.com.
Data Availability
The anonymized data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Zhu W, Wang D, Xiong J, et al. Study on clinical application of nano-hydroxyapatite bone in bone defect repair. Artif Cells Nanomed Biotechnol. 2015;43(6):361–365. doi: 10.3109/21691401.2014.893521. [DOI] [PubMed] [Google Scholar]
- 2. Johnson EO, Troupis T, Soucacos PN. Tissue-engineered vascularized bone grafts: basic science and clinical relevance to trauma and reconstructive microsurgery. Microsurgery. 2011;31(3):176–182. doi: 10.1002/micr.20821. [DOI] [PubMed] [Google Scholar]
- 3. Nair MB, Kretlow JD, Mikos AG, Kasper FK. Infection and tissue engineering in segmental bone defects--a mini review. Curr Opin Biotechnol. 2011;22(5):721–725. doi: 10.1016/j.copbio.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiiski J, Kuokkanen HO, Kääriäinen M, Kaartinen IS, Pakarinen T-K, Laitinen MK. Clinical results and quality of life after reconstruction following sacrectomy for primary bone malignancy. J Plast Reconstr Aesthet Surg. 2018;71(12):1730–1739. doi: 10.1016/j.bjps.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 5. Lagaras A, Kontogeorgakos V, Varitimidis S, Malizos KN. Treatment outcomes for infected juxta-articular knee nonunions. Hippokratia. 2018;22(4):183–187. [PMC free article] [PubMed] [Google Scholar]
- 6. Zekry KM, Yamamoto N, Hayashi K, et al. Reconstruction of intercalary bone defect after resection of malignant bone tumor. J Orthop Surg (Hong Kong) 2019;27(1):2309499019832970. doi: 10.1177/2309499019832970. [DOI] [PubMed] [Google Scholar]
- 7. Baldwin P, Li DJ, Auston DA, Mir HS, Yoon RS, Koval KJ. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J Orthop Trauma. 2019;33(4):203–213. doi: 10.1097/BOT.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 8. Sharifi M, Kheradmandi R, Salehi M, Alizadeh M, Ten Hagen TLM, Falahati M. Criteria, challenges, and opportunities for acellularized allogeneic/xenogeneic bone grafts in bone repairing. ACS Biomater Sci Eng. 2022;8(8):3199–3219. doi: 10.1021/acsbiomaterials.2c00194. [DOI] [PubMed] [Google Scholar]
- 9. Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bharadwaz A, Jayasuriya AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110698. doi: 10.1016/j.msec.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raslan A, Saenz Del Burgo L, Ciriza J, Pedraz JL. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int J Pharm. 2020;580:119226. doi: 10.1016/j.ijpharm.2020.119226. [DOI] [PubMed] [Google Scholar]
- 12. Yadav S, Singh Raman AP, Meena H, et al. An update on graphene oxide: applications and toxicity. ACS Omega. 2022;7(40):35387–35445. doi: 10.1021/acsomega.2c03171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holt BD, Wright ZM, Arnold AM, Sydlik SA. Graphene oxide as a scaffold for bone regeneration. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(3) doi: 10.1002/wnan.1437. [DOI] [PubMed] [Google Scholar]
- 14. Peng Z, Zhao T, Zhou Y, Li S, Li J, Leblanc RM. Bone tissue engineering via carbon-based nanomaterials. Adv Healthc Mater. 2020;9(5):e1901495. doi: 10.1002/adhm.201901495. [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Liao C, Tjong SC. Synthetic biodegradable aliphatic polyester nanocomposites reinforced with nanohydroxyapatite and/or graphene oxide for bone tissue engineering applications. Nanomater (Basel) 2019;9(4):590. doi: 10.3390/nano9040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Govindarajan D, Saravanan S, Sudhakar S, Vimalraj S. Graphene: a multifaceted carbon-based material for bone tissue engineering applications. ACS Omega. 2024;9(1):67–80. doi: 10.1021/acsomega.3c07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alazab MH, Abouelgeit SA, Aboushelib MN. Histomorphometric evaluation of 3D printed graphene oxide-enriched poly(ε-caprolactone) scaffolds for bone regeneration. Heliyon. 2023;9(5):e15844. doi: 10.1016/j.heliyon.2023.e15844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan Q-C, Jiang X-S, Chen L, et al. Bioactive graphene oxide-functionalized self-expandable hydrophilic and osteogenic nanocomposite for orthopaedic applications. Mater Today Bio. 2023;18:100500. doi: 10.1016/j.mtbio.2022.100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lalwani G, Henslee AM, Farshid B, et al. Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering. Biomacromolecules. 2013;14(3):900–909. doi: 10.1021/bm301995s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seonwoo H, Choung H-W, Park S, et al. Reduced graphene oxide-incorporated calcium phosphate cements with pulsed electromagnetic fields for bone regeneration. RSC Adv. 2022;12(9):5557–5570. doi: 10.1039/d1ra05717k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nasrollahi N, Nourian Dehkordi A, Jamshidizad A, Chehelgerdi M. Preparation of brushite cements with improved properties by adding graphene oxide. Int J Nanomedicine. 2019;14:3785–3797. doi: 10.2147/IJN.S196666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abedin Dargoush S, Hanaee-Ahvaz H, Irani S, Soleimani M, Khatami SM, Sohi AN. A composite bilayer scaffold functionalized for osteochondral tissue regeneration in rat animal model. J Tissue Eng Regen Med. 2022;16(6):559–574. doi: 10.1002/term.3297. [DOI] [PubMed] [Google Scholar]
- 23. Ryu SB, Park KM, Park KD. In situ graphene oxide-gelatin hydrogels with enhanced mechanical property for tissue adhesive and regeneration. Biochem Biophys Res Commun. 2022;592:24–30. doi: 10.1016/j.bbrc.2022.01.010. [DOI] [PubMed] [Google Scholar]
- 24. Qin W, Li C, Liu C, et al. 3D printed biocompatible graphene oxide, attapulgite, and collagen composite scaffolds for bone regeneration. J Biomater Appl. 2022;36(10):1838–1851. doi: 10.1177/08853282211067646. [DOI] [PubMed] [Google Scholar]
- 25. Zhang J, Eyisoylu H, Qin XH, Rubert M, Müller R. 3D bioprinting of graphene oxide-incorporated cell-laden bone mimicking scaffolds for promoting scaffold fidelity, osteogenic differentiation and mineralization. Acta Biomater. 2021;121:637–652. doi: 10.1016/j.actbio.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 26. Khan MUA, Razak SIA, Ansari MNM, Zulkifli RM, Ahmad Zawawi N, Arshad M. Development of biodegradable bio-based composite for bone tissue engineering: synthesis, characterization and in vitro biocompatible evaluation. Polymers (Basel) 2021;13(21):3611. doi: 10.3390/polym13213611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Devi G V Y, Nagendra AH, Shenoy P S, Chatterjee K, Venkatesan J. Fucoidan-Incorporated Composite Scaffold Stimulates Osteogenic Differentiation of Mesenchymal Stem Cells for Bone Tissue Engineering. Mar Drugs. 2022;20(10):589. doi: 10.3390/md20100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu W, Dong X, Qin H, Sui L, Wang J. Three-dimensional porous reduced graphene oxide/hydroxyapatite membrane for guided bone regeneration. Colloids Surf B Biointerfaces. 2021;208:112102. doi: 10.1016/j.colsurfb.2021.112102. [DOI] [PubMed] [Google Scholar]
- 29. Bahrami S, Baheiraei N, Shahrezaee M. Biomimetic reduced graphene oxide coated collagen scaffold for in situ bone regeneration. Sci Rep. 2021;11(1):16783. doi: 10.1038/s41598-021-96271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu S, Zhou C, Mou S, et al. Biocompatible graphene oxide-collagen composite aerogel for enhanced stiffness and in situ bone regeneration. Mater Sci Eng C Mater Biol Appl. 2019;105:110137. doi: 10.1016/j.msec.2019.110137. [DOI] [PubMed] [Google Scholar]
- 31. Dinescu S, Ionita M, Ignat S-R, Costache M, Hermenean A. Graphene oxide enhances chitosan-based 3d scaffold properties for bone tissue engineering. IJMS. 2019;20(20):5077. doi: 10.3390/ijms20205077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. López Tenorio D, Valencia CH, Valencia C, et al. Evaluation of the biocompatibility of CS-Graphene Oxide compounds in vivo. Int J Mol Sci. 2019;20(7):1572. doi: 10.3390/ijms20071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou C, Liu S, Li J, et al. Collagen functionalized with graphene oxide enhanced biomimetic mineralization and in situ bone defect repair. ACS Appl Mater Interfaces. 2018;10(50):44080–44091. doi: 10.1021/acsami.8b17636. [DOI] [PubMed] [Google Scholar]
- 34. Liu S, Mou S, Zhou C, et al. Off-the-shelf biomimetic graphene oxide–collagen hybrid scaffolds wrapped with osteoinductive extracellular matrix for the repair of cranial defects in rats. ACS Appl Mater Interfaces. 2018;10(49):42948–42958. doi: 10.1021/acsami.8b11071. [DOI] [PubMed] [Google Scholar]
- 35. Yu Z, Xiao C, Huang Y, et al. Enhanced bioactivity and osteoinductivity of carboxymethyl chitosan/nanohydroxyapatite/graphene oxide nanocomposites. RSC Adv. 2018;8(32):17860–17877. doi: 10.1039/C8RA00383A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawamoto K, Miyaji H, Nishida E, et al. Characterization and evaluation of graphene oxide scaffold for periodontal wound healing of class II furcation defects in dog. Int J Nanomedicine. 2018;13:2365–2376. doi: 10.2147/IJN.S163206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valencia-Llano CH, Solano MA, Grande-Tovar CD. Nanocomposites of Chitosan/Graphene Oxide/Titanium Dioxide nanoparticles/blackberry waste extract as potential bone substitutes. Polymers (Basel) 2021;13(22):3877. doi: 10.3390/polym13223877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qi C, Deng Y, Xu L, et al. A sericin/ graphene oxide composite scaffold as a biomimetic extracellular matrix for structural and functional repair of calvarial bone. Theranostics. 2020;10(2):741–756. doi: 10.7150/thno.39502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karimi N, Kharaziha M, Raeissi K. Electrophoretic deposition of chitosan reinforced graphene oxide-hydroxyapatite on the anodized titanium to improve biological and electrochemical characteristics. Mat Sci Eng C. 2019;98:140–152. doi: 10.1016/j.msec.2018.12.136. [DOI] [PubMed] [Google Scholar]
- 40. Liang J, Li W, Zhuang N, et al. Experimental study on bone defect repair by bmscs combined with a light-sensitive material: g-C(3)N(4)/rgo. J Biomater Sci Polym Ed. 2021;32(2):248–265. doi: 10.1080/09205063.2020.1827923. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y, Hu J. Isocyanate modified GO shape-memory polyurethane composite. Polymers (Basel) 2020;12(1):118. doi: 10.3390/polym12010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, Hu J, Zhao X, Xie R, Qin T, Ji F. Mechanically robust shape memory polyurethane nanocomposites for minimally invasive bone repair. ACS Appl Bio Mater. 2019;2(3):1056–1065. doi: 10.1021/acsabm.8b00655. [DOI] [PubMed] [Google Scholar]
- 43. Biru EI, Necolau MI, Zainea A, Iovu H. Graphene oxide-protein-based scaffolds for tissue engineering: recent advances and applications. Polymers (Basel) 2022;14(5):1032. doi: 10.3390/polym14051032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duran M, Luzo ACM, de Souza JG, Favaro WJ, Garcia P, Duran N. Graphene oxide as scaffolds for stem cells: an overview. Curr Mol Med. 2017;17(9):619–626. doi: 10.2174/1566524018666180308111915. [DOI] [PubMed] [Google Scholar]
- 45. Jiang Y, Zhou D, Yang B. 3D bioprinted GelMA/GO composite induces osteoblastic differentiation. J Biomater Appl. 2022;37(3):527–537. doi: 10.1177/08853282221098235. [DOI] [PubMed] [Google Scholar]
- 46. Khan MUA, Rizwan M, Razak SIA, Hassan A, Rasheed T, Bilal M. Electroactive polymeric nanocomposite BC-g-(fe(3)O(4)/GO) materials for bone tissue engineering: in vitro evaluations. J Biomater Sci Polym Ed. 2022;33(11) doi: 10.1080/09205063.2022.2054544. [DOI] [PubMed] [Google Scholar]
- 47. Hussein KH, Abdelhamid HN, Zou X, Woo HM. Ultrasonicated graphene oxide enhances bone and skin wound regeneration. Mater Sci Eng C Mater Biol Appl. 2019;94:484–492. doi: 10.1016/j.msec.2018.09.051. [DOI] [PubMed] [Google Scholar]
- 48. Șelaru A, Herman H, Vlăsceanu GM, et al. Graphene-oxide porous biopolymer hybrids enhance in vitro osteogenic differentiation and promote ectopic osteogenesis in vivo. Int J Mol Sci. 2022;23(1):491. doi: 10.3390/ijms23010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu D, Wang C, Wu J, et al. Effects of low-concentration graphene oxide quantum dots on improving the proliferation and differentiation ability of bone marrow mesenchymal stem cells through the Wnt/β-Catenin signaling pathway. ACS Omega. 2022;7(16):13546–13556. doi: 10.1021/acsomega.1c06892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Halim A, Liu L, Ariyanti AD, Ju Y, Luo Q, Song G. Low-dose suspended graphene oxide nanosheets induce antioxidant response and osteogenic differentiation of bone marrow-derived mesenchymal stem cells via JNK-dependent FoxO1 activation. J Mater Chem B. 2019;7(39):5998–6009. doi: 10.1039/c9tb01413f. [DOI] [PubMed] [Google Scholar]
- 51. Jang HJ, Kang MS, Kim W-H, et al. 3D printed membranes of polylactic acid and graphene oxide for guided bone regeneration. Nanoscale Adv. 2023;5(14):3619–3628. doi: 10.1039/d3na00112a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sharma A, Gupta S, Sampathkumar TS, Verma RS. Modified graphene oxide nanoplates reinforced 3D printed multifunctional scaffold for bone tissue engineering. Biomater Adv. 2022;134:112587. doi: 10.1016/j.msec.2021.112587. [DOI] [PubMed] [Google Scholar]
- 53. Khoramgah MS, Ghanbarian H, Ranjbari J, et al. Repairing rat calvarial defects by adipose mesenchymal stem cells and novel freeze-dried three-dimensional nanofibrous scaffolds. Bioimpacts. 2023;13(1):31–42. doi: 10.34172/bi.2021.23711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saravanan S, Vimalraj S, Anuradha D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomed Pharmacother. 2018;107:908–917. doi: 10.1016/j.biopha.2018.08.072. [DOI] [PubMed] [Google Scholar]
- 55. Jiao D, Zheng A, Liu Y, et al. Bidirectional differentiation of BMSCs induced by a biomimetic procallus based on a gelatin-reduced graphene oxide reinforced hydrogel for rapid bone regeneration. Bioact Mater. 2021;6(7):2011–2028. doi: 10.1016/j.bioactmat.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Purohit SD, Bhaskar R, Singh H, Yadav I, Gupta MK, Mishra NC. Development of a nanocomposite scaffold of gelatin-alginate-graphene oxide for bone tissue engineering. Int J Biol Macromol. 2019;133:592–602. doi: 10.1016/j.ijbiomac.2019.04.113. [DOI] [PubMed] [Google Scholar]
- 57. Sun X, Yang J, Ma J, et al. Three-dimensional bioprinted BMSCs-laden highly adhesive artificial periosteum containing gelatin-dopamine and graphene oxide nanosheets promoting bone defect repair. Biofabrication. 2023;15(2):Epub 20230210. doi: 10.1088/1758-5090/acb73e. [DOI] [PubMed] [Google Scholar]
- 58. Jampilek J, Kralova K. Advances in biologically applicable graphene-based 2D nanomaterials. Int J Mol Sci. 2022;23(11):6253. doi: 10.3390/ijms23116253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu X, Li L, Gaihre B, et al. Scaffold-free spheroids with two-dimensional heteronano-layers (2DHNL) enabling stem cell and osteogenic factor codelivery for bone repair. ACS Nano. 2022;16(2):2741–2755. doi: 10.1021/acsnano.1c09688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li Y, Zhang X, Dai C, et al. Bioactive three-dimensional graphene oxide foam/polydimethylsiloxane/zinc silicate scaffolds with enhanced osteoinductivity for bone regeneration. ACS Biomater Sci Eng. 2020;6(5):3015–3025. doi: 10.1021/acsbiomaterials.9b01931. [DOI] [PubMed] [Google Scholar]
- 61. Wang W, Liu Y, Yang C, et al. Mesoporous bioactive glass combined with graphene oxide scaffolds for bone repair. Int J Biol Sci. 2019;15(10):2156–2169. doi: 10.7150/ijbs.35670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Y, Hu X, Dai J, et al. A 3D graphene coated bioglass scaffold for bone defect therapy based on the molecular targeting approach. J Mater Chem B. 2017;5(33):6794–6800. doi: 10.1039/C7TB01515A. [DOI] [PubMed] [Google Scholar]
- 63. Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu Rev Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 64. Zeng Y, Zhou M, Chen L, et al. Alendronate loaded graphene oxide functionalized collagen sponge for the dual effects of osteogenesis and anti-osteoclastogenesis in osteoporotic rats. Bioact Mater. 2020;5(4):859–870. doi: 10.1016/j.bioactmat.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dou C, Ding N, Luo F, et al. Graphene-based microRNA transfection blocks preosteoclast fusion to increase bone formation and vascularization. Adv Sci (Weinh) 2018;5(2):1700578. doi: 10.1002/advs.201700578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jiao D, Wang J, Yu W, et al. Biocompatible reduced graphene oxide stimulated BMSCs induce acceleration of bone remodeling and orthodontic tooth movement through promotion on osteoclastogenesis and angiogenesis. Bioact Mater. 2022;15:409–425. doi: 10.1016/j.bioactmat.2022.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang H, Wu Q, Lai Y, Cai Y. Effect of graphene-oxide-modified osteon-like concentric microgrooved surface on the osteoclastic differentiation of macrophages. Hua Xi Kou Qiang Yi Xue Za Zhi. 2023;41(2):165–174. doi: 10.7518/hxkq.2023.2022354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xiong K, Wu T, Fan Q, Chen L, Yan M. Novel reduced graphene oxide/zinc silicate/calcium silicate electroconductive biocomposite for stimulating osteoporotic bone regeneration. ACS Appl Mater Interfaces. 2017;9(51):44356–44368. doi: 10.1021/acsami.7b16206. [DOI] [PubMed] [Google Scholar]
- 69. Yang Y, Li M, Zhou B, Jiang X, Zhang D, Luo H. Graphene oxide/gallium nanoderivative as a multifunctional modulator of osteoblastogenesis and osteoclastogenesis for the synergistic therapy of implant-related bone infection. Bioact Mater. 2023;25:594–614. doi: 10.1016/j.bioactmat.2022.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chumakova N, Kokorin A. Graphene oxide membranes-synthesis, properties, and applications. Membranes (Basel) 2023;13(9):771. doi: 10.3390/membranes13090771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yi L, Zhang Y, Shi X, et al. Recent progress of functionalised graphene oxide in cancer therapy. J Drug Target. 2019;27(2):125–144. doi: 10.1080/1061186X.2018.1474359. [DOI] [PubMed] [Google Scholar]
- 72. Huang C-Y, Lin Y-C, Chung JHY, et al. Enhancing cementitious composites with functionalized graphene oxide-based materials: surface chemistry and mechanisms. Int J Mol Sci. 2023;24(13):10461. doi: 10.3390/ijms241310461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ding H, Peng X, Yu X, et al. The construction of a self-assembled coating with chitosan-grafted reduced graphene oxide on porous calcium polyphosphate scaffolds for bone tissue engineering. Biomed Mater. 2022;17(4) doi: 10.1088/1748-605X/ac6eab. [DOI] [PubMed] [Google Scholar]
- 74. Pahlevanzadeh F, Bakhsheshi-Rad HR, Hamzah E. In-vitro biocompatibility, bioactivity, and mechanical strength of PMMA-PCL polymer containing fluorapatite and graphene oxide bone cements. J Mech Behav Biomed Mater. 2018;82:257–267. doi: 10.1016/j.jmbbm.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 75. Silva E, Vasconcellos L de, Rodrigues BVM, et al. PDLLA honeycomb-like scaffolds with a high loading of superhydrophilic graphene/multi-walled carbon nanotubes promote osteoblast in vitro functions and guided in vivo bone regeneration. Mater Sci Eng C Mater Biol Appl. 2017;73:31–39. doi: 10.1016/j.msec.2016.11.075. [DOI] [PubMed] [Google Scholar]
- 76. S Medeiros J, Oliveira AM, Carvalho J de, et al. Nanohydroxyapatite/graphene nanoribbons nanocomposites induce in vitro osteogenesis and promote in vivo bone neoformation. ACS Biomater Sci Eng. 2018;4(5):1580–1590. doi: 10.1021/acsbiomaterials.7b01032. [DOI] [PubMed] [Google Scholar]
- 77. Peng S, Feng P, Wu P, et al. Graphene oxide as an interface phase between polyetheretherketone and hydroxyapatite for tissue engineering scaffolds. Sci Rep. 2017;7:46604. doi: 10.1038/srep46604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee JH, Shin YC, Lee S-M, et al. Enhanced osteogenesis by reduced graphene oxide/hydroxyapatite nanocomposites. Sci Rep. 2015;5:18833. doi: 10.1038/srep18833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang J, Tong D, Song H, et al. Osteoimmunity‐regulating biomimetically hierarchical scaffold for augmented bone regeneration. Adv Mater Weinheim. 2022;34(36) doi: 10.1002/adma.202202044. [DOI] [PubMed] [Google Scholar]
- 80. Su N, Villicana C, Barati D, Freeman P, Luo Y, Yang F. Stem cell membrane-coated microribbon scaffolds induce regenerative innate and adaptive immune responses in a critical-size cranial bone defect model. Adv Mater. 2023;35(10):e2208781. doi: 10.1002/adma.202208781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu X, Gaihre B, Park S. 3D-printed scaffolds with 2D hetero-nanostructures and immunomodulatory cytokines provide pro-healing microenvironment for enhanced bone regeneration. Bioact Mater. 2023;27:216–230. doi: 10.1016/j.bioactmat.2023.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fu M, Li J, Liu M, et al. Sericin/Nano-Hydroxyapatite hydrogels based on graphene oxide for effective bone regeneration via immunomodulation and osteoinduction. Int J Nanomedicine. 2023;18:1875–1895. doi: 10.2147/IJN.S399487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xue H, Zhang Z, Lin Z, et al. Enhanced tissue regeneration through immunomodulation of angiogenesis and osteogenesis with a multifaceted nanohybrid modified bioactive scaffold. Bioact Mater. 2022;18:552–568. doi: 10.1016/j.bioactmat.2022.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guo B, Feng X, Wang Y, Wang X, He Y. Biomimetic and immunomodulatory baicalin-loaded graphene oxide-demineralized bone matrix scaffold for in vivo bone regeneration. J Mater Chem B. 2021;9(47):9720–9733. doi: 10.1039/d1tb00618e. [DOI] [PubMed] [Google Scholar]
- 85. Sun J, Li L, Xing F, et al. Graphene oxide-modified silk fibroin/nanohydroxyapatite scaffold loaded with urine-derived stem cells for immunomodulation and bone regeneration. Stem Cell Res Ther. 2021;12(1):591. doi: 10.1186/s13287-021-02634-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhao S, Dong W, Wang Y, et al. Construction of multifunctional zinc ion sustained-release biocoating on the surface of carbon fiber reinforced polyetheretherketone with enhanced anti-inflammatory activity, angiogenesis, and osteogenesis. ACS Appl Mater Interfaces. 2023;15(26):31256–31272. doi: 10.1021/acsami.3c04948. [DOI] [PubMed] [Google Scholar]
- 87. Zheng Z, Chen Y, Hong H, et al. The “Yin and Yang” of immunomodulatory magnesium-enriched graphene oxide nanoscrolls decorated biomimetic scaffolds in promoting bone regeneration. Adv Healthc Mater. 2021;10(2):e2000631. doi: 10.1002/adhm.202000631. [DOI] [PubMed] [Google Scholar]
- 88. Zhou J, Li Y, He J, et al. ROS scavenging graphene-based hydrogel enhances type H vessel formation and vascularized bone regeneration via ZEB1/Notch1 mediation. Macromol Biosci. 2023;23(4):e2200502. doi: 10.1002/mabi.202200502. [DOI] [PubMed] [Google Scholar]
- 89. Li Y, Yang L, Hou Y, et al. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact Mater. 2022;18:213–227. doi: 10.1016/j.bioactmat.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Radunovic M, De Colli M, De Marco P, et al. Graphene oxide enrichment of collagen membranes improves DPSCs differentiation and controls inflammation occurrence. J Biomed Mater Res. 2017;105(8):2312–2320. doi: 10.1002/jbm.a.36085. [DOI] [PubMed] [Google Scholar]
- 91. Yu K, Huangfu H, Qin Q, et al. Application of bone marrow-derived macrophages combined with bone mesenchymal stem cells in dual-channel three-dimensional bioprinting scaffolds for early immune regulation and osteogenic induction in rat calvarial defects. ACS Appl Mater Interfaces. 2022;14(41):47052–47065. doi: 10.1021/acsami.2c13557. [DOI] [PubMed] [Google Scholar]
- 92. Kim J-W, Shin YC, Lee J-J, et al. The effect of reduced graphene oxide-coated biphasic calcium phosphate bone graft material on osteogenesis. Int J Mol Sci. 2017;18(8):1725. doi: 10.3390/ijms18081725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Grijalvo S, Díaz DD. Graphene-based hybrid materials as promising scaffolds for peripheral nerve regeneration. Neurochem Int. 2021;147:105005. doi: 10.1016/j.neuint.2021.105005. [DOI] [PubMed] [Google Scholar]
- 94. Wang S-X, Lu Y-B, Wang X-X, et al. Graphene and graphene-based materials in axonal repair of spinal cord injury. Neural Regen Res. 2022;17(10):2117. doi: 10.4103/1673-5374.335822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Aleemardani M, Zare P, Seifalian A, Bagher Z, Seifalian AM. Graphene-based materials prove to be a promising candidate for nerve regeneration following peripheral nerve injury. Biomedicines. 2021;10(1):73. doi: 10.3390/biomedicines10010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang Y, Yang B, Huang Z, et al. Progress and mechanism of graphene oxide-composited materials in application of peripheral nerve repair. Colloids Surf B Bio. 2024;234:113672. doi: 10.1016/j.colsurfb.2023.113672. [DOI] [PubMed] [Google Scholar]
- 97. Kim TH, Lee T, El-Said WA, Choi JW. Graphene-based materials for stem cell applications. Materials (Basel) 2015;8(12):8674–8690. doi: 10.3390/ma8125481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang X, Zhang H, Zhang Y, et al. 3D printed reduced graphene oxide-GelMA hybrid hydrogel scaffolds for potential neuralized bone regeneration. J Mater Chem B. 2023;11(6):1288–1301. doi: 10.1039/D2TB01979E. [DOI] [PubMed] [Google Scholar]
- 99. Yan Z, Li K, Shao D, et al. Visible-light-responsive reduced graphene oxide/g-C3N4/TiO2composite nanocoating for photoelectric stimulation of neuronal and osteoblastic differentiation. RSC Adv. 2022;12(15):8878–8888. doi: 10.1039/D2RA00282E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang W, Fang X-X, Li Q-C, Pi W, Han N. Reduced graphene oxide-embedded nerve conduits loaded with bone marrow mesenchymal stem cell-derived extracellular vesicles promote peripheral nerve regeneration. Neural Regen Res. 2023;18(1):200–206. doi: 10.4103/1673-5374.343889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang J, Cheng Y, Wang H, et al. Biomimetic and hierarchical nerve conduits from multifunctional nanofibers for guided peripheral nerve regeneration. Acta Biomater. 2020;117:180–191. doi: 10.1016/j.actbio.2020.09.037. [DOI] [PubMed] [Google Scholar]
- 102. Qian Y, Song J, Zhao X, et al. 3D fabrication with integration molding of a graphene oxide/polycaprolactone nanoscaffold for neurite regeneration and angiogenesis. Adv Sci (Weinh) 2018;5(4):1700499. doi: 10.1002/advs.201700499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Awaja F, Speranza G, Kaltenegger H, Coraça-Huber D, Lohberger B. Surface modification and characterization of GO/polymer thin coatings as excellent bio-active platforms for tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2018;84:130–139. doi: 10.1016/j.msec.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 104. Li T, Zhang T. The application of nanomaterials in angiogenesis. Curr Stem Cell Res Ther. 2021;16(1):74–82. doi: 10.2174/1574888X15666200211102203. [DOI] [PubMed] [Google Scholar]
- 105. Liu W, Luo H, Wei Q, et al. Electrochemically derived nanographene oxide activates endothelial tip cells and promotes angiogenesis by binding endogenous lysophosphatidic acid. Bioact Mater. 2022;9:92–104. doi: 10.1016/j.bioactmat.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dantas PCL, Martins-Júnior PA, Coutinho DCO, et al. Nanohybrid composed of graphene oxide functionalized with sodium hyaluronate accelerates bone healing in the tibia of rats. Mater Sci Eng C Mater Biol Appl. 2021;123:111961. doi: 10.1016/j.msec.2021.111961. [DOI] [PubMed] [Google Scholar]
- 107. Chopra V, Thomas J, Sharma A, et al. Synthesis and evaluation of a zinc eluting rGO/Hydroxyapatite nanocomposite optimized for bone augmentation. ACS Biomater Sci Eng. 2020;6(12):6710–6725. doi: 10.1021/acsbiomaterials.0c00370. [DOI] [PubMed] [Google Scholar]
- 108. Zhang W, Chang Q, Xu L, et al. Graphene oxide‐copper nanocomposite‐coated porous CaP scaffold for vascularized bone regeneration via activation of Hif‐1α. Adv Healthc Mater. 2016;5(11):1299–1309. doi: 10.1002/adhm.201500824. [DOI] [PubMed] [Google Scholar]
- 109. Kiew SF, Kiew LV, Lee HB, Imae T, Chung LY. Assessing biocompatibility of graphene oxide-based nanocarriers: a review. J Control Release. 2016;226:217–228. doi: 10.1016/j.jconrel.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 110. Yang K, Feng L, Shi X, Liu Z. Nano-graphene in biomedicine: theranostic applications. Chem Soc Rev. 2013;42(2):530–547. doi: 10.1039/C2CS35342C. [DOI] [PubMed] [Google Scholar]
- 111. Qin H, Ji Y, Li G, et al. MicroRNA-29b/graphene oxide-polyethyleneglycol-polyethylenimine complex incorporated within chitosan hydrogel promotes osteogenesis. Front Chem. 2022;10:958561. doi: 10.3389/fchem.2022.958561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ou L, Lan Y, Feng Z, et al. Functionalization of SF/HAP scaffold with GO-PEI-miRNA inhibitor complexes to enhance bone regeneration through activating transcription factor 4. Theranostics. 2019;9(15):4525–4541. doi: 10.7150/thno.34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wu J, Zheng A, Liu Y, et al. Enhanced bone regeneration of the silk fibroin electrospun scaffolds through the modification of the graphene oxide functionalized by BMP-2 peptide. Int J Nanomedicine. 2019;14:733–751. doi: 10.2147/IJN.S187664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. La W, Park S, Yoon H, et al. Delivery of a therapeutic protein for bone regeneration from a substrate coated with graphene oxide. Small. 2013;9(23):4051–4060. doi: 10.1002/smll.201300571. [DOI] [PubMed] [Google Scholar]
- 115. Wang B, Yuan S, Xin W, et al. Synergic adhesive chemistry-based fabrication of BMP-2 immobilized silk fibroin hydrogel functionalized with hybrid nanomaterial to augment osteogenic differentiation of rBMSCs for bone defect repair. Int J Biol Macromol. 2021;192:407–416. doi: 10.1016/j.ijbiomac.2021.09.036. [DOI] [PubMed] [Google Scholar]
- 116. Zou M, Sun J, Xiang Z. Induction of M2‐type macrophage differentiation for bone defect repair via an interpenetration network hydrogel with a GO‐based controlled release system. Adv Healthc Mater. 2021;10(6) doi: 10.1002/adhm.202001502. [DOI] [PubMed] [Google Scholar]
- 117. Zou M, Sun J, Xiang Z. Early effect of graphene oxide-carboxymethyl chitosan hydrogel loaded with interleukin 4 and bone morphogenetic protein 2 on bone immunity and repair. Zhong Xiu Fu Chong Jian Wai Ke Za Zhi. 2020;34(8):1044–1051. doi: 10.7507/1002-1892.201911068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang Y, Wang C, Fu L, Ye S, Wang M, Zhou Y. Fabrication and application of novel porous scaffold in situ-loaded graphene oxide and osteogenic peptide by cryogenic 3D printing for repairing critical-sized bone defect. Molecules. 2019;24(9):1669. doi: 10.3390/molecules24091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ren X, Liu Q, Zheng S, et al. Synergistic delivery of bFGF and BMP-2 from poly(l-lactic-co-glycolic acid)/graphene oxide/hydroxyapatite nanofibre scaffolds for bone tissue engineering applications. RSC Adv. 2018;8(56):31911–31923. doi: 10.1039/C8RA05250F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang W, Liu Y, Yang C, et al. Delivery of salvianolic acid B for efficient osteogenesis and angiogenesis from silk fibroin combined with graphene oxide. ACS Biomater Sci Eng. 2020;6(6):3539–3549. doi: 10.1021/acsbiomaterials.0c00558. [DOI] [PubMed] [Google Scholar]
- 121. Chen Y, Zheng Z, Zhou R, et al. Developing a strontium-releasing graphene oxide-/collagen-based organic–inorganic nanobiocomposite for large bone defect regeneration via MAPK signaling pathway. ACS Appl Mater Interfaces. 2019;11(17):15986–15997. doi: 10.1021/acsami.8b22606. [DOI] [PubMed] [Google Scholar]
- 122. McPherson EJ, Stavrakis AI, Chowdhry M, Curtin NL, Dipane MV, Crawford BM. Biphasic bone graft substitute in revision total hip arthroplasty with significant acetabular bone defects: a retrospective analysis. Bone Jt Open. 2022;3(12):991–997. doi: 10.1302/2633-1462.312.BJO-2022-0094.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tsang S-TJ, Ferreira N, Simpson AHRW. The reconstruction of critical bone loss: the holy grail of orthopaedics. Bone Joint Res. 2022;11(6):409–412. doi: 10.1302/2046-3758.116.BJR-2022-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sadek AA, Abd-Elkareem M, Abdelhamid HN, Moustafa S, Hussein K. Repair of critical-sized bone defects in rabbit femurs using graphitic carbon nitride (g-C3N4) and graphene oxide (GO) nanomaterials. Sci Rep. 2023;13(1):5404. doi: 10.1038/s41598-023-32487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rashkow JT, Talukdar Y, Lalwani G, Sitharaman B. In Vivo Hard and soft tissue response of two-dimensional nanoparticle incorporated biodegradable polymeric scaffolds. ACS Biomater Sci Eng. 2017;3(10):2533–2541. doi: 10.1021/acsbiomaterials.7b00425. [DOI] [PubMed] [Google Scholar]
- 126. Feng W, Wang Z. Biomedical applications of chitosan-graphene oxide nanocomposites. i Sci. 2022;25(1):103629. doi: 10.1016/j.isci.2021.103629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Li Y, He J, Zhou J, et al. A conductive photothermal non-swelling nanocomposite hydrogel patch accelerating bone defect repair. Biomater Sci. 2022;10(5):1326–1341. doi: 10.1039/D1BM01937F. [DOI] [PubMed] [Google Scholar]
- 128. Zhou K, Yu P, Shi X, et al. Hierarchically porous hydroxyapatite hybrid scaffold incorporated with reduced graphene oxide for rapid bone ingrowth and repair. ACS Nano. 2019;13(8):9595–9606. doi: 10.1021/acsnano.9b04723. [DOI] [PubMed] [Google Scholar]
- 129. Zhou T, Li G, Lin S, et al. Electrospun Poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/Graphene Oxide Scaffold: enhanced properties and promoted in vivo bone repair in rats. ACS Appl Mater Interfaces. 2017;9(49):42589–42600. doi: 10.1021/acsami.7b14267. [DOI] [PubMed] [Google Scholar]
- 130. Hermenean A, Codreanu A, Herman H, et al. Chitosan-Graphene Oxide 3D scaffolds as promising tools for bone regeneration in critical-size mouse calvarial defects. Sci Rep. 2017;7(1):16641. doi: 10.1038/s41598-017-16599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Saravanan S, Chawla A, Vairamani M, Sastry TP, Subramanian KS, Selvamurugan N. Scaffolds containing chitosan, gelatin and graphene oxide for bone tissue regeneration in vitro and in vivo. Int J Biol Macromol. 2017;104(Pt B):1975–1985. doi: 10.1016/j.ijbiomac.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 132. Huang Z, Xu J, Chen J, et al. Photoacoustic stimulation promotes the osteogenic differentiation of bone mesenchymal stem cells to enhance the repair of bone defect. Sci Rep. 2017;7(1):15842. doi: 10.1038/s41598-017-15879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tan B, Tang Q, Zhong Y, et al. Biomaterial-based strategies for maxillofacial tumour therapy and bone defect regeneration. Int J Oral Sci. 2021;13(1):9. doi: 10.1038/s41368-021-00113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Itoo AM, Vemula SL, Gupta MT, et al. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J Control Release. 2022;350:26–59. doi: 10.1016/j.jconrel.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 135. Ge Y-W, Liu X-L, Yu D-G, et al. Graphene-modified CePO4 nanorods effectively treat breast cancer-induced bone metastases and regulate macrophage polarization to improve osteo-inductive ability. J Nanobiotechnology. 2021;19(1):11. doi: 10.1186/s12951-020-00753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yan F, Liu Z, Zhang T, et al. Biphasic injectable bone cement with Fe3O4/GO Nanocomposites for the minimally invasive treatment of tumor-induced bone destruction. ACS Biomater Sci Eng. 2019;5(11):5833–5843. doi: 10.1021/acsbiomaterials.9b00472. [DOI] [PubMed] [Google Scholar]
- 137. Zhang Y, Zhai D, Xu M, Yao Q, Chang J, Wu C. 3D-printed bioceramic scaffolds with a Fe3O4/graphene oxide nanocomposite interface for hyperthermia therapy of bone tumor cells. J Mater Chem B. 2016;4(17):2874–2886. doi: 10.1039/C6TB00390G. [DOI] [PubMed] [Google Scholar]
- 138. Xu C, Ma B, Peng J, et al. Tricalcium silicate/graphene oxide bone cement with photothermal properties for tumor ablation. J Mater Chem B. 2019;7(17):2808–2818. doi: 10.1039/C9TB00246D. [DOI] [PubMed] [Google Scholar]
- 139. Li D, Nie W, Chen L, et al. Self-assembled hydroxyapatite-graphene scaffold for photothermal cancer therapy and bone regeneration. J Biomed Nanotechnol. 2018;14(12):2003–2017. doi: 10.1166/jbn.2018.2646. [DOI] [PubMed] [Google Scholar]
- 140. Caplin JD, García AJ. Implantable antimicrobial biomaterials for local drug delivery in bone infection models. Acta Biomater. 2019;93(2–11):2–11. doi: 10.1016/j.actbio.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Behzadi S, Luther GA, Harris MB, Farokhzad OC, Mahmoudi M. Nanomedicine for safe healing of bone trauma: opportunities and challenges. Biomaterials. 2017;146:168–182. doi: 10.1016/j.biomaterials.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yang Y, Li M, Luo H, Zhang D. Surface-decorated graphene oxide sheets with copper nanoderivatives for bone regeneration: an in vitro and in vivo study regarding molecular mechanisms, osteogenesis, and anti-infection potential. ACS Infect Dis. 2022;8(3):499–515. doi: 10.1021/acsinfecdis.1c00496. [DOI] [PubMed] [Google Scholar]
- 143. Fu C, Jiang Y, Yang X, Wang Y, Ji W, Jia G. Mussel-inspired gold nanoparticle and PLGA/L-Lysine-g-graphene oxide composite scaffolds for bone defect repair. Int J Nanomedicine. 2021;16:6693–6718. doi: 10.2147/IJN.S328390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Weng W, Li X, Nie W, et al. One-step preparation of an AgNP-nHA@RGO three-dimensional porous scaffold and its application in infected bone defect treatment. Int J Nanomedicine. 2020;15(5027–42):5027–5042. doi: 10.2147/IJN.S241859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhang Y, Zhai D, Xu M, et al. 3D-printed bioceramic scaffolds with antibacterial and osteogenic activity. Biofabrication. 2017;9(2):025037. doi: 10.1088/1758-5090/aa6ed6. [DOI] [PubMed] [Google Scholar]
- 146. Doughty ACV, Hoover AR, Layton E, Murray CK, Howard EW, Chen WR. Nanomaterial applications in photothermal therapy for cancer. Materials (Basel) 2019;12(5):779. doi: 10.3390/ma12050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Li B, Shu R, Dai W, et al. Bioheterojunction‐engineered polyetheretherketone implants with diabetic infectious micromilieu twin‐engine powered disinfection for boosted osteogenicity. Small. 2022;18(45) doi: 10.1002/smll.202203619. [DOI] [PubMed] [Google Scholar]
- 148. Wang S, Duan C, Yang W, et al. Two-dimensional nanocoating-enabled orthopedic implants for bimodal therapeutic applications. Nanoscale. 2020;12(22):11936–11946. doi: 10.1039/D0NR02327B. [DOI] [PubMed] [Google Scholar]
- 149. Yang Y, Zan J, Shuai Y, et al. In situ growth of a metal–organic framework on graphene oxide for the chemo-photothermal therapy of bacterial infection in bone repair. ACS Appl Mater Interfaces. 2022;14(19):21996–22005. doi: 10.1021/acsami.2c04841. [DOI] [PubMed] [Google Scholar]
- 150. Wu S, Weir MD, Lei L, Liu J, Xu HHK. Novel nanographene oxide-calcium phosphate cement inhibits Enterococcus faecalis biofilm and supports dental pulp stem cells. J Orthop Surg Res. 2021;16(1):580. doi: 10.1186/s13018-021-02736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 152. Chen C, Zhang W, Wu H, Chen C, Ma B. Study on the damage resistance and deterioration behavior of GO concrete under the harsh environment. PLoS One. 2023;18(4):e0284186. doi: 10.1371/journal.pone.0284186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Xu G, Zhao Y, Geng Y, et al. Nano-hybrid gradient scaffold for articular repair. Colloids Surf B Biointerfaces. 2021;208:112116. doi: 10.1016/j.colsurfb.2021.112116. [DOI] [PubMed] [Google Scholar]
- 154. Gong M, Sun J, Liu G, Li L, Wu S, Xiang Z. Graphene oxide-modified 3D acellular cartilage extracellular matrix scaffold for cartilage regeneration. Mater Sci Eng C Mater Biol Appl. 2021;119:111603. doi: 10.1016/j.msec.2020.111603. [DOI] [PubMed] [Google Scholar]
- 155. Satapathy MK, Manga YB, Ostrikov KK, et al. Microplasma cross-linked graphene oxide-gelatin hydrogel for cartilage reconstructive surgery. ACS Appl Mater Interfaces. 2020;12(1):86–95. doi: 10.1021/acsami.9b14073. [DOI] [PubMed] [Google Scholar]
- 156. Xu H, Qiu Y, Xiong Z, Shao W, Zhang Q, Tang G. Tracking mesenchymal stem cells with Ir(III) complex-encapsulated nanospheres in cranium defect with postmenopausal osteoporosis. Mat Sci Eng C. 2021;122:111842. doi: 10.1016/j.msec.2020.111842. [DOI] [PubMed] [Google Scholar]
- 157. Oh W-T, Yang Y-S, Xie J, et al. WNT-modulating gene silencers as a gene therapy for osteoporosis, bone fracture, and critical-sized bone defects. Mol Ther. 2023;31(2):435–453. doi: 10.1016/j.ymthe.2022.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Dong W, Postlethwaite BC, Wheller PA, et al. Beta-caryophyllene prevents the defects in trabecular bone caused by Vitamin D deficiency through pathways instated by increased expression of klotho. Bone Joint Res. 2022;11(8):528–540. doi: 10.1302/2046-3758.118.BJR-2021-0392.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ensrud KE, Crandall CJ. Osteoporosis. Ann Intern Med. 2017;167(3):ITC17–ITC32. doi: 10.7326/AITC201708010. [DOI] [PubMed] [Google Scholar]
- 160. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115(12):3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mai X, Kang Z, Wang N, Qin X, Xie W, Song F. Oxygen plasma technology-assisted preparation of three-dimensional reduced graphene oxide/polypyrrole/strontium composite scaffold for repair of bone defects caused by osteoporosis. Molecules. 2021;26(15):4451. doi: 10.3390/molecules26154451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Arbo MD, Altknecht LF, Cattani S, et al. In vitro cardiotoxicity evaluation of graphene oxide. Mutat Res Genet Toxicol Environ Mutagen. 2019;841:8–13. doi: 10.1016/j.mrgentox.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 163. Hashemi E, Akhavan O, Shamsara M, et al. Graphene oxide negatively regulates cell cycle in embryonic fibroblast cells. Int J Nanomedicine. 2020;15:6201–6209. doi: 10.2147/IJN.S260228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Grilli F, Hajimohammadi Gohari P, Zou S. Characteristics of graphene oxide for gene transfection and controlled release in breast cancer cells. Int J Mol Sci. 2022;23(12):6802. doi: 10.3390/ijms23126802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Audira G, Lee J-S, Siregar P, et al. Comparison of the chronic toxicities of graphene and graphene oxide toward adult zebrafish by using biochemical and phenomic approaches. Environ Pollut. 2021;278:116907. doi: 10.1016/j.envpol.2021.116907. [DOI] [PubMed] [Google Scholar]
- 166. Aguado-Henche S, Escudero ML, García-Alonso MC, Lozano-Puerto RM, Clemente de Arriba C. Biological responses in the blood and organs of rats to intraperitoneal inoculation of graphene and graphene oxide. Mat Basel. 2022;15(8):2898. doi: 10.3390/ma15082898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Qu G, Wang X, Liu Q, et al. The ex vivo and in vivo biological performances of graphene oxide and the impact of surfactant on graphene oxide’s biocompatibility. J Environ Sci (China) 2013;25(5):873–881. doi: 10.1016/S1001-0742(12)60252-6. [DOI] [PubMed] [Google Scholar]
- 168. Makvandi P, Ghomi M, Ashrafizadeh M, et al. A review on advances in graphene-derivative/polysaccharide bionanocomposites: therapeutics, pharmacogenomics and toxicity. Carbohydr Polym. 2020;250:116952. doi: 10.1016/j.carbpol.2020.116952. [DOI] [PubMed] [Google Scholar]
- 169. Zhu J, Li B, Xu M, et al. Graphene oxide promotes cancer metastasis through associating with plasma membrane to promote TGF-β Signaling-dependent epithelial-mesenchymal transition. ACS Nano. 2020;14(1):818–827. doi: 10.1021/acsnano.9b07891. [DOI] [PubMed] [Google Scholar]
- 170. Orecchioni M, Cabizza R, Bianco A, Delogu LG. Graphene as cancer theranostic tool: progress and future challenges. Theranostics. 2015;5(7):710–723. doi: 10.7150/thno.11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized data used and/or analyzed during the current study are available from the corresponding author on reasonable request.