Abstract
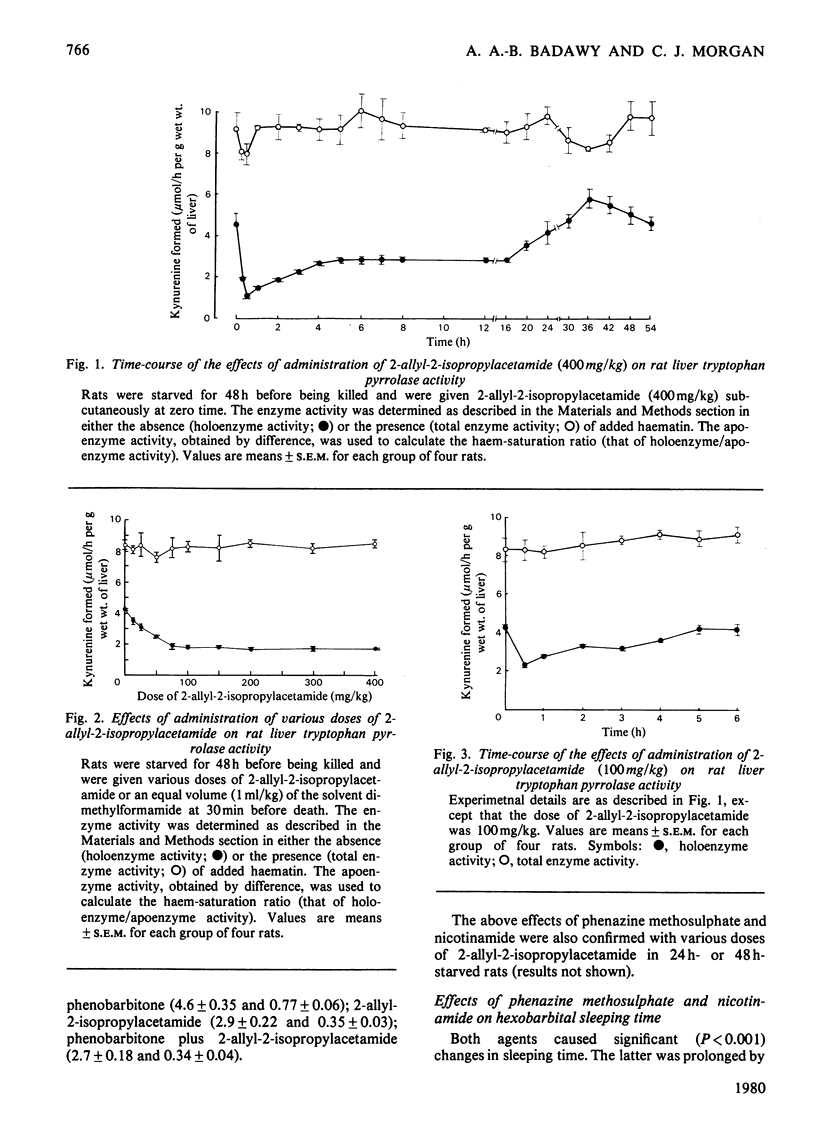
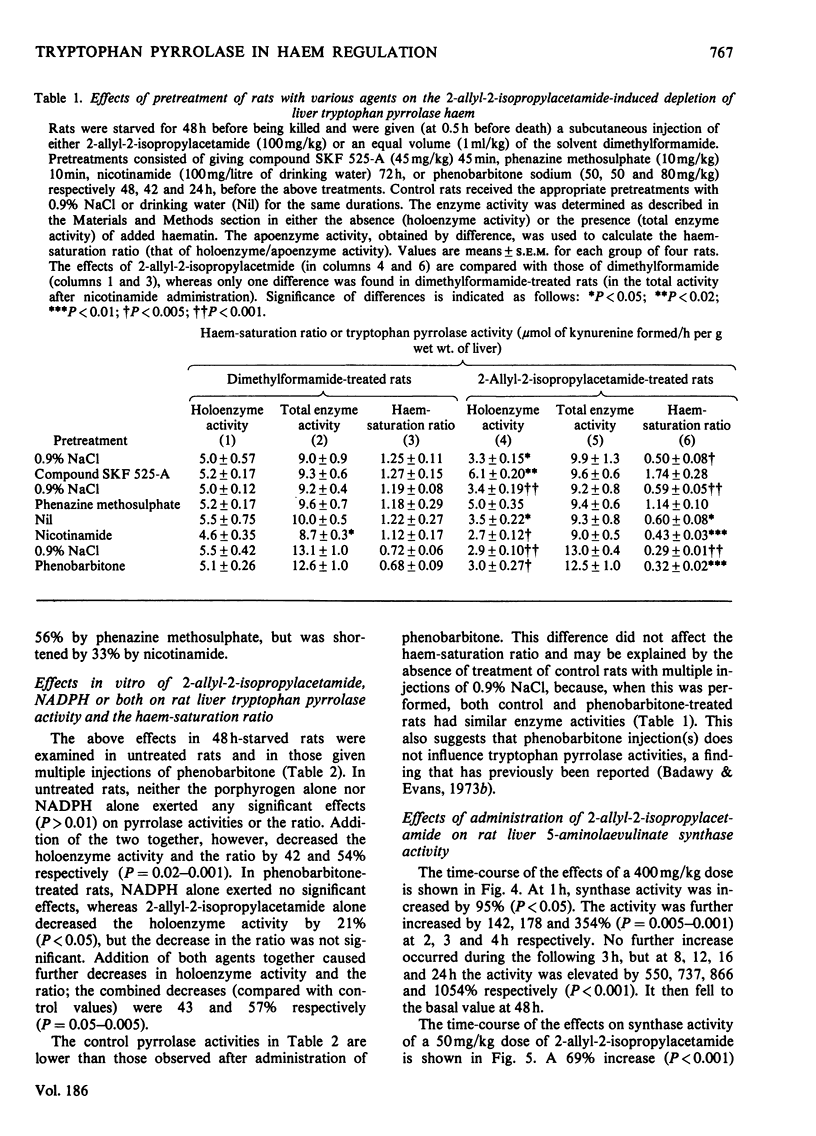
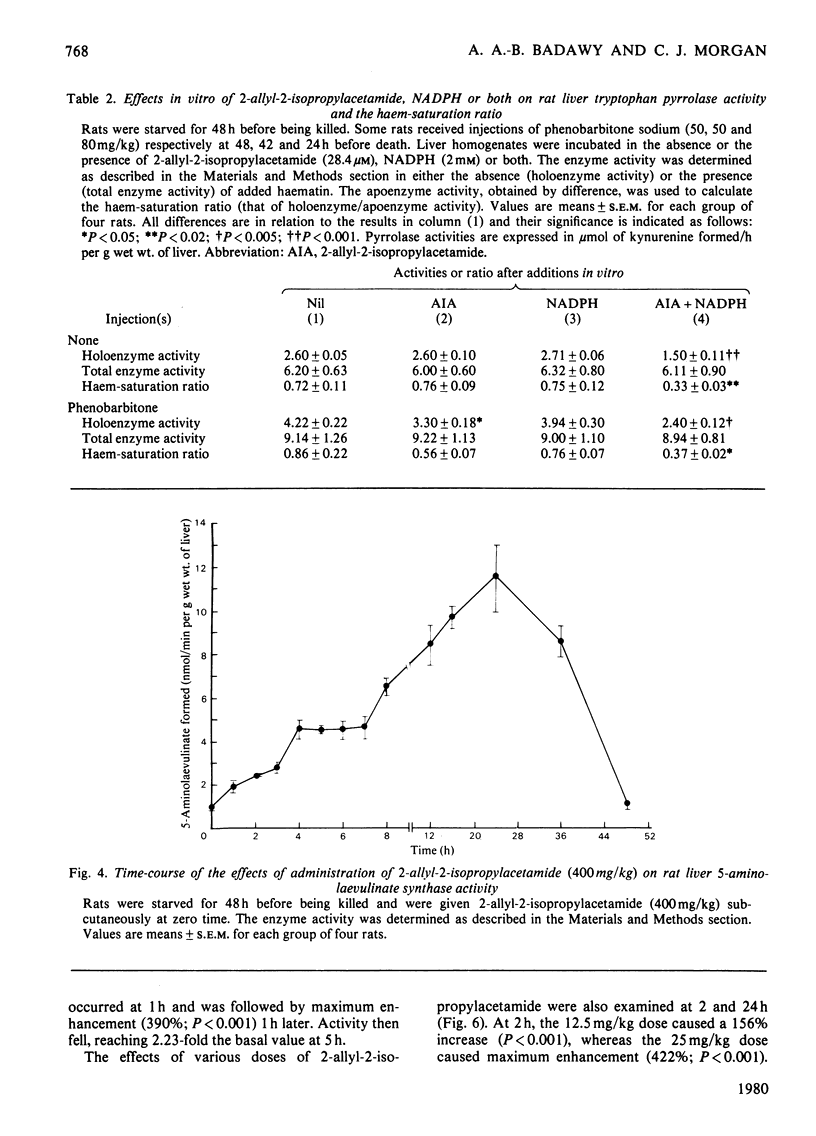
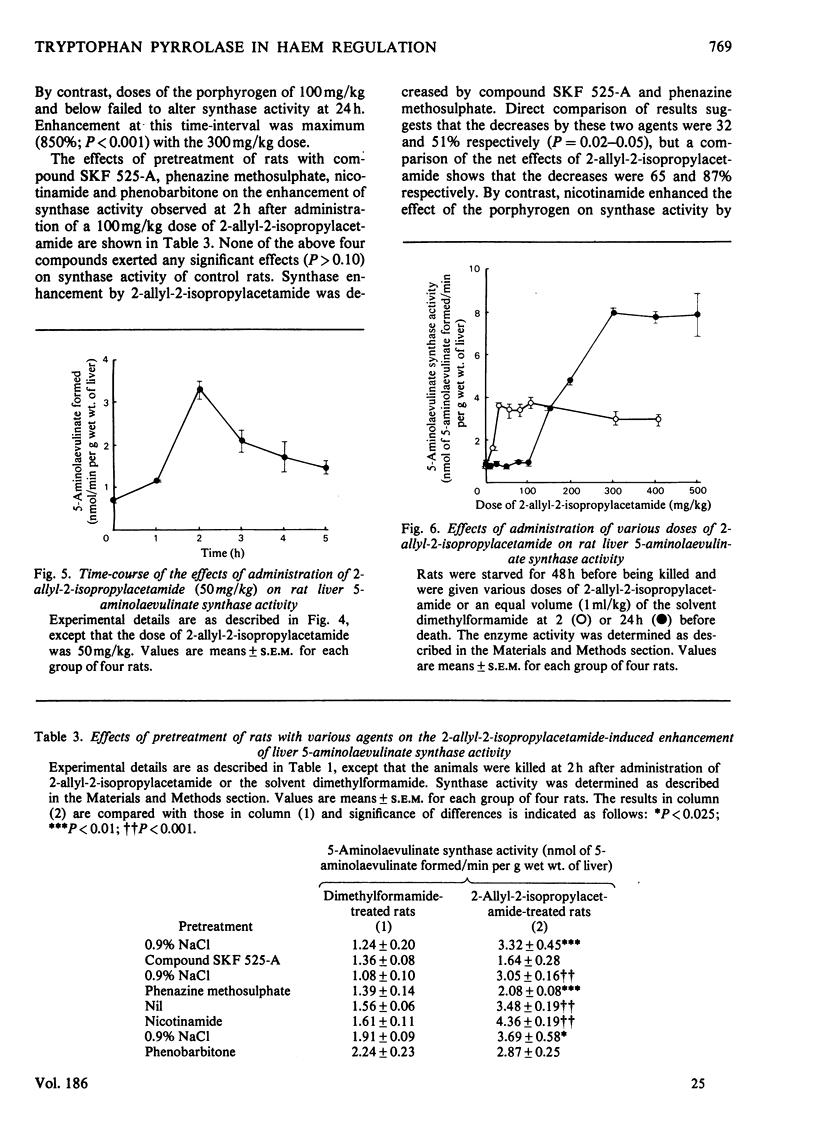
Rat liver tryptophan pyrrolase haem is maximally depleted at 30 min after administration of a 400 mg/kg dose of 2-allyl-2-isopropylacetamide. This depletion lasts for 24 h, by which time 5-aminoleevulinate synthase activity becomes maximally enhanced. 2. though the above maximum depletion of pyrrolase haem (at 0.5h) is also produced by a 100 mg/kg dose of the porphyrogen, this does not enhance synthase activity at 24 h. It and smaller doses, however, cause a smaller but earlier enhancement of synthase activity (maximum at 2 h) and produce a similarly short-lived deplation of pyrrolase haem. 3. The depletion of pyrrolase haem and the enhancement of synthase activity by the porphyrogen are inhibited by compound SKF 525-A and phenazine methosulphate, and are potentiated by nicotinamide but not by phenobarbitone. Phenazine methosulphate and nicotinamide also exert opposite effects on hexobarbital sleeping-time. 4. 2-Allyl-2-isopropylacetamde also the depletes pyrrolase haem in vitro. It does so in liver homogenates of control rats in the presence, and in those of phenobarbitone-treated rats in the absence of added NADPH. 5. A discussion of the present results in relation to previous work with other haemoproteins suggests that, whereas cytochrome P-450 (haem) is primarily involved in the production of the active (porphyrogenic) metabolite(s) of 2-allyl-2-isopropylacetamide, the haem pool used by tryptophan pyrrolase may play an important role in the effects of this compound on haem biosynthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badawy A. A. Central role of tryptophan pyrrolase in haem metabolism. Biochem Soc Trans. 1979 Jun;7(3):575–583. doi: 10.1042/bst0070575. [DOI] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. Regulation of rat liver tryptophan pyrrolase by its cofactor haem: Experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem J. 1975 Sep;150(3):511–520. doi: 10.1042/bj1500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The effects of chemical porphyrogens and drugs on the activity of rat liver tryptophan pyrrolase. Biochem J. 1973 Dec;136(4):885–892. doi: 10.1042/bj1360885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The effects of chronic phenobarbitone administration and subsequent withdrawal on the activity of rat liver tryptohan pyrrolase and their resemblance to those of ethanol. Biochem J. 1973 Nov;135(3):555–557. doi: 10.1042/bj1350555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The effects of ethanol on tryptophan pyrrolase activity and their comparison with those of phenobarbitone and morphine. Adv Exp Med Biol. 1975;59:229–251. doi: 10.1007/978-1-4757-0632-1_15. [DOI] [PubMed] [Google Scholar]
- Badawy A. A., Evans M. The regulation of rat liver tryptophan pyrrolase activity by reduced nicotinamide-adenine dinucleotide (phosphate). Experiments with glucose and nicotinamide. Biochem J. 1976 May 15;156(2):381–390. doi: 10.1042/bj1560381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A. The functions and regulation of tryptophan pyrrolase. Life Sci. 1977 Sep 15;21(6):755–768. doi: 10.1016/0024-3205(77)90402-7. [DOI] [PubMed] [Google Scholar]
- Badawy A. A. Tryptophan pyrrolase, the regulatory free haem and hepatic porphyrias. Early depletion of haem by clinical and experimental exacerbators of porphyria. Biochem J. 1978 Jun 15;172(3):487–494. doi: 10.1042/bj1720487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock K. W., Weiner R., Fröhling W. Regulation of delta-aminolevulinic acid synthetase by drugs and steroids in isolated perfused rat liver. Enzyme. 1973;16(1):295–301. doi: 10.1159/000459393. [DOI] [PubMed] [Google Scholar]
- Bond E. J., De Matteis F. Biochemical changes in rat liver after administration of carbon disulphide, with particular reference to microsomal changes. Biochem Pharmacol. 1969 Oct;18(10):2531–2549. doi: 10.1016/0006-2952(69)90368-2. [DOI] [PubMed] [Google Scholar]
- Creighton J. M., Marks G. S. Drug-induced porphyrin biosynthesis. VII. Species, sex, and developmental differences in the generation of experimental porphyria. Can J Physiol Pharmacol. 1972 Jun;50(6):485–489. doi: 10.1139/y72-074. [DOI] [PubMed] [Google Scholar]
- De Matteis F. Drug interactions in experimental hepatic porphyria. A model for the exacerbation by drugs of human variegate porphyria. Enzyme. 1973;16(1):266–275. doi: 10.1159/000459390. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. Stimulation of liver 5-aminolaevulinate synthetase by drugs and its relevance to drug-induced accumulation of cytochrome P-450. Studies with phenylbutazone and 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Biochem J. 1972 Mar;126(5):1149–1160. doi: 10.1042/bj1261149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. The effect of drugs on 5-aminolaevulinate synthetase and other enzymes in the pathway of liver haem biosynthesis. Basic Life Sci. 1975;6:185–205. doi: 10.1007/978-1-4615-8954-9_7. [DOI] [PubMed] [Google Scholar]
- Dhar G. J., Bossenmaier I., Petryka Z. J., Cardinal R., Watson C. J. Effects of hematin in hepatic porphyria. Further studies. Ann Intern Med. 1975 Jul;83(1):20–30. doi: 10.7326/0003-4819-83-1-20. [DOI] [PubMed] [Google Scholar]
- FOUTS J. R., BRODIE B. B. Inhibition of drug metabolic pathways by the potentiating agent, 2, 4-dichloro-6-phenyl-phenoxyethyl diethylamine. J Pharmacol Exp Ther. 1955 Sep;115(1):68–73. [PubMed] [Google Scholar]
- Granick S., Sinclair P., Sassa S., Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975 Dec 25;250(24):9215–9225. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]
- Marver H. S., Tschudy D. P., Perlroth M. G., Collins A. Coordinate synthesis of heme and apoenzyme in the formation of tryptophan pyrrolase. Science. 1966 Oct 28;154(3748):501–503. doi: 10.1126/science.154.3748.501. [DOI] [PubMed] [Google Scholar]
- Rao M. R., Malathi K., Padmanaban G. The relationship between delta-aminolaevulinate synthetase induction and the concentration of cytochrome P-450 and catalase in rat liver. Biochem J. 1972 Apr;127(3):553–559. doi: 10.1042/bj1270553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unseld A., de Matteis F. Destruction of endogenous and exogenous haem by 2-allyl-2-isopropylacetamide: role of the liver cytochrome P-450 which is inducible by phenobarbitone. Int J Biochem. 1978;9(12):865–869. doi: 10.1016/0020-711x(78)90061-7. [DOI] [PubMed] [Google Scholar]
- White I. N., Muller-Eberhard U. Decreased liver cytochrome P-450 in rats caused by norethindrone or ethynyloestradiol. Biochem J. 1977 Jul 15;166(1):57–64. doi: 10.1042/bj1660057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Matteis F. Disturbances of liver porphyrin metabolism caused by drugs. Pharmacol Rev. 1967 Dec;19(4):523–557. [PubMed] [Google Scholar]


